Abstract
Assays for embryonic stem cells (ESCs) of the blastocyst are needed to quantify stress-induced decreases of potent subpopulations. High-throughput screens (HTSs) of stressed ESCs quantify embryonic stress, diminishing laboratory animal needs. Normal or stress-induced ESC differentiation is marked by Rex1 potency factor loss. Potency reporter ESC assays were developed, using low-stress techniques to create transgenic ESCs. Rex1 and Oct4 promoters drove RFP and green fluorescent protein (GFP) expression, respectively. Lentivirus infection and fluorescence-activated cell sorting selection of ESCs obviated the need for stressful electroporation and antibiotic selection, respectively. We showed using immunoblots, microscopic analysis, flow cytometry, and fluorescence microplate reader that the response to stress of potency-reporter ESCs is similar to parental ESCs assayed by biochemical means. Stress caused a dose-dependent decrease in bright Rex1-RFP+ ESCs and increase in Rex1 dim ESCs. At highest stress, ∼20% of bright Rex1-RFP cells are lost coinciding with a 2.8-fold increase in Rex1-RFP dim cells that approach 20%. This conversion of bright to dim cells tested by flow cytometry is commensurate with about 60% loss in fluorescence measured by microplate reader. Dose-dependent stress-induced Rex1-RFP and endogenous Rex1 protein decreases are similar. The data show that Rex1 reporter ESCs accurately report stress in a microplate reader-based HTS. The increasing dim Rex1 subpopulation size is balanced by the decreasing total ESC number during culture at multiple sorbitol doses. This is consistent with previous observations that stress forces potency decrease and differentiation increase to compensate for stress-induced diminished stem cell population growth.
Introduction
Stress and common responses of stem cells of the implanting embryo
The embryo is exposed to stress both in vivo and in vitro. This decreases embryonic developmental rates and stem cell growth rates and causes potency loss affecting fetal growth, function, and viability [1,2]. Embryonic stem cells (ESCs) respond to hyperosmotic stress with a transient loss of Oct4 and long-term-loss of Rex1 proteins in a proteasome-dependent manner [3]. This correlates with stress-induced first cell lineage differentiation and suppression of later lineages. Early embryos at the two-cell and blastocyst stage also undergo stress-induced potency loss as do placental trophoblast stem cells (TSCs) derived from the blastocyst [4,5]. In all these circumstances, ESCs, TSCs, and embryos were cultured under potency-maintaining conditions and therefore stress breaks potency.
Oct4 and Rex1
Oct4 is a DNA-binding transcription factor that mediates stemness in gametes and early embryos and in pluripotent cells through the start of gastrulation, [6] Oct4 null lethality occurs at the blastocyst stage when inner cell mass (ICM) loses pluripotency and fails to synthesize fibroblast growth factor (FGF)4 [7] that maintains adjacent polar trophectoderm [8]. Oct4 is also transiently required for extraembryonic endoderm development from the ICM [9,10]. Oct4 promoter methylation is diminished in oocytes undergoing in vitro maturation [11] and Oct4 expression is decreased in embryos derived from smoke-exposed mouse females [12]. This suggests that environmental stimuli can change potency state and cause potency loss.
Oct1 and Oct4 have been used to test for toxic stress in ESCs [13] and both transcription factors have stress domains that prepare stem cells for stress and are phosphorylated and regulate the stress response [14]. Rex1 is also a transcriptional factor that is lost from ICM as stem cells there differentiate to either extraembryonic primitive endoderm or to embryonic primitive ectoderm [15]. The Rex1 null is not lethal at the blastocyst stage and not required to initiate and maintain pluripotency of ESCs [16]. We previously showed that Oct4, Sox2, and Nanog transcription factor protein underwent stress-induced transient loss at 4 h, returning to baseline by 24 h.
However, Rex1 protein loss due to stress is not transient, but terminal [3]. Together these data suggest that Rex1-driven fluorescence reporters should be more useful than Oct4-driven reporters to create mESCs which enable high-throughput screens (HTSs) for toxicants or other stressful stimuli, which could negatively affect embryos by impacting stem cell potency and differentiation.
However stress-induced loss of potency is not the current dogma and there are other reports showing variability in the change in potency with stress [17]. One deficiency is that no other studies have used Rex1 to assay stress responses. This is important since Oct4, Nanog, and Sox2 rebound after transient loss at 4 h of stress, but only Rex1 stays low from 1 to 3 days of stress [3]. Most stresses are studied after several days of ESC exposure [17–19] and not in the early hours where transient potency factor loss is observed [3,20]. Although one report showed transient changes in expression of proliferation and checkpoint genes that would transiently slow growth [20] in agreement with our study [3], this report did not assay transient loss of potency factors [20].
Many ESC toxicological stress studies remove leukemia inhibitory factor (LIF) to start potency loss and then add stress to test affects on later lineages such as cardiomyocytes, after they have begun differentiation [17,19]. Since these studies did not observe early effects of stress on potency loss, they did not test for effects of stress with LIF present on later differentiation events. However, stress affects differentiation of ESCs in monolayer at 1 day [3], and these effects continue and amplify during 7 days of embryoid body culture after stress in monolayer (unpublished data). Development of Rex1-RFP and Oct4-green fluorescent protein (GFP) ESC reporters should further define the continuing effects of stress-induced potency loss in subpopulations of mouse ESCs.
To produce transgenic reporter mESCs without the stress of electroporation or cationic lipids, lentivirus infection of parental ESCs is used [21]. Instead of antibiotic selection, transgenic ESCs are passaged in a normal manner, but fluorescence-activated cell sorting (FACS) is used to select stem cells reporting pluripotency. This creates viable potency reporter ESC lines with normal growth rates and without cell loss during infection and selection. These mESCs also report potency factor activity loss using (1) visual microscopic inspection, (2) microplate readers, (3) FACS, and (4) immunoblots. The assays provide similar results as previously reported [3].
The analysis by flow cytometry also provides important new information on the size of the bright (potent) subpopulation that decreases with stress and the near-zero potency dim subpopulation that increases. The microplate reader approach provides a high-throughput platform for the analysis of toxicants and the underlying mechanisms that may induce potency loss and enable stress-forced differentiation.
Materials and Methods
Materials
Germline competent mESC-D3 cells were purchased from ATCC (Manassas, VA). DMEM medium was obtained from HyClone (Logan, UT). mESC-D3 ESCs were tested for and confirmed for ability to make all three germ layers in embryoid body differentiation culture (unpublished data). Gibco™ (Grand Island, NY) glutamine and sodium pyruvate supplement solutions were from Life Technologies (Grand Island, NY). ESC-qualified EmbryoMax fetal bovine serum, 0.1% gelatin solution and ESGRO™ Mouse LIF medium supplement were from EMD Millipore (Billerica, MA).
Oct4 promoter reporter lentiviral particles that express the green fluorescent protein copGFP and TranDux™ transduction reagent were from System Biosciences (Mountain View, CA). The Oct4-GFP reporter construct uses the mouse 2.3-kb minimal response promoter for ESC and mouse blastocyst ICM defined previously [22]. Rex1 promoter reporter lentiviral particles that express the red fluorescent protein mApple were from Allele Biotechnology (San Diego, CA).
The Rex1-RFP reporter construct uses the mouse 0.7-kb minimal response promoter for F9 teratocarcinoma [23], used in human ESC to drive GFP [24], and modified to replace GFP with mApple red reporter and packaged in lentivirus in these studies [25]. Rabbit anti-Rex1 antibody was from Abcam (Cambridge, MA). Rabbit anti-β-actin antibody and HRP-conjugated second antibodies were from Cell Signaling Technology (Danvers, MA). Pierce RIPA lysis buffer, protease inhibitor cocktail, and BCA protein assay reagents were from Thermo Scientific (Rockford, IL). Enhanced chemiluminescence (ECL) chemiluminescence reagent was from GE Healthcare Bio-Sciences (Pittsburgh, PA). MEM nonessential amino acid solution, sorbitol, 2-mercaptoethanlol, and other chemicals were from Sigma (St. Louis, MO).
Low stress effects on single- and double-viable potency activity reporter mouse ESCs
mESCs were cultured in 24-well plates precoated with 0.1% gelatin. The starting confluence of cells was ∼20% or 100,000 cells per well. The cells were incubated in 37°C for 2–3 h for attachment and readiness for virus infection. A virus infection medium was made by mixing Oct4 and/or Rex1 promoter reporter lentiviral particles into a regular growth medium supplemented with TransDux. The final concentration of the viral particles was 106/mL. The infection medium was applied to mESCs at 400 μL per well.
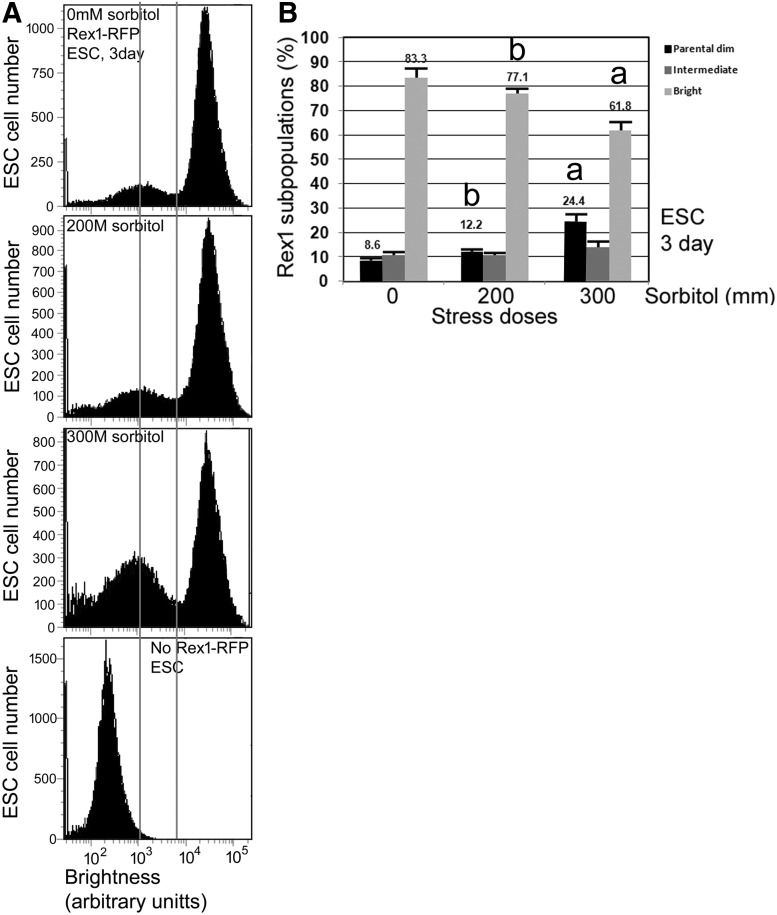
After infection, the cells were cultured for 3 days to allow the expression of the fluorescent reporter proteins. After expansion of the total cell number by passaging, the infected cells were subjected to FACS sorting using a BD FACS Vantage SE cell sorter (BD Biosciences, San Jose, CA) at the Wayne State University Microscopy, Imaging, and Cytometry Resources (MICR) core facility. Pure, fluorescent cells were obtained after two FACS repeats. However, as indicated in Fig. 4 and as previously reported by Toyooka et al. [15], heterogeneity reestablishes after sorting and culture and normally a small population becomes Rex1 dim.
FIG. 4.
Rex1 promoter RFP reporter ESCs respond to stress treatment with a dose-dependent 2.8-fold increase in the low Rex1 activity subpopulation and a 20% decrease in high activity Rex1 subpopulation. Rex1-RFP mESCs expressing Rex1 promoter reporter were cultured in 12-well plates until 30% confluent, treated with 0, 200, and 300 mM sorbitol for 3 days, trypsinized, and cell suspensions were assayed by flow cytometry. Arbitrary thresholds (vertical lines) were drawn at 1 × 103 on the X-axis and between the middle of the two peaks and at two standard deviations (approximately 5 × 103 on the X-axis) above the mean of the parental ESC dim population shown in the bottom panel. Areas in parental dim, intermediate dim, and bright were defined by the two vertical lines and quantified as pixels using Image J from data in (A) to give histograms in (B). See detailed description of analyses in Materials and Methods section. The histogram bars (B) are derived from three independent experiments (Supplementary Fig. S2) with errors bars showing standard error of the mean (SEM), one-way ANOVA was performed followed by Dunnett's post hoc test; (a) indicates significantly smaller bright cell subpopulation and greater parental dim cells at 300 mM sorbitol (a) (P < 0.05), but no significant decrease in bright cell subpopulation or increase in parental dim cells at 200 mM sorbitol (b).
ESC culture and stimulation
Germline-competent mESC-D3 cells (ATCC [26]) were cultured in the absence of feeder cells in DMEM (Gibco) supplemented with 15% mESC-screened fetal bovine serum (HyClone), 2 mM l-glutamine, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol (Sigma), and 1,000 U/mL murine LIF (Millipore, Temecula, CA) on 0.1% gelatin-coated dishes at 37°C in humidified air with 5% CO2 [27]. mESCs were cultured overnight after passaging before stimulation with sorbitol. Osmolality of ESC media with and without added 200 mM sorbitol was determined previously [3].
The excitation and detection wavelengths for Rex1-mApple were 568 nm and 611 nm, respectively. The optimal day for measurement of Rex1-RFP was identified before the dose testing started. Although the specific half-life of mApple in mouse D3 ESC is not clear, the average half-life of wild-type fluorescent proteins in mouse cells has been reported to be ∼26 h [28]. Sorbitol at 200 mM caused Rex1-RFP fluorescent reduction measured by microplate reading from day 1 to 3 (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd).
The theoretical maximal decreased activity based on a 24-h half-life for RFP is shown in Supplementary Fig. S1B for comparison. Before testing, it was anticipated that 3 days would be required to obtain a sensitive assay as maximal loss at day 2 is 75%, and a 87.5% loss is possible at 3 days. The experimental data showed a large RFP loss by day 3, but an incremental loss each day, and the total loss by day 3 demonstrated that the loss was not maximal. Since day 2 had a higher standard deviation and less magnitude loss, and culture >3 days was postconfluent at “zero” stress and crowded (data not shown), day 3 was confirmed as the optimal day for dose testing.
The excitation and detection wavelengths for Oct4-copGFP were 485 nm and 528 nm, respectively. After live-cell reading, the cells were lysed with 50 μL of lysis buffer (1% NP-40, 0.5% TX-100, 100 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl, and pH 7.5) and measured for fluorescence with the same parameters above. After fluorescence determination, the cell lysates were further measured for total protein concentrations by adding 100 μL of Pierce BCA protein assay reagent (Thermo Scientific, Inc.) following the manufacturer's instructions. Finally, the fluorescence readings from either live cells or lysates were normalized to total protein concentration readings, in which higher readings represent higher pluripotency of the cells.
Flow cytometric analysis of mESCs expressing pluripotency reporters
Fluorescence determination by the plate reader reported an averaged biochemical result from a population of cells inside the tissue culture vessel. To get insight into the heterogenic nature of the cells that respond to the same stress condition, we used flow cytometry which provides quantification of cells that show different levels of pluripotency under stress. mESCs expressing potency reporter Oct4-CopGFP or Rex1-mApple were plated at a starting confluence of 10% and grown for 24 h until reaching ∼25% confluence. This time point was designated as time zero.
The cells were then treated with different sorbitol concentrations for 1–3 days. At each time point, cells were trypsinized and resuspended in ice-cold D-PBS and acquired using an BD LSR II flow cytometer (BD biosciences) and the FACSDiva Software version 6.0 (BD biosciences). Nonstressed mESCs that express Oct4-copGFP and/or Rex1-mApple and the nontransgenic parental mESCs were used to set up the flow cytometry parameters that define the fluorescent and nonfluorescent cells.
In the histogram of the flow cytometry figure, two peaks are seen (Fig. 4a and Supplementary Fig. S2). The right peak represents the bright fluorescent potent Rex1-RFP ESCs. The left peak represents the parental (near zero) dim and intermediate, dim fluorescent low-potency Rex1-RFP ESCs. The vertical line on the left was drawn at the ∼1 × 103 position on the X-axis to identify the near-zero low-potency transgenic ESCs that overlap in dimness with 97.73% of the parental nontransgenic ESCs (Fig. 4a, bottom curve). This vertical line was placed at two standard deviations above the mean of the parental ESC curve at far bottom, with the third standard deviation and greater (2.27% of peak area at far right) excluded. The vertical line on the right was placed closest to the low point between the bright and dim peaks for all six curves in Supplementary Fig. S2. Three areas, identified as parental dim, potent bright on far left and far right, and intermediate dim in the middle, were identified by the two vertical lines. The three areas were calculated using Image J 1.48v (NIH, Bethesda, MD).
Immunoblot
After treatment, the ESCs were rinsed with PBS, lysed with the RIPA buffer supplemented with protease inhibitors, sonicated briefly, total protein concentration was determined using Pierce BCA protein assay reagent admixed with Laemmli sample buffer, and boiled for 5 min. Equal total protein amounts from the lysates were size fractionated by 5%–20% gradient SDS-PAGE and transferred on a nitrocellulose membrane. Rex1 immunofluorescent signals were detected using ECL reagent with X-ray films and quantified using ImageJ 1.48v software (NIH).
Statistics
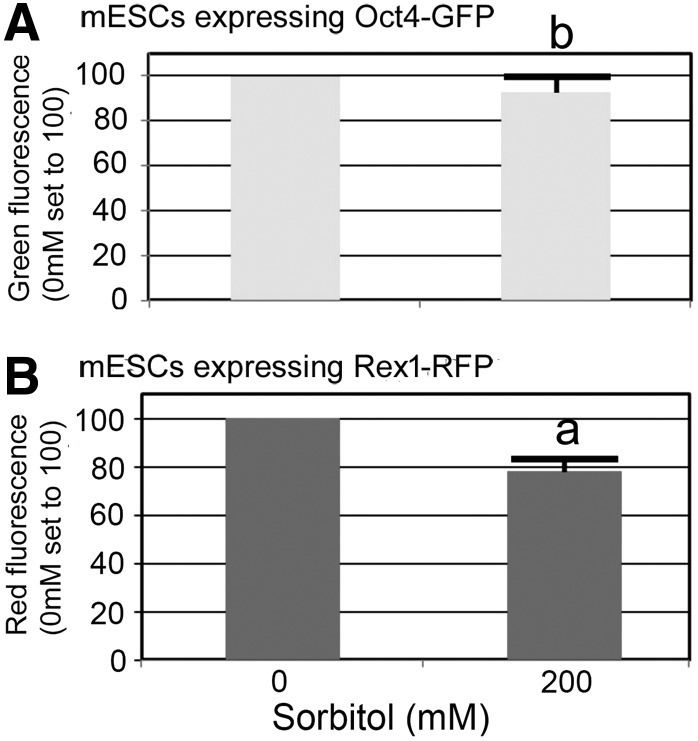
Data collected over at least three independent biological experiments were analyzed using SPSS Version 22.0 and presented as mean ± standard error of the mean. Data distributions met the assumptions of independent t-test and one-way ANOVA. Dunnett's post hoc test was performed following significant ANOVA test. For Fig. 2, Independent t-tests were performed to examine the difference between control and treatment for Oct and Rex. Significance was detected for Rex (P < 0.05), but not for Oct.
FIG. 2.
Stress induces loss of Rex1-RFP, but not Oct4-GFP after days of 200 mM sorbitol. Viable pluripotency reporter Rex1-RFP ESCs (A) or Oct4-GFP ESCs (B) were cultured in 94-well plates to 30% confluence. Cells were then treated with 200 mM of sorbitol as an osmotic stress for 3 days. Fluorescence was determined from live ESCs using a plate reader from 200 mM sorbitol-treated cells and was normalized with the nonsorbitol-treated control cells. Four independent experiments were carried out and plotted. By t-test, Oct4-GFP fluorescence in (A) was not significantly different (b) with stress P = 0.327 and in B Rex1-RFP fluorescence was significantly different with stress (a) P < 0.05.
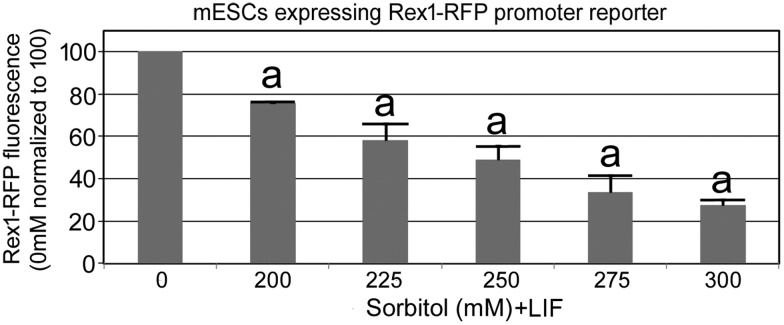
For Fig. 3, one-way ANOVA was performed followed by Dunnett's post hoc test using 0 mM as reference. All treatments were significantly different from the control. For Fig. 4 and Supplementary Fig. S2, one-way ANOVA was performed followed by Dunnett's post hoc test using 0 mM as reference for parental dim, intermediate dim, and bright separately. For Fig. 5a, b, one-way ANOVA was performed followed by Dunnett's post hoc test using 0 mM as reference. Differences between treatments and/or groups were considered significant if P < 0.05.
FIG. 3.
Viable Rex1 promoter reporter responds with stress treatment in a dose-dependent manner in flow cytometry assay. mESCs expressing Rex1 promoter reporter were cultured in 12-well plates till 30% confluence. Cells were then treated with 200–300 mM of sorbitol in 25 mM increments for 3 days. After trypsinization, cell suspensions were assayed by microplate reader. Histograms shown in this study are from three independent experiments and (a) indicates significant decrease in Rex1-RFP fluorescence at all sorbitol doses from 200 to 300 mM as determined by ANOVA followed by Dunnett two-sided post hoc test, n = 3, P < 0.05.
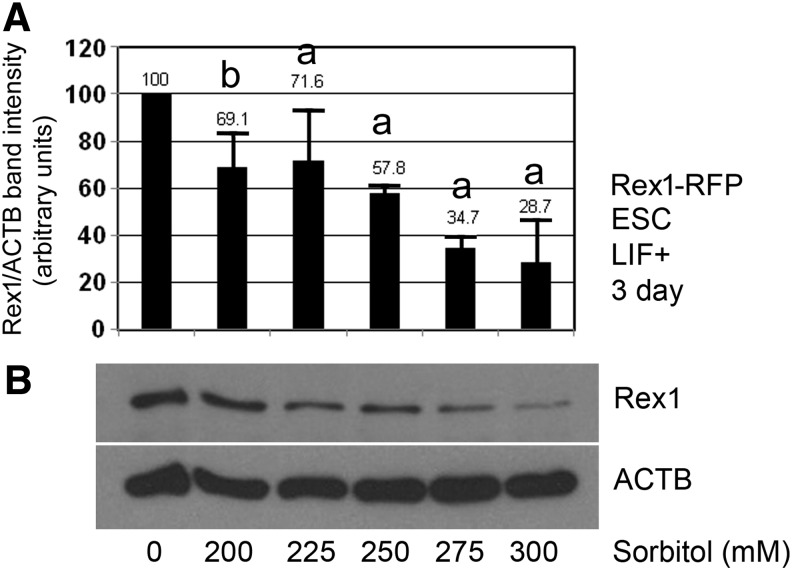
FIG. 5.
Rex1 promoter RFP have a stress dose-dependent loss of endogenous Rex1 protein corresponding to dose-dependent loss of Rex1 activity shown in Fig. 3. (A) Rex1-RFP mESCs were cultured until 30% confluent, treated with 0, 200–300 mM sorbitol in 25 mM increments for 3 days (as in Fig. 3), lysed, size fractionated by SDS-PAGE, blotted, probed for Rex1 and ACTB proteins, and analyzed for changes compared to time zero and stress dose responses. The histogram bars are derived from three independent experiments, one of which is shown in (A), shown are X ± SEM, n = 3, (a) is P < 0.05 and (b) indicates not significant. (B) One-way ANOVA was performed followed by Dunnett's post hoc test; (a) indicates significantly lower endogenous Rex1 at 225–300 mM sorbitol (P < 0.05), but not at 200 mM sorbitol (b).
Results
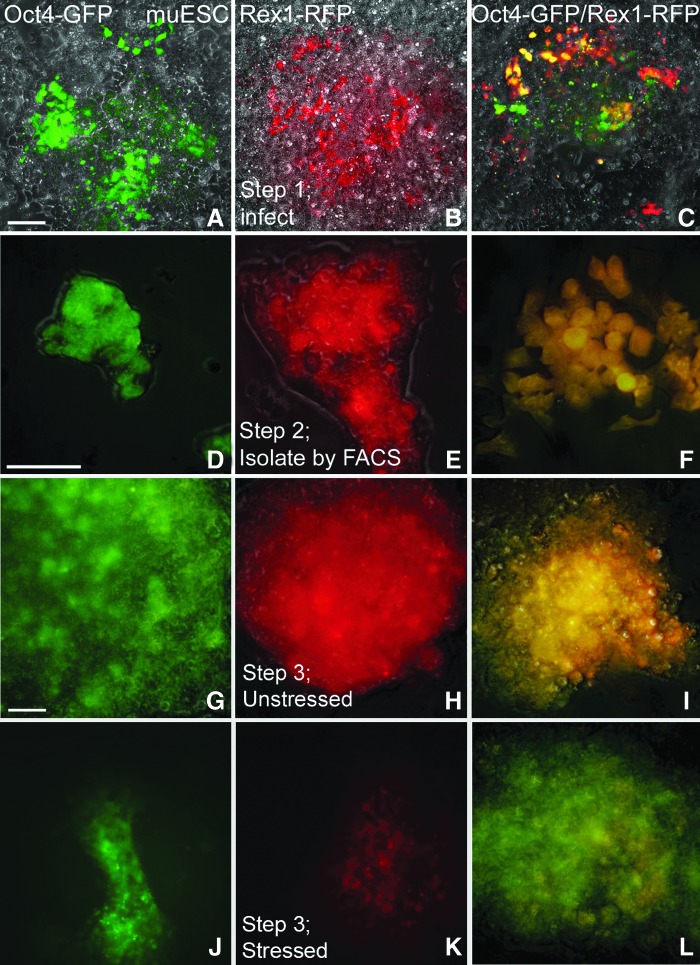
In this study, we sought to develop Rex1-RFP ESCs and test their kinetic and dose-dependent responses to stress. To create the Rex1-RFP ESCs and use them to compare to the Oct4-GFP stress response model, we infected ESCs with lentivirus with either single vector (Rex1-RFP or Oct-4-GFP) or both vectors (Fig. 1A–C). Note that single infected cells were red for Rex1-RFP or green for Oct4-GFP, but expression of both produced yellow emissions (Fig. 1C). This technology was used with no cell loss, which is commonly observed using either electroporation or cationic lipids [29]. In a second step, we trypsinized transgenic ESCs, as for a normal passage, and used a FACS to purify Rex1-RFP (Fig. 1D), Oct4-GFP (Fig. 1E), and dual Rex1-RFP/Oct4-GFP expressing ESCs (Fig. 1F).
FIG. 1.
Viable pluripotency activity reporters respond to stress treatment as previously reported for endogenous pluripotency reporter protein levels. ESCs were lentivirus infected (Step 1; A–C), FACS-selected (Step 2; D–F), and stress tested (Step 3) without (G–I) or with 200 mM sorbitol for 3 days (J–L) using pluripotency reporter Oct4-GFP ESCs (A, D, G, and J), Rex1-RFP ESCs (B, E, H, and K), or ESCs with both reporters (C, F, I, and L). ESCs were imaged by epifluorescence microscopy and transmitted light (phase contrast) with fluorescence superimposed. Micron bars in (A, D, and G) indicate 100 μm as (A–C, G–L) were micrographed at 10× and D–F were micrographed at 20×. ESC, embryonic stem cell; FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein.
To test for the stress response of these three transgenic ESCs, we incubated them for 3 days with LIF and with or without 200 mM sorbitol. The 3-day exposure was optimized in tests from 1 to 6 days (see Materials and Methods section and Supplementary Fig. S1). The choice of day 3 avoided low stimulation indices of stress-induced RFP decrease in early days due to relatively long half-life of RFP reporters and avoided the morbidity due to cell crowding in later days.
At this submorbid 200 mM sorbitol stress dose, Rex1 protein expression was significantly reduced as reported previously [3]. Stress did not affect Oct4-GFP expression (Fig. 1G, J). Rex1-RFP fluorescence was reduced in response to stress (Fig. 1K) compared to control (Fig. 1H). In a validation experiment of Rex1-RFP/Oct4-GFP dual expressing cells (yellow) (Fig. 1I), stress decreased Rex1-RFP expression (red). Stress did not change Oct4-GFP expression, and thus, the loss of red changed yellow (dual expression) to only the green fluorescence of Oct4 (Fig. 1L). In summary, the visual data show that Rex1-RFP activity reporter, but not Oct4-GFP, is decreased in viable ESCs after 3 days of hyperosmotic stress.
Next, the amount of stress-induced loss of Rex1-RFP or Oct4-GFP was quantified using a microplate reader. Stress mediated by 200 mM sorbitol, despite the presence of LIF, induces a significant loss of Rex1-RFP, but not Oct4-GFP (Fig. 2A, B). This observation is in general agreement with the loss of endogenous Rex1 protein, but not endogenous Oct4 protein, after 24 h of stress exposure ([3], Fig. 5).
In the test of Oct4-GFP and Rex1-RFP reporter ESCs, we used 200 mM sorbitol as defined previously by low toxicity, yet strong induction of transient Oct4 loss [3]. To test the effects of high dose, we used increments of 25 mM from 200 to 300 mM sorbitol for 3 days in ESC culture with LIF present. We found a significant dose-dependent increase in Rex1-RFP loss in triplicate biological experiments in which stress was dominant over potency and the Rex1 activity was decreased as measured by the microplate reader (Fig. 3). Although the Rex1-RFP activity loss at 200 mM sorbitol, in this study, was less than the endogenous Rex1 protein loss previously reported [3] by 300 m, the 60% loss of Rex1 activity measured in this study is as high as the loss of endogenous Rex1 protein reported previously.
To further define the nature of the response of ESCs to hyperosmotic stress, we tested for changes in the potency of ESC subpopulations using flow cytometry. We found that there was an increase in the dim subpopulation after 3 days of stress that was at 102 arbitrary intensity units, the level of autofluorescence of nontransgenic parental ESCs (Fig. 4 and Supplementary Fig. S2; “parental dim”). The dim subpopulation signifies that this transgenic Rex1-RFP is the same level of fluorescence as nontransgenic parental ESCs and thus has lost all activity that drives the transgenic Rex1 promoter.
Unstressed ESCs had 8.6% ± 1.0% dim cells, but this increased to 24.4% ± 2.9% by 300 mM sorbitol, despite the presence of LIF. In contrast, 83.3% ± 3.9% of unstressed cells were in the bright group (104 arbitrary intensity units), but the number decreased to 61.8% ± 3.7% at 300 mM. There was a small increase in intermediate dim cells (the population of ESCs between the two vertical lines, Fig. 4) from 10.7% ± 1.2% to 13.8% ± 2.1%. For methods of placing vertical lines and analysis of areas, see Materials and Methods. Thus, the stress induced a significant conversion of bright Rex1 activity cells to dim cells.
There was an ∼60% decrease in total Rex1-RFP from 0 to 300 mM sorbitol determined by microplate reader in Fig. 3. However, there was less than a 60% change in subpopulation sizes. Instead, there was a total change of 40.4% of cell subpopulations: a 3.1% increase in intermediate dim ESCs, a 15.8% increase in parental dim ESCs, and a decrease of 21.5% in the bright group (Fig. 4 and Supplementary Fig. S2). The additional decrease in brightness determined by microplate reader compared with subpopulation change producing decreased brightness may be a result of increased changes in brightness/cell.
Finally, we tested whether endogenous Rex1 protein levels decreased commensurately with decreases in Rex1-RFP activity levels assayed in Fig. 3. Rex1-RFP ESCs were cultured for 3 days with 0–300 mM sorbitol and assayed by immunoblot for endogenous Rex1 levels normalized to ACTB loading controls and with time zero unstressed Rex1 set to 100 as a baseline level.
Stress-induced decreases in the Rex1 activity in Fig. 3 were ∼20% and ∼70% at 200 mM and 300 mM sorbitol. Reductions in endogenous Rex1 protein expression were comparable at ∼30% and ∼70% for similar levels of sorbitol exposure. (Fig. 5A). Both, the Rex1 activity and endogenous protein had less decrease at 200 mM sorbitol than endogenous at 3 days compared with Rex1 protein loss at 1 day reported previously [3], Throughout the series of 25 mM increments from 200 to 300 mM sorbitol, there was generally a good agreement between the Rex1 activity level and endogenous protein level for Rex1 at 3 days of stress.
To correlate stress-induced Rex1 potency loss with stress-induced diminished cell growth rates we performed cell counts at time zero and at 3 day over the same sorbitol dose range as in Figs. 5A and 3A and Supplementary Fig. S2. As shown in the past [1,30], potency loss was directly proportional to diminished growth. To derive the relative number of dim cells in each dose of sorbitol stress, stress-diminished total cell counts were multiplied by stress-increased subpopulation fractions of intermediate dull + parental dim cells.
For several doses from 200 to 250 mM sorbitol the product, which equaled total numbers of cells which lost potency, was constant within 1% (Tables 1, 2). Interestingly, in the 200–250 mM sorbitol range cell doubling rates decreased, but in a shallow linear range between 34.1 and 39.3 h, but between 275 and 300 mM sorbitol doubling rates fell off greatly to 51.4–103.4 h. In this high sorbitol range, the potency also fell off greatly and the size of subpopulation with potency loss was not constant, but also fell off. Taken together, the Rex1 expression is regulated by stress in a dose-dependent manner, which is manifested in decreased cell growth, but a near constant production of cells with Rex1 loss in the nonmorbid dose range.
Table 1.
Stress Induces % RFP Stem Cell/Potency Loss and Slows Doubling Rate
| Sorbitol duration | Total dim cellsa,c | |||||
|---|---|---|---|---|---|---|
| mM | h | Totala,bdoubling rateacell (×100) | Brightc(h) | Total dimc(%) | % | Arbitrary cell numberb |
| 0 | 0 | 100 | ||||
| 0 | 72 | 460 | 32.7 | 83.3 | 16.7 | 7,682 |
| 200 | 72 | 432 | 34.1 | 77.1 | 22.9 | 9,893 |
| 225 | 72 | 407 | 35.6 | 75.6 | 24.4 | 9,931 |
| 250 | 72 | 356 | 39.3 | 72 | 28 | 9,968 |
| 275 | 72 | 264 | 51.4 | 71.9 | 28.1 | 7,418 |
| 300 | 72 | 162 | 103.4 | 61.8 | 38.2 | 6,150 |
See group 2 in Table 2.
% total dim cells were determined in one triplicate optimized for speed from trypsinization to flow cytometry without counting cell number. Total cell number was determined from a separate biological triplicate. Thus, we labeled “Total Dim Cells” an arbitrary number since triplicates for the multiplied factors were from different triplicates.
See group 1 in Table 2. (Fig. 4 and Supplementary Fig. S2).
Table 2.
Stress Induces Threefold Total Potency Loss but Total Differentiated Subpopulation Remains Similar During Stress Responses with Mildly Diminished Growth Rates
| Sorbitol (mM) | 0 (%) | 200 (%) | 225 (%) | 250 (%) | 275 (%) | 300 (%) |
|---|---|---|---|---|---|---|
| LIF + bright | 83.3 | 77.1 | 75.6 | 72 | 71.9 | 61.8 |
| Intermediate | 8.1 | 10.7 | 10.7 | 11.4 | 11 | 13.8 |
| Dim + parental dim | 8.6 | 12.2 | 13.7 | 16.6 | 17.1 | 24.4 |
| % Total dima | 16.7 | 22.9 | 24.4 | 28 | 28.1 | 38.2 |
| Total cellb (×100) | 460 | 432 | 407 | 356 | 264 | 161 |
| % Total dima × total cellb (×100) = total dim cellsa,b (arbitrary cell number)c | ||||||
| 16.7 × 460 = 7,682 |
22.9 × 432 = 9,893 |
24.4 × 407 = 9,931 |
28 × 356 = 9,968 |
28.1 × 264 = 7,418 |
38.2 × 161 = 6,150 |
|
Derived from triplicate biological experiments with a constant fraction of the volume of trypsinized Rex1-RFP ESCs for each treatment group assayed for fluorescence intensity by flow cytometry (Fig. 4 and Supplementary Fig. S2).
Derived from triplicate biological experiments (separate from the triplicates assayed by flow cytometry1) with trypsinized Rex1-RFP ESCs with same treatment groups as assayed by flow cytometry, but in this study the entire treatment group was counted by hemocytometer.
Total cell number was determined from a separate biological triplicate from which % total dim subpopulation was assayed and thus, we labeled “Total Dim Cells” an arbitrary number (since triplicates for the multiplied factors were from different triplicates).
ESCs, embryonic stem cells; LIF, leukemia inhibitory factor.
Discussion
The goals of this research were to (1) test whether the potency factor activity reporter ESCs and biochemical data for stress-induced potency loss are consistent using various assays, and (2) to validate these reporter ESCs for use in an HTS for future toxicology assessment.
Several molecular approaches confirmed Rex1-RFP potency activity reporter ESCs show consistency between micrographic analysis, biochemical averages from immunoblots, and microplate reader results, as well as subpopulation studies using flow cytometry. Endogenous Rex1 protein levels are consistent with Rex1-RFP activity levels measured in the same cells under the same stress exposures.
Mouse ESC colonies were heterogeneous for Rex1+ and Rex1− cells as was reported previously [15]. The Rex1 fluorescent cells studied previously interconverted to Rex1-negative cells after FACS isolation and culture. However, it is likely that stress-induced Rex1-negative activity cells in this study remain so and either become FGF5+ or primitive ExEndo as indicated by increases in Dab2/LRP2 mRNA [3] and induction from ESCs of first lineage ESC reporter-GFP cells that are also Dab2 protein positive (Li et al., unpublished data). Since stress decreases FGF5 expression and increases LRP2/DAB2 ([3], Li et al., unpublished), it is likely that stress-induced ExEndo induction is increased relative to ExEcto induction.
As suggested in a previous study [3], Rex1-RFP, but not Oct4-GFP, is a sensitive stress reporter and will enable an HTS for developmental toxicology of manufactured compounds, environmental pollutants, diet supplements, maternal stress hormones, new pharma, and cosmetics, as well as fluid collected during IVF and ART protocols. These include follicular, vaginal, uterine, amniotic fluids, and spent media.
The limitation of these studies is that, although considered to be the gold standard, only a single hyperosmotic stressor was used [2]. There is an exemplar set of toxicants used for the only government-validated ESC toxicology assay [18] and these will be used to further test the validity of the Rex1-RFP HTS. In addition, it will be important to test whether the stressors in this study cause potency loss in vivo and whether HTS ESC report potency loss in vivo in immunocompromised recipient mice. An important limitation is the inconclusive nature of this HTS to report differentiation gain, which should be remedied by a second HTS with a first lineage promoter driving GFP.
The theory of stress-induced compensatory differentiation [1–2] states that stress first diminishes normal stem cell population expansion and then compensates for fewer stem cells by differentiating more of the remaining stem cells to produce essential first lineages. This occurs despite the presence of growth factors that would normally maintain proliferation and potency: thus stress breaks potency. There are two lines of evidence for the theory. One is that dose-dependent stress diminishes stem cell growth as was observed previously in ESCs [3] and TSCs, as well as in the current study. A second previously established line of evidence is that stress increases the stress enzyme activity in proportion to reduction in potency factors and increases in differentiation factors, as measured in various assays. It can be expected that there is a putative subpopulation of stem cells that decreases potency and increases differentiation in response to stress.
Our group [1] and others have also shown [31] that various stressors can induce differentiation in placental and ESCs of the early rodent embryo. The Rex1-RFP and future potency and first differentiated lineage ESC HTSs should be invaluable in rapidly compiling quantitative data on stress-induced differentiation from large sets of stressors of interest to toxicologists, dieticians, and reproductive scientists.
Supplementary Material
Acknowledgments
Thanks to Drs. Jill Slater, Sichang Zhou, Ali Faqi, Mike Caudle, and members of our laboratory for analysis and comments on the article. We also thank Drs. Kami Moin and Jessica Back at the WSU MICR facility for helpful discussions of FACS isolation of stem cells and quantitative immunofluorescence. This research was supported by grants to DAR from NIH (1R03HD061431 02), the Mary Iacobelli endowed chair (EEP), and from the Office of the Vice President for Research at Wayne State University. Dr. Gomez-Lopez is funded by the Wayne State University Research Initiative in Maternal, Perinatal, and Child Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NICHD/NIH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Puscheck EE, Awonuga AO, Yang Y, Jiang Z. and Rappolee DA. (2015). Molecular biology of the stress response in the early embryo and its stem cells. In: Cell Signalling During Mammalian Early Embryo Development. Leese H, Brison D, eds. New York, NY: Springer; Chapter 4, pp. 77–128 [DOI] [PubMed] [Google Scholar]
- 2.Xie Y, Awonuga AO, Zhou S, Puscheck EE. and Rappolee DA. (2011). Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol 287:43–95 [DOI] [PubMed] [Google Scholar]
- 3.Slater JA, Zhou S, Puscheck EE. and Rappolee DA. (2014). Stress-induced enzyme activation primes murine embryonic stem cells to differentiate toward the first extraembryonic lineage. Stem Cells Dev 23:3049–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE. and Rappolee DA. (2013). Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells. Stem Cells Dev 22:1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, Puscheck EE. and Rappolee DA. (2010). Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction 140:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pesce M. and Scholer HR. (2000). Oct-4: control of totipotency and germline determination. Mol Reprod Dev 55:452–457 [DOI] [PubMed] [Google Scholar]
- 7.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H. and Smith A. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379–391 [DOI] [PubMed] [Google Scholar]
- 8.Chai N, Patel Y, Jacobson K, McMahon J, McMahon A. and Rappolee DA. (1998). FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol 198:105–115 [DOI] [PubMed] [Google Scholar]
- 9.Frum T, Halbisen MA, Wang C, Amiri H, Robson P. and Ralston A. (2013). Oct4 cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev Cell 25:610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Bin GC, Munoz-Descalzo S, Kurowski A, Leitch H, Lou X, Mansfield W, Etienne-Dumeau C, Grabole N, Mulas C, et al. (2014). Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst. Development 141:1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milroy C, Liu L, Hammoud S, Hammoud A, Peterson CM. and Carrell DT. (2011). Differential methylation of pluripotency gene promoters in in vitro matured and vitrified, in vivo-matured mouse oocytes. Fertil Steril 95:2094–2099 [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Okuka M, McLean M, Keefe DL. and Liu L. (2009). Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil Steril 92:1456–1465 [DOI] [PubMed] [Google Scholar]
- 13.Pal R, Mamidi MK, Das AK. and Bhonde R. (2011). Human embryonic stem cell proliferation and differentiation as parameters to evaluate developmental toxicity. J Cell Physiol 226:1583–1595 [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Gemberling M, Nakamura M, Whitby FG, Handa H, Fairbrother WG. and Tantin D. (2009). A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev 23:208–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyooka Y, Shimosato D, Murakami K, Takahashi K. and Niwa H. (2008). Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135:909–918 [DOI] [PubMed] [Google Scholar]
- 16.Rezende NC, Lee MY, Monette S, Mark W, Lu A. and Gudas LJ. (2011). Rex1 (Zfp42) null mice show impaired testicular function, abnormal testis morphology, and aberrant gene expression. Dev Biol 356:370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandler KJ, Barrier M, Jeffay S, Nichols HP, Kleinstreuer NC, Singh AV, Reif DM, Sipes NS, Judson RS, et al. (2011). Evaluation of 309 environmental chemicals using a mouse embryonic stem cell adherent cell differentiation and cytotoxicity assay. PLoS One 6:e18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genschow E, Spielmann H, Scholz G, Seiler A, Brown N, Piersma A, Brady M, Clemann N, Huuskonen H, et al. (2002). The ECVAM international validation study on in vitro embryotoxicity tests: results of the definitive phase and evaluation of prediction models. European Centre for the Validation of Alternative Methods. Altern Lab Anim 30:151–176 [DOI] [PubMed] [Google Scholar]
- 19.Schulpen SH, Robinson JF, Pennings JL, van Dartel DA. and Piersma AH. (2013). Dose response analysis of monophthalates in the murine embryonic stem cell test assessed by cardiomyocyte differentiation and gene expression. Reprod Toxicol 35:81–88 [DOI] [PubMed] [Google Scholar]
- 20.Guo YL, Chakraborty S, Rajan SS, Wang R. and Huang F. (2010). Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev 19:1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman MR, Albers RE, Keoni C, Kulkarni-Datar K, Natale DR. and Brown TL. (2014). Important aspects of placental-specific gene transfer. Theriogenology 82:1043–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumura-Nakanishi S, Saito M, Niwa H. and Ishikawa F. (2005). Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem 280:5307–5317 [DOI] [PubMed] [Google Scholar]
- 23.Hosler BA, Rogers MB, Kozak CA. and Gudas LJ. (1993). An octamer motif contributes to the expression of the retinoic acid-regulated zinc finger gene Rex-1 (Zfp-42) in F9 teratocarcinoma cells. Mol Cell Biol 13:2919–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J. and Benvenisty N. (2001). Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol 11:514–518 [DOI] [PubMed] [Google Scholar]
- 25.Chang LJ. and Gay EE. (2001). The molecular genetics of lentiviral vectors—current and future perspectives. Curr Gene Ther 1:237–251 [DOI] [PubMed] [Google Scholar]
- 26.Doetschman TC, Eistetter H, Katz M, Schmidt W. and Kemler R. (1985). The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27–45 [PubMed] [Google Scholar]
- 27.Masui S, Ohtsuka S, Yagi R, Takahashi K, Ko MS. and Niwa H. (2008). Rex1/Zfp42 is dispensable for pluripotency in mouse ES cells. BMC Dev Biol 8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corish P. and Tyler-Smith C. (1999). Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng 12:1035–1040 [DOI] [PubMed] [Google Scholar]
- 29.Ausubel FM. (2002). Short Protocols in Molecular Biology: A Compendium of Methods from Current Protocols in Molecular Biology. Wiley, New York [Google Scholar]
- 30.Mansouri L, Xie Y. and Rappolee DA. (2012). Adaptive and pathogenic responses to stress by stem cells during development. Cells 1:1197–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soares MJ, Chakraborty D, Kubota K, Renaud SJ. and Rumi MA. (2014). Adaptive mechanisms controlling uterine spiral artery remodeling during the establishment of pregnancy. Int J Dev Biol 58:247–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.