Abstract
Background
In Ontario, current treatment for eligible patients who have an acute ischemic stroke is intravenous thrombolysis (IVT). However, there are some limitations and contraindications to IVT, and outcomes may not be favourable for patients with stroke caused by a proximal intracranial occlusion. An alternative is mechanical thrombectomy with newer devices, and a number of recent studies have suggested that this treatment is more effective for improving functional independence and clinical outcomes. The objective of this health technology assessment was to evaluate the clinical effectiveness and cost-effectiveness of new-generation mechanical thrombectomy devices (with or without IVT) compared to IVT alone (if eligible) in patients with acute ischemic stroke.
Methods
We conducted a systematic review of the literature, limited to randomized controlled trials that examined the effectiveness of mechanical thrombectomy using stent retrievers and thromboaspiration devices for patients with acute ischemic stroke. We assessed the quality of the evidence using the GRADE approach. We developed a Markov decision-analytic model to assess the cost-effectiveness of mechanical thrombectomy (with or without IVT) versus IVT alone (if eligible), calculated incremental cost-effectiveness ratios using a 5-year time horizon, and conducted sensitivity analyses to examine the robustness of the estimates.
Results
There was a substantial, statistically significant difference in rate of functional independence (GRADE: high quality) between those who received mechanical thrombectomy (with or without IVT) and IVT alone (odds ratio [OR] 2.39, 95% confidence interval [CI] 1.88–3.04). We did not observe a difference in mortality (GRADE: moderate quality) (OR 0.80, 95% CI 0.60–1.07) or symptomatic intracerebral hemorrhage (GRADE: moderate quality) (OR 1.11, 95% CI 0.66–1.87).
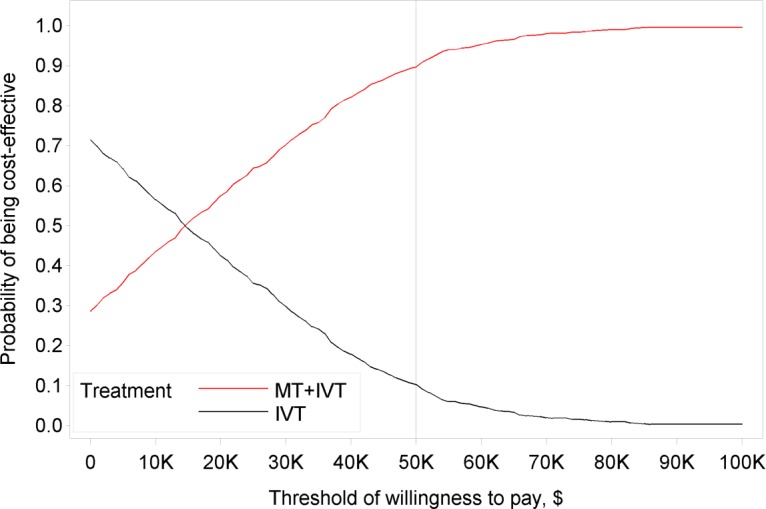
In the base-case cost-utility analysis, which had a 5 year time horizon, the costs and effectiveness for mechanical thrombectomy were $126,939 and 1.484 quality-adjusted life-years (QALYs) (2.969 life-years). The costs and effectiveness for IVT alone were $124,419 and 1.273 QALYs (2.861 life-years), respectively. Mechanical thrombectomy was associated with an incremental cost-effectiveness ratio of $11,990 per QALY gained. Probabilistic sensitivity analysis showed that the probability of mechanical thrombectomy being cost-effective was 57.5%, 89.7%, and 99.6%, at thresholds of $20,000, $50,000, and $100,000 per QALY gained, respectively. We estimated that adopting mechanical thrombectomy would lead to a cost increase of approximately $1 to 2 million.
Conclusions
High quality evidence showed that mechanical thrombectomy significantly improved functional independence and appeared to be cost-effective compared to IVT alone for patients with acute ischemic stroke.
BACKGROUND
Clinical Need and Target Population
Description of Disease/Condition
Acute ischemic stroke comprises 85% of all strokes and is caused by occlusion of a cerebral artery.1 Risk factors for ischemic stroke include hypertension, diabetes mellitus, cigarette smoking, alcohol consumption, atrial fibrillation, carotid artery stenosis.2 Ischemic stroke is characterized by the sudden loss of blood flow to an area of the brain, resulting in a loss of neurologic function. Signs and symptoms of stroke include muscular weakness or paralysis on one side of the body (including facial droop, arm drift, or leg weakness), impaired speech, or vision loss in one or both eyes. This condition comes with a high burden of disability and death.
Prevalence and Incidence
In Canada, there are 62,000 new strokes per year and more than 300,000 stroke survivors (1.1% of the population).3 In the United States each year, approximately 795,000 people experience a stroke (ischemic or hemorrhagic); about 610,000 are first-time (incidence) events and 185,000 are recurrent. In 2009, stroke caused approximately one in every 19 deaths in the United States, and in Canada, it was the third leading cause of death, with over 14,000 Canadians dying from stroke (6% of all deaths in Canada).4,5
Technology
In patients who are brought to medical attention promptly, acute treatment of acute ischemic stroke may include efforts to open the occluded blood vessels and re-establish blood flow. Reperfusion means reflow into the arterial tree and evidence of flow at the capillary level. This can be attained using intravenous thrombolysis (IVT) or endovascular treatment via mechanical thrombectomy with retrievable stents, thrombus aspiration, retraction, wire disruption, or direct intra-arterial administration of thrombolytic drugs. For the purposes of this report, we will focus only on new mechanical thrombectomy devices (i.e., retrievable stents and thrombus aspiration devices), since randomized controlled trials have demonstrated a highly significant difference between older and newer devices.6,7
In Ontario, IVT is the current standard of care. The only approved thrombolytic agent for acute ischemic stroke in Canada is recombinant tissue plasminogen activator, or alteplase. It is recommended that IVT be first-line therapy and should be administered within 4.5 hours of the onset of stroke symptoms.8 However, there are some limitations to this treatment, including a narrow therapeutic time window and contraindications such as recent surgery, active bleeding, coagulation abnormalities, and history of trauma and intracranial hemorrhage.9 Patients who are ineligible for IVT due to contraindications receive best medical care. Although overall recanalization (opening of the blocked artery) rates for IVT are approximately 46%, rates are lower when the blockage is in a large artery (middle cerebral and carotid terminus). Published recanalization rates for large arteries range from 4% to 68% and depend on the location of occlusion and the particular study. Further, the key clinical outcome of interest is early recanalization with full reperfusion of the distal arterial bed; if recanalization occurs too slowly or not at all, poor clinical outcomes may result, due to irreversible infarction.10–17
Efforts to improve recanalization rates in patients with a large-vessel occlusion have been explored in several large randomized controlled trials (RCTs). Intra-arterial therapy and endovascular treatment with older mechanical thrombectomy devices have been examined, but early trials have failed to show clinical benefit.18–20 This may be due to three factors: use of less-effective, older-generation thrombectomy devices; slow process times (specifically time to reperfusion); and a more heterogeneous group of patients, some of whom were unlikely to benefit (established stroke, poor collaterals, no confirmation of proximal artery occlusion on imaging).21 Newer-generation mechanical thrombectomy devices have the potential to improve clinical and functional outcomes, either alone or in addition to IVT.
An example of the procedure with a retrievable stent is explained below:
A balloon-guided catheter is placed proximal to the intracranial thrombus. A guide-wire is passed through the thrombus and then a microcatheter is passed over the guide-wire through the thrombus. The guide-wire is withdrawn and the stent retriever is passed through the micro-catheter to position the distal end a few millimeters distal to the thrombus. The microcatheter is then withdrawn while the retrievable stent device is held in place and the stent opens within the thrombus, allowing the tines of the stent to capture the thrombus. At this point, contrast can be injected through the balloon guide catheter to assess for distal perfusion. After a short period (5 minutes), the balloon is inflated proximally to achieve flow arrest and the microcatheter and stent is retracted gradually into the guiding catheter while aspirating the guide catheter. The balloon is then deflated and a control angiogram confirms if the clot has been removed. If not, this process can be repeated several times.22
Ontario Context
In fiscal year 2012/13, our best estimate is that 1.1% of patients in Ontario with acute ischemic stroke had mechanical.23 Approximately 70 mechanical thrombectomy cases were completed at 11 sites.
Mechanical thrombectomy is done only in comprehensive stroke centres with neurointerventional services and physicians with expertise in this procedure (mainly neuroradiologists in Canada, but also some neurosurgeons and some neurologists with specialty training in interventional neuroradiology, angiography, and mechanical thrombectomy). The 11 sites in Ontario where this procedure is completed are located in urban areas.
Regulatory Status
Four devices are currently approved by Health Canada for mechanical thrombectomy, but the Merci Retriever and the first-generation Penumbra device are no longer on the market or in use in Canada; they have been excluded from the analysis. For the purposes of this report, we will focus on the retrievable stents and thrombus aspiration devices described in Table 1.
Table 1:
Mechanical Thrombectomy Devices Approved by Health Canada
| Device Name | Manufacturer | Licence Number | Description |
|---|---|---|---|
| Penumbra System MAX | Penumbra | 93596 | Intended for the revascularization of patients with acute ischemic stroke secondary to intracranial large-vessel occlusive disease (internal carotid, middle cerebral M1 and M2 segments, basilar, and vertebral arteries) within 8 hours of symptom onset |
| Trevo Retriever | Stryker | 62603 | Intended to restore blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke within 8 hours of symptom onset. Patients who are ineligible for IV tPA or who fail IV tPA therapy are candidates for treatment |
| Solitaire FR Revascularization Device | Covidiena | 89137 | Intended to restore blood flow by removing thrombus from a large intracranial vessel in patients experiencing ischemic stroke within 8 hours of symptom onset. Patients who are ineligible for IV tPA or who fail IV tPA therapy are candidates for treatment |
Abbreviation: IV tPA, intravenous tissue plasminogen activator.
Covidien is now owned by Medtronic.
Research Questions
What is the clinical effectiveness and safety of endovascular treatment via new-generation mechanical thrombectomy devices (with or without IVT) compared to IVT alone (if eligible) in patients with acute ischemic stroke caused by a proximal intracranial occlusion in the anterior circulation?
What is the cost-effectiveness of mechanical thrombectomy (with or without IVT) versus IVT alone?
What is the budget impact of adopting mechanical thrombectomy in Ontario?
CLINICAL EVIDENCE REVIEW
Objective of Analysis
The objective of this analysis was to evaluate the clinical effectiveness and safety of endovascular treatment employing stent retrievers and thromboaspiration in patients with acute ischemic stroke.
Methods
Literature Search
Search Strategy
A literature search was performed on March 11, 2015, using All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database for studies published from January 1, 2005, to March 11, 2015. (Appendix 1 provides details of the search strategies.) Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria
English-language full-text publications
Published between January 1, 2005, and March 11, 2015
Health technology assessments, RCTs, systematic reviews, and meta-analyses
> 3 months of follow-up
Studies that used imaging-based methods to triage patients
Patients with acute ischemic stroke caused by proximal anterior circulation intracranial occlusion in the internal carotid artery, M1 or M2 middle cerebral artery, or A1-anterior cerebral artery
Patients treated with mechanical thrombectomy (with or without IVT)
Comparator group treated with IVT or best medical care
Patients who presented in hospital up to 12 hours after symptom onset
Exclusion Criteria
Observational studies, case reports, and editorials
Occlusions in other parts of the brain (outside of the anterior circulation)
Studies examining “off-label” mechanical devices for endovascular treatment
Studies using older devices (Merci Retriever and first-generation Penumbra) that are no longer available in Canada or in use
Outcomes of Interest
Primary outcomes: functional independence using the modified Rankin Scale (mRS), mortality, adverse events (symptomatic intracerebral hemorrhage)
Secondary outcomes: quality of life using the EuroQoL Group 5-Dimension Self-Report Questionnaire (EQ-5D), reperfusion rates, recanalization rates
Statistical Analysis
Where appropriate, we undertook a meta-analysis for reported outcomes to determine the pooled estimate of effect of mechanical thrombectomy compared with IVT alone, using Review Manager Version 5.2.24 For continuous scores, we calculated the mean difference; for binary data, we used odds ratios as the pooled summary estimates because they accurately represented the data from the individual studies.
We assessed the degree of statistical heterogeneity among studies using the I2 statistic for each outcome. An I2 > 50% was considered to be substantial heterogeneity. We used random- or fixed-effects models for meta-analysis following the guidance of the Cochrane handbook.25
We completed three sensitivity analyses to establish trends in prespecified, clinically meaningful patient populations for the outcome of functional independence as measured by the mRS:
Age of the patient (< 70 years versus > 70 years)
Status of IVT (IVT-eligible versus IVT-ineligible)
Location of occlusion (internal carotid artery versus middle cerebral artery)
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria.26 The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that RCTs are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were then taken into account. Limitations in these areas resulted in downgrading the quality of evidence. Finally, three main factors that may raise the quality of evidence were considered: the large magnitude of effect, the dose response gradient, and any residual confounding factors.26 For more detailed information, please refer to the latest series of GRADE articles.26
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | High confidence in the effect estimate—the true effect lies close to the estimate of the effect |
| Moderate | Moderate confidence in the effect estimate—the true effect is likely to be close to the estimate of the effect, but may be substantially different |
| Low | Low confidence in the effect estimate—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very low confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of the effect |
Results
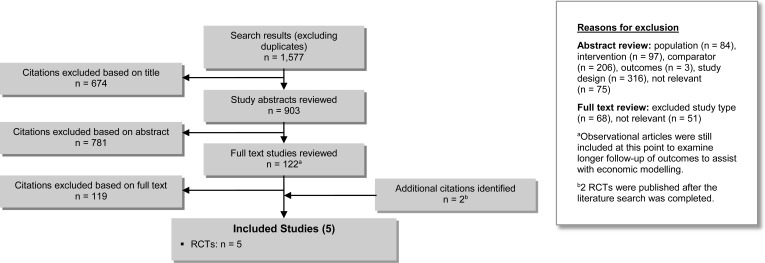
The database search yielded 1,577 citations published between January 1, 2005, and March 11, 2015, (with duplicates removed). We excluded articles based on information in the title and abstract. We obtained the full texts of potentially relevant articles for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded from the analysis.
Figure 1: Citation Flow Chart.
Abbreviation: RCT, randomized controlled trial.
Three RCTs27–29 met the inclusion criteria. We hand-searched the reference lists of the included studies and consulted with experts to identify other relevant studies; two additional RCTs30,31 were included (published after the literature search was completed), for a total of 5.
All RCTs were conducted at multiple sites, across 14 countries in total. Inclusion criteria were similar across RCTs: adults 18 and over, functionally independent prior to stroke, majority of patients with an occlusion of the distal internal carotid artery or middle cerebral artery (M1 or M2). Baseline characteristics in the intervention and control arms were evenly distributed. All RCTs had a 90-day follow-up.
Table 2 presents the characteristics of the included studies, and Tables 3 and 4 present the baseline characteristics for the intervention and control study samples, respectively.
Table 2:
Study Characteristics of Included RCTs
| Author, Year | Country | Objective | Eligibility Criteria | Sample Size (Intervention/Control) | Number of Sites | Randomization Method |
|---|---|---|---|---|---|---|
| Berkhemer et al, 201527 | Netherlands | To assess whether mechanical thrombectomy plus IVT would be more effective than IVT alone in patients with a proximal arterial occlusion in the anterior cerebral circulation that could be treated within 6 hours after symptom onset | Eligible patients were 18 years of age or older (no upper age limit) with AIS caused by an intracranial occlusion in the anterior circulation artery. Initiation of endovascular treatment had to be possible within 6 hours of stroke onset. Patients had to have an occlusion of the distal intracranial carotid artery, middle cerebral artery (M1 or M2), or anterior cerebral artery (A1 or A2), and an NIHSS score of 2 or higher | 500 (233/267) | 16 | Web-based, with permuted blocks. Stratified randomization according to medical centre, use of IVT, planned treatment, and stroke severity |
| Campbell et al, 201528 | Australia New Zealand |
To assess whether mechanical thrombectomy after IVT administration, compared with IVT alone, would improve reperfusion in patients with anterior circulation ischemic stroke within 4.5 hours after stroke onset | Eligible patients were included if they could receive IVT within 4.5 hours after the onset of anterior circulation ischemic stroke and had occlusion of the internal carotid artery or of the first or second segment of the middle cerebral artery, as seen on CT angiography. Endovascular treatment had to be initiated (groin puncture) within 6 hours after stroke onset and completed within 8 hours after onset. There were no restrictions on age or clinical severity, as assessed according to the NIHSS score. Patients were required to have functional independence before the stroke episode, which was defined as a score of < 2 on the mRS | 70 (35/35) | 10 | Centralized website and stratified according to the site of arterial occlusion: the internal carotid artery or the first or second segment of the middle cerebral artery |
| Goyal et al, 201529 | Canada United States South Korea Ireland United Kingdom |
To assess whether patients with AIS would benefit from rapid mechanical thrombectomy with or without IVT compared to IVT alone | Eligible participants were adults (no upper age limit) with a disabling ischemic stroke who had been functioning independently in the community (score on the Barthel Index [range, 0 to 100, with higher scores indicating a greater ability to complete activities of daily living] ≥ 90) before the stroke. Enrollment could occur up to 12 hours after the onset of stroke symptoms | 315 (165/150) | 22 | Real-time, dynamic, Internet-based, randomized minimization procedure (minimal sufficient balance method) to achieve distribution balance with regard to age, sex, baseline NIHSS score, site of arterial occlusion, baseline ASPECTS, and IVT status |
| Jovin et al, 201530 | Spain | To assess the safety and efficacy of mechanical thrombectomy with or without IVT versus IVT alone among patients with AIS that could be treated within 8 hours after stroke onset | Eligible patients were between the ages of 18 and 80 years, had an occlusion in the proximal anterior circulation that could be treated within 8 hours after symptom onset, had a prestroke functional ability of 1 or less on the mRS, and had a baseline score of at least 6 points on the NIHSS. After enrollment of 160 patients, the inclusion criteria were modified to include patients up to the age of 85 years with an ASPECTS score of > 8 | 206 (103/103) | 4 | Real-time computerized randomization procedure that was stratified according to age (≤ 70 or > 70 years), baseline NIHSS score (6 to 16 or ≥ 17), therapeutic window (≤ 4.5 or > 4.5 hours), occlusion site (intracranial internal carotid artery or M1 segment [main trunk] of the middle cerebral artery), and participating centre |
| Saver et al, 201531 | United States Europe |
To assess the efficacy and safety of rapid mechanical thrombectomy in conjunction with IVT versus IVT alone in patients with AIS | Eligible patients who had acute ischemic stroke with moderate-to-severe neurologic deficits; had imaging-confirmed occlusion of the intracranial internal carotid artery, the first segment of the middle cerebral artery, or both; met the imaging eligibility requirements; were receiving or had received IVT; and were able to undergo initiation of endovascular treatment within 6 hours after the time they were last known to be well before the onset of acute stroke symptoms | 196 (98/98) | 39 | Minimization algorithm to balance the numbers of patients in the two treatment groups with respect to four factors: investigational site; baseline severity according to the NIHSS score (≤ 17 vs. > 17, on a scale of 0–42, with higher scores indicating greater severity); age (< 70 years vs. ≥ 70 years); and occlusion location (middle cerebral artery vs. internal carotid artery). |
Abbreviations: AIS, acute ischemic stroke; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; CT, computed tomography; IVT, intravenous thrombolysis, mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; RCT, randomized controlled trial.
Table 3:
Baseline Characteristics of Intervention Group in Included RCTs
| Author, Year | Treatment Protocol | Agea Male, n (%) | Type of Occlusion, n (%) | Prestroke mRS, n (%) | NIHSS (range) | ASPECTS (range) | Status of IVT, n (%) |
|---|---|---|---|---|---|---|---|
| Berkhemer et al, 201527 | Endovascular treatment consisted of arterial catheterization with a microcatheter and delivered a thrombolytic agent, mechanical thrombectomy, or both. Mechanical treatment was performed in 195 (83.7%) patients. Retrievable stents were used in 190 (81.5%) patients, and other devices in 5 (2.1%) patients | 65.8 (54.5–76.0) 135 (57.9) |
Intracranial ICA: 1 (0.4) ICA with involvement of M1 MCA: 59 (25.3) M1 MCA: 154 (66.1) M2 MCA: 18 (7.7) A1 or A2: 1 (0.4) Extracranial ICA occlusion was included based on physician judgement Extracranial ICA: 75 (32.2) |
mRS 0: 190 (81.5) mRS 1: 21 (9.0) mRS 2: 12 (5.2) mRS > 2: 10 (4.3) |
17 (14–21) | 9 (7–10) | IVT: 203 (87.1) No IVT: 30 (12.9) |
| Campbell et al, 201528 | All patients received IVT at a dose of 0.9 mg/kg as standard care. Patients in the intervention group also had standard of care plus mechanical thrombectomy with the Solitaire device | 68.6 ± 12.3 17 (49) |
ICA: 11 (31) M1 MCA: 20 (57) M2 MCA: 4 (11) |
All patients had to be functionally independent with an mRS score of ≤ 2 prior to AIS | 17 (13–20) | NR | All patients received IVT |
| Goyal et al, 201529 | The neurointerventionist used available thrombectomy devices to achieve reperfusion. The use of retrievable stents was recommended. During thrombus retrieval, suction through a balloon guide catheter in the relevant internal carotid artery was also recommended. Retrievable stents were used in 130/151 patients (86.1 %) who underwent an endovascular procedure; 100/130 (77.0%) received a Solitaire stent. | 71 (60–81) 79 (47.9) |
ICA with involvement of the M1 MCA segment: 45/163 (27.6) M1 or all M2 MCA segments: 111/163 (68.1) Single M2 MCA segment: 6/163 (3.7) Ipsilateral cervical carotid occlusion plus one of the above: 21 (12.7) |
All patients had to be functionally independent prior to AIS with a score on the Barthel Index of ≥ 90 | 16 (13–20) | 9 (8–10) | IVT: 119 (72.7) No IVT: 45 (27.3) |
| Jovin et al, 201530 | Medical therapy (including IVT when eligible) and endovascular treatment with the Solitaire stent retriever | 65.7 ± 11.3 55 (53.4) |
Intracranial ICA without involvement of M1: 0 (0) Terminal internal carotid artery with involvement of M1 MCA: 26/102 (25.5) M1 MCA: 66/102 (64.7) Single M2 MCA: 10/102 (9.8) Ipsilateral cervical carotid occlusion: 19/102 (18.6) |
All patients had to have a prestroke mRS of 0–1 | 17 (14–20) | 7 (6–9) | IVT: 70 (68.0) No IVT: 32 (32.0) |
| Saver et al, 201531 | Mechanical thrombectomy with the Solitaire FR (Flow Restoration) or Solitaire 2 device. Concomitant stenting of the cervical internal carotid artery was not permitted, although angioplasty could be performed to permit intracranial access | 65.0 ± 12.5 54/98 (55.1) |
ICA: 17/93 (18.0) M1 MCA: 62/93 (67.0) M2 MCA: 13/93 (14.0) |
mRS 0 or 1: 96/98 (98) | 17 (13–20) | 9 (7–10) | All patients received IVT |
Abbreviations: AIS, acute ischemic stroke; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; IVT, intravenous thrombolysis; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; RCT, randomized controlled trial; SD, standard deviation.
Age is reported as mean ± SD or median (interquartile range).
Table 4:
Baseline Characteristics of Control Group in Included RCTs
| Author, Year | Treatment Protocol | Agea Male, n (%) | Type of Occlusion, n (%) | Prestroke mRS, n (%) | NIHSS (range) | ASPECTS (range) | Status of IVT, n (%) |
|---|---|---|---|---|---|---|---|
| Berkhemer et al, 201527 | The use of alteplase or urokinase for intra-arterial thrombolysis was allowed in this trial, with a maximum dose of 90 mg of alteplase or 1,200,000 IU of urokinase. The dose was restricted to 30 mg of alteplase or 400,000 IU of urokinase if intravenous alteplase was given | 65.7 (55.5–76.4) 157 (58.8) |
Intracranial ICA: 3/266 (1.1%) ICA with involvement of M1: 75/266 (28.2%) M1: 165/266 (62.0%) M2: 21/266 (7.9%) A1 or A2: 2/266 (0.8%) Extracranial ICA occlusion was included based on physician judgement Extracranial ICA: 70 (26.3%) |
mRS 0: 214 (80.1) mRS 1: 29 (10.9) mRS 2: 13 (4.9) mRS > 2: 11 (4.1) |
18 (14–22) | 9 (8–10) | IVT: 242 (90.6) No IVT: 25 (9.4) |
| Campbell et al, 201528 | All patients received alteplase at a dose of 0.9 mg/kg as standard care. Patients assigned to the control group received only IVT | 70.2 ± 11.8 17 (49) |
ICA: 11 (31) M1 MCA: 18 (51) M2 MCA: 6 (17) |
All patients had to be functionally independent with an mRS score of ≤ 2 prior to AIS | 13 (9–19) | NR | All patients received IVT |
| Goyal et al, 201529 | The control group received the current standard of care as described in the Canadian or local guidelines for the management of acute stroke | 70 (60–81) 71 (47.3) |
ICA with involvement of the M1 MCA segment: 39/147 (26.5) M1 or all M2 MCA segments: 105/147 (71.4) Single M2 MCA segment: 3/147 (2.0) Ipsilateral cervical carotid occlusion plus one of the above: 19 (12.9) |
All patients had to be functionally independent prior to AIS with a score on the Barthel Index of ≥ 90 | 17 (12–20) | 9 (8–10) | IVT: 118 (78.7) No IVT: 32 (21.3) |
| Jovin et al, 201530 | IVT alone or best medical therapy | 67.2 ± 9.5 54 (52.4) |
Intracranial ICA without involvement of M1: 1/101(1.0) Terminal internal carotid artery with involvement of M1 MCA: 27/101 (26.7) M1 MCA: 65/101 (64.4) Single M2 MCA: 8/101 (7.9) Ipsilateral cervical carotid occlusion: 13/101 (12.9) |
All patients had to have a prestroke mRS of 0–1 | 17 (12–19) | 8 (6–9) | IVT: 80 (77.7) No IVT: 21 (22.3) |
| Saver et al, 201531 | IV tPA alone | 66.3 ± 11.3 45/96 (47.0) |
ICA: 15/94 (16.0) M1 MCA: 72/94 (77.0) M2 MCA: 6/94 (6.0) |
mRS 0 or 1: 93/94 (99) | 17 (13–19) | 9 (8–10) | All patients received IVT |
Abbreviations: AIS, acute ischemic stroke; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; IVT, intravenous thrombolysis; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NR, not reported; RCT, randomized controlled trial; SD, standard deviation.
Age is reported as mean ± SD or median (interquartile range).
Functional Independence
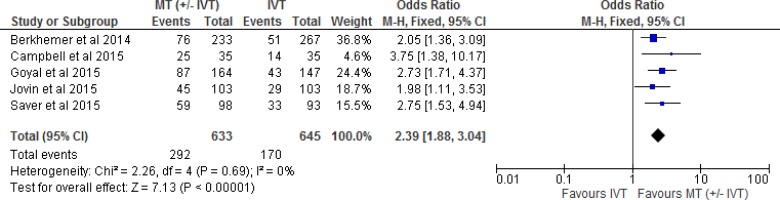
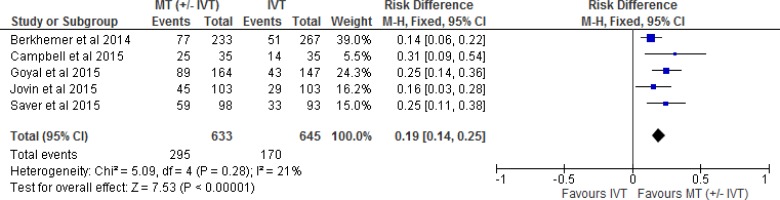
All five included RCTs reported functional independence as an outcome measured by the modified Rankin Scale. (The mRS is a 7-point scale ranging from 0 [no symptoms] to 6 [death]. A score of 2 or less indicates functional independence.) We conducted a meta-analysis for this outcome, comparing intervention and control arms for the proportion of patients with an mRS of 0 to 2. The effect of mechanical thrombectomy on functional independence was examined by pooling data from five studies with 1,278 participants using a fixed-effects model (Figure 2). There was a significant difference for functional independence between those who received mechanical thrombectomy (+/- IVT) and those who received IVT We also conducted a meta-analysis to show the risk difference between the two groups (Appendix 3, Figure A1). The meta-analysis showed that the absolute risk reduction is approximately 19% (95% confidence interval 14% to 25%), therefore, the number needed to treat is 5. The quality of evidence was “high” for functional independence according to the GRADE system.
Figure 2: Mechanical Thrombectomy Versus IVT on the Proportion of Functionally Independent Patients at 90-Day Follow-up.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
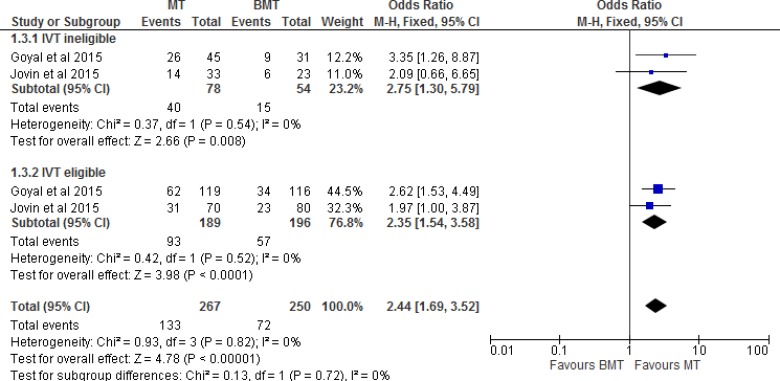
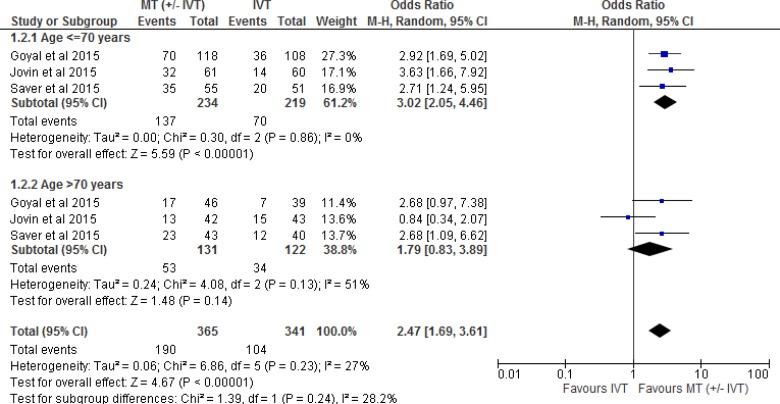
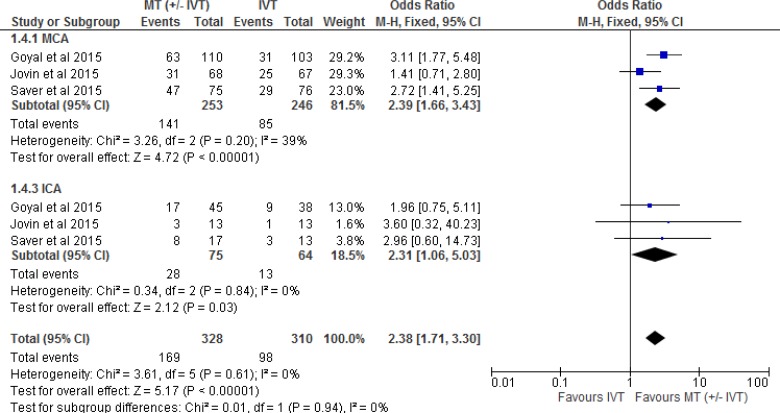
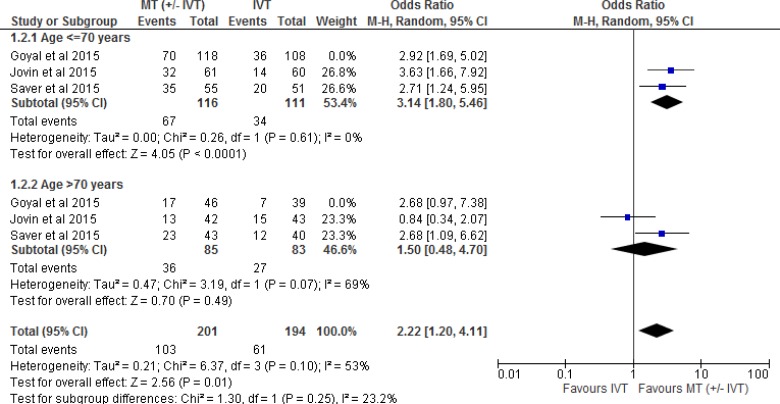
Two of the five RCTs28,31 included patients only if they could receive IVT. The other three RCTs included a combination of patients who were eligible or ineligible for IVT. Tables 3 and 4 show the percentage of patients who received or did not receive IVT in all five included RCTs. Therefore, we performed a separate meta-analysis to examine the effect of mechanical thrombectomy in patients who were IVT-eligible and -ineligible (Figure 3). We also completed two other sensitivity analyses to determine the proportion of patients with a score of 0 to 2 on the mRS by age (Figure 4) and occlusion site (Figure 5).
Figure 3: Mechanical Thrombectomy Versus BMT on the Proportion of Functionally Independent Patients at 90-Day Follow-up by Status of IVT.
Abbreviations: BMT, best medical therapy; CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
Figure 4: Mechanical Thrombectomy Versus IVT on the Proportion of Functionally Independent Patients at 90-Day Follow-up by Age.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
Note: Goyal et al used < 80 years and > 80 years in the age sensitivity analysis.
Figure 5: Mechanical Thrombectomy Versus IVT on the Proportion of Functionally Independent Patients at 90-Day Follow-up by Occlusion Site.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
Figure 3 shows the effect of mechanical thrombectomy and best medical therapy on functional independence by status of IVT. This was examined by pooling data from two studies with 132 IVT-ineligible patients and 385 IVT-eligible patients using a fixed-effects model. There was still a significant difference for functional independence in favour of those who received mechanical thrombectomy compared to best medical therapy, regardless of eligibility for IVT.
Figure 4 shows the effect of mechanical thrombectomy and IVT on functional independence by age. This was examined by pooling data from three studies with 453 patients age < 70 years and 253 patients age > 70 years using a random-effects model (chosen to comply with the I2 statistic of > 50%). There was still a significant difference for functional independence in favour of mechanical thrombectomy compared to IVT in patients age < 70 years, but that significant effect disappeared in patients age > 70 years. However, overall the effect estimate still favoured mechanical thrombectomy, regardless of age. We performed a meta-analysis without the Goyal et al (25) study because of the difference in age division (< 80 years vs. > 80 years), but the findings did not change (Appendix 3, Figure A2).
Figure 5 shows the effect of mechanical thrombectomy and IVT on functional independence by occlusion site. This was examined by pooling data from three studies with 499 patients with a middle cerebral artery (M1 or M2) occlusion and 139 patients with an internal carotid artery occlusion using a fixed-effects model. There was still a significant difference for functional independence in favour of those who received mechanical thrombectomy, regardless of occlusion site.
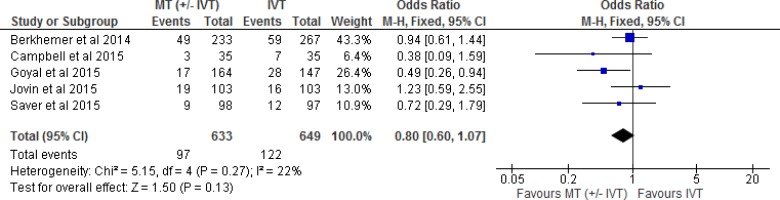
Mortality
All five included RCTs reported mortality as an outcome at 90-day follow-up. We examined the effect of mechanical thrombectomy on mortality by pooling data from five studies with 1,282 participants using a fixed-effects model (Figure 6). We did not observe a statistically significant difference in mortality for those who received mechanical thrombectomy or IVT. Goyal et al29 was the only RCT that showed a significant reduction in mortality in the mechanical thrombectomy group compared to IVT alone. That study had very similar inclusion criteria to the other RCTs, but the imaging-related selection criteria focused on a population with a small infarct core at baseline and moderate-to-good collateral circulation distal to the occlusion. Goyal et al29 also emphasized rapid endovascular treatment with quick process-time targets. This trial achieved shorter interval times than previous trials, with a median time from CT to reperfusion of 84 minutes. These factors may have contributed to the significant reduction in mortality in this RCT. The quality of evidence was “moderate” for mortality according to the GRADE system.
Figure 6: Mechanical Thrombectomy Versus IVT on Mortality at 90-Day Follow-up.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
Symptomatic Intracerebral Hemorrhage
All five included RCTs reported symptomatic intracerebral hemorrhage (SICH) as an adverse event outcome.
Berkhemer et al27 defined SICH as neurologic deterioration (an increase of 4 or more points in the score on the National Institutes of Health Stroke Scale [NIHSS]) and evidence of intracranial hemorrhage on imaging studies.
Jovin et al30 provided two sets of criteria for SICH: the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) and the second European-Australasian Acute Stroke Study (ECASS II). We chose to use the ECASS II criteria in this analysis because they were more aligned with those set out in the other RCTs; ECASS II defines SICH as any symptomatic intracranial hemorrhage and neurologic worsening of at least 4 points on the NIHSS.
Campbell et al28 defined symptomatic intracranial hemorrhage as a large parenchymal hematoma (blood clot occupying > 30% of infarct volume with mass effect) and an increase of 4 points or more in the NIHSS score.
Goyal et al29 and Saver et al31 stated that SICH was clinically determined at the study site as new intracranial hemorrhage proven on imaging and associated with and causing any degree of clinical neurological worsening.
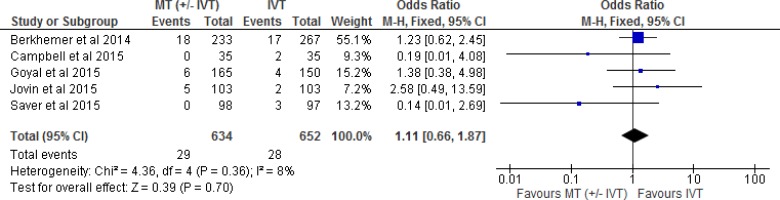
We examined the effect of mechanical thrombectomy on SICH by pooling data from five studies with 1,286 participants using a fixed-effects model (Figure 7). We did not observe a statistically significant difference for SICH between those who received mechanical thrombectomy or IVT. The quality of evidence was “moderate” for SICH according to the GRADE system.
Figure 7: Mechanical Thrombectomy Versus IVT on Symptomatic Intracerebral Hemorrhage.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
Quality of Life
Three25,27,28 of the five included RCTs reported quality of life as an outcome measured by EQ-5D, a standardized instrument for the measurement of health status. Scores range from –0.33 to 1.00, with higher scores indicating a better quality of life. However, Goyal et al29 reported only the EQ-5D visual analogue scale score, a continuous scale measure of self-reported quality of life; scores range from 0 to 100, with 0 indicating the worst possible quality of life and 100 the best possible quality of life. The three RCTs that measured quality of life reported the median and interquartile range (IQR) for both groups. To analyze the results of quality of life via meta-analysis, we would have had to convert the median and IQR to mean and standard deviation. Assuming normal distribution of the variable, the mean and median would be the same, and one can subtract the maximum and minimum value of the reported IQR and divide it by 1.34898 to get the standard deviation. However, authors often report medians because the data are skewed. Therefore, it was not appropriate to pool the results of this outcome; they are reported individually in Table 5.
Table 5:
Quality of Life (EQ-5D) in Included RCTs
| Author, Year | Intervention | Control | Effect Variable | Unadjusted Value (95% CI) | Adjusted Value (95% CI) |
|---|---|---|---|---|---|
| Berkhemer et al, 201527 | 0.69 (0.33–0.85)a | 0.66 (0.30–0.81) | Betab | 0.08 (0.00–0.15) | 0.06 (−0.01 to 0.13) |
| Goyal et al, 201529 | 80 (60–90)c | 65 (50–80) | Betab | 9.4 (3.5–15.2) | 9.9 (3.8–16.0) |
| Jovin et al, 201530 | 0.65 (0.21–0.79)a | 0.32 (0.13–0.70) | Betab | 0.13 (0.03–0.23) | 0.11 (0.02–0.21) |
Abbreviations: CI, confidence interval; EQ-5D, EuroQoL Group 5-Dimension Self-Report Questionnaire; IQR, interquartile range; RCT, randomized controlled trial.
EQ-5D index score presented as median (IQR).
Regression was used analyze the effect.
EQ-5D visual-analogue scale score presented as median (IQR).
Quality of life was measured in a linear regression model in the three RCTs in Table 5. Only Goyal et al29 stated that they had conducted a simple linear regression for quality of life. No P-values were reported for this outcome in any study. When looking at the 95% confidence intervals in the “Adjusted Value” column, Goyal29 and Jovin30 showed statistically significantly higher quality of life scores associated with mechanical thrombectomy. The quality of evidence was “moderate” for quality of life according to the GRADE system.
Recanalization and Reperfusion
Three of the five included RCTs reported recanalization as an outcome measure. Both Berkhemer et al27 and Goyal et al29 used the modified Arterial Occlusion Lesion score, where a score of 2 or 3 indicates partial or complete recanalization. Campbell et al28 defined recanalization as a Thrombolysis in Myocardial Infarction (TIMI) score of 2 or 3 (partial or complete restoration of flow at the site of arterial occlusion). In Berkhemer et al,27 recanalization rates were significantly higher in the intervention group (no P-value provided). In Campbell et al,28 recanalization rates were also significantly higher in the intervention group (P < 0.001). In Goyal et al,29 recanalization was measured only in the control group, so no comparison between groups could be made. The quality of evidence was “low” for recanalization according to the GRADE system. The results for recanalization are shown in Table 6.
Table 6:
Recanalization in Included RCTs
| Author, Year | Intervention | Control | Effect Variable | Unadjusted Value (95% CI) | Adjusted Value (95% CI) |
|---|---|---|---|---|---|
| Berkhemer et al, 201527 | 141/187 (75.4)a | 68/207 (32.9)a | Odds ratio | 6.27 (4.03–9.74) | 6.88 (4.34–10.94) |
| Campbell et al, 201528 | 33 (94)b | 15 (43)b | Odds ratio | 22.0 (4.5–106.0)c | 29.0 (5.4–155.0)c |
| Goyal et al, 201529 | NR | 43/138 (31.2)d: 41/110 (37.3) with IVT 2/28 (7) without IVT |
NR | NR | NR |
Abbreviations: AOL, Arterial Occlusive Lesion; CI, confidence interval; IVT, intravenous thrombolysis; NR, not reported; RCT, randomized controlled trial; TIMI, Thrombolysis in Myocardial Infarction.
Measured as number of patients with no intracranial occlusion on follow-up CT angiography: number/total number (%). Data for follow-up computed tomography angiography were not available for 106 patients, owing to imminent death or death (24 patients), decreased kidney function (13 patients), insufficient scan quality (5 patients), and other reasons (64 patients).
Recanalization was defined as a TIMI score of 2 or 3 (partial or complete restoration of flow at the site of arterial occlusion) and measured at 24 hours. This analysis was adjusted for the site of vessel occlusion at baseline.
P < 0.001.
Recanalization was measured by a modified AOL score. A score of 2 or 3 indicates partial or complete recanalization: number/total number (%).
All five included RCTs reported reperfusion as an outcome measure. Campbell et al28 defined reperfusion as the percentage reduction in the perfusion-lesion volume between initial imaging and 24-hour imaging. This value can be negative if hypoperfusion becomes more severe over time. Berkhemer et al,27 Goyal et al,29 Jovin et al,30 and Saver et al31 measured reperfusion using the Thrombolysis in Cerebral Infarction (TICI) score. TICI categories span from no perfusion (grade 0) to complete perfusion (grade 3). The “partial perfusion” category (grade 2) is defined as cases in which contrast passes the obstruction but with rates of entry and washout slower than normal and is subdivided into two subcategories (2a and 2b). A score of 2b or 3 indicated successful reperfusion in this study. Berkhemer et al,27 Goyal et al,29 and Jovin et al30 measured reperfusion in the intervention arm only, so no comparison could be made between groups. In Campbell et al,28 reperfusion rates were significantly higher in the intervention arm (89%) than in the control arm (43%) (P < 0.001). In Saver et al,31 reperfusion rates were significantly higher in the intervention arm (83%) than in the control group (40%) (P < 0.001). The quality of evidence was “moderate” for reperfusion according to the GRADE system. The results for reperfusion are presented in Table 7.
Table 7:
Reperfusion in Included RCTs
| Author, Year | Intervention | Control | Effect Variable | Unadjusted Value (95% CI) | Adjusted Value (95% CI) |
|---|---|---|---|---|---|
| Berkhemer et al, 201527 | 115/196 (58.7)a | NR | NR | NR | NR |
| Campbell et al, 201528 | 100 (100–100)b | 37 (−0.5 to 96) | Odds ratio | 4.9 (2.5–9.5)c | 4.7 (2.5–9.0)c |
| Goyal et al, 201529 | 113/156 (72.4)a: 79/112 (70.5) with IVT 34/44 (77) without IVT |
NR | NR | NR | NR |
| Jovin et al, 201530 | 67/102 (65.7)a | NR | NR | NR | NR |
| Saver et al, 201531 | 73/83 (88)a 53/64 (83)d |
NR 21/52 (40) |
NR Risk ratio |
NR 2.05 (1.45–2.91)c |
NR NR |
Abbreviations: CI, confidence interval, IVT, intravenous thrombolysis, NR, not reported; RCT, randomized controlled trial; TICI, Thrombolysis in Cerebral Infarction.
Reperfusion was measured by the TICI score, where a score of 2b or 3 indicated complete filling of the expected vascular territory: number/total number (%).
Reperfusion was defined as the percentage reduction in the perfusion-lesion volume between initial imaging and 24-hour imaging (interquartile range). This value can be negative if hypoperfusion becomes more severe over time. This analysis was adjusted for the site of vessel occlusion at baseline.
P<0.001.
Saver et al (32) also reported successful reperfusion at 27 hours: number/total number (%). Successful reperfusion was defined as reperfusion of at least 90%, as assessed with the use of perfusion computed tomography or magnetic resonance imaging. Data on successful reperfusion were not obtained for all patients after the adoption of the protocol amendment making penumbral imaging optional.
Limitations
In the literature there was variability in the choice of revascularization scales (in this case, reperfusion and recanalization). Reperfusion means reflow into the arterial tree and evidence of flow at the capillary level; recanalization means opening of the blocked major artery. A patient can have recanalization without reperfusion. The TICI scale is meant to measure reperfusion and the Arterial Occlusive Lesion scale is meant to measure recanalization, but these scales are inconsistently described and applied in the literature.32 Also, the timing of measurement is critical. Reperfusion should be measured soon after treatment, because reporting it early is clinically meaningful. Some studies report reperfusion rates up to 24 hours after treatment takes place, which can be misleading, since rates can be high (upwards of 80%) but the patient may not have a favourable functional outcome.
Four of the five included RCTs were stopped early based on prespecified boundaries of efficacy that had been crossed28,29,31 or the emerging results of other studies.30 This can be problematic, as early termination of studies can overestimate effect size.
Discussion
Process Times
Table 8 describes important process times when conducting endovascular treatment. Time to reperfusion was lower in the included RCTs than in earlier trials18–20 (except for Jovin et al,30 where time to reperfusion was longer: 355 minutes). Although times to groin puncture were not significantly different from the Interventional Management of Stroke (IMS III) study (186 to 210 minutes), times to reperfusion were lower in Goyal et al29 (241 minutes) and Campbell et al28 (248 minutes) compared to IMS III (324 minutes).21
Table 8:
Process Times for Endovascular Treatmenta
| Author, Year | Stroke Onset to IVT Initiation, Intervention/Control | Stroke Onset to Groin Puncture | Stroke Onset to Reperfusion | Hospital Arrival to Groin Puncture | Imaging to Groin Puncture | Imaging to Reperfusion |
|---|---|---|---|---|---|---|
| Berkhemer et al, 201527 | 85 (67–110)/87 (65–116) | 260 (210–313) | NR | NR | NR | NR |
| Campbell et al, 201528 | NR | 210 (166–251) | 248 (204–277) | 113 (83–159) | 93 (71–138) | NR |
| Goyal et al, 201529 | 110 (80–142)/125 (89–183) | NR | 241 (176–359) | NR | 51 (39–68) | 84 (65–115) |
| Jovin et al, 201530 | 117.5 (90–150)/105.0 (86–137.5) | 269 (201–340) | 355 (269–430) | NR | NR | NR |
| Saver et al, 201531 | NR | 224 (165–275) | NR | 90 (69–120) | 57 (40–80) | NR |
Abbreviations: IVT, intravenous thrombolysis; NR, not reported.
All process times were measured in minutes and are presented as median (interquartile range).
The fact that Jovin et al30 had a time to reperfusion that was comparable to earlier trials may explain some of the observed heterogeneity in outcome effects compared to the other included RCTs (since the inclusion criteria were very similar to the other included RCTs). In some of the meta-analyses, the effect estimates from this RCT were closer to no effect for mechanical thrombectomy than in the other RCTs.
Importance of Imaging Prior to Mechanical Thrombectomy
To improve patient selection, the included RCTs stated the importance of imaging. Sites in the included RCTs used non-contrast computed tomography or magnetic resonance imaging (MRI). Sites also required the following imaging for patient assessment: diffusion weighted imaging, perfusion weighted imaging, magnetic resonance angiography, MRI or computed tomography angiography, perfusion CT.
Imaging is used to identify the following:
Location of occlusion and extent of penumbra.
Infarct extension (based on the Alberta Stroke Program Early Computed Tomography Score [ASPECTS] in four out of the five RCTs where patients with a hypodensity area greater than one-third of the middle cerebral artery territory or with ASPECTS < 7 should not be treated).
Presence of collaterals.
Extent of tissue-at risk or irreversible ischemia (for patients with symptom onset to qualifying imaging of > 4.5 hours).
Goyal et al29 used a novel technique—multi-phase computed tomography angiography—to identify patients in a simple, timely manner. The authors addressed the imaging criteria above and interpreted the data in less than 10 minutes.
Potential Increase in Mechanical Thrombectomy Cases
Only 1.1% of patients with acute ischemic stroke received endovascular treatment in Ontario in the 2012/13 fiscal year.23 This is because endovascular treatment was used primarily in research trials or for rare cases when IVT was contraindicated. However, experts have stated that since the technology has demonstrated a beneficial effect in the RCTs examined in this report, the number of eligible patients has increased, and that as many as 10% of all acute ischemic stroke patients may have intracranial artery occlusion that could be considered for endovascular treatment.
Conclusions
-
Compared with IVT:
-
○
High quality evidence showed a significant difference in functional independence among patients with acute ischemic stroke who received mechanical thrombectomy (with or without IVT).
-
○
Moderate quality evidence showed no significant difference in mortality among patients with acute ischemic stroke who received mechanical thrombectomy (with or without IVT).
-
○
Moderate quality evidence showed no significant difference in symptomatic intracranial hemorrhage among patients with acute ischemic stroke who received mechanical thrombectomy (with or without IVT).
-
○
Moderate quality evidence showed higher quality-of-life scores in the mechanical thrombectomy group (with or without IVT) in two RCTs.
-
○
Moderate quality evidence showed higher reperfusion rates in the mechanical thrombectomy group (with or without IVT) in two RCTs.
-
○
Low quality evidence showed higher recanalization rates in the mechanical thrombectomy group (with or without IVT) in two RCTs.
-
○
REVIEW OF THE ECONOMIC LITERATURE
Objective
The objective of this analysis was to review the published economic evidence on the cost-effectiveness of mechanical thrombectomy (with or without IVT) versus IVT or medical therapy in patients with acute ischemic stroke.
Methods
Sources
We performed an economic literature search on March 23, 2015, using Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid Embase, and the Cochrane Library for studies published up to March 23, 2015. Reference lists were also examined for any additional relevant studies not identified through the search. We also carried out an informal search using Google and PubMed for additional economic studies. The date of the last informal search was June 1, 2015.
Search Strategy
We based our search terms on those used in the clinical evidence review, above, and applied economic filters to the search results. Study eligibility criteria for the literature search are listed below. Appendix 4 provides details of the search strategies.
Inclusion Criteria
English-language full-text publications
Studies published up to March 23, 2015
Studies comparing mechanical thrombectomy (with or without IVT) with IVT alone or medical therapy
Cost-utility analyses, regardless of location
Any type of economic studies (i.e., cost-utility analyses, cost-effectiveness analyses, cost-benefit analyses, budget impact analyses, and cost analyses) in Canada
Study follow-up time (or the time horizon in the modelling study) of 1 year or greater
Exclusion Criteria
Abstracts, letters, editorials, and unpublished studies
Literature Screening
A single reviewer reviewed abstracts and, for those studies meeting the eligibility criteria, we obtained full-text articles.
Results
After removing duplicates (n = 15), the database search yielded 162 citations. Eleven full-text articles were retrieved for review, and four met the inclusion criteria.33–36 One more article (published after the date of formal literature search) was identified during the informal literature search.37 The five included articles were all cost-utility analyses: four from the United States,34–37 and one from the Netherlands.33 There were no Canadian HTA reports or economic analyses. Table 9 provides a summary of the included five studies.
Table 9:
Results of Economic Literature Review—Summary
| Name, Year | Study Design and Perspective | Population | Interventions Comparators | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| Leppert et al, 201537 | Type of analysis: CUA Study design: decision-analytic model Perspective: payer, United States Time horizon: lifetime |
Adults with an acute large-artery ischemic stroke; see MR CLEAN study for details38 | IV tPA IV tPA plus MT |
QALY gained: 0.70 Total QALYs: 3.10 (IV tPA); 3.80 (IV tPA plus MT) Annual discount rate: 3% |
Cost year: 2012 Incremental cost: $9,911 USD Total costs: $130,144 USD (IV tPA); $140,055 USD (IV tPA plus MT) Annual discount rate: 3% |
ICER: $14,137 USD per QALY gained |
| Bouvy et al, 201333 | Type of analysis: CUA Study design: decision-analytic model Perspective: health sector, Netherlands Time horizon: lifetime |
Patients with a clinical diagnosis of ischemic stroke, and no contraindications for IVT or MT | Medical therapy IVT IA thrombolysisa IV-IA thrombolysis |
QALY gained: 0.28 (IA thrombolysis vs. medical therapy); 0.11 (IV-IA thrombolysis vs. IVT) Total QALYs: 3.39 (medical therapy); 3.61 (IVT); 3.67 (IA thrombolysis); 3.72 (IV-IA thrombolysis) Annual discount rate: 3% |
Cost year: 2010 Incremental cost: –€1,983 (IA thrombolysis vs. medical therapy); €222 (IV-IA thrombolysis vs. IVT) Total costs: €34,182 (medical therapy); €32,113 (IVT); €32,199 (IA thrombolysis); €32,335 (IV-IA thrombolysis) Annual discount rate: 3% |
ICER: dominant (IA thrombolysis vs. medical therapy); €1,922 per QALY gained (IV-IA thrombolysis vs. IVT) |
| Nguyen-Huynh et al, 201134 | Type of analysis: CUA Study design: decision-analytic model Perspective: society, United States Time horizon: lifetime |
65-year-old men or women with acute ischemic stroke and an occlusion of a major intracranial artery, but not eligible for IV tPA | Best medical therapy Neurointerventional radiology, typically MT |
QALY gained: 0.82 Total QALYs: NA Annual discount rate: 3% |
Cost year: 2009 Incremental cost: $7,718 USD Total costs: NA Annual discount rate: 3% |
ICER: $9,386 USD per QALY gained |
| Kim et al, 201135 | Type of analysis: CUA Study design: decision-analytic model Perspective: payer, United States Time horizon: lifetime |
Hypothetical 68-year-old patient with an acute large-artery ischemic stroke who was eligible for IV tPA | IV tPA IV tPA plus MT |
QALY gained: 0.68 Total QALYs: NA Annual discount rate: 3% |
Cost year: 2009 Incremental cost: $10,840 USD Total costs: NA Annual discount rate: 3% |
ICER: $16,001 USD per QALY gained |
| Patil et al, 200936 | Type of analysis: CUA Study design: decision-analytic model Perspective: payer, United States Time horizon: 20 years |
Hypothetical 67-year-old patient with a large-artery ischemic stroke who was ineligible for IV tPA | Best medical therapy MT | QALY gained: 0.54 Total QALYs: 1.83 (best medical therapy); 2.37 (MT) Annual discount rate: 3% |
Cost year: 2008 Incremental cost: $6,600 USD Total costs: $142,000 USD (best medical therapy); $148,600 USD (MT) Annual discount rate: 3% |
ICER: $12,120 USD per QALY gained |
Abbreviations: CUA, cost-utility analysis; IA, intra-arterial; ICER, incremental cost-effectiveness ratio; IV, intravenous; IVT, intravenous thrombolysis; IV tPA, intravenous tissue plasminogen activator; MT, mechanical thrombectomy; NA, not applicable; QALY, quality-adjusted life-year.
50% of patients underwent treatment using a retrievable stent.
All five studies used modelling approaches to estimate the cost-effectiveness of mechanical thrombectomy. Three33,34,36 compared mechanical thrombectomy with medical therapy for those who were not eligible for intravenous tissue plasminogen activator, and three33,35,37 compared mechanical thrombectomy (with or without IVT) with IVT alone. The Dutch study33 included both comparisons. The estimated efficacy of mechanical thrombectomy was based on a single randomized controlled trial (RCT)38 in the most recent cost-utility analysis37; efficacy in the other four analyses came from observational studies.33–36 Although not reported explicitly, mechanical thrombectomy devices in the four earlier studies33–36 were likely to be older-generation ones; only the most recent study37 used newer-generation devices. Nevertheless, the conclusions in all five economic studies were similar: compared with IVT or medical therapy, mechanical thrombectomy (with or without IVT) was cost-effective.
Discussion and Conclusions
The four earlier studies investigated the cost-effectiveness of mechanical thrombectomy,33–36 but they used older-generation devices and the health benefit was based on observational studies (no RCTs were available at that time). Later RCTs have failed to demonstrate the benefits of the older-generation mechanical thrombectomy devices.39–41 Although the results of the four earlier studies also showed that mechanical thrombectomy was cost-effective compared to IVT or medical therapy (from dominant to an ICER of $16,000 USD per QALY gained), the health outcomes in these models contradicted the evidence from RCTs for old generation MT.39–41
In summary, a single health economic study of mechanical thrombectomy with new-generation devices showed the cost-effectiveness of this treatment in the United States.
PRIMARY ECONOMIC EVALUATION
The published economic evaluations identified in the literature review addressed the interventions of interest, but none of them took a Canadian perspective. Also, the efficacy of mechanical thrombectomy treatment in published health economic studies was based on either a single RCT38 or on earlier observational studies. Five RCTs of new-generation mechanical thrombectomy devices versus IVT were published in 201528–31,38; using this updated high-level evidence, we conducted a cost-utility analysis.
Objective
The objective of this analysis was to assess the cost-effectiveness (incremental cost per quality-adjusted life-year [QALY] gained) of mechanical thrombectomy (with or without IVT) compared with IVT alone within the context of the Ontario Ministry of Health and Long-Term Care.
Methods
Type of Analysis
We conducted a cost-utility analysis. We developed a Markov decision-analytic model to capture the long-term clinical and economic outcomes of mechanical thrombectomy and IVT. Clinical outcomes for the first 90 days were based on the clinical evidence review above. Long-term outcomes (after 3 months) were based on a large cohort of stroke patients in the United Kingdom.42,43 The inputs for health utility44 and costs45 were estimated from published data.
Target Population
The target population was adults who had acute large-artery ischemic stroke with moderate-to-severe neurologic deficits. According to the baseline characteristics of the five recent RCTs, the mean age of the target population was 65 to 70 years old, and about 50% were male. Patients must have had the occlusion confirmed by imaging, and have been functioning independently before the stroke.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Interventions
The intervention of interest was endovascular treatment via new-generation mechanical thrombectomy devices, with or without IVT, for acute large-artery ischemic stroke patients in Ontario. Mechanical thrombectomy can be performed using stent retrievers and thromboaspiration. IVT was selected as the comparator because it is the first-line therapy in Ontario at present. In our model, mechanical thrombectomy (with or without IVT) or IVT alone were the expected treatments. More than 70% of patients in the RCTs received IVT in both study arms, and more than 80% of patients received mechanical thrombectomy in the mechanical thrombectomy arm.
Discounting and Time Horizon
We applied an annual discount rate of 5% to both costs and QALYs, following the guidelines from Canadian Agency for Drugs and Technologies in Health.46
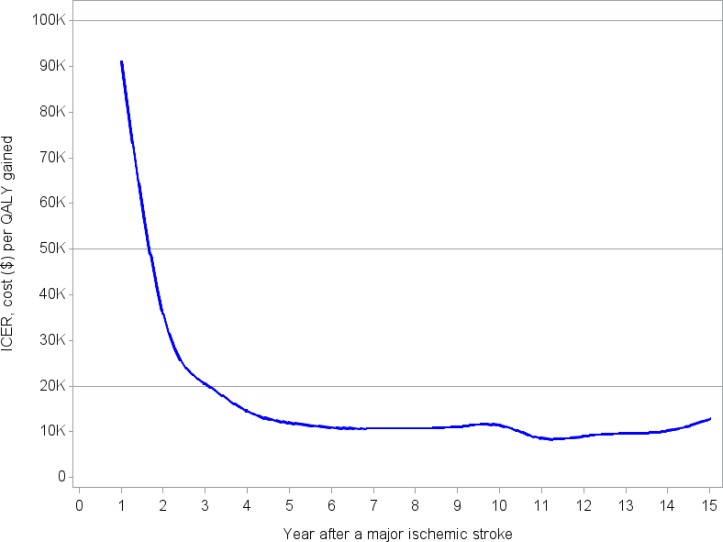
Follow-up time in the five recent RCTs was only 90 days, but the preferred time horizon for economic evaluations is lifetime. Since there were considerable uncertainties related to the long-term outcomes of both treatment strategies, we selected a time horizon of 5 years for the base-case analysis, and 10 and 15 years for the sensitivity analysis (15 years being close to a lifetime time horizon). All costs are expressed in 2015 Canadian dollars.47
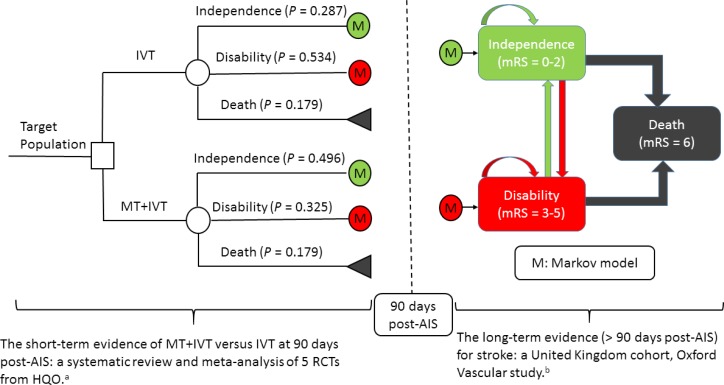
Model Structure
We developed a Markov decision-analytic model to assess the cost-effectiveness of mechanical thrombectomy (with or without IVT) versus IVT alone (Figure 8). The model combined a decision tree for the first 3 months post-stroke and a Markov model for those at risk after 3 months. The Markov model consists of three mutually exclusive health states: functional independence (modified Rankin Scale [mRS] 0 to 2), disability (mRS 3 to 5), and death (mRS 6). Target patients would receive mechanical thrombectomy (with or without IVT) or IVT alone, and they would be functionally independent, disabled, or dead at 90 days. Survivors at 90 days would join the corresponding health state in the Markov model. Patients could transfer between health states or stay in the same health state at the end of the monthly cycle, with assigned probabilities. Arrows indicate pathways. In the model, patients could recover from disability to functional independence during the first year following a stroke, but not after the first year.
Figure 8: Mechanical Thrombectomy (With or Without IVT) Versus IVT Alone for Acute Ischemic Stroke, Decision-Analytic Model.
Abbreviations: AIS, acute ischemic stroke; HQO, Health Quality Ontario; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; MT, mechanical thrombectomy; RCT, randomized controlled trial.
aSee clinical evidence review, above.
Main Assumptions
The goal of this analysis was to estimate the differences in costs and utility between the two treatments and calculate the resulting incremental cost-effectiveness ratios (ICERs). For simplicity, we ignored symptomatic intracerebral hemorrhage and related health care resource use, which were likely to be similar in both arms (see clinical evidence review, above). We assumed the following:
Compared with IVT, mechanical thrombectomy can reduce the risk of disability at 90 days, but not mortality, based on the clinical evidence review, above. (Note: we released this assumption in the sensitivity analysis.)
Patients’ long-term health outcomes (i.e., more than 3 months after a major stroke) would be conditional on their health status at 3 months (i.e., functional independence or disability).
Disability is associated with increased risk of mortality and reduced health-related quality of life. Mechanical thrombectomy would lead to life-years gained and QALYs gained over the long term, because it is associated with a lower risk of disability.
Model Input Parameters
We obtained data from the best available evidence, with an emphasis on results from the clinical evidence review, above. When necessary, we contacted authors to clarify details from their publications. When we could not obtain the desired estimates, we supplemented and/or adapted available data after discussion with clinical experts. We also consulted experts to validate our parameter estimates.
Intervention Summary Estimates (First 3 Months)
All-cause mortality: The clinical evidence review showed a nonsignificant reduction in mortality for mechanical thrombectomy versus IVT (odds ratio [OR] 0.80; 95% confidence interval [CI]: 0.60–1.07).28–31,38 There was insufficient evidence to demonstrate a survival benefit for mechanical thrombectomy versus IVT at 90 days, so we assigned the same mortality rate to both arms (17.86%), based on the pooled mortality rate in the control arms of the five recent RCTS (Table 10).
Functional independence: The clinical evidence review showed that compared with IVT, mechanical thrombectomy substantially increased the likelihood of functional independence at 90 days, with an OR of 2.39 (95% CI, 1.88–3.04).28–31,38 The pooled estimate of the proportion of functionally independent patients in the IVT group was 0.2874 (95% CI, 0.2180–0.3567). Given the OR of 2.39, we estimated that the proportion of functionally independent patients in the mechanical thrombectomy group to be 0.4908 (Table 10).
Health utility: Two RCTs reported the EuroQoL Group 5-Dimension Self-Report Questionnaire (EQ-5D) utility at 90 days after a stroke, but none reported baseline utility or QALYs. The beta coefficient in the linear regression model of utility was the expected mean difference between two treatments. The adjusted beta coefficients (95% CI) in Berkhemer et al 201538 and Jovin et al 201530 were 0.06 (−0.01 to 0.13) and 0.11 (0.02–0.21), respectively, favouring mechanical thrombectomy. The pooled estimate showed that mechanical thrombectomy increased health utility by 0.0735 (95% CI, 0.014–0.133) at 90 days (Table 10). We assumed that the two arms had the same baseline utility, but over time, the difference would increase linearly to 0.0735 at 90 days post-stroke. Thus, we expected that the mechanical thrombectomy arm would lead to 0.008 QALYs gained in the first 3 months (((0+0.0735)/2) × 0.25) × (1–0.1786).
Table 10:
Mechanical Thrombectomy Versus IVT 90 Days After Acute Ischemic Stroke
| Treatment | All-Cause Mortality, % | Functional Independence, % | Mean Difference in Health Utility | Reference |
|---|---|---|---|---|
| IVT | 17.86 | 28.74 | — | 28–31,38 |
| MT + IVT | 17.86 | 49.08 | 0.0735 | 28–31,38 |
Abbreviations: MT, mechanical thrombectomy; IVT, intravenous thrombolysis.
Natural History (3 Months Post-Stroke)
Since the follow-up time of the direct scientific evidence from the five recent RCTs was only 90 days,28–31,38 we used evidence from other sources to project longer-term outcomes. We assumed that patients’ health outcomes at > 3 months after an acute ischemic stroke would be independent of earlier treatments but conditional on their health status at 90 days after the stroke.
Reliable evidence of long-term outcomes post-stroke is relatively sparse. For our model inputs, we used evidence from the Oxford Vascular Study, a large cohort study from the United Kingdom.42,43 However, although this study presented a survival curve for 5 years and the proportion of survivor disability at different time points, it did not provide accurate estimates of the transition probabilities between health states (disability to functional independence, disability to death, and functional independence to disability) that would contribute to changes in proportions over time. For this reason, we used a calibration approach to estimate the time-dependent monthly transition probabilities.
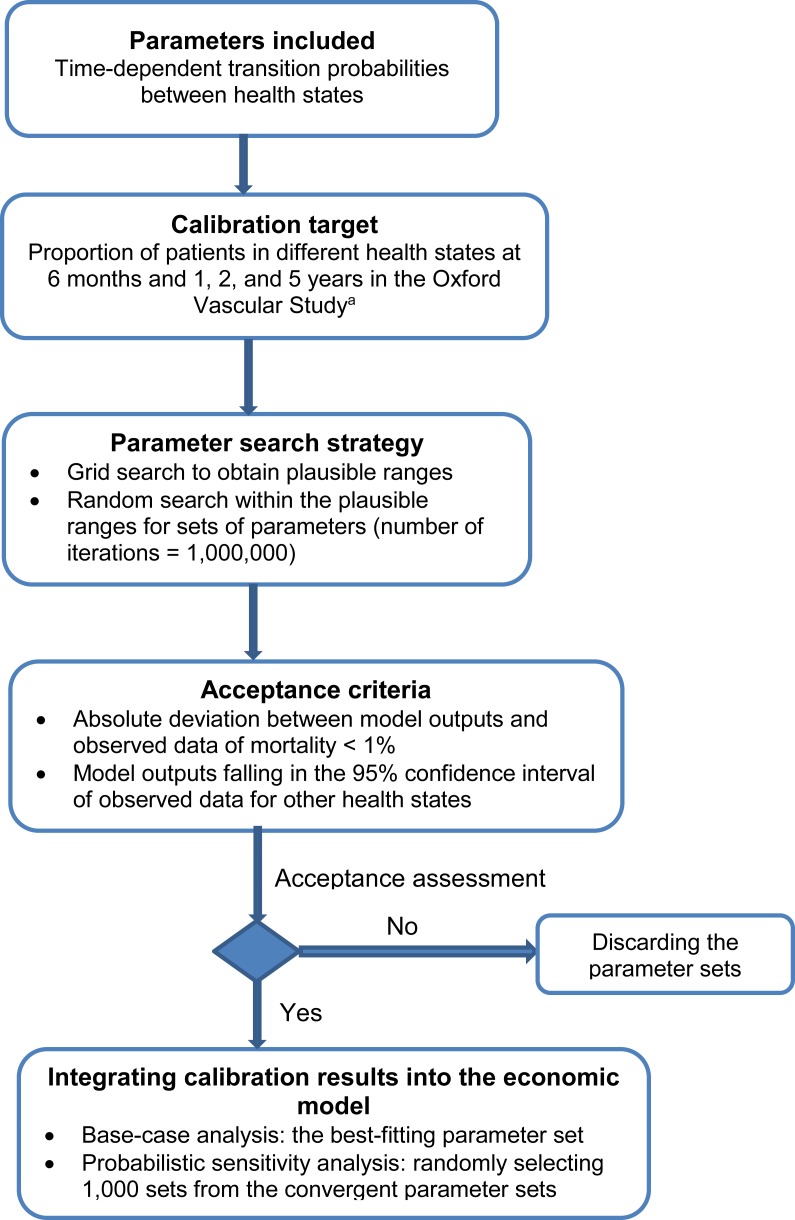
We calibrated the parameters for the Markov model using the seven-step approach introduced by Vanni et al.48 A brief summary of the calibration process is provided in Figure 9. See Appendix 5 for a full description.
Figure 9: Calibration for Time-Dependent Transition Probabilities: Summary.
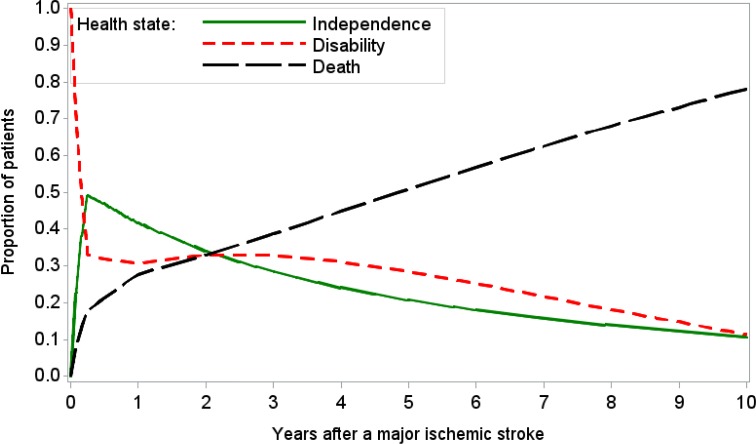
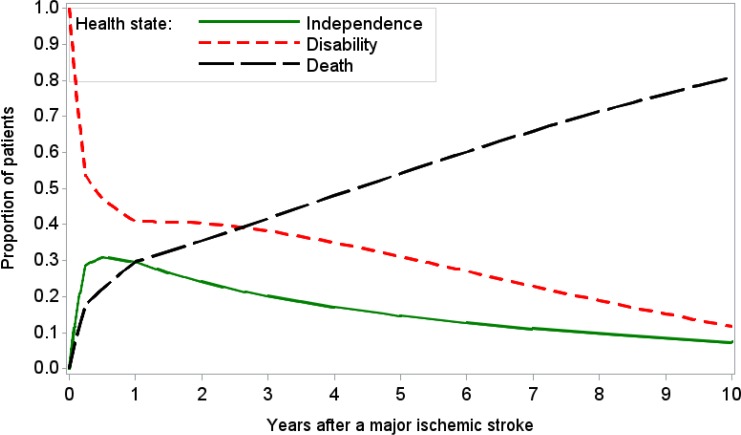
First, we defined the parameters for estimating time-dependent transition probabilities and selected the proportions of mortality, functional independence, and disability at 6 months, 1 year, 2 years, and 5 years in the moderate stroke group of the Oxford Vascular Study as the calibration targets.42,43 We used a grid search to obtain plausible ranges for each parameter, and then simulated 1,000,000 parameter sets by sampling values from plausible ranges. We assessed the goodness of fit (i.e., absolute deviation and sum of squares due to error) for the model output produced by each parameter set. We used the best-fitting parameter set (i.e., minimal sum of squares due to error) as the base case, and randomly selected 1,000 parameter sets from those meeting the acceptance criteria in the probabilistic sensitivity analysis. See Table 11 for the transition probabilities of the best-fitting model. Appendix 5 provides more details about the calibration process.
Table 11:
Calibrated Monthly Transition Probabilities, Natural History of Stroke
| Transition | Probability Per Cycle Per Month | References |
|---|---|---|
| Functional independence to disability | ||
| 4–6 months | 0.0321 | 42,43,49,50 |
| 7–12 months | 0.0220 | 42,43,49,50 |
| 13–24 months | 0.0134 | 42,43,49,50 |
| 25–36 months | 0.0111 | 42,43,49,50 |
| 37–48 months | 0.0093 | 42,43,49,50 |
| 49–60 months | 0.0077 | 42,43,49,50 |
| Disability to functional independence | ||
| 4–6 months | 0.0372 | 49 |
| 7–12 months | 0.0156 | 49 |
| 13–60 months | 0 | Assumptiona |
| Functional independence to death | ||
| 4–12 months | 0.0080 | 42,43,49,50 |
| 13–24 months | 0.0034 | 42,43,49,50 |
| 25–36 months | 0.0039 | 42,43,49,50 |
| 37–48 months | 0.0043 | 42,43,49,50 |
| 49–60 months | 0.0047 | 42,43,49,50 |
| Disability to death | ||
| 4–12 months | 0.0229 | 42,43,49,50 |
| 13–24 months | 0.0096 | 42,43,49,50 |
| 25–36 months | 0.0108 | 42,43,49,50 |
| 37–48 months | 0.0122 | 42,43,49,50 |
| 49–60 months | 0.0131 | 42,43,49,50 |
Evidence suggested that the chance of patients recovering from disability to functional independence 1 year after an acute stroke was small.49
Costs of the Disease and Intervention Under Evaluation
Staff at one hospital in Ontario estimated their costs per procedure to be approximately $10,000. However, it was difficult to make an exact estimate of the additional cost of mechanical thrombectomy relative to IVT, since besides materials and staffing, mechanical thrombectomy might also affect intensive care unit time, angiography suite time, recovery room time, screening imagery, and physician fees (e.g., neuroradiologist). We reviewed the published health economic studies to determine specific intervention costs. We assumed that extra hospitalization costs due to mechanical thrombectomy were the difference in hospitalization costs between mechanical thrombectomy and IVT groups. We converted international costs to Canadian dollars for the corresponding year, and then adjusted them to 2015 Canadian dollars.47 Except for an apparent outlier,33 which assumed that 50% of patients used a retrievable stent for intra-arterial thrombolysis, the incremental hospitalization cost for mechanical thrombectomy versus control in most studies ranged from $10,000 to $20,00034–37,51–53 (Appendix 6). Therefore, we estimated that compared with IVT, mechanical thrombectomy led to an additional $15,000 ($10,000 to $20,000 in sensitivity analyses) in hospitalization costs for Ontario.
Costs of stroke were based on the Economic Burden of Ischemic Stroke (BURST) study,45 a prospective cohort study of ischemic stroke patients in 12 Canadian stroke centres. Authors divided costs into direct costs (such as emergency services, hospitalizations, rehabilitation, physician services, diagnostics, medications, etc.) and indirect costs (such as productivity loss and resource use for unpaid caregivers). They stratified the costs for disability status measured at discharge—nondisabling stroke (mRS 0–2) and disabling stroke (mRS 3–5)—and reported the average costs for preadmission and hospitalization to 3 months, 4 to 6 months, and 7 to 12 months. We considered direct costs from the BURST study in the base-case analysis, and direct plus indirect costs as the costs from a societal perspective in the sensitivity analysis. We also adapted the results from the BURST study to fit our model (for example, combining 4 to 6 months and 7 to 12 months to estimate the average monthly cost from 4 to 12 months) (Table 12). We did not assign explicit probabilities of recurrent ischemic stroke in our model, but the monthly cost in two health states would include the hospitalization costs for stroke recurrence.
Table 12:
Costs in the Economic Modela
| Health Care Costs, $ | Costs From a Societal Perspective, $ | Reference | |
|---|---|---|---|
| First 3 months after stroke | |||
| Functional independence (mRS 0–2) | 18,852 | 21,471 | 45 |
| Disability (mRS 3–6) | 57,382 | 65,355 | 45 |
| IVT alone | 46,308 (18,852 × 0.2874 + 57,382 × 0.7126) | 52,743 | 34–37,45,51–53 |
| MT+IVT | 53,471 (15,000 + 18,852 × 0.4908 + 57,382 × 0.5092) | 58,817 | 34–37,45,51–53 |
| More than 3 months after strokeb | |||
| Functional independence (mRS 0–2) | 1,384 per month | 2,647 per month | 45 |
| Disability (mRS of 3–5) | 3,080 per month | 5,913 per month | 45 |
Abbreviations: IVT, intravenous thrombolysis; mRS, modified Rankin Scale; MT, mechanical thrombectomy.
All costs in 2015 Canadian dollars.
Costs included home care, rehabilitation, hospitalization (e.g., for recurrence of stroke), physician fees, medications, etc.
Health Utilities
Some factors significantly affected health utilities for stroke patients, including stroke severity, comorbidity, and age.43 For simplicity, we considered stroke severity only for functional independence and disability, and used the EQ-5D utility from Dorman et al44 for > 3 months post-stroke (Table 13). The estimates of the difference in utility and QALYs gained for the first 3 months are described in Model Input Parameters, above.
Table 13:
Utility for > 3 Months After Stroke
| Mean Utility (95% CI) | Reference | |
|---|---|---|
| Functional independence (mRS 0–2) | 0.71 (0.68–0.74) | 44 |
| Disability (mRS 3–5) | 0.31 (0.29–0.34) | 44 |
Abbreviation: CI, confidence interval; mRS, modified Rankin Scale.
Inputs From the ESCAPE Trial for Scenario Analysis
The patient management flow and the population in the Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE) trial were slightly different from the other four RCTs. The ESCAPE trial also demonstrated statistically significant survival benefits of mechanical thrombectomy treatment at 90 days after an acute ischemic stroke (19% for intervention versus 10.4% for control).29 The proportions of patients with an mRS of 0 to 2 were 53% and 29.3% for the intervention and control arms, respectively. The first 3 months of health care costs were $51,961 for the intervention and $46,093 for control.
Distribution of Model Inputs for Probabilistic Sensitivity Analysis
We conducted the Monte Carlo simulation (1,000 iterations) for the probabilistic sensitivity analysis. Distributions of the major parameters are listed in Table 14; additional details are available on request.
Table 14:
Parameter Distributions
| Parameter | Distribution |
|---|---|
| Mortality and functional independence at 90 days | Beta |
| Effectiveness of mechanical thrombectomy (OR of MT+IVT vs. IVT alone) | Log normal |
| Natural disease history after 3 months of stroke | Calibration |
| Costsa | Gamma |
| Utility | Beta |
Abbreviations: IVT, intravenous thrombolysis; MT, mechanical thrombectomy; OR, odds ratio.
The BURST study45 did not report 95% confidence intervals or standard errors for cost estimates. We assumed that the standard error was equal to a 20% mean in the probabilistic sensitivity analysis.
Variability and Uncertainty
We conducted a scenario analysis based on the ESCAPE trial.29 Eleven health centres in Canada participated in this trial, so it was closer to the stroke work flow in Ontario than the other trials. It was also one of the only studies that included patients with proximal occlusions and contraindications to intravenous tissue plasminogen activator, an important population that is likely to receive the most benefit from mechanical thrombectomy.
We also conducted one-way and multi-way sensitivity analyses to assess factors that affected the incremental cost per QALY gained, including the following:
Time horizon
Reduced mortality risk at 90 days with mechanical thrombectomy
Extra hospitalization costs due to mechanical thrombectomy, relative to IVT or medical therapy
Costs from a societal perspective, including productivity loss and unpaid caregivers
Age group, < 70 years and > 70 years
Health utility for stroke patients
Discounting rate
Extra costs for end-of-life care
Finally, we conducted a probabilistic sensitivity analysis by considering inputs as random variables associated with probability distributions.
Generalizability
The findings of this economic analysis cannot be generalized to all patients with acute ischemic stroke. They may, however, be used to guide decision-making for patients with acute large-artery ischemic stroke in hospitals that have the vascular imaging technology to detect occlusions and the techniques for mechanical thrombectomy in Ontario.
Software
Economic analyses and calibration were conducted using SAS 9.4 (SAS, Cary, NC). We also used R 3.1.2 (R Development Core Team, Vienna, Austria) for meta-analysis (“metafor” package54 in R) and simultaneous confidence intervals for multinomial proportion (“MultinomialCI”55 and “CoinMinD”56 packages in R).
Results
Validation of the Economic Model
The plots of the health state probabilities (i.e., the probabilities of functional independence, disability, and mortality over time) following mechanical thrombectomy (with or without IVT) and IVT alone based on our Markov model are shown in Figures 10a and 10b. These plots reflect our model inputs and assumptions, and assume that the model captures the different health state probabilities appropriately.
Figure 10a: Health State Probabilities After Mechanical Thrombectomy (With or Without IVT).
Abbreviations: IVT, intravenous thrombolysis.
Figure 10b: Health State Probabilities Following IVT Alone.
Abbreviation: IVT, intravenous thrombolysis.
Base-Case Analysis
Based on the model proposed in Figure 8 and using the parameter estimates in Tables 10 to 13, we calculated the cost and effectiveness of mechanical thrombectomy (with or without IVT) versus IVT alone over 5 years (Table 15). Mechanical thrombectomy was associated with an ICER of $11,990 per QALY gained. Although there is no universally accepted maximum willingness to-pay threshold in Canada, $50,000 and $100,000/QALY are the common thresholds in practice. Compared with IVT alone, mechanical thrombectomy was cost-effective for acute ischemic stroke at 5 years’ follow-up.
Table 15:
Base-Case Analysis Results (Time Horizon, 5 Years)a
| Strategy | Average Total Cost, $ | Incremental Cost, $ | Life-Years | Life-Years Gained | QALYs | QALYs Gained | ICER (QALY),b $ |
|---|---|---|---|---|---|---|---|
| IVT | 124,419 | — | 2.861 | — | 1.273 | — | — |
| MT+IVT | 126,939 | 2,520 | 2.969 | 0.107 | 1.484 | 0.210 | 11,990 |
Abbreviations: MT, mechanical thrombectomy; ICER, incremental cost-effectiveness ratio; IVT, intravenous thrombolysis; QALY, quality-adjusted life-year.
All costs are in Canadian dollars.
Incremental cost per QALY gained.
Note: numbers may appear inexact due to rounding.
Scenario Analysis (Based on ESCAPE Trial)
Table 16 presents the results of the scenario analysis using the inputs from the ESCAPE trial.29 Mechanical thrombectomy resulted in an ICER of $26,815 per QALY gained. If we extended the time horizon to 10 years, the effectiveness would be as much as 0.5 QALY gained, and the corresponding ICER would be $27,885 per QALY gained.
Table 16:
Scenario Analysis Results, Based on ESCAPE Trial (Time Horizon, 5 Years)a
| Strategy | Average Total Cost, $ | Incremental Cost, $ | QALYs | QALYs Gained | ICER (QALY),b $ |
|---|---|---|---|---|---|
| IVT | 122,901 | — | 1.265 | — | — |
| MT+IVT | 132,224 | 9,323 | 1.613 | 0.348 | 26,815 |
Abbreviations: MT, mechanical thrombectomy; ICER, incremental cost-effectiveness ratio; IVT, intravenous thrombolysis; QALY, quality-adjusted life-year.
All costs are in Canadian dollars.
Incremental cost per QALY gained.
Deterministic Sensitivity Analysis
We examined several factors that could affect the ICER of mechanical thrombectomy (with or without IVT) versus IVT alone. When the model inputs were varied, mechanical thrombectomy remained cost-effective in most scenarios (Table 17). The main factors influencing ICERs were the time horizon, the additional hospitalization cost of MT, the age group of patients, and the perspective of the analysis.
Table 17:
One-Way or Two-Way Sensitivity Analysis Results
| Scenarios | Incremental Cost Per QALY Gained, $ |
|---|---|
| Base case analysis (reference) | 11,990 |
| Time horizon | |
| 1 year | 91,080 |
| 3 years | 20540 |
| 10 years | 11,491 |
| 15 yearsa | 12,877 |
| MT with reduced mortality risk (OR of mortality, MT+IVT vs. IVT alone, 0.80) | 22,891 |
| Extra hospitalization cost of MT+IVT vs. IVT | |
| $10,000 | Dominant |
| $20,000 | 35,779 |
| Costs from a societal perspective | Dominant |
| Age groups | |
| ≤ 70 years old (OR of functional independence, MT+IVT vs. IVT alone, 3.02) | 4,429 |
| > 70 years old (OR of functional independence, MT+IVT vs. IVT alone, 1.79) | 29,899 |
| Health utility in functional independence and disability states | |
| Lower limits of 95% CI | 12,366 |
| Upper limits of 95% CI | 11,809 |
| No discounting for both cost and utility | 10,028 |
| Including cost for end-of-life care ($50,892 per patient57) for those who survived at 90 days after an acute ischemic stroke | 4,212 |
Abbreviations: CI, confidence interval; MT, mechanical thrombectomy; IVT, intravenous thrombolysis; OR, odds ratio; QALY, quality adjusted life-year.
About 6.9% and 5.4% of patients survived in the IVT+MT and IVT alone arms, respectively, at 15 years’ follow-up.
The ICER decreased dramatically with longer follow-up time in the first 4 years (Figure 11), and was relatively stable at a follow-up time of 5 years or longer.
Figure 11: ICERs by Follow-up Time, Mechanical Thrombectomy (With or Without IVT) Versus IVT Alone.
Abbreviations: ICER, incremental cost-effectiveness ratio; IVT, intravenous thrombolysis; MT, mechanical thrombectomy; QALY, quality-adjusted life-year.
When we conducted the analysis under the assumption that mechanical thrombectomy was associated with a reduced risk of mortality, we kept the probability of functional independence at 90 days post-stroke constant (i.e., the same as base case), so mechanical thrombectomy increased the likelihood of disability (1– pfunctional independence – pmortality) compared with the base case. Disability was associated with higher costs and relatively lower health utility. Under these assumptions, the total cost and effectiveness were $130,072 and 1.52 QALYs for mechanical thrombectomy at 5 years, and the corresponding ICER was $22,891 per QALY gained (incremental cost $5,654; incremental effectiveness 0.247 QALY)—higher than the base case.
Probabilistic Sensitivity Analysis
The results of the Monte Carlo simulations were consistent with those of the base case (Figure 12). The triangle indicates the base-case scenario. Each circle surrounding the triangle represents a single result from the simulation, presenting the incremental effects and incremental costs of mechanical thrombectomy (with or without IVT) relative to IVT alone. The probability of mechanical thrombectomy dominating IVT alone was 0.286 (mechanical thrombectomy with lower costs and higher QALYs).
Figure 12: Cost-Effectiveness Plane: Incremental Costs and QALYs Gained, Mechanical Thrombectomy (With or Without IVT) Versus IVT Alone.
Abbreviations: IVT, intravenous thrombosis, MT, mechanical thrombectomy; QALY, quality-adjusted life-year.
The cost-effectiveness acceptability curve (Figure 13) shows that the probability of mechanical thrombectomy being cost-effective was 57.5%, 89.7%, and 99.6%, at thresholds of $20,000, $50,000, and $100,000 per QALY gained, respectively.
Figure 13: Cost-Effectiveness Acceptability Curve, Mechanical Thrombectomy (With or Without IVT) Versus IVT Alone.
Abbreviations: IVT, intravenous thrombolysis; MT, mechanical thrombectomy.
Discussion and Conclusions
The results of our economic analysis demonstrate that MT is highly likely to be cost-effective according to commonly used cost-effectiveness thresholds. This is concordant with the large clinical effect size observed in the randomized trials, and sensitivity analyses suggest that these findings are robust.
Our findings are consistent with the most recent published economic evaluation from the United States,37 which used newer-generation devices. The efficacy of mechanical thrombectomy came from a single RCT.38 In that evaluation, mechanical thrombectomy (with or without IVT) resulted in an ICER of $14,137 USD per QALY gained.
Among the strengths of our economic evaluation:
Our cost-utility analysis was based on high-level evidence from a meta-analysis of five RCTs.28–31,38
We used monthly cycles in the Markov model. Compared with yearly cycles, the smaller cycles may have modelled the disease progression more accurately.
We considered both forward and backward transitions for progression from functional independence to disability and recovery from disability to functional independence. Compared with a one-way (forward) transition model, we modelled the progression of stroke patients more naturally.
We used a calibration approach to provide relatively reliable parameter estimates for the economic model.
Model assumptions and inputs were verified by experts.
Our economic evaluation also had the following limitations:
The follow-up time was only 90 days in the five recent RCTs that we identified.28–31,38 The expected long-term benefits from mechanical thrombectomy in our model were calculated by combining results from the RCTs with a cohort study under some assumptions.
It was challenging to make a precise estimate of the incremental hospitalization costs of mechanical thrombectomy (with or without IVT) versus IVT alone. Our estimate was based largely on published studies from the United States.
We have not found reliable evidence of long-term clinical outcomes for post-stroke patients in Canada. Model inputs used in this analysis were based largely on the Oxford Vascular study from the United Kingdom,42,43 and there were some differences between our target population and the population of that study, including age and stroke severity level.
We used three health states (functional independence, disability, and death) in the Markov model, since the available data (clinical outcomes, costs, and health utility) are often categorized using those three health states. In reality, the categories were inadequate to describe severity levels for post-stroke patients.
We did not attempt to evaluate the cost-effectiveness of a specific type of mechanical thrombectomy device. The efficacy and the cost of interventions can differ by device, although the difference is probably not substantial.
The role of imaging techniques in patient selection and management strategies was not included in our model.
Mechanical thrombectomy as an adjunct to intravenous thrombolysis appears to be cost-effective compared with intravenous thrombolysis alone for patients with large-artery acute ischemic stroke.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis from the perspective of the Ontario Ministry of Health and Long-Term Care to determine the estimated cost burden in 2015 and over the following 4 years (2016 to 2019) under the assumption of the gradual diffusion of mechanical thrombectomy. All costs are reported in 2015 Canadian dollars.47
Objective
The objective of this analysis was to assess the potential budget impact of adopting mechanical thrombectomy in Ontario.
Methods
Target Population
According to the Ontario Stroke Registry Database, about 6,500 people had an acute ischemic stroke in the fiscal year 2012/13 in Ontario, and about 1.1% (70) received endovascular treatment during the same period (different data sources and/or inclusion criteria may result in slightly different estimates). Since mechanical thrombectomy has demonstrated a substantially beneficial effect in randomized controlled trials (RCTs), experts have suggested that about 10% of all acute ischemic stroke patients may receive it. We assumed that the total number of acute ischemic stroke patients was constant from 2015 to 2019 (6,500 annually), and that the uptake rate was about 3% in 2015 (N = 200) and 4% in 2016, then increasing by 2% yearly and reaching 10% in 2019 (Table 18).
Table 18:
Expected Number of Mechanical Thrombectomy Procedures, 2015 to 2019, Ontario
| Year | Patients With Acute Ischemic Stroke, n | Uptake, % | Mechanical Thrombectomy Procedures, n |
|---|---|---|---|
| 2015 | 6,500 | 3% | 200 |
| 2016 | 6,500 | 4% | 260 |
| 2017 | 6,500 | 6% | 390 |
| 2018 | 6,500 | 8% | 520 |
| 2019 | 6,500 | 10% | 650 |
Canadian Costs
Based on undiscounted results from the model in the cost-utility analysis, we estimated the average cost in Canadian dollars for each year post-stroke (up to the fifth year) (Table 19).
Table 19:
Average Cost Per Patient for Each Year Post-stroke, All Patients
| Therapy | Year Post-stroke | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| MT+IVT | $67,903 | $18,049 | $17,362 | $16,184 | $14,730 |
| IVT | $62,786 | $19,474 | $18,211 | $16,612 | $14,852 |
Abbreviations: IVT, intravenous thrombolysis; MT, mechanical thrombectomy.
We also used the model to determine the expected percentage of patients who survived at the beginning of each year (Table 20).
Table 20:
Percentage of Patients Who Survived at the Beginning of Each Year
| Therapy | Year Post-stroke | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| MT+IVT | 100% | 72.3% | 67.0% | 61.3% | 55.2% |
| IVT | 100% | 70.4% | 64.5% | 58.4% | 52.0% |
Abbreviations: IVT, intravenous thrombolysis; MT, mechanical thrombectomy.
Then, we calculated the average cost in Canadian dollars for each year post-stroke (up to the fifth year) for those at risk (Table 21).
Table 21:
Average Cost Per Patient in Each Year Post-stroke, Patients at Risk
| Therapy | Year Post-stroke | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| MT+IVT | $67,903 | $24,973 | $25,903 | $26,400 | $26,667 |
| IVT | $62,786 | $27,680 | $28,221 | $28,449 | $28,536 |
Abbreviations: IVT, intravenous thrombolysis; MT, mechanical thrombectomy.
Based on the information in Tables 18 and 20, we estimated the number of patients who would be at risk over next 5 years (Table 22). For simplicity, we ignored a small number of patients treated with new mechanical thrombectomy techniques before 2015.
Table 22:
Expected Number of Patients at Risk After Adopting Mechanical Thrombectomy in Ontario, 2015 to 2019
| Year | Therapy | Year Post-stroke | Total Number of Patients at Risk | ||||
|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||
| 2015 | MT+IVT | 200 | — | — | — | — | 200 |
| IVT | 200 | — | — | — | — | 200 | |
| 2016 | MT+IVT | 260 | 145 | — | — | — | 405 |
| IVT | 260 | 141 | — | — | — | 401 | |
| 2017 | MT+IVT | 390 | 188 | 134 | — | — | 712 |
| IVT | 390 | 183 | 129 | — | — | 702 | |
| 2018 | MT+IVT | 520 | 282 | 174 | 123 | — | 1,099 |
| IVT | 520 | 274 | 168 | 117 | — | 1,079 | |
| 2019 | MT+IVT | 650 | 376 | 261 | 159 | 110 | 1,556 |
| IVT | 650 | 366 | 252 | 152 | 104 | 1,524 | |
Abbreviations: MT, mechanical thrombectomy; IVT, intravenous thrombolysis.
When estimating the budget impact, we included both new patients and survivors treated previously. Based on the information in Tables 21 and 22, we used the following formula to estimate the net budget impact by mechanical thrombectomy, relative to IVT:
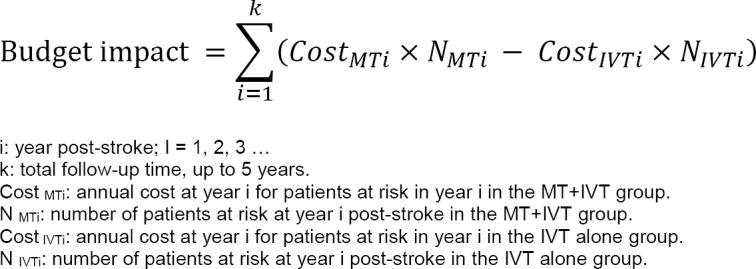 |
We conducted the budget impact analysis using Excel 2013 (Microsoft, Redmond, WA).
Results
Using the assumptions outlined above, we estimated that adopting mechanical thrombectomy would lead to a moderate cost increase—about $1 million in 2015 at an uptake rate of 3% (Table 23).
Table 23:
Budget Impact ($) of Adopting Mechanical Thrombectomy in Ontario, 2015 to 2019
| Year | Strategy | Year Post-stroke | Sum | ||||
|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||
| 2015 | MT+IVT | 13,580,594 | 13,580,594 | ||||
| IVT | 12,557,104 | 12,557,104 | |||||
| NBI | 1,023,490 | 1,023,490 | |||||
| 2016 | MT+IVT | 17,654,772 | 3,621,018 | 21,275,790 | |||
| IVT | 16,324,235 | 3,902,869 | 20,227,104 | ||||
| NBI | 1,330,537 | −281,851 | 1,048,686 | ||||
| 2017 | MT+IVT | 26,482,158 | 4,694,837 | 3,470,977 | 34,647,972 | ||
| IVT | 24,486,353 | 5,065,426 | 3,640,525 | 33,192,303 | |||
| NBI | 1,995,806 | −370,589 | −169,548 | 1,455,669 | |||
| 2018 | MT+IVT | 35,309,544 | 7,042,255 | 4,507,090 | 3,247,194 | 50,106,083 | |
| IVT | 32,648,470 | 7,584,299 | 4,741,148 | 3,328,528 | 48,302,446 | ||
| NBI | 2,661,074 | −542,044 | −234,059 | −81,334 | 1,803,637 | ||
| 2019 | MT+IVT | 44,136,931 | 9,389,674 | 6,760,634 | 4,197,592 | 2,933,345 | 67,418,176 |
| IVT | 40,810,588 | 10,130,852 | 7,111,723 | 4,324,241 | 2,967,729 | 65,345,133 | |
| NBI | 3,326,343 | −741,178 | −351,088 | −126,650 | −34,383 | 2,073,043 | |
Abbreviations: IVT, intravenous thrombolysis; MT, mechanical thrombectomy; NBI, net budget impact. Note: numbers may appear inexact due to rounding.
Due to the lower risk of disability, the cost in the mechanical thrombectomy arm in years 2 to 5 post-stroke was slightly lower than in the IVT-alone arm, but savings could not adequately compensate for the extra cost of mechanical thrombectomy in the first year. It was expected that the uptake rate would be approximately 10% in 2019. When considering the budget impact for both new patients in 2019 (n = 650) and survivors treated in 2015–2018 (n = 906 in the mechanical thrombectomy arm; n = 874 in the IVT-alone arm), mechanical thrombectomy would have a budget impact of about $2 million in 2019.
Discussion and Conclusions
Our budget impact analysis has one limitation. It does not include the costs of screening necessary patients with computerized tomographic angiography.
Mechanical thrombectomy would lead to a moderate cost increase: about $1 million in 2015 at an uptake rate of 3%, and about $2 million in 2019 at an uptake rate of 10%.
Acknowledgments
The medical editor was Elizabeth Jean Betsch. Others involved in the development and production of this report were Irfan Dhalla, Nancy Sikich, Andree Mitchell, Claude Soulodre, Merissa Mohamed, and Jessica Verhey.
We gratefully acknowledge Dr. Jeffrey Turnbull for his support and expert consultation.
Glossary
LIST OF ABBREVIATIONS
- ASPECTS
Alberta Stroke Program Early Computed Tomography Score
- BURST
Economic Burden of Ischemic Stroke
- CI
Confidence interval
- ECASS II
Second European-Australasian Acute Stroke Study
- EQ-5D
EuroQoL Group 5-Dimension Self-Report Questionnaire
- ESCAPE
Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- IVT
Intravenous thrombolysis
- MRI
Magnetic resonance imaging
- mRS
Modified Rankin Scale
- MT
Mechanical thrombectomy
- NIHSS
National Institutes of Health Stroke Scale
- OR
Odds ratio
- QALY
Quality-adjusted life-year
- RCT
Randomized controlled trial
- SICH
Symptomatic intracerebral hemorrhage
- TICI
Thrombolysis in Cerebral Infarction
APPENDICES
Appendix 1: Clinical Literature Search Strategies
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <February 2015>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to January 2015>, EBM Reviews -Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews - Health Technology Assessment <1st Quarter 2015>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2015 Week 10>, All Ovid MEDLINE(R) <1946 to Present> Search Strategy:
| 1 | exp Brain Ischemia/ (198337) |
| 2 | ((isch?emi* adj3 (stroke* or apoplex* or cerebr* or brain or encephalopath* or neur*)) or AIS).tw. (198076) |
| 3 | exp Stroke/ (189703) |
| 4 | (stroke* adj3 (acute or cerebr* or attack* or accident* or lacunar or cardioembol*)).tw. (76907) |
| 5 | Intracranial Arteriosclerosis/ (10347) |
| 6 | exp “Intracranial Embolism and Thrombosis”/ (367169) |
| 7 | Carotid Artery Thrombosis/ (5356) |
| 8 | ((occlus* or block* or infarct* or clot* or termination) adj6 (carotid or cerebr* or MCA or ACA)).tw. (96218) |
| 9 | or/1–8 (786703) |
| 10 | exp Thrombectomy/ (15008) |
| 11 | Embolectomy/ (4255) |
| 12 | ((Mechanical adj3 (thromb* or embol* or clot disruption* or clot retrieval*)) or ((clot* or thromb* or embol*) adj3 (retriev* or disruption* or fragmentation)) or ((stent* or stent-assisted) adj3 retriev*) or stentriever*).tw. (9056) |
| 13 | ((Merci or Trevo or Penumbra or Solitaire) adj3 (retriever* or system* or device*)).mp. (1233) |
| 14 | or/10–13 (24914) |
| 15 | 9 and 14 (14515) |
| 16 | exp Animals/ not (exp Animals/ and Humans/) (8045601) |
| 17 | 15 not 16 (14213) |
| 18 | (case reports or congresses).pt. (1780093) |
| 19 | 17 not 18 (13866) |
| 20 | limit 19 to english language [Limit not valid in CDSR,DARE; records were retained] (12321) |
| 21 | limit 20 to yr=“2005 -Current” [Limit not valid in DARE; records were retained] (9737) |
| 22 | 21 use pmoz,cctr,coch,dare,clhta,cleed (970) |
| 23 | exp Brain Ischemia/ (198337) |
| 24 | ((isch?emi* adj3 (stroke* or apoplex* or cerebr* or brain or encephalopath* or neur*)) or AIS).tw. (198076) |
| 25 | exp Cerebrovascular Accident/ (189703) |
| 26 | Stroke Patient/ (13478) |
| 27 | (stroke* adj3 (acute or cerebr* or attack* or accident* or lacunar or cardioembolic)).tw. (76838) |
| 28 | exp Occlusive Cerebrovascular Disease/ (26483) |
| 29 | exp Carotid Artery Obstruction/ (25862) |
| 30 | Brain Embolism/ (8515) |
| 31 | ((occlus* or block* or infarct* or clot* or termination) adj6 (carotid or cerebr* or MCA or ACA)).tw. (96218) |
| 32 | or/23–31 (478286) |
| 33 | Mechanical Thrombectomy/ (1828) |
| 34 | Thrombectomy/ (10732) |
| 35 | Embolectomy/ (4255) |
| 36 | ((Mechanical adj3 (thromb* or embol* or clot disruption* or clot retrieval*)) or ((clot* or thromb* or embol*) adj3 (retriev* or disruption* or fragmentation)) or ((stent* or stent-assisted) adj3 retriev*) or stentriever*).tw. (9056) |
| 37 | ((Merci or Trevo or Penumbra or Solitaire) adj3 (retriever* or system* or device*)).mp. (1233) |
| 38 | or/33–37 (22583) |
| 39 | 32 and 38 (4742) |
| 40 | exp animal experimentation/ or exp models animal/ or exp animal experiment/ or nonhuman/ or exp vertebrate/ (38090949) |
| 41 | exp humans/ or exp human experimentation/ or exp human experiment/ (29700691) |
| 42 | 40 not 41 (8416467) |
| 43 | 39 not 42 (4642) |
| 44 | case report/ or conference abstract.pt. (5381946) |
| 45 | 43 not 44 (2728) |
| 46 | limit 45 to english language [Limit not valid in CDSR,DARE; records were retained] (2466) |
| 47 | limit 46 to yr=“2005 -Current” [Limit not valid in DARE; records were retained] (2248) |
| 48 | 47 use emez (1362) |
| 49 | 22 or 48 (2332) |
| 50 | remove duplicates from 49 (1624) |
Appendix 2: Evidence Quality Assessment
Table A1:
GRADE Evidence Profile for Comparison of Mechanical Thrombectomy and IVT on Clinical Outcomes
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Functional Independence (mRS) | |||||||
| 5 (RCTs) | No serious limitations | No serious limitations | No serious limitations | No serious limitationsa | Undetected | No other considerations | ⊕⊕⊕⊕ High |
| Mortality | |||||||
| 5 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| SICH | |||||||
| 5 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Quality of Life | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (−1)a | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Reperfusion | |||||||
| 5 (RCTs) | No serious limitations | No serious limitations | No serious limitationsb | Serious limitations (−1)a | Undetected | No other considerations | ⊕⊕⊕ Moderate |
| Recanalization | |||||||
| 3 (RCTs) | No serious limitations | No serious limitations | Serious limitations (−1)c | Serious limitations (−1)a | Undetected | No other considerations | ⊕⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; SICH, symptomatic intracerebral hemorrhage; RCT, randomized controlled trial.
Optimal information size may not be met for this outcome, as four out of five RCTs were stopped early.
Reperfusion can be considered a surrogate outcome.
Recanalization can be considered a surrogate outcome.
Table A2:
Risk of Bias Among Randomized Controlled Trials for the Comparison of Mechanical Thrombectomy and Clinical Outcomes
| Author, Year | Allocation Concealments | Blindingb | Complete Accounting of Patients and Outcome Events | Selective Reporting Bias | Other Limitationsc |
|---|---|---|---|---|---|
| Berkhemer et al, 201527 | No limitations | No limitations | No limitations | Limitationsd | No limitations |
| Campbell et al, 201528 | No limitations | No limitations | No limitations | No limitations | Limitationse |
| Goyal et al, 201529 | No limitations | No limitations | No limitations | Limitationsd | Limitationse |
| Jovin et al, 201530 | No limitations | No limitations | No limitations | No limitations | Limitationse |
| Saver et al, 201531 | No limitations | No limitations | No limitations | No limitations | Limitationse |
Abbreviations: RCT, randomized controlled trial.
All five RCTs used a web-based randomized minimization procedure.
All five included RCTs had blind outcome evaluation, but physicians conducting the intervention were aware of treatment assignment. This was appropriate, as a sham procedure was not ethical, and the standard of care is intravenous thrombolysis, which is a more appropriate comparator.
All five studies had grant support from Covidien/ev3 (the company that manufactures the Solitaire FR stent retriever) and/or other manufacturers/industry support.
Protocol states the functional outcome measured by the Academic Linear Disability Scale would be collected at 90 days, but this outcome was not reported in the published article.
After the Berkhemer et al (28) study was published, the four following RCTs stopped early based on prespecified boundaries for efficacy during interim analysis.
Protocol states the functional outcome measured by miFUNCTION scale would be collected, but this outcome was not reported in the published article.
Appendix 3: Supplementary Results
Figure A1: Mechanical Thrombectomy Versus IVT on the Proportion of Functionally Independent Patients at 90-Day Follow-up, Risk Difference.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy.
Figure A2: Mechanical Thrombectomy Versus IVT on Proportion of Functionally Independent Patients at 90-Day Follow-up by Age, Secondary Analysis.
Abbreviations: CI, confidence interval; IVT, intravenous thrombolysis; M-H, Mantel-Haenszel; MT, mechanical thrombectomy. Note: Goyal et al estimate was not included in this analysis.
Appendix 4: Economic Literature Search Strategies Databases searched:
Ovid MEDLINE(R)
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations
Ovid Embase
Cochrane Library:
Cochrane Central Register of Controlled Trials
Cochrane Database of Systematic Reviews
Database of Abstracts of Reviews of Effects
CRD Health Technology Assessment Database
NHS Economic Evaluation Database
(NEW) HTA Database Canadian Search Interface: http://www.crd.york.ac.uk/PanHTA/
1. MEDLINE SEARCH
Search date: March 23, 2015
Databases searched: Ovid MEDLINE(R) 1946 to March Week 3 2015, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations March 20, 2015
Limits: English Language, 2005 –Current, conference abstract/letter/commentary
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Brain Ischemia/ or exp Stroke/ or Intracranial Arteriosclerosis/ or exp “Intracranial Embolism and Thrombosis”/ or Carotid Artery Thrombosis/ or ((isch?emi* adj3 (stroke* or apoplex* or cerebr* or brain or encephalopath* or neur*)) or AIS or (stroke* adj3 (acute or cerebr* or attack* or accident* or lacunar or cardioembol*)) or ((occlus* or block* or infarct* or clot* or termination) adj6 (carotid or cerebr* or MCA or ACA))).tw. | 197957 |
| 2 | exp Thrombectomy/ or Embolectomy/ or ((Mechanical adj3 (thromb* or embol* or clot disruption* or clot retrieval*)) or ((clot* or thromb* or embol*) adj3 (retriev* or disruption* or fragmentation)) or ((stent* or stent-assisted) adj3 retriev*) or stentriever*).tw. or ((Merci or Trevo or Penumbra or Solitaire) adj3 (retriever* or system* or device*)).mp. | 7279 |
| 3 | 1 and 2 | 1448 |
| 4 | economics/ or exp “costs and cost analysis”/ or economics, dental/ or exp “economics, hospital”/ or economics, medical/ or economics, nursing/ or economics, pharmaceutical/ or (economics or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$ or (expenditure$ not energy) or (value adj1 money) or budget$).ti,ab. | 623674 |
| 5 | (((energy or oxygen) adj cost) or (metabolic adj cost) or ((energy or oxygen) adj expenditure)).ti,ab. | 20983 |
| 6 | (letter or editorial or historical article).pt. | 1537200 |
| 7 | exp Animals/ not (exp Animals/ and Humans/) | 4002853 |
| 8 | 4 not (5 or 6 or 7) | 554706 |
| 9 | 3 and 8 | 29 |
| 10 | (congresses or comment).pt. | 678064 |
| 11 | 9 not 10 | 28 |
| 12 | limit 11 to (english language and yr=“2005 -Current”) | 22 |
2. EMBASE SEARCH
Search date: March 23, 2015
Databases searched: Embase 1980 to 2015 Week 12
Limits: English Language, 2005 -Current, conference abstract/letter/commentary
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Brain Ischemia/ or exp Cerebrovascular Accident/ or Stroke Patient/ or exp Occlusive Cerebrovascular Disease/ or exp Carotid Artery Obstruction/ or Brain Embolism/ or ((isch?emi* adj3 (stroke* or apoplex* or cerebr* or brain or encephalopath* or neur*)) or AIS or (stroke* adj3 (acute or cerebr* or attack* or accident* or lacunar or cardioembolic)).tw. or ((occlus* or block* or infarct* or clot* or termination) adj6 (carotid or cerebr* or MCA or ACA))).tw. | 281182 |
| 2 | Mechanical Thrombectomy/ or Thrombectomy/ or Embolectomy/ or ((Mechanical adj3 (thromb* or embol* or clot disruption* or clot retrieval*)) or ((clot* or thromb* or embol*) adj3 (retriev* or disruption* or fragmentation)) or ((stent* or stent-assisted) adj3 retriev*) or stentriever*).tw. or ((Merci or Trevo or Penumbra or Solitaire) adj3 (retriever* or system* or device*)).mp. | 15231 |
| 3 | 1 and 2 | 3402 |
| 4 | (health economics/ or exp economic evaluation/ or exp health care cost/ or exp pharmacoeconomics/ or (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. or (expenditure$ not energy).ti,ab. or (value adj2 money).ti,ab. or budget$.ti,ab.) not ((metabolic adj cost) or ((energy or oxygen) adj cost) or ((energy or oxygen) adj expenditure)).ti,ab. | 931742 |
| 5 | exp Animals/ not (exp Animals/ and Humans/) | 4046944 |
| 6 | 4 not 5 | 892754 |
| 7 | (conference abstract or editorial or letter or note).pt. | 3705856 |
| 8 | 6 not 7 | 714587 |
| 9 | 3 and 8 | 85 |
| 10 | limit 9 to (english language and yr=“2005 -Current”) | 72 |
| 11 | 3 and 6 | 138 |
3. COCHRANE LIBRARY SEARCH
Databases searched: Cochrane Library Databases (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Database of Abstracts of Reviews of Effects, Health Technology Assessment NHS Economic Evaluation Database)
Filters: Health Technology Assessment Filter: NHS EED MEDLINE, best sensitivity validated filter from Glanville2009
Search Name: HQO_MechanicalThrombectomy_LitSearchStrategy_JB_Mar2015
Date Run: 23/03/15 22:08:24.167
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor: [Brain Ischemia] explode all trees | 2330 |
| #2 | MeSH descriptor: [Stroke] explode all trees | 5532 |
| #3 | MeSH descriptor: [Intracranial Arteriosclerosis] this term only | 129 |
| #4 | MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees | 260 |
| #5 | MeSH descriptor: [Carotid Artery Thrombosis] this term only | 18 |
| #6 | (((isch?emi* near/3 (stroke* or apoplex* or cerebr* or brain or encephalopath* or neur*)) or AIS) or (stroke* near/3 (acute or cerebr* or attack* or accident* or lacunar or cardioembol*)) or ((occlus* or block* or infarct* or clot* or termination) near/6 (carotid or cerebr* or MCA or ACA))):ti,ab,kw (Word variations have been searched) | 8778 |
| #7 | #1 or #2 or #3 or #4 or #5 or #6 | 13191 |
| #8 | MeSH descriptor: [Thrombectomy] explode all trees | 141 |
| #9 | MeSH descriptor: [Embolectomy] this term only | 10 |
| #10 | ((Mechanical near/3 (thromb* or embol* or clot disruption* or clot retrieval*)) or ((clot* or thromb* or embol*) adj3 (retriev* or disruption* or fragmentation)) or ((stent* or stent-assisted) near/3 retriev*) or stentriever*):ti,ab,kw (Word variations have been searched) | 168 |
| #11 | ((Merci or Trevo or Penumbra or Solitaire) near/3 (retriever* or system* or device*)):ti,ab,kw (Word variations have been searched) | 43 |
| #12 | #8 or #9 or #10 or #11 | 302 |
| #13 | #7 and #12 | 88 |
| #14 | MeSH descriptor: [Economics] this term only | 58 |
| #15 | MeSH descriptor: [Costs and Cost Analysis] explode all trees | 23270 |
| #16 | MeSH descriptor: [Economics, Dental] this term only | 3 |
| #17 | MeSH descriptor: [Economics, Hospital] explode all trees | 1655 |
| #18 | MeSH descriptor: [Economics, Medical] this term only | 38 |
| #19 | MeSH descriptor: [Economics, Nursing] this term only | 17 |
| #20 | MeSH descriptor: [Economics, Pharmaceutical] this term only | 236 |
| #21 | (economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic*) or (expenditure* not energy) or (value near/1 money) or budget*:ti,ab,kw (Word variations have been searched) | 48053 |
| #22 | #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 | 48139 |
| #23 | ((energy or oxygen) near cost) or (metabolic near cost) or ((energy or oxygen) near expenditure):ti,ab,kw (Word variations have been searched) | 2475 |
| #24 | #22 not #23 | 47615 |
| #25 | letter or editorial or historical article:pt (Word variations have been searched) | 6747 |
| #26 | #24 not #25 | 47507 |
| #27 | MeSH descriptor: [Animals] explode all trees | 6890 |
| #28 | MeSH descriptor: [Humans] explode all trees | 1190 |
| #29 | #27 not (#27 and #28) | 5700 |
| #30 | #26 not #29 | 47330 |
| #31 | #13 and #30 | 9 |
4. Canadian HTA within International HTA Database
| Search | Hits | |
|---|---|---|
| 1 | MeSH DESCRIPTOR Brain Ischemia EXPLODE ALL TREES IN PCHTA | 4 |
| 2 | MeSH DESCRIPTOR Stroke EXPLODE ALL TREES IN PCHTA | 31 |
| 3 | MeSH DESCRIPTOR Intracranial Embolism and Thrombosis EXPLODE ALL TREES IN PCHTA | 1 |
| 4 | MeSH DESCRIPTOR Intracranial Arteriosclerosis EXPLODE 1 2 3 IN PCHTA | 0 |
| 5 | MeSH DESCRIPTOR Carotid Artery Thrombosis EXPLODE 1 2 IN PCHTA | 0 |
| 6 | ((((isch?emi* near3 (stroke* or apoplex* or cerebr* or brain or encephalopath* or neur*)) or AIS) or (stroke* near3 (acute or cerebr* or attack* or accident* or lacunar or cardioembol*)) or ((occlus* or block* or infarct* or clot* or termination) near6 (carotid or cerebr* or MCA or ACA)))) IN PCHTA | 12 |
| 7 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 | 36 |
| 8 | MeSH DESCRIPTOR Thrombectomy EXPLODE ALL TREES IN PCHTA | 0 |
| 9 | MeSH DESCRIPTOR Embolectomy IN PCHTA | 0 |
| 10 | ((((Mechanical near3 (thromb* or embol* or clot disruption* or clot retrieval*)) or ((clot* or thromb* or embol*) near3 (retriev* or disruption* or fragmentation)) or ((stent* or stent-assisted) near3 retriev*) or stentriever*) or ((Merci or Trevo or Penumbra or Solitaire) near3 (retriever* or system* or device*)))) IN PCHTA | 1 |
| 11 | #8 OR #9 OR #10 | 1 |
| 12 | #7 AND #11 | 1 |
Appendix 5: Calibration of Natural History, Post-stroke Patients
Using data from the Oxford Vascular Study, we estimated the proportion of acute ischemic stroke patients who were functionally independent (mRS 0–2), disabled (mRS 3–5), and dead (mRS 6) at different follow-up points,42,43 but we could not obtain transition probabilities between two states since more than one transition contributes to a change in the proportion of patients in the three health states. For example, we know the proportion increase of death in a given time interval, but we do not know for certain whether patients died when they were in functional independence or disability. For this reason, we calibrated the parameters for the Markov model using a seven-step approach introduced by Vanni et al.48 We aimed to obtain calibrated parameters with the following features:
They are the most common measures or statistics (e.g., relative risk and odds ratio) in epidemiology studies.
The values of calibrated parameters are consistent with the natural biological system (e.g., relative risk of mortality for post-stroke patients versus general population >1).
Model outputs and the observed data (i.e., Oxford Vascular Study) must be consistent.
The values of calibrated parameters (e.g., relative risk) are consistent with external data (e.g., the Australian study).
Parameters should be reasonable for projection of long-term outcomes beyond the observed period.
The calibration was performed with SAS, version 9.4 (SAS Institute, Cary, North Carolina).
Methods
Step 1: Parameters Included
We divided the follow-up time (> 3 months) into three phases: 4 to 6 months, 7 to 12 months, and 13 months or more. Parameters used in each phase were addressed separately. The potential parameters included in months 4 and 6 post-stroke are shown in Table A3.
Table A3:
Parameters for Months 4 to 6 Post-stroke
| Parameter | Definition |
|---|---|
| Rab4–6 | Annual disability rate from functional independence to disability (e.g., disability following recurrent ischemic stroke) |
| Rba4–6 | Annual recovery rate from disability to functional independence: 0.455 per patient-year |
| RRac4–6 | Relative risk of mortality versus the age-specific general population for patients in functional independence |
| RRbc4–6 | Relative risk of mortality versus the age-specific general population for patients in disability |
If Rab4–6 and Rba4–6 changed simultaneously, their values would be balanced (at least partially), but the exact value of each parameter was unobservable from the summarized data. Thus, we fixed Rba4–6 using an estimated annual recovery rate of 0.455 from months 4 to 6 in the mRS 4 group, as reported by Hankey et al.49 According the Kaplan-Meier curve of time to recovery, we approximated patient-years and number of patients recovered in a given time interval by assuming no censoring, and then we calculated the recovery rate.49 We used the formula below to translate the rate into transition probability.
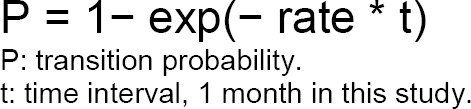 |
For example, the monthly transition probability from disability to functional independence from months 4 to 6 was 1– exp(− 0.455/12) = 0.037.
In addition, the risk of mortality per month was assumed to be equal to the risk of age-specific mortality for the general population, multiplied by the relative risk for a given health state. The age-specific monthly risk of mortality was based on the United Kingdom population in 2004 (Table A4), because our calibration target was based on a cohort study from the United Kingdom (adults 75 years old recruited between 2002 and 2007).50 Sex was not a significant predictor of long-term mortality for stroke patients, so we did not consider it in this analysis.42,58
Table A4:
Life Tables, United Kingdom, 2004
| Age | Monthly Probability of Mortalitya (Both Sexes, 2004) |
|---|---|
| 75 | 0.003027 |
| 76 | 0.003328 |
| 77 | 0.003724 |
| 78 | 0.004202 |
| 79 | 0.004532 |
| 80 | 0.005064 |
| 81 | 0.005607 |
| 82 | 0.006284 |
| 83 | 0.006774 |
| 84 | 0.008258 |
| 85 | 0.008288 |
| 86 | 0.009484 |
| 87 | 0.010762 |
| 88 | 0.011789 |
| 89 | 0.013033 |
The monthly probability of mortality for 75- to 79-year-olds was used in the model calibration, and the probability for 80- to 89-year-olds was used to project long-term outcomes.
The parameters for months 7 to 12 post-stroke were similar as those for months 4 to 6 (Table A5). The recovery rate (Rba7–12) was 0.188 per patient-year, and the corresponding monthly transition probability was 0.0156.49
Table A5:
Parameters for Months 7 to 12 Post-stroke
| Parameter | Definition |
|---|---|
| Rab7–12 | Annual disability rate from functional independence to disability (e.g., disability following recurrent ischemic stroke) |
| Rba7–12 | Annual recovery rate from disability to functional independence: 0.188 per patient-year |
| RRac7–12 | Relative risk of mortality versus the age-specific general population for patients in functional independence |
| RRbc7–12 | Relative risk of mortality versus the age-specific general population for patients in disability |
The parameters for months 13 to 60 are presented in Table A6. We assumed that patients in the disability state could not recover to functional independence after 1 year post-stroke (Rba13–60 = 0), while patients in functional independence could still transition to disability over time (risk related to age). ORab_age denoted the odds ratio of age for risk of disability (an increment of 12 cycles was equivalent to 1 year in the model) and Pab13–24 (derived from Rab13–24) denoted the risk of disability at age 76 years or the second year post-stroke. We could then calculate the risk of disability at different follow-up times.
Table A6:
Parameters for Months 13 to 60 Post-stroke
| Parameter | Definition |
|---|---|
| Rab13–24 | Annual disability rate from functional independence to disability (e.g., disability following recurrent ischemic stroke) |
| ORab_age | Odds ratio of age for risk of disability |
| RRac13–60 | Relative risk of mortality versus the age-specific general population for patients in functional independence |
| RRbc13–60 | Relative risk of mortality versus the age-specific general population for patients in disability |
In summary, excluding two fixed parameters, Rba4–6 and Rba7–12, we calibrated 10 parameters in total.
Step 2: Calibration Target
Good-quality Canadian data should provide the best calibration targets. However, the evidence for long-term outcomes in acute ischemic stroke is relatively sparse in Canada, and for this reason we selected the Oxford Vascular Study to use for our target population.42,43 This study had a large sample size and was well conducted; the United Kingdom population is similar to the Canadian population; and the evidence from this study was more recent than some others, because the stroke patients’ long-term outcomes substantially improved over the previous two decades.59
The United Kingdom cohort (about 83% were ischemic stroke) had three subgroups: minor stroke (National Institutes of Health Stroke Scale [NIHSS] 0–3), moderate stroke (NIHSS 4–10), and major stroke (NIHSS > 10).42,43 Theoretically, our target population would be similar to the major stroke group, but in this subgroup, the 3-month mortality rate was as high as 56.5%, and about 95% of survivors were disabled at 1 month and 6 months post-stroke. We determined that this subgroup had much more severe stroke than our target population. In contrast, the moderate stroke group had a 3-month mortality rate of about 22%, and about 60% of survivors were disabled. Outcomes were similar to those of the control arms in the five RCTs (mortality rate of 18% and disability rate of about 60% for survivors).28–31,38 As a result, we used the moderate subgroup (n = 169 patients) for our calibration targets (Table A7). Treatments for patients in the Oxford Vascular study have not been reported in the articles published. Because intravenous thrombolysis treatment was recommended by The National Institute for Health and Care Excellence in 2007,60 most patients in the Oxford Vascular Study might not have received IVT therapy.
Table A7:
Expected Percentage of Patients in Three Health States
| Time Post-stroke | Functional Independence, % (mRS 0–2) | Disability, % (mRS 3–5) | Death, % (mRS 6) |
|---|---|---|---|
| 3 monthsa | 30.3 | 47.5 | 22.2 |
| 6 months | 31.7 | 42.0 | 26.3 |
| 1 year | 29.9 | 36.5 | 33.6 |
| 2 years | 23.6 | 38.4 | 38.0 |
| 5 years | 15.4 | 28.6 | 56.0 |
Abbreviation: mRS, modified Rankin Scale.
We started with month 4 in calibration, so the targets were observations in month 6 or later. A total of 68% and 57% of survivors were in the disability state at the end of months 1 and 6, respectively, but the authors did not report the percentage who were disabled at 3 months.42 We assumed that the proportion of patients in disability at month 3 should be between the values in months 1 and 6, but closer to that of month 6, so we estimated that 61 % of survivors were disabled at 3 months.
Mortality at different follow-up points was the primary calibration target, since the mortality data were accurate, and there were missing mRS data for survivors at years 2 and 5. Mortality was estimated using the Kaplan-Meier curve in Luengo-Fernandez et al.43 Secondary calibration targets were the percentages of patients from the entire cohort in functional independence and disability at different follow-up times; this was estimated by multiplying the percentage of disabled patients by the percentage of survival.42,43
Step 3: Measure of Goodness-of-Fit
We set multiplex calibration targets in step 2. For the primary target of mortality, we used absolute deviations to assess goodness of fit.
 |
When the absolute deviations of mortality were within the acceptable range for all four follow-up times (6 months, 1 year, 2 years, and 5 years), we evaluated goodness of fit using the sum of squares due to error for the proportion of the three health states at the four observation times. A smaller sum of squares due to error indicates a better-fitting parameter set.
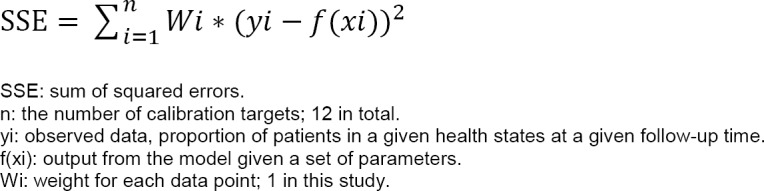 |
Step 4: Parameter Search Strategy
We started with calibrations for parameters from the 4 to 6 months post-stroke (Rab4–6, RRac4–6 and RRbc4–6), because these values were not affected by the parameters used in the > 6 months model. Initially, we set wide ranges and used a grid-search method to gradually narrow the possible parameter space. When this range of parameters was fairly stable, we moved on to the calibrations for 7 to 12 months post-stroke, and then the parameters for 13 to 60 months post-stroke. After obtaining plausible ranges for all, we used a random search method to generate numerous sets of parameters with sampling values from the plausible ranges.
Step 5: Acceptance Criteria
There is no consensus on the most appropriate convergence or acceptance criteria. We set the minimum acceptable level of accuracy as follows: a) the absolute deviation of mortality between observed data and the model output was < 1% at 6 months, and 1, 2 and 5 years; and b) the model outputs falling in the 95% confidence intervals of observed data for the proportion of patients in functional independence and disability states at each follow-up point. Parameter sets that met these acceptance criteria were considered to be good-fitting.
Based on Table A7, and assuming no censoring of the 169 patients, we calculated the expected number of patients in each health state at each time point. Then we estimated 95% simultaneous confidence intervals for the multinomial distribution of the three classes (functional independence, disability, and death) using the method by Sison and Glaz in 1995.61 (Table A8).
Table A8:
95% Simultaneous Confidence Intervals for Patients in Three Health States
| Time Post-stroke | Functional Independence, % (mRS 0–2) | Disability, % (mRS 3–5) | Death, % (mRS 6) |
|---|---|---|---|
| 3 months | 22.5–38.5 | 40.0–55.6 | 14.8–30.8 |
| 6 months | 24.3–40.5 | 34.3–50.5 | 18.3–34.6 |
| 1 year | 21.9–38.2 | 29.0–45.3 | 26.0–42.3 |
| 2 years | 16.0–32.2 | 30.8–47.0 | 30.2–46.4 |
| 5 years | 8.3–23.6 | 21.3–36.6 | 49.1–64.4 |
Abbreviation: mRS, modified Rankin Scale.
Step 6: Stopping Rule
We generated 1,000,000 unique parameter sets using a random search strategy. The search strategy and number of iterations in simulation could be changed in the event that we obtained no adequate good-fitting sets of parameters.
Step 7: Integrating Calibration Results Into the Economic Model
We used the best-fitting parameter set as the base case in the economic model and randomly selected 1,000 good-fitting parameter sets for the probabilistic sensitivity analysis.
Results
The values for the best-fitting parameter set and ranges for 1,000 good-fitting parameter sets are shown in Table A9. The corresponding monthly transition probabilities using the best fitting parameter set can be found in Table 12 of the main text.
Table A9:
Values of the Best-Fitting and Good-Fitting Parameter Sets
| Parameter | Value in best-fitting (range of 1,000 good-fitting) parameter set | Definition |
|---|---|---|
| Rab4–6 | 0.392 (0.34, 0.44) per patient-year | Annual disability rate from functional independence to disability for months 4 to 6 post-stroke |
| Rab7–12 | 0.267 (0.23, 0.28) per patient-year | Annual disability rate from functional independence to disability for months 7 to 12 post-stroke |
| Rab13–24 | 0.161 (0.16, 0.20) per patient-year | Annual disability rate from functional independence to disability for months 13 to 24 post-stroke; i.e., at 76 years old |
| ORab_age | 0.830 (0.83, 0.92) | Odds ratio of age for risk of disability |
| RRac4–12a | 2.646 (2.1, 2.9) | Relative risk of mortality versus the age-specific general population for patients with functional independence for months 4 to 12 post-stroke |
| RRbc4–12a | 7.57 (7.5, 8.2) | Relative risk of mortality versus the age-specific general population for patients with disability for months 4 to 12 post-stroke |
| RRac13–60 | 1.035 (1.0, 1.1) | Relative risk of mortality versus the age-specific general population for patients with functional independence for months 13 to 60 post-stroke |
| RRbc13–60 | 2.899 (2.6, 3.0) | Relative risk of mortality versus the age-specific general population for patients with disability for months 13 to 60 post-stroke |
Because values of time-dependent parameters in 4- to 6-month and 7- to 12-month groups were fairly close, we combined them.
Table A10 presents the percentage of patients in different health states at various follow-up times using the best-fitting parameter set. The calibrated results were very close to the observed data (Table A7).
Table A10:
Percentage of Patients in Three Health States, Best-Fitting Model
| Time Post-stroke | Functional Independence, % (mRS 0–2) | Disability, % (mRS 3–5) | Death, % (mRS 6) |
|---|---|---|---|
| 3 months | 30.3 | 47.5 | 22.2 |
| 6 months | 31.7 | 42.2 | 26.1 |
| 1 year | 29.9 | 37.1 | 33.1 |
| 2 years | 24.4 | 37.1 | 38.5 |
| 5 years | 14.9 | 29.1 | 56.0 |
Abbreviation: mRS, modified Rankin Scale.
To assess external consistency, we compared the calibrated relative risks of mortality (post-stroke patients versus the age-specific general population) with that of the Perth Community Stroke Study in Australia.58 On the basis of the calibrated relative risk of mortality for the general population versus risk for functional independence and disability patients in Table A7, and the percentage of patients of functional independence and disability in the best-fitting model in Table A8, we estimated that the relative risks weighted by the functional status were approximately 2.07, 2.16, and 2.27 at 1, 2, and 5 years after stroke, respectively. These relative risks were very close to that reported in Australia, which ranged from 2 to 2.3 between years 2 and 5. Also, the trend of risk of mortality over time in our calibrated results was the same as that in a study of the Swedish population.62
Appendix 6: Cost of Mechanical Thrombectomy
Table A11:
Literature Review of Hospitalization Costs for Mechanical Thrombectomy—Summary
| Author, Year | Currency, Cost Year | Mean Hospitalization Cost for Mechanical Thrombectomy | Mean Hospitalization Cost for Control | Incremental Hospitalization Cost, Mechanical Thrombectomy Versus Control,a 2015 Canadian Dollars |
|---|---|---|---|---|
| Leppert et al, 201537 | USD, 2012 | $14,405 (additional cost of MT, relative to IVT) | NA | $14,572 |
| Rai et al, 201551 | USD, cost year unclear | $23,698 (favourable outcome) $31,500 (poor outcome) |
$13,688 (favourable outcome) $20,934 (poor outcome) |
$12,359 (favourable outcome) $13,046 (poor outcome) |
| Simpson et al, 201452 | USD, 2012 | $35,130 | $25,630 | $9,610 |
| Bouvy et al, 201333 | Euro, 2010 | €4,171 (additional costs at 6 months IVT relative to conservative treatment, 50% patients used retrievable stent) |
€971 (additional costs at 6 months IVT relative to conservative treatment) |
$4,558 |
| Chen, 201253 | USD, 2012 | At least $10,000 more per patient (estimate) | Not report explicitly | >$10,116 |
| Nguyen-Huynh et al, 201134 | USD, 2009 | $19,210 (without SICH) $28,087 (with SICH) |
$4,686 (without SICH) $10,245 (with SICH) |
$17,681 (without SICH) $21,720 (with SICH) |
| Kim et al, 201135 | USD, 2009 | $20,657 (without SICH) $29,534 (with SICH) |
$8,408 (without SICH) $15,945 (with SICH) |
$14,910 (without SICH) $16,543 (with SICH) |
| Patil et al, 200936 | USD, 2008 | $24,154 | $6,749 | $20,349 |
| University Health Network (UHN), 2015 | CAD, 2015 | $41,941 (entire episode of care, excluding physician fee) $16,965 (device) |
NA | NA |
| Ottawa Hospital, 2015 | CAD, 2015 | $10,473 (assuming 1.3 devices per patient) | NA | NA |
| Turk et al, 201463 | USD, 2013 | Traditional Penumbra aspiration system with separator: $33,611 (total); $7,421 (device) Stent retriever with local aspiration: $51,599 (total); $10,263 (device) direct aspiration first-pass technique: $54,700 (total); $15,798 |
NA | NA |
| Bing et al, 201364 | Euro, 2010 CAD, 2010 |
€5,018 (cost of materials, wires, catheters, femoral introducers, carotid stents, et al) or $6,936 CAD | NA | NA |
| Brinjikji et al, 201165 | USD, 2008 | $36,999 (median, with good outcomes) $50,628 (median, with severe disability) $35,109 (median, with mortality) |
NA | NA |
Abbreviations: MT, mechanical thrombectomy; IVT, intravenous thrombolysis; SICH, symptomatic intracerebral hemorrhage.
Author contributions
This report was developed by a multi-disciplinary team from Health Quality Ontario. The lead clinical epidemiologist was Vania Costa, the medical librarian was Corinne Holubowich
KEY MESSAGES
More than eight in 10 strokes happen because an artery becomes blocked, causing a sudden loss of blood flow to part of the brain. Ongoing lack of blood flow can lead to permanent brain damage. Treating these types of strokes involves trying to open the blocked blood vessels so the blood can flow again. The blockages can be removed using drugs or different types of surgery. Still, when the blockage is in the large artery, current treatments are not always enough to clear it. Newer devices may do a better job of clearing large blood vessels and helping patients recover from stroke.
This study investigated how safe, effective, and cost-effective these new devices are. We found that they improved patients’ ability to live independently after a stroke caused by a blockage in a large artery. They were as safe as current treatment options, and they were also cost-effective.
Contributor Information
Health Quality Ontario:
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are.
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do.
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters.
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).van der Worp BH, van Gijn J. Acute Ischemic Stroke. New Engl J Med. 2007(357): 572–9. [DOI] [PubMed] [Google Scholar]
- (2).Association AS. Guidelines for the prevention of stroke in patients with ischemic stroke. Circulation. 2006(113): 409–49. [Google Scholar]
- (3).Canada S. Canadian community health survey - overall stroke statistics. 2009.
- (4).Association AH. Executive summary: heart disease and stroke statistics – 2013 update. Circulation. 2013(127): 143–52. [DOI] [PubMed] [Google Scholar]
- (5).Canada S. Canada health measures survey - cholesterol levels of Canadians, 2009–2011. 2012.
- (6).Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. The Lancet. 2012; 380(9849): 1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. The Lancet. 2012; 380(9849): 1241–9. [DOI] [PubMed] [Google Scholar]
- (8).American Stroke Association. Guidelines for the early management of patients with ischemic stroke. Stroke. 2003; 34: 1056–83. [DOI] [PubMed] [Google Scholar]
- (9).Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384(9958): 1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bhatia R, Hill MD, Shobha N, Menon B, Bal S. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010; 41: 2254–8. [DOI] [PubMed] [Google Scholar]
- (11).Christou I, Felberg RA, Demchuk AM, Burgin WS, Malkoff M, Grotta C, et al. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging. 2002; 12: 119–23. [DOI] [PubMed] [Google Scholar]
- (12).del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. Stroke. 1998; 29: 4–11. [DOI] [PubMed] [Google Scholar]
- (13).Endo S, Kuwayama N, Hirashima Y, Akai T, Nishijima M. Results of urgent thrombolysis in patients with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. Am J Neuroradiol. 1998; 19: 1169–75. [PMC free article] [PubMed] [Google Scholar]
- (14).Rabinstein AA, Wijdicks EF, Nichols DA. Complete recovery after early intraarterial recombinant tissue plasminogen activator thrombolysis of carotid T occlusion. Am J Neuroradiol. 2002; 23: 1596–9. [PMC free article] [PubMed] [Google Scholar]
- (15).Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z. Site of arterial occlusion identified by transcranial doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007; 38: 948–54. [DOI] [PubMed] [Google Scholar]
- (16).Tomsick T, Brott T, Barsan W, Broderick J, Haley EC. Prognostic value of the hyperdense middle cerebral artery sign and stroke scale before ultra early thrombolytic therapy. Am J Neuroradiol. 1996; 17: 79–85. [PMC free article] [PubMed] [Google Scholar]
- (17).Zangerle A, Kiechi S, Spiegel M, Furtner M, Knoflach M, Werner P, et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology. 2007; 68: 39–44. [DOI] [PubMed] [Google Scholar]
- (18).Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013; 368(10): 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. The New England journal of medicine. 2013; 368(10): 904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kidwell CS, Jahan R, Saver JL. Endovascular treatment for acute ischemic stroke. The New England journal of medicine. 2013; 368(25): 2434–5. [DOI] [PubMed] [Google Scholar]
- (21).Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: a systematic review. JAMA - Journal of the American Medical Association. 2015; 313(14): 1451–62. [DOI] [PubMed] [Google Scholar]
- (22).Beadell NC, Lutsep H. New stent retriever devices. Current atherosclerosis reports. 2013; 15(6): 333. [DOI] [PubMed] [Google Scholar]
- (23).Ontario Stroke Registry Database [Internet]. Toronto, ON. [cited 2015 April 16].
- (24).Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen (DK): The Nordic Cochrane Centre TCC. [Google Scholar]
- (25).Collaboration TC. Cochrane Handbook for Systematic Reviews of Interventions. Higgins JP. Tag S., editor. England: The Cochrane Collaboration and John Wiley & Sons Ltd.; 2008. [Google Scholar]
- (26).Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64(4): 380–2. [DOI] [PubMed] [Google Scholar]
- (27).Berkhemer OA, Fransen PSS, Beumer D, Van Den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372(1): 11–20. [DOI] [PubMed] [Google Scholar]
- (28).Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372(11): 1009–18. [DOI] [PubMed] [Google Scholar]
- (29).Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372(11): 1019–30. [DOI] [PubMed] [Google Scholar]
- (30).Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372(24): 2296–306. [DOI] [PubMed] [Google Scholar]
- (31).Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372(24): 2285–95. [DOI] [PubMed] [Google Scholar]
- (32).Fugate JE, Klunder AM, Kallmes DF. What is meant by “TICI”? Am J Neuroradial. 2013; 34(9): 1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Bouvy JC, Fransen PS, Baeten SA, Koopmanschap MA, Niessen LW, Dippel DW. Cost-effectiveness of two endovascular treatment strategies vs intravenous thrombolysis. Acta Neurol Scand. 2013; 127(5): 351–9. [DOI] [PubMed] [Google Scholar]
- (34).Nguyen-Huynh MN, Johnston SC. Is mechanical clot removal or disruption a cost-effective treatment for acute stroke? AJNR Am J Neuroradiol. 2011; 32(2): 244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kim AS, Nguyen-Huynh M, Johnston SC. A cost-utility analysis of mechanical thrombectomy as an adjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke. Stroke. 2011; 42(7): 2013–8. [DOI] [PubMed] [Google Scholar]
- (36).Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg. 2009; 110(3): 508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Leppert MH, Campbell JD, Simpson JR, Burke JF. Cost-Effectiveness of Intra-Arterial Treatment as an Adjunct to Intravenous Tissue-Type Plasminogen Activator for Acute Ischemic Stroke. Stroke. 2015; 46(7): 1870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372(1): 11–20. [DOI] [PubMed] [Google Scholar]
- (39).Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. The New England journal of medicine. 2013; 368(10): 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. The New England journal of medicine. 2013; 368(10): 904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. The New England journal of medicine. 2013; 368(10): 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Luengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013; 44(10): 2854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013; 81(18): 1588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Dorman P, Dennis M, Sandercock P. Are the modified “simple questions” a valid and reliable measure of health related quality of life after stroke? United Kingdom Collaborators in the International Stroke Trial. J Neurol Neurosurg Psychiatry. 2000; 69(4): 487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Mittmann N, Seung SJ, Hill MD, Phillips SJ, Hachinski V, Cote R, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci. 2012; 39(6): 793–800. [DOI] [PubMed] [Google Scholar]
- (46).Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006. [Google Scholar]
- (47).Statistics Canada. Consumer Price Index, health and personal care [Internet]. Statistics Canada; 2015. Available from: http://www.statcan.gc.ca
- (48).Vanni T, Karnon J, Madan J, White RG, Edmunds WJ, Foss AM, et al. Calibrating models in economic evaluation: a seven-step approach. Pharmacoeconomics. 2011; 29(1): 35–49. [DOI] [PubMed] [Google Scholar]
- (49).Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007; 68(19): 1583–7. [DOI] [PubMed] [Google Scholar]
- (50).Life tables in 2004, United Kingdom [Internet]. 2013. Available from: http://www.mortality.org
- (51).Rai AT, Evans K. Hospital-based financial analysis of endovascular therapy and intravenous thrombolysis for large vessel acute ischemic strokes: The ‘bottom line’. J Neurointerv Surg. 2015; 7(2): 150–6. [DOI] [PubMed] [Google Scholar]
- (52).Simpson KN, Simpson AN, Mauldin PD, Hill MD, Yeatts SD, Spilker JA, et al. Drivers of costs associated with reperfusion therapy in acute stroke: The interventional management of stroke III trial. Stroke. 2014; 45(6): 1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Chen M. Cost-effectiveness of endovascular therapy for acute ischemic stroke. Neurology. 2012; 79(SUPPL. 1): S16–S21. [DOI] [PubMed] [Google Scholar]
- (54).Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010; 36(3): 48. [Google Scholar]
- (55).Villacorta P. MultinomialCI: Simultaneous confidence intervals for multinomial proportions according to the method by Sison and Glaz. R package version 1.0. 2012. Available from: http://CRAN.R-project.org/package=MultinomialCI
- (56).Subbiah M. CoinMinD: Simultaneous Confidence Interval for Multinomial Proportion. R package version 1.1.2013. Available from: http://CRAN.R-project.org/package=CoinMinD
- (57).Pham B, Krahn M. End-of-life care interventions: an economic analysis. Ontario health technology assessment series. 2014; 14(18): 1–70. [PMC free article] [PubMed] [Google Scholar]
- (58).Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS, et al. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke. 2000; 31(9): 2080–6. [DOI] [PubMed] [Google Scholar]
- (59).Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011; 377(9778): 1681–92. [DOI] [PubMed] [Google Scholar]
- (60).National Institute for Health and Clinical Excellence. Technology appraisal guidance: Alteplase for treating acute ischaemic stroke. London and Manchester; 2012. [cited 2015 Nov 16].
- (61).Sison C, Glaz J. Simultaneous confidence intervals and sample size determination for multinomial proportions. J Am Stat Assoc. 1995; 90: 66–9. [Google Scholar]
- (62).Olai L, Omne-Ponten M, Borgquist L, Svardsudd K. Survival, hazard function for a new event, and healthcare utilization among stroke patients over 65 years old. Stroke. 2009; 40(11): 3585–90. [DOI] [PubMed] [Google Scholar]
- (63).Turk AS, Turner R, Spiotta A, Vargas J, Holmstedt C, Ozark S, et al. Comparison of endovascular treatment approaches for acute ischemic stroke: cost effectiveness, technical success, and clinical outcomes. J Neurointerv Surg. 2014. [DOI] [PubMed]
- (64).Bing F, Jacquin G, Poppe A, Roy D, Raymond J, Weill A. The cost of materials for intra-arterial thrombectomy. Interv Neuroradiol. 2013; 19(1): 83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Brinjikji W, Kallmes DF, Rabinstein AA, Lanzino G, Cloft HJ. Hospitalization costs for patients with acute ischemic stroke treated with endovascular embolectomy in the United States. Stroke. 2011; 42(11): 3271–3. [DOI] [PubMed] [Google Scholar]
- (66).Canadian Forex Foreign Exchange Services. Yearly Average Exchange Rates [Internet]. 2015. [cited 2015]. Available from: http://www.canadianforex.ca/forex-tools/historical-rate-tools/yearly-average-rates