Abstract
Background
Evidence comparing premixed insulin analogues with other antidiabetic agents is urgently required to guide appropriate therapy.
Purpose
To summarize the English-language literature on the effectiveness and safety of premixed insulin analogues as compared with other antidiabetic agents in adults with type 2 diabetes.
Data Sources
We searched MEDLINE®, EMBASE®, CINAHL, and the Cochrane Central Register of Controlled Trials from inception to February 2008, and unpublished data from U.S. Food and Drug Administration, European Medicines Agency, and industry.
Study Selection
Studies with control arms that compared premixed insulin analogues to another antidiabetic medication in adults with type 2 diabetes
Data Extraction
Serial abstraction by 2 reviewers using standardized protocols
Data Synthesis
Evidence from clinical trials was inconclusive for clinical outcomes, such as mortality. Therefore, the review focused on intermediate outcomes. Premixed analogues were similar to premixed human insulin in lowering fasting glucose, hemoglobin A1c, and the incidence of hypoglycemia but were more effective in lowering postprandial glucose (mean difference = -1.1 mmol/L; 95% CI = -1.4 to -0.7 mmol/L [-19.2 mg/dL; 95% CI=-25.9 to -12.5 mg/dL]). As compared to long-acting insulin analogues, premixed analogues were superior in lowering postprandial glucose (mean difference= -1.5 mmol/L; 95%CI = -1.9 to -1.2 mmol/L [-27.9 mg/dL; 95% CI=-34.3 to -21.5 mg/dL]) and hemoglobin A1c (mean difference=-0.39%; 95% CI=-0.50% to -0.28%) but inferior in lowering fasting glucose (mean difference=0.7 mmol/L; 95%CI = 0.3 to 1.0 mmol/L [12.0 mg/dL; 95% CI=6.0 to 18.1 mg/dL]) and had higher incidence of hypoglycemia. When compared to noninsulin antidiabetic agents, premixed analogues were more effective in lowering fasting glucose (mean difference= -1.1mmol/L; 95%CI = -1.7 to 0.6 mmol/L [-20.5 mg/dL; 95% CI=-29.9 to -11.2 mg/dL]), postprandial glucose (mean difference= -2.1 mmol/L; 95%CI = -3.4 to -0.8 mmol/L [-37.4 mg/dL; 95% CI=-61.0 to -13.7 mg/dL]), and hemoglobin A1c (mean difference=-0.49%; 95% CI=-0.86% to -0.12%) but had higher incidence of hypoglycemia.
Limitations
Searching was restricted to studies published in English. Data on clinical outcomes was limited. The small number of studies for each comparison limited assessment of between-study heterogeneity.
Conclusions
Premixed insulin analogues provide glycemic control similar to premixed human insulin and may provide tighter glycemic control than long-acting insulin analogues and noninsulin antidiabetic agents.
According to the National Health Interview Survey, 28% of patients with type 2 diabetes are using insulin either alone (16%) or in combination with oral antidiabetic agents (12%) (1). In the management of type 2 diabetes, the place of premixed insulin analogues in relation to other insulin regimens and noninsulin antidiabetic agents is unclear. Premixed insulin analogues may allow patients flexible meal times, since these insulin preparations can be administered from 15 minutes before meals to immediately following a meal. Given the increasing prevalence of type 2 diabetes (2), the number of patients who use insulin for glycemic control (1), and the importance of glycemic control in decreasing mortality and morbidity (3), it is imperative to establish the weight of evidence for the safety and effectiveness of these relatively newer insulin preparations, as compared to those of traditional insulin preparations. Therefore, the Agency for Healthcare Research and Quality commissioned a systematic review of published studies on the comparative effectiveness and safety of all the premixed insulin analogues that are approved by the Food and Drug Administration and are available in the U.S.
Methods
Data Sources and Selection
We searched MEDLINE®, EMBASE®, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Cumulative Index to Nursing & Allied Health Literature (CINAHL) from inception to February 2008 (complete search strategy available at http://effectivehealthcare.ahrq.gov/). We also reviewed reference lists of included articles, recent issues of 13 medical journals, the Food and Drug Administration and European Medicines Agency websites for the premixed insulin analogues, unpublished data from premixed insulin analogue manufacturers (Eli Lilly and Company [Indianapolis, IN], Sanofi-Aventis [Bridgewater, NJ], and Novo Nordisk [Bagsvaerd, Denmark]), and websites of public registries of clinical trials (clinicaltrials.gov and clinicalstudyresults.org).
Study Selection
We included studies that compared a premixed insulin analogue approved by the Food and Drug Administration as of February 2008 to any other drug for adults with type 2 diabetes and evaluated clinical outcomes (e.g., mortality), intermediate outcomes (e.g., hemoglobin A1c level), or adverse events (e.g., hypoglycemia). We included randomized controlled trials (RCTs), controlled clinical trials, and observational studies with control groups, regardless of their duration or sample size. However, we used data from crossover studies only for intermediate outcomes and hypoglycemia. We excluded crossover trials from the quantitative evaluation of outcomes that were either progressive (e.g., retinopathy) or irreversible (e.g., mortality). For the evaluation of hemoglobin A1c, we included crossover trials with at least 12 weeks of follow-up before and after the crossover phase. We aimed to use within-individual comparisons from crossover trials if trials had reported data in such detail, but no study presented data in such detail. As all crossover studies reported results for each intervention and no trial reported a statistically significant carryover effect, we ignored the crossover design and used reported estimates as if they came from a parallel trial. We excluded non-English articles, editorials, comments, letters, and abstracts.
Data Extraction and Quality Assessment
Two investigators reviewed the titles, abstracts, and full articles independently for inclusion and abstracted data using standardized forms. We developed a study quality assessment tool based on the Jadad criteria (4), the Newcastle-Ottawa Scale (5), and questions from Agency for Healthcare Research and Quality’s Guide for Conducting Comparative Effectiveness Reviews (6). We adapted the evidence grading scheme recommended by the Grading of Recommendations Assessment, Development and Evaluation Working Group (7) to classify the strength of the body of evidence on each comparison as high, moderate, low, or insufficient.
Data Synthesis and Analysis
We conducted meta-analyses for outcomes when data were sufficient (two or more trials). For intermediate outcomes (fasting glucose, postprandial glucose, and hemoglobin A1c) and the adverse outcome of weight change, we recorded the mean difference between groups, along with its measure of dispersion. If this was not reported, we calculated the point estimate using the mean difference from baseline for each group. If the mean difference from baseline was not reported, we calculated this from the baseline and final values for each group. If no measure of dispersion was reported for the between-group difference, we then calculated it using the sum of the variances for the mean difference from baseline for each group. If there were no measures of dispersion for the mean difference from baseline for each group, we then calculated the variance using the standard deviation of the baseline and final values, assuming a correlation between baseline and final values of 0.5. We pooled the results of the plasma and blood glucose levels from different studies, since blood glucose measurements accurately reflect plasma glucose levels (8). For hypoglycemia, we used two strategies to synthesize data. If a trial reported the incidence of hypoglycemia, we calculated an odds ratio (OR) using the incidence of hypoglycemia in each study group. If a trial did not report the incidence of hypoglycemia but reported event rates in episodes per patient per 30 days, we calculated the rate ratio by dividing the event rate in the premixed insulin analogue arm by the event rate in the comparator arm. If a trial reported the number of episodes in each arm or reported an event rate in a form other than episodes per patient per 30 days, we converted this information into episodes per patient per 30 days. We pooled the results of individual studies using a random-effects model. These analyses were conducted using Comprehensive Meta-Analysis (version 2.2.046) software (Biostat, Englewood, NJ).
For clinical outcomes, we included all studies that reported any information about clinical events (all-cause mortality and cardiovascular mortality and morbidity). All analyses followed the intention-to-treat principle. We combined results from the premixed insulin analogue arm of different trials assuming that the results were similar enough between premixed insulin analogues. In the study with three arms and comparing a premixed insulin analogue with two different insulin preparations (9), we chose the most relevant comparison to include in the meta-analyses (premixed insulin analogue versus long-acting insulin analogue). Pooled ORs and 95% confidence intervals (CIs) were calculated using a Mantel-Haenszel fixed-effects model (with a 0.1 continuity correction) using Stata Intercooled version 9.2 (StataCorp, College Station, TX) (10;11). For analysis of clinical outcomes, we used a fixed-effects model because it is less biased with rare event data (12). For sensitivity analyses, we used the following meta-analytic methods: Peto’s method, the Mantel-Haenszel fixed-effects model (with a 0.5 and 0.01 continuity correction), and a Bayesian analysis (13). Heterogeneity among the trials was tested with a standard chi-squared test, using a significance level less than or equal to 0.10. We also examined inconsistency among studies with an I2 statistic (14). A value greater than 50% represented substantial variability.
For all outcomes, we conducted sensitivity analyses by omitting one study at a time. We assessed publication bias visually by examining the symmetry of funnel plots and statistically by Begg’s (15) and Egger’s (16) tests.
Role of the Funding Source
Agency for Healthcare Research and Quality suggested the initial questions and provided copyright release for this manuscript but did not participate in the literature search, data-analysis or interpretation of the results.
Results
Study Characteristics
Appendix Figure (available at www.annals.org) shows the results of the literature search. We found 45 studies that reported on at least one of the intermediate clinical outcomes or adverse events (Appendix Table, available at www.annals.org). All were RCTs except two (17;18). In one study (17), patients were enrolled consecutively and followed prospectively, while in the other study (18), data were obtained from the medical record database of a large employer. Among the RCTs, 23 were parallel-arm (9;19-40) and 20 were crossovers (41-60). The median duration of follow-up in these trials was 16 weeks (range of 1 day to 2 years).
These trials enrolled a total of 14,603 patients (median number per trial = 93; range: 8 to 8,166 patients). The enrolled populations in the studies had a median age of 59 years (range: 51 to 68 years), and most patients were male (median = 52 percent, range: 16 to 92 percent; see Appendix Table, available at www.annals.org). The study populations had a median hemoglobin A1c of 8.7 percent (range: 7.3 to 10.7 percent), a median body mass index of 29.4 kg/m2 (range: 24 to 37 kg/m2), and a median duration of diabetes of 11 years (range: 4 to 16 years). Eleven trials enrolled insulin-naïve patients (9;18;19;27;29;33;34;36;38;43;44), 26 enrolled insulin-treated patients (23;25;28;32;34;35;37;39-42;45-55;57-60), and nine did not specify history of insulin treatment (17;20-22;24;26;30;31;56).
Study Quality and Applicability
Randomization methods were described in 17 studies (9;20-22;24;27-30;35-37;39;41;44;46;59) and were adequate in all except one (20). Five trials used blinding for patients and providers (32;41;49;57;60), and two trials used blinding for outcome assessors (9;28). It is difficult to achieve blinding of patients and providers because premixed insulin analogues need to be given with meals, while the other insulin preparations are generally given at other times or with different frequency. The funding source was the pharmaceutical industry in all except two trials; one was funded jointly by the National Institutes of Health and Eli Lilly (56), and the other was funded by the Japan Diabetes Foundation (40). Six trials did not report their funding source (29;38;44;45;52;60).
Most studies, except 5 (20;34;41;47;57), enrolled patients similar in age to the general U.S. diabetic population. Women were underrepresented in five trials (17;19;30;35;57), and in two trials there were more women than men (27;36). In most trials, the spectrum of diabetic complications and co-morbid conditions among the enrolled participants was limited. All trials either excluded patients with cardiac, renal, or hepatic disease or did not report whether or not such patients were included, thus limiting our ability to generalize the results to these subpopulations.
Publication Bias
Using the Egger’s test, we found evidence of possible publication bias for the comparison between premixed insulin analogues and noninsulin antidiabetic agents and between insulin aspart 70/30 and noninsulin antidiabetic agents for fasting glucose (1-sided p-values 0.07 and 0.05, respectively) and for mild hypoglycemia (1-sided p-value 0.03 and 0.07, respectively). Not enough studies were available to assess possible bias for other comparisons.
Premixed insulin analogues versus long-acting insulin analogues (see Table 1 and Figures 1-4)
Table 1.
Summary of the Strength of Evidence* Comparing Premixed Insulin Analogues to Other Antidiabetic Agents for Intermediate Outcomes and Adverse Events
| Premixed Insulin Analogues vs. Long- Acting Insulin Analogues |
Premixed Insulin Analogues vs. Premixed Human Insulin |
Premixed Insulin Analogues vs. Other Insulin Regimens |
Premixed Insulin Analogues vs. Noninsulin Antidiabetic Agents |
|
|---|---|---|---|---|
| Fasting glucose | Moderate SOE favoring long-acting insulin analogues |
Moderate SOE suggesting similar effectiveness |
Low SOE; unable to make a conclusion |
Moderate SOE favoring premixed insulin analogues |
|
Postprandial
glucose |
High SOE favoring premixed insulin analogues |
High SOE favoring premixed insulin analogues |
Low SOE unable to make a conclusion |
Moderate SOE favoring premixed insulin analogues |
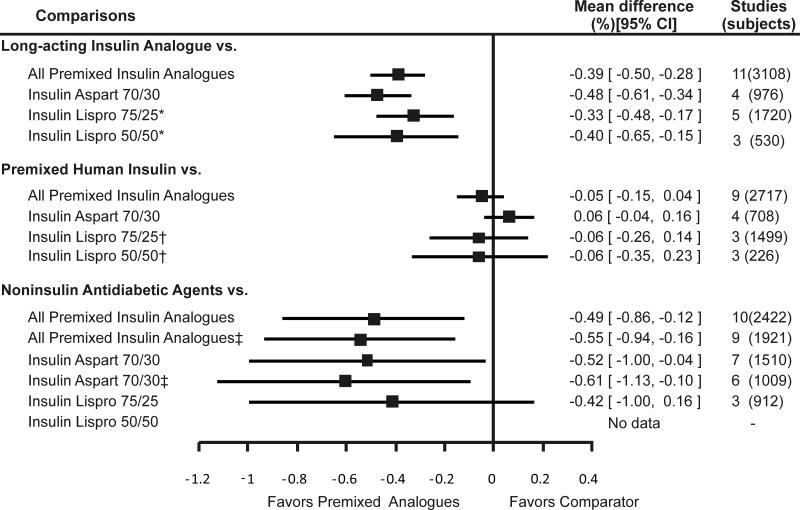
| Hemoglobin A1c | High SOE favoring premixed insulin analogues |
High SOE suggesting similar effectiveness |
Low SOE; unable to make a conclusion |
Moderate SOE favoring premixed insulin analogues |
| Hypoglycemia | High SOE favoring long- acting insulin analogues |
High SOE favoring premixed human insulin |
Low SOE; unable to make a conclusion |
High SOE favoring noninsulin antidiabetic agents |
| Weight | Moderate SOE favoring long-acting insulin analogues |
Moderate SOE suggesting similar effectiveness |
Low SOE; unable to make a conclusion |
Moderate SOE favoring noninsulin antidiabetic agents |
|
All-cause
mortality, CVD mortality and morbidity |
Low SOE; unable to make a conclusion | |||
Definitions for strength of evidence: High = further research is very unlikely to change our confidence in the estimates; Moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low = further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
CVD = cardiovascular disease; NA = not applicable; SOE = strength of evidence
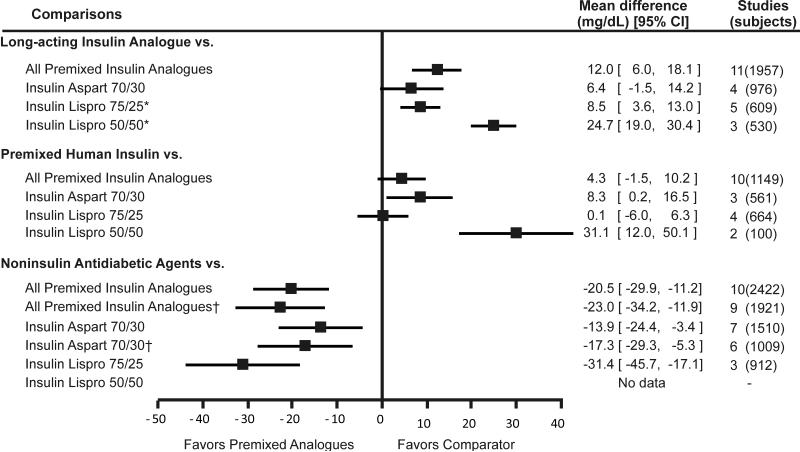
Figure 1. Weighted mean difference of change in fasting glucose comparing premixed insulin analogues with other antidiabetic medications.
Footnote: error bars represent 95% confidence intervals.
* pooled results include a study by Jacober et al (43) that administered insulin lispro 50/50 in the morning and afternoon and insulin lispro 75/25 in the evening.
† Nauck et al (21) excluded.
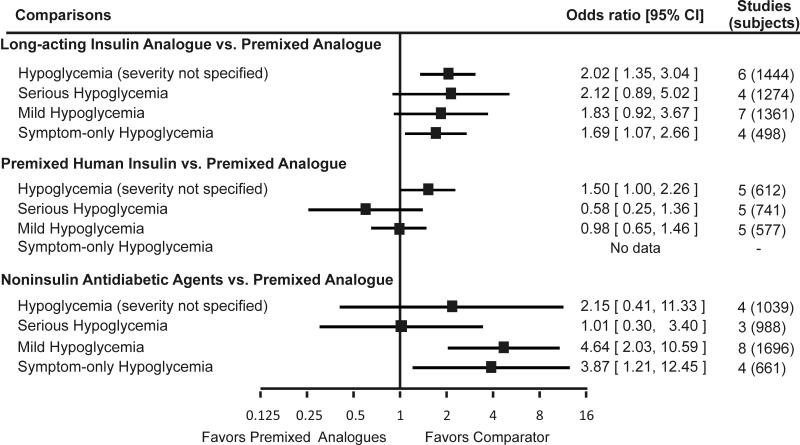
Figure 4. Incidence of unclassified hypoglycemia, serious hypoglycemia, mild hypoglycemia, and symptom-only hypoglycemia with premixed insulin analogues as compared to other antidiabetic medications.
Footnote: Error bars represent 95% confidence intervals.
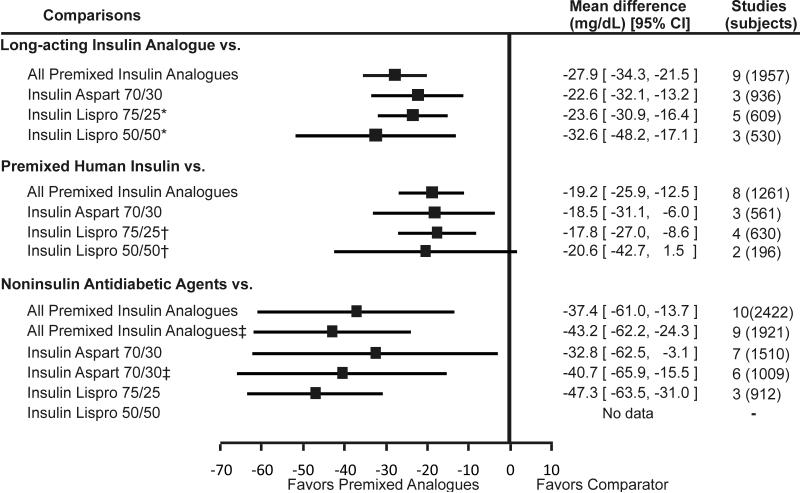
Premixed insulin analogues were less effective than long-acting insulin analogues (administered alone) in lowering fasting glucose (pooled difference = 0.7mmol/L; 95%CI = 0.3 to 1.0 mmol/L [12.0 mg/dL; 95% CI = 6.0 to 18.1mg/dL]). Individually, insulin lispro 75/25 and insulin lispro 50/50 were less effective in lowering fasting glucose than long-acting insulin. While the difference between insulin aspart 70/30 and long-acting insulin was not statistically significant, the direction of the effect was in favor of the long-acting insulin analogues. In contrast to fasting glucose, premixed insulin analogues were more effective than long-acting insulin analogues in lowering postprandial glucose (pooled difference = -1.5 mmol/L; 95%CI = -1.9 to -1.2 mmol/L [-27.9 mg/dL; 95% CI = -34.3 to -21.5 mg/dL]) and A1c (pooled difference = -0.39%; 95% CI = -0.5% to -0.3%). When compared individually to long-acting insulin analogues, all three premixed insulin analogues remained statistically significantly better in lowering postprandial glucose and A1c.
While effective in lowering postprandial glucose and A1c, premixed insulin analogues may increase the incidence of hypoglycemia (severity not specified) and the amount of weight gain to a greater extent than the long-acting insulin analogues (OR = 2.0; 95% CI = 1.3 to 3.0; and pooled difference = 2.0 kg; 95% CI = 1.1 to 3.0 kg, respectively). Insulin aspart 70/30 was associated with a higher incidence of hypoglycemia (severity not specified) and minor hypoglycemia and statistically significantly more weight gain (pooled difference = 2.5 kg; 95% CI: 1.6 to 3.4 kg). Although the incidence of hypoglycemia was neither consistent nor statistically significant across all trials, the direction of the individual study effect sizes suggested that both insulin lispro 75/25 and insulin lispro 50/50 may increase the incidence of hypoglycemia when compared to long-acting insulin analogues. In two studies, use of insulin lispro 50/50 resulted in a larger weight gain than did long-acting insulin analogues; this difference was statistically significant in only one study. None of the studies reported the comparative effects of insulin lispro 75/25 and long-acting insulin analogues on weight change.
Premixed insulin analogues versus premixed human insulin (see Table 1 and Figures 1-4)
We found 16 studies that compared premixed insulin analogues with premixed human insulin. The pooled analysis suggested that premixed insulin analogues may be less effective than premixed human insulin in lowering fasting glucose (pooled difference = 0.2 mmol/L; 95%CI = -0.1 to 0.6 mmol/L [4.3 mg/dL; 95% CI = -1.5 to 10.2 mg/dL]), but this difference was not statistically significant. However, these analogues were more effective in lowering postprandial glucose (pooled difference = -1.1 mmol/L; 95%CI = -1.4 to -0.7 mmol/L [-19.2 mg/dL; 95% CI = -25.9 to -12.5 mg/dL]). Premixed insulin analogues were similar to premixed human insulin in lowering A1c levels (pooled difference = -0.05%; 95% CI = -0.14% to 0.04%). Insulin aspart 70/30 was less effective while insulin lispro 75/25 and 50/50 were similar to premixed human insulin in lowering fasting glucose. All three premixed insulin analogues were more effective in lowering postprandial glucose than premixed human insulin. None of the premixed insulin preparations was better than premixed human insulin in lowering A1c.
Premixed insulin analogues, as group or individually, were similar to premixed human insulin in the incidence of major and minor hypoglycemia (OR = 0.6; 95% CI = 0.2 to 1.3; and OR = 1.0; 95% CI = 0.6 to 1.5, respectively). Similarly, insulin aspart 70/30 and insulin lispro 50/50 were similar to premixed human insulin in weight gain (28;35).
Premixed insulin analogues versus other insulin regimens
We found only two studies that compared a premixed insulin analogue with a rapid-acting insulin analogue (9;23), two studies that compared a premixed insulin analogue with a combination regimen of long-acting and rapid-acting insulin analogues (17;39), two studies that compared a premixed insulin analogue to intermediate-acting insulin (32;33), and one study that compared a premixed insulin analogue with a combination rapid-acting insulin analogues with intermediate-acting human insulin (40). Due to the sparseness of the data, we are unable to draw firm conclusions about these comparisons.
Premixed insulin analogues versus noninsulin antidiabetic agents (see Table 1 and Figures 1-4)
Ten studies evaluated this comparison. Premixed insulin analogues were more effective than noninsulin antidiabetic agents in lowering fasting glucose (pooled difference = -1.1 mmol/L; 95% CI = -1.7 to 0.6 mmol/L [-20.5 mg/dL; 95% CI = -29.9 to -11.2 mg/dL]), postprandial glucose (pooled difference = -2.1 mmol/L; 95%CI = -3.4 to -0.8 mmol/L [-37.4 mg/dL; 95% CI = -61.0 to -13.7 mg/dL]), and hemoglobin A1c levels (pooled difference = -0.5%; 95% CI = -0.9% to -0.1). These results did not change when the only study that compared premixed insulin analogue (insulin aspart 70/30) to exenatide was excluded. Insulin aspart 70/30 was more effective than noninsulin antidiabetic agents in lowering fasting glucose, postprandial glucose, and hemoglobin A1c. However, when only oral antidiabetic agents were kept in the meta-analysis, the pooled results were no longer statistically significant for hemoglobin A1c. Insulin lispro 75/25 was also more effective than oral antidiabetic agents in lowering fasting glucose, postprandial glucose and A1c, although the latter effect did not reach statistical significance.
As a group, premixed insulin analogues were associated with an increased risk of minor hypoglycemia (OR = 4.6; 95% CI = 2.0 to 10.6) and weight gain (pooled difference = 2.3 kg; 95% CI = 0.8 to 3.9 kg) as compared to noninsulin antidiabetic agents, while there was no difference in major hypoglycemia risk (OR = 1.0; 95% CI = 0.3 to 3.4). Patients on insulin aspart 70/30 had a higher incidence of minor hypoglycemia and symptom-only hypoglycemia and experienced a larger weight gain than did those on oral antidiabetic agents. Patients on exenatide lost weight, in contrast to the weight gain experienced by patients on premixed insulin analogues. Insulin lispro 75/25 was associated with a higher rate (measured as episode/patient/30-day) of overall hypoglycemia (rate ratio = 4.86; 95% CI: 0.5 to 49.5) and larger weight gain (pooled mean difference = 1.88 kg; 95% CI: 1.35 to 2.41 kg) when compared to oral antidiabetic agents. There were no studies that compared insulin lispro 50/50 with oral antidiabetic agents.
Premixed insulin analogues versus other antidiabetic medications: clinical outcomes (see Table 1 and Figure 5)
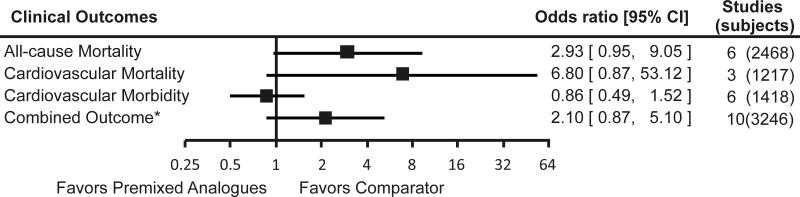
Figure 5. Incidence of clinical outcomes with premixed insulin analogues as compared to other antidiabetic medications.
Footnote: Error bars represent 95% confidence intervals.
* Combined outcome include all-cause mortality, and cardiovascular morbidity
We found 16 studies that evaluated clinical outcomes. Eleven studies were parallel-arm RCTs (9;21;22;24;26;28;30;31;37;38;40), and the remaining five were crossover RCTs. Two studies reported one death but did not state in which arm the event occurred (29;49). No statistically significant differences were found between premixed insulin analogues and their comparators in terms of all-cause mortality, cardiovascular mortality, or cardiovascular morbidity. While a suggestion of harm was seen in the pooled ORs for all-cause mortality, cardiovascular mortality, and the combined outcome of cardiovascular morbidity and mortality when premixed insulin analogues were compared to other antidiabetic medications, these point estimates were based on few absolute events in only a few studies, in which clinical outcomes were not the primary endpoints. Insufficient or no evidence was found with regard to microvascular outcomes.
No statistically significant heterogeneity was found in these studies with I-squared statistics less than 50 percent for all analyses. Sensitivity analyses using different meta-analytic techniques did not markedly affect the results, although less conservative techniques such as Peto’s method did reach borderline statistical significance for potential harm comparing premixed insulin analogues with active comparators.
Discussion
The ultimate goal of treatment of diabetes mellitus is improvement in clinical outcomes, particularly microvascular and macrovascular complications and mortality. We did not find any study that was specifically designed to evaluate these clinical outcomes. All studies were designed to evaluate intermediate outcomes (e.g., A1c, fasting glucose, and postprandial glucose), although some studies did report clinical outcomes as adverse events. Due to the sparse data on clinical outcomes, estimates of the comparative efficacy of premixed insulin analogues with other insulin preparations and noninsulin antidiabetic agents were inconclusive. Although pooled data suggested the possibility of worse clinical outcomes with premixed insulin analogues than with other antidiabetic medications, the data were too weak to support any firm conclusion about such a possibility. Due to the paucity of evidence on clinical outcomes, we evaluated the effects of premixed insulin analogues on intermediate outcomes. Although not ideal, these outcomes are commonly used clinically to optimize glycemic control and are known to predict clinical outcomes.
For intermediate outcomes, we found that premixed insulin analogues were similar to premixed human insulin in lowering fasting glucose and hemoglobin A1c but more effective in lowering postprandial glucose. Premixed insulin analogues appeared better than long-acting insulin analogues and noninsulin antidiabetic agents in lowering hemoglobin A1c and postprandial glucose. Premixed insulin analogues seemed less effective than long-acting insulin analogues but more effective than oral antidiabetic agents in lowering fasting glucose. Control of fasting and postprandial glucose levels in diabetic patients is important to bring and keep hemoglobin A1c within the desired range (61). Hemoglobin A1c is the standard of care for monitoring long-term glycemic control and in turn reflects both fasting and postprandial glucose control (62). A lower hemoglobin A1c is associated with a decrease in diabetic complications, such as retinopathy, nephropathy, and neuropathy (3;63), while cardiovascular complications of diabetes such as coronary heart disease, stroke, cardiovascular mortality, sudden cardiac death, and all cause mortality are closely related to fasting and postprandial glucose levels (64-68). Whether the observed effectiveness of premixed insulin analogues in lowering fasting and postprandial hyperglycemia translates into decreased mortality and morbidity remains unclear.
An important clinical consideration in the treatment of diabetic patients is to strike a balance between optimal glycemic control and treatment-associated side-effects such as hypoglycemia and weight gain. This consideration is particularly important as more recent data suggests that intensive glycemic control (i.e., target A1c less than 6%) may be associated with poorer clinical outcomes than with standard glycemic control (69;70). Premixed insulin analogues were more likely than noninsulin antidiabetic agents and long-acting insulin analogues to cause hypoglycemia while the incidence of hypoglycemia was similar between premixed insulin analogues and other insulin preparations. Data demonstrated a trade-off between tighter glucose control and more hypoglycemic events. This was evident in comparisons between the premixed insulin analogues and the long-acting insulin analogues or noninsulin antidiabetic agents where A1c is more effectively reduced by the premixed insulin analogues at the expense of an increased incidence of hypoglycemia. A similar trade-off was seen between tighter glycemic control and treatment-associated weight gain, although the paucity of data limited our evaluation of change in weight to only one of the premixed insulin analogues, insulin aspart 70/30.
For individual premixed insulin preparations, the number of comparative studies and the strength of evidence were variable, thereby affecting the precision of estimates of the direction and magnitude of effect size. Study design characteristics may, at least partially, explain the differences between premixed insulin analogues and premixed human insulin and long-acting insulin analogues. For example, late administration (less than 30 minutes before meals) of the premixed human insulin preparations may be responsible for the observed benefit of the premixed insulin analogues over the premixed human insulin preparations in lowering postprandial glucose, as less than half of the studies (25;28;33;47;48;51;55;71) administered human insulin at least 30 minutes before meals. Although premixed insulin analogues appear better than long-acting insulin analogues in lowering hemoglobin A1c, this finding may reflect the fact that the total daily dose of the long-acting insulin analogues was lower than that of the daily premixed insulin analogue dose in several studies (20;22;23;27;42-45;72). Similarly, the dose of premixed insulin analogues was titrated to achieve optimal glycemic control in several studies while the dose of oral antidiabetic agents was kept constant. This difference in dosing of the drugs may be responsible for the observed benefit of premixed insulin analogue preparations (24;30;34;36;38).
There are limitations to this systematic review. Several of these limitations are due to the constraints of limited reporting of data in the trials. Several studies presented blood glucose data in figures only, and we abstracted data from these figures when possible. Cross-over studies did not report data in a manner that could be used in a quantitative synthesis without some assumptions being made. These assumptions may have affected the quantitative synthesis of the evidence. We could not explore heterogeneity due to the relatively small number of studies available for each comparison. We addressed this limitation by using a random-effects model for all analyses of intermediate outcomes, regardless of the presence or absence of statistical heterogeneity. The small number of studies also precluded our ability to fully assess the potential for publication bias.
As most of the studies excluded patients with diabetic complications or other co-morbid conditions, findings of this systemic review cannot be generalized to all diabetic patients. Moreover, due to limited duration of follow-up in most studies, we cannot draw conclusions about the long-term comparative effectiveness of premixed insulin analogues. Finally, as we limited our search to English language articles, we may have missed some studies published in other languages.
In conclusion, premixed analogues provide glycemic control similar to premixed human insulin and may provide better glycemic control than long-acting insulin analogues and noninsulin antidiabetic agents, but the data on clinical outcomes is very limited. Studies with longer follow-up are needed to determine whether the effects observed early in treatment are sustainable long-term. Moreover, as improvement in intermediate clinical outcomes may not always result in improvement in clinical outcomes, studies specifically designed to evaluate clinical outcomes are needed.
Figure 2. Weighted mean difference of change in postprandial glucose comparing premixed insulin analogues with other antidiabetic medications.
Footnote: Error bars represent 95% confidence intervals.
* pooled results include a study by Jacober et al (43) that administered insulin lispro 50/50 in the morning and afternoon and insulin lispro 75/25 in the evening.
† Nauck et al (21) excluded. ‡ pooled results include a study by Roach et al (55) that administered insulin lispro 50/50 in the morning and insulin lispro 75/25 in the evening.
Figure 3. Weighted mean difference of change in hemoglobin A1c comparing premixed insulin analogues with other antidiabetic medications.
Footnote: Error bars represent 95% confidence intervals.
* pooled results include a study by Jacober et al (43) that administered insulin lispro 50/50 in the morning and afternoon and insulin lispro 75/25 in the evening.
† Nauck et al (21) excluded. ‡ pooled results include a study by Roach et al (55) that administered insulin lispro 50/50 in the morning and insulin lispro 75/25 in the evening.
Acknowledgments
Grant Support: This article is based on research conducted by the Johns Hopkins Evidence-based Practice Center under contract number 290-02-0018 with the Agency for Healthcare Research and Quality, Rockville, MD.
Appendix
Appendix Table.
Population characteristics of the included studies
| Mean A1c in % | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year Study Duration |
Group, N | Mean Age in Years |
Male, % | Mean BMI in kg/m2 Mean Weight in kg |
Mean Duration of Diabetes in Years |
Mean FG in mg/dL* |
Previous Treatment, n (%) |
| Randomized Controlled Trials | |||||||
| Abrahamian, 2005(25) |
Insulin aspart 70/30, 89 | 62.6 | 52 | BMI: 28 | 12.7 | A1c: 9.8 | Insulin naive: No |
| NPH/regular 70/30, 88 | 62.3 | 35 | BMI: 28.3 | 9.5 | A1c: 9.85 | Insulin naive: No | |
| 24 weeks | |||||||
| Bebakar, 2007(19) | Insulin aspart 70/30 + OAAs, 128 |
55 | 92 | BMI: 26.2 | 4.4 | A1c: 8.6 | Insulin naive: Yes |
| 24 weeks | OAAs: 128 (100) | ||||||
| OAAs, 63 | 52.7 | 69 | BMI: 25.4 | 4.3 | A1c: 8.5 | Insulin naive: Yes | |
| OAAs: 63 (100) | |||||||
| Boehm, 2004(28) Boehm, 2002(73)‡ |
Insulin aspart 70/30, 58 | 62.8 | 55† | BMI: 29.1 | 15.5 | A1c: 8.11 | Insulin naive: No |
| NPH/regular 70/30, 67 | 62.6 | 51† | BMI: 27.2 | 12.9 | A1c: 8.21 | Insulin naive: No | |
| 104 weeks | |||||||
| Christiansen, 2003(32) |
Insulin aspart 70/30, 201 | 59.3 | 47 | BMI: 28 | 9.2 | A1c: 8.8 | Insulin: 66 (33) |
| OAAs: 78 (39) | |||||||
| 16 weeks | |||||||
| Insulin and OAAs: 55 (27) |
|||||||
| NPH insulin, 202 | 59.6 | 50 | BMI: 28.4 | 10.5 | A1c: 8.8 | Insulin: 66 (33) | |
| OAAs: 75 (37) | |||||||
| Insulin and OAAs: 59 (29) |
|||||||
| Herz, 2002(34) | Insulin lispro 75/25, 71 | 68.1 | 52.1 | BMI: 28 | 11.4 | A1c: 9.82 | Insulin naive: No |
| 16 weeks | Glyburide, 72 | 67.7 | 44.4 | BMI: 27.8 | 12.4 | A1c: 9.9 | Insulin naive: No |
| Hirao, 2008(40) | Insulin aspart 70/30, 80 | 58.5 | 59† | BMI: 23.7 | 9.5 | A1c: 10.5 | Insulin naive: No |
| 6 months | Weight: 62.5 | OAAs: 41 (51†) | |||||
| Insulin aspart + NPH insulin, 80 |
57.9 | 61† | BMI:23.7 | 12.2 | A1c: 10.7 | Insulin naive: No | |
| Weight: 62.1 | OAAs: 39 (49†) | ||||||
| Holman, 2007(9) | Insulin aspart 70/30,** 235 | 61.7 | 67.7 | BMI: 30.2 | 9 median (IQR: 6 - 12) |
A1c: 8.6 | Insulin naive: Yes |
| 52 weeks | Weight: 86.9 | FG: 175 | OAAs: 221 (94†) | ||||
| Insulin aspart,** 239 | 61.6 | 63.6 | BMI: 29.6 | 9 median (IQR: 6 - 14) |
A1c: 8.6 | Insulin naive: Yes | |
| Weight: 84.9 | FG: 173 | OAAs: 227 (95†) | |||||
| Insulin detemir,** 234 | 61.9 | 61.9 | BMI: 29.7 | 9 median (IQR: 6 - 12) |
A1c: 8.4 | Insulin naive: Yes | |
| Weight: 85.5 | FG: 171 | OAAs: 224 (96†) | |||||
| Total, 708 | 61.7 | 64.1 | BMI: 29.8 | 9 median (IQR: 6 - 13) |
A1c: 8.5 | Insulin naive: Yes | |
| Weight: 85.8 | FG: 173 | OAAs: 672 (95†) | |||||
| Kann, 2006(22) | Insulin aspart 70/30 + metformin, 128 |
61.5 | 54† | BMI: 29.9 | 10.3 | A1c: 9.21 | NR |
| 26 weeks | Weight: 84.2 | ||||||
| Insulin glargine + glimepiride, 127 |
61 | 49† | BMI: 30.6 | 10.2 | A1c: 8.9 | NR | |
| Weight: 86.6 | |||||||
| Kazda, 2006(23) | Insulin lispro 50/50, 54 | 58.7 | 59† | BMI: 31 | 5.9 | A1c: 8.1 | Insulin naive: No Insulin: 0 in last 3 months |
| 24 weeks | FG: 167.4 | ||||||
| Insulin lispro, 52 | 60.4 | 62† | BMI: 31.7 | 5.3 | A1c: 8.2 | Insulin naive: No Insulin: 0 in last 3 months |
|
| FG: 176.4 | |||||||
| Insulin glargine, 53 | 59.1 | 43† | BMI: 30.1 | 5.5 | A1c: 8.1 | Insulin naive: No Insulin: 0 in last 3 months |
|
| FG: 172.8 | |||||||
| Kilo, 2003(33) | Insulin aspart 70/30,¶ 46 | 57.2 | 54 | BMI: 30.4 | 10.4 | A1c: 9.5 | Insulin naive: Yes |
| 12 weeks | FG: 241.8 | OAAs: 46 (100) | |||||
| NPH insulin,¶ 47 | 55.1 | 40 | BMI: 30.4 | 10.7 | A1c: 9.5 | Insulin naive: Yes | |
| FG: 242.7 | OAAs: 47 (100) | ||||||
| NPH/regular 70/30,¶ 47 | 55.4 | 52 | BMI: 30.6 | 8.4 | A1c: 9.3 | Insulin naive: Yes | |
| FG: 227.2 | OAAs: 47 (100) | ||||||
| Kvapil, 2006(24) | Insulin aspart 70/30, 107 | 55.2 | 47† | BMI: 30.9 | 8.2 | A1c: 9.6 | Insulin naive: NR |
| 16 weeks | Weight: 87.3 | ||||||
| Insulin aspart 70/30,¶ 108 | 56.4 | 49† | BMI: 30.4 | 6.7 | A1c: 9.3 | Insulin naive: NR | |
| Weight: 85.1 | |||||||
| Glibenclamide,¶ 114 | 58.1 | 46† | BMI: 30.5 | 8.1 | A1c: 9.4 | Insulin naive: NR | |
| Weight: 84 | |||||||
| Malone, 2003(31) | Insulin lispro 75/25,¶ 296 | 58 | 57 | BMI: 29.8 | 8 | A1c: 9.17 | Insulin naive: NR |
| 16 weeks | Weight: 83 | OAAs: 296 (100) | |||||
| Glibenclamide,¶ 301 | 59 | 49 | BMI: 29.6 | 7.4 | A1c: 9.27 | Insulin naive: NR | |
| Weight: 81.7 | OAAs: 301 (100) | ||||||
| Nauck, 2007(21) | Insulin aspart 70/30,** 248 | 58 | 51 | BMI: 30.2 | 10 | A1c: 8.6 | Insulin naive: NR |
| 52 weeks | Weight: 83.4 | FG: 203.4 | OAAs: 248 (100) | ||||
| Exenatide,** 253 | 59 | 47 | BMI: 30.6 | 9.8 | A1c: 8.6 | Insulin naive: NR | |
| Weight: 85.5 | FG: 198 | OAAs: 253 (100) | |||||
| Raskin, 2005(27) | nsulin aspart 70/30,‡‡ 117 | 52.6 | 53 | BMI: 31.5 | 9.5 | A1c: 9.7 | Insulin naive: Yes |
| OAAs: 117 (100) | |||||||
| Brod, 2007(74) | Weight: 90.6 | FG: 252 | |||||
| 28 weeks | Insulin glargine,‡‡ 116 | 52.3 | 56 | BMI: 31.4 | 8.9 | A1c: 9.8 | Insulin naive: Yes |
| Weight: 89.9 | FG: 243 | OAAs: 116 (100) | |||||
| Raskin, 2007(72)§ | Insulin aspart 70/30,¶ 79 | 52 | 51.9 | BMI: 31.2 | NR | A1c: 9.9 | Insulin naive: Yes |
| 28 weeks | Weight: 88.7 | FG: 255.6 | Insulin: 0 (0) | ||||
| OAAs: 79 (100) | |||||||
| Insulin glargine,¶ 78 | 51.7 | 53.8 | BMI: 30.8 | NR | A1c: 9.9 | Insulin naive: Yes | |
| Weight: 86.2 | FG: 239.4 | Insulin: 0 (0) | |||||
| OAAs: 78 (100) | |||||||
| Raskin, 2007(38) | Insulin aspart 70/30,‡‡ 102 | 53.4 | 45† | BMI: 32.4 | 9.2 | A1c: 8.1 | Insulin naive: Yes |
| 34 weeks | OAAs: 102 (100) | ||||||
| Metformin + pioglitazone, 98 | 54.2 | 39† | BMI: 33.4 | 8.3 | A1c: 8.1 | Insulin naive: Yes | |
| OAAs: 98 (100) | |||||||
| Raz, 2003(30) | Insulin aspart 70/30,§§ 26 | 60.3 | 73.1 | BMI: 27.7 | 10.9 | A1c: 9.9 | Insulin naive: NR |
| 6 weeks | FG: 259.8 | OAAs: 26 (100) | |||||
| Glibenclamide,§§ 23 | 57.8 | 56.5 | BMI: 27.6 | 10.3 | A1c: 10.3 | Insulin naive: NR | |
| FG: 265.2 | OAAs: 23 (100) | ||||||
| Raz, 2005(26) | Insulin aspart 70/30, 97 | 55.2 | 65 | BMI: 29.5 | 10 | A1c: 9.5 | Insulin naive: NR |
| 18 weeks | OAAs: 97† (100) | ||||||
| Insulin aspart 70/30,∥∥ 93 | 56.7 | 53 | BMI: 29.4 | 9.2 | A1c: 9.6 | Insulin naive: NR | |
| OAAs: 93† (100) | |||||||
| Glibenclamide,∥∥ 91 | 55.8 | 62 | BMI: 29.5 | 9.9 | A1c: 9.4 | Insulin naive: NR | |
| OAAs: 91† (100) | |||||||
| Robbins, 2007(37) | Insulin lispro 50/50,¶ 157 | 57.4 | 50.3 | BMI:32.1 | 11.3 | A1c: 7.8 | Insulin naive: No |
| 24 weeks | Weight: 89.1 | Insulin: 125 (79.6) | |||||
| Insulin glargine,¶ 158 | 58.1 | 49.4 | BMI: 32 | 12.5 | A1c: 7.8 | Insulin naive: No | |
| Weight: 88.1 | Insulin: 123 (77.8) | ||||||
| Rosenstock, 2008(39) | Insulin lispro 50/50,¶¶ 187 | 55.4 | 53 | BMI:34.1 | 10.9 | A1c: 8.83 | Insulin naive: No |
| 24 weeks | Weight: 99.1 | FG: 171.81 | Insulin: 187 (100) | ||||
| Insulin and OAAs: 185 (98.9) |
|||||||
| Insulin glargine + insulin lispro,¶¶ 187 |
54 | 52 | BMI: 34.8 | 11.2 | A1c: 8.89 | Insulin naive: No | |
| Weight: 99.8 | FG: 181.48 | ||||||
| Insulin: 187 (100) | |||||||
| Insulin and OAAs: 184 (98.5) |
|||||||
| Tamemoto, 2007(20) | Insulin aspart 70/30, 14 | 55.9 | 54 | BMI: 23.9 | 9.8 | A1c: 9.13 | Insulin naive: NR |
| 24 weeks | FG: 183.3 | OAAs: 14 (100) | |||||
| Insulin glargine, 20 | 61.7 | 68 | BMI: 25.5 | 10.4 | A1c: 8.45 | Insulin naive: NR | |
| FG: 184.1 | OAAs: 19 (100) | ||||||
| Tirgoviste, 2003(29) | Insulin lispro 75/25, 85 | 58.7 | 35† | BMI: 26.8 | 10.3 | A1c: 9.85 | Insulin naive: Yes |
| Roach, 2001(75) | Weight: 74.1 | FG: 208.8 | OAAs: 85 (100) | ||||
| 16 weeks | Glibenclamide, 87 | 60.3 | 36† | BMI: 27.6 | 10.2 | A1c: 10.07 | Insulin naive: Yes |
| Weight: 75.8 | FG: 219.6 | OAAs: 87 (100) | |||||
| Total, 172 | 59.5 | 35† | Weight: 75 | 10.2 | NR | Insulin naive: Yes | |
| OAAs: 172 (100) | |||||||
| Ushakova, 2007(36) | Insulin aspart 70/30 TID, 104 | 58 | 16.3 | BMI: 29.8 | 9.9 | A1c: 10.4 | Insulin naive: Yes |
| Weight: 79.3 | |||||||
| 8 weeks titration; 8 | OAAs: 104 (100) | ||||||
| weeks maintenance | Insulin aspart 70/30 BID + metformin, 100 |
58.4 | 27 | BMI: 29.2 | 8.4 | A1c: 10.4 | Insulin naive: Yes |
| Weight: 78.4 | OAAs: 100 (100) | ||||||
| OAAs, 104 | 58.4 | 20.2 | BMI: 29.3 | 8.3 | A1c: 10.1 | Insulin naive: Yes | |
| Weight: 78 | |||||||
| OAAs: 104 (100) | |||||||
| Yamada, 2007(35) | Insulin lispro 50/50, 15 | 66 | 80† | BMI: 27 | 13.7 | A1c: 7.59 | Insulin naive: No |
| 4 months | FG: 130.3 | Insulin: 15 (100) | |||||
| NPH/regular 70/30 + NPH/regular 50/50, 15 |
66.3 | 73† | BMI: 23.8 | 15.9 | A1c: 7.33 | Insulin naive: No | |
| FG: 141.8 | Insulin: 15 (100) | ||||||
| Crossover Trials With No Washout Periods | |||||||
| Coscelli, 2003(48) | Insulin lispro 75/25,∥ 18 | 59.1 | 39 | BMI: 29.5 | 14.9 | FG: 154.2 | Insulin naive: No |
| 12 days/period | Weight: 79 | Insulin: 18 (100) | |||||
| NPH/regular 70/30,∥ 15 | 59.2 | 53 | BMI: 30.1 | 13.8 | FG: 150.9 | Insulin naive: No | |
| Weight: 80.2 | Insulin: 15 (100) | ||||||
| Total, 33 | 59.1 | 45 | BMI: 29.8 | 14.4 | FG: 152.5 | Insulin naive: No | |
| Weight: 79.5 | Insulin: 33 (100) | ||||||
| Cox, 2007(56) | Insulin lispro 75/25,¶ 28 | NR | NR | NR | NR | NR | NR |
| 12 weeks/period | Insulin glargine,¶ 28 | NR | NR | NR | NR | NR | NR |
| Total, 45 | 52.6 | NR | BMI: 35.08 | 11.9 | NR | Insulin naive: NR | |
| OAAs: 45 (100) | |||||||
| Herz, 2002(52) | Insulin lispro 75/25, 19 | 56.3 | 63† | BMI: 27 | 8.9 | NR | Insulin naive: No |
| 4 weeks/period | Weight: 76 | Insulin: 19† (100) | |||||
| NPH/regular 70/30, 18 | 55.3 | 33† | BMI: 26.3 | 7.5 | NR | Insulin naive: No | |
| Weight: 75.8 | Insulin: 18† (100) | ||||||
| Herz, 2003(50) | Insulin lispro 75/25, 13 | 54.8 | 77† | BMI: 29.2 | NR | A1c: 7.81 | Insulin naive: No |
| 4 weeks/period | Insulin: 13† (100) | ||||||
| NPH/regular 70/30, 12 | 53.6 | 58† | BMI: 29.3 | NR | A1c: 7.6 | Insulin naive: No | |
| Insulin: 12† (100) | |||||||
| Jacober, 2006(43) | Insulin lispro 75/25 + insulin lispro 50/50,†† 59 |
NR | NR | NR | NR | NR | NR |
| 16 weeks/period | |||||||
| Insulin glargine,†† 59 | NR | NR | NR | NR | NR | NR | |
| Total, 60 | 54.9 | 56.7 | BMI: 32.9 | 8.4 | A1c: 9.21 | Insulin naive: Yes | |
| Weight: 95.1 | OAAs: 60 (100) | ||||||
| Malone, 2004(44) | Insulin lispro 75/25,¶ 52 | 54.5 | 63.5 | BMI: 30.1 | 8.1 | A1c: 8.7 | Insulin naive: Yes |
| 16 weeks/period | Weight: 88.5 | FG: 150.2 | OAAs: 52 (100) | ||||
| Insulin glargine,¶ 53 | 55.3 | 62.3 | BMI: 31.7 | 9.8 | A1c: 8.7 | Insulin naive: Yes | |
| Weight: 94.4 | FG: 155.3 | OAAs: 53 (100) | |||||
| Malone, 2005(45) | Insulin lispro 75/25,¶ 50 | 59.18 | 50 | BMI: 29.41 | 13.52 | A1c: 8.5 | Insulin naive: No |
| 16 weeks/period | Weight: 77.82 | FG: 155.34 | Insulin: 50† (100) | ||||
| OAAs: 26 (52†) | |||||||
| Insulin glargine,¶ 47 | 59.63 | 38 | BMI: 29.64 | 11.9 | A1c: 8.48 | Insulin naive: No | |
| Weight: 77.21 | FG: 147.78 | Insulin: 47† (100) | |||||
| OAAs: 28 (60†) | |||||||
| Mattoo, 2003(51) | Insulin lispro 75/25, 72 | 54 | 47.2 | BMI: 26.9 | 13.2 | NR | Insulin naive: No |
| 2 weeks/period | Weight: 71 | Insulin: 72† (100) | |||||
| NPH/regular 70/30, 79 | 52 | 44.3 | BMI: 26.5 | 11.8 | NR | Insulin naive: No | |
| Weight: 71 | Insulin: 79† (100) | ||||||
| Total, 151 | 53 | 45.7 | BMI: 26.7 | 12.5 | NR | Insulin naive: No | |
| Weight: 71 | Insulin: 151† (100) | ||||||
| McNally, 2007(41) | Insulin aspart 70/30, 80 | 61.8 | 61† | BMI: 29.7 | 11.5 | A1c: 7.5 | Insulin naive: No |
| 16 weeks/period | Weight: 83.3 | Insulin: 80 (100) | |||||
| NPH/regular 70/30, 80 | 62.7 | 79† | BMI: 30.5 | 12.1 | A1c: 7.5 | Insulin naive: No | |
| Weight: 89.1 | Insulin: 80 (100) | ||||||
| Total, 160 | 62.3 | 70† | BMI: 30.1 | 11.8 | A1c: 7.5 | Insulin naive: No | |
| Weight: 86.2 | Insulin: 160 (100) | ||||||
| McSorley, 2002(53) | Insulin aspart 70/30, 13 | NR | NR | NR | NR | NR | NR |
| 2 weeks/period | NPH/regular 70/30, 13 | NR | NR | NR | NR | NR | NR |
| Total, 13 | 64 | 62† | BMI: 28.1 | 13 | A1c: 7.7 | Insulin naive: No | |
| Insulin: 13† (100) | |||||||
| Niskanen, 2004(46) | Insulin aspart 70/30, 132 | NR | NR | NR | NR | NR | NR |
| 12 weeks/period | Insulin lispro 75/25, 132 | NR | NR | NR | NR | NR | NR |
| Total, 133 | 62.3 | 59† | BMI: 28.1 | 12.1 | A1c: 8.5 | Insulin naive: No | |
| Insulin: 133† (100) | |||||||
| Roach, 1999(54) | Insulin lispro 75/25, 44 | 56.5 | 52† | BMI: 28.3 | 12.8 | NR | Insulin naive: No |
| 13 weeks | Insulin: 44† (100) | ||||||
| NPH/regular 70/30, 45 | 57.4 | 42† | BMI: 29.4 | 11.5 | NR | Insulin naive: No | |
| Insulin: 45† (100) | |||||||
| Roach, 1999(55) | Insulin lispro 50/50 + insulin lispro 75/25, 34 |
58 | 53† | BMI: 28.4 | 12.2 | NR | Insulin naive: No |
| 12 weeks/period | Insulin: 34† (100) | ||||||
| NPH/regular 50/50 + NPH/ regular 70/30, 29 |
60.2 | 41† | BMI: 28.4 | 13.1 | NR | Insulin naive: No | |
| Insulin: 29† (100) | |||||||
| Roach, 2003(49) | Insulin lispro 75/25, 57 | 53.9 | 40 | Weight: 62.8 | 12.4 | NR | Insulin naive: No |
| 8 weeks/period | Insulin: 57† (100) | ||||||
| Insulin lispro 50/50 + insulin lispro 75/25, 58 |
54.2 | 40 | Weight: 65.1 | 13.1 | NR | Insulin naive: No | |
| Insulin: 58† (100) | |||||||
| Roach, 2006(42) | Insulin lispro 75/25,†† 20 | NR | NR | NR | NR | NR | NR |
| 12 weeks/period | Insulin glargine,†† 20 | NR | NR | NR | NR | NR | NR |
| Total, 20 | 53.5 | 50 | BMI: 36.7 | NR | A1c: 8.4 | Insulin naive: No | |
| Weight: 108 | |||||||
| Schernthaner, 2004(47) |
Insulin lispro 50/50,∥ 18 | 66.1 | 17† | BMI: 29.5 | 16.2 | A1c: 8.3 | Insulin naive: No |
| Insulin: 18† (100) | |||||||
| 12 weeks/period | |||||||
| NPH/regular 70/30,∥ 17 | 67.8 | 29† | BMI: 28.8 | 14.2 | A1c: 8.5 | Insulin naive: No | |
| Insulin: 17† (100) | |||||||
| Total, 35 | 67 | 23† | BMI: 29.2 | 15.3 | NR | Insulin naive: No | |
| Insulin: 35† (100) | |||||||
| Crossover Trials With Washout Period | |||||||
| Hermansen, 2002(59) | Insulin aspart 70/30, 41 | NR | NR | NR | NR | NR | NR |
| 1 day/period, washout period at least 5 days |
Insulin lispro 75/25, 42 | NR | NR | NR | NR | NR | NR |
| NPH/regular 70/30, 44 | NR | NR | NRN | NR | NR | NR | |
| Total, 61 | 60.1 | 66† | BMI: 27.3 | 11.6 | A1c: 8.3 | Insulin naive: No | |
| Insulin: 61† (100) | |||||||
| Kapitza, 2004(58) | Insulin aspart 70/30 (15 min. after test meal), 31 |
NR | NR | NR | NR | NR | NR |
| 1 day/period, washout period 3 - 21 days |
Insulin aspart 70/30 (before meal), 31 |
NR | NR | NR | NR | NR | NR |
| NPH/regular 70/30 (15 min. before test meal), 31 |
NR | NR | NR | NR | NR | NR | |
| NPH/regular 70/30 (before test meal), 31 |
NR | NR | NR | NR | NR | NR | |
| Total, 31 | 57 | 68† | BMI: 29 | 12 | A1c: 8.7 | Insulin naive: No | |
| Insulin: 31† (100) | |||||||
| Malone, 2000(60) | Insulin lispro 75/25, 41 | 59.2 | 63† | BMI: 29.1 | 14 | NR | Insulin naive: No |
| Malone, 2000(76) | Insulin: 41† (100) | ||||||
| 1 day/period, washout period 3 – 11 days |
NPH/regular 70/30, 43 | 60.5 | 63† | BMI: 29.2 | 16.2 | NR | Insulin naive: No |
| Insulin: 43† (100) | |||||||
| Total, 84 | 59.9 | 63† | BMI: 29.2 | 15.1 | NR | Insulin naive: No | |
| Insulin: 84† (100) | |||||||
| Schwartz, 2006(57) | Insulin lispro 50/50, 7 | NR | NR | NR | NR | NR | NR |
| 1 day/period, washout period 3 – 11 days |
Insulin lispro 75/25, 8 | NR | NR | NR | NR | NR | NR |
| NPH/regular, 8 | NR | NR | NR | NR | NR | NR | |
| Total, 23 | 61.3 | 73.9 | BMI: 33 | NR | A1c: 8.1 | Insulin naive: No | |
| Weight: 98.5 | FG: 158.7 | Insulin: 23 (100) | |||||
| Other Study Designs | |||||||
| Joshi, 2005(17) | Insulin aspart 70/30, 114 | 52.41 | 67† | Weight: 70.4 | 9.53 | A1c: 8.79 | Insulin naive: NR |
| Insulin: 62 (54.39) | |||||||
| 12 weeks | FG: 186.59 | ||||||
| OAAs: 102 (89.47) | |||||||
| Insulin aspart + insulin glargine, 31 |
51.1 | 77† | Weight: 69.63 | 11.98 | A1c: 8.53 | Insulin naive: NR | |
| Insulin: 21 (67.74) | |||||||
| FG: 190.23 | |||||||
| OAAs: 25 (80.65) | |||||||
| Sun, 2007(18) | Insulin lispro 75/25, 895 | 62.8 | 49.1 | Weight: 93.9 | 20.5 | A1c: 8.6 | Insulin naive: Yes |
| 18 months | Insulin glargine, 3624 | 58.4 | 48.5 | Weight: 93.3 | 24.7 | A1c: 8.6 | Insulin naive: Yes |
| NPH/regular 70/30, 3647 | 65.7 | 44.7 | Weight: 92.3 | 18.3 | A1c: 8.4 | Insulin naive: Yes | |
All numbers have been converted from mmol/L to mg/dL. To convert from mg/dL to mmol/L, divide by 18.
Number has been imputed.
The study population for Boehm 2002(73) was patients with either type 1 or type 2 diabetes. The type 2 diabetic population was the same study population used for Boehm 2004. (28) Boehm 2002(73) reported results after 12 weeks of followup.
Raskin 2007(72) was conducted among a subpopulation of Raskin 2005(27) who were not using thiazolidinediones.
Additionally, the study group received diet and exercise.
Additionally, the study group received metformin.
Additionally, the study group received metformin and a sulfonylurea.
Additionally, the study group received oral antidiabetic agents.
Additionally, the study group received metformin and pioglitazone.
Additionally, the study group received rosiglitazone.
Additionally, the study group received pioglitazaone.
Additionally, the study group received existing oral antidiabetic agents, except for sulfonylureas and glinides.
A1c = hemoglobin A1c; BMI = body mass index; BID = twice daily; dL = deciliter; FG = fasting glucose; IQR = interquartile range; kg = kilogram; kg/m2 = kilogram per square meter; mg/dL = milligram per deciliter; NPH = neutral protamine Hagedorn; NR = not reported; OAAs = oral antidiabetic agents; TID = thrice daily
Footnotes
Disclaimer:
This project was funded under Contract No. 290-02-0018 from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Potential Financial Conflicts of Interest:
None.
Reference List
- (1).Centers for Disease Control and Prevention . National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: U.S.: 2005. [Google Scholar]
- (2).Gregg EW, Cadwell BL, Cheng YJ, Cowie CC, Williams DE, Geiss L, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27:2806–12. doi: 10.2337/diacare.27.12.2806. [DOI] [PubMed] [Google Scholar]
- (3).Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- (4).Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- (5).Wells GA, Shea B, O’Connel D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2007. [accessed on August 4, 2008]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Available online at.
- (6).Guide for Conducting Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality; Rockville, MD: 2007. [accessed on August 4, 2008]. http://effectivehealthcare.ahrq.gov/repFiles/2007_10DraftMethodsGuide.pdf Available online at. [Google Scholar]
- (7).Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295:1688–97. doi: 10.1001/jama.295.14.1688. [DOI] [PubMed] [Google Scholar]
- (9).Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy C, 4-T Study Group Addition of Biphasic, Prandial, or Basal Insulin to Oral Therapy in Type 2 Diabetes. N Engl J Med. 2007;357:1716–30. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- (10).Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- (11).Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124:719–23. doi: 10.1093/oxfordjournals.aje.a114447. [DOI] [PubMed] [Google Scholar]
- (12).Bradburn MJ, Deeks JJ, Berlin JA, Localio A Russell. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- (13).Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–71. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- (14).Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- (16).Egger M, Smith G Davey, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Joshi SR, Kalra S, Badgandi M, Rao YS, Chawla M. Designer insulins regimens in clinical practice--pilot multicenter Indian study. J Assoc Physicians India. 2005;53:775–9. [PubMed] [Google Scholar]
- (18).Sun P, Wang R, Jacober S. The effectiveness of insulin initiation regimens in patients with type 2 diabetes mellitus: a large national medical records review study comparing a basal insulin analogue to premixed insulin. Curr Med Res Opin. 2007;23:3017–23. doi: 10.1185/030079907X242845. [DOI] [PubMed] [Google Scholar]
- (19).Bebakar WM, Chow CC, Kadir KA, Suwanwalaikorn S, Vaz JA, Bech OM. Adding biphasic insulin aspart 30 once or twice daily is more efficacious than optimizing oral antidiabetic treatment in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:724–32. doi: 10.1111/j.1463-1326.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- (20).Tamemoto H, Ikoma A, Saitoh T, Ishikawa SE, Kawakami M. Comparison of once-daily glargine plus sulfonylurea with twice-daily 70/30 aspart premix in insulin-naive Japanese patients with diabetes. Diabetes Technol Ther. 2007;9:246–53. doi: 10.1089/dia.2006.0016. [DOI] [PubMed] [Google Scholar]
- (21).Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–67. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- (22).Kann PH, Wascher T, Zackova V, Moeller J, Medding J, Szocs A, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes. 2006;114:527–32. doi: 10.1055/s-2006-949655. [DOI] [PubMed] [Google Scholar]
- (23).Kazda C, Hulstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications. 2006;20:145–52. doi: 10.1016/j.jdiacomp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- (24).Kvapil M, Swatko A, Hilberg C, Shestakova M. Biphasic insulin aspart 30 plus metformin: an effective combination in type 2 diabetes. Diabetes Obes Metab. 2006;8:39–48. doi: 10.1111/j.1463-1326.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- (25).Abrahamian H, Ludvik B, Schernthaner G, Prager R, Zellenka U, Knudsen L, et al. Improvement of glucose tolerance in type 2 diabetic patients: traditional vs. modern insulin regimens (results from the Austrian Biaspart Study) Horm Metab Res. 2005;37:684–9. doi: 10.1055/s-2005-870579. [DOI] [PubMed] [Google Scholar]
- (26).Raz I, Stranks S, Filipczak R, Joshi P, Lertoft B, Rastam J, et al. Efficacy and safety of biphasic insulin aspart 30 combined with pioglitazone in type 2 diabetes poorly controlled on glibenclamide (glyburide) monotherapy or combination therapy: an 18-week, randomized, open-label study. Clin Ther. 2005;27:1432–43. doi: 10.1016/j.clinthera.2005.09.001. [DOI] [PubMed] [Google Scholar]
- (27).Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, et al. Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–5. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- (28).Boehm BO, Vaz JA, Brondsted L, Home PD. Long-term efficacy and safety of biphasic insulin aspart in patients with type 2 diabetes. Eur J Intern Med. 2004;15:496–502. doi: 10.1016/j.ejim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- (29).Tirgoviste CI, Strachinariu R, Farcasiu E, Milicevic Z, Teodorescu G. Humalog Mix 25 in patients with type 2 diabetes which do not achieve acceptable glycemic control with oral agents: results from a phase III, randomized, parallel study. Rom J Intern Med. 2003;41:153–62. [PubMed] [Google Scholar]
- (30).Raz I, Mouritzen U, Vaz J, Hershkovitz T, Wainstein J, Harman-Boehm I. Addition of biphasic insulin aspart 30 to rosiglitazone in type 2 diabetes mellitus that is poorly controlled with glibenclamide monotherapy. Clin Ther. 2003;25:3109–23. doi: 10.1016/s0149-2918(03)90095-6. [DOI] [PubMed] [Google Scholar]
- (31).Malone JK, Beattie SD, Campaigne BN, Johnson PA, Howard AS, Milicevic Z. Therapy after single oral agent failure: adding a second oral agent or an insulin mixture? Diabetes Res Clin Pract. 2003;62:187–95. doi: 10.1016/j.diabres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- (32).Christiansen JS, Vaz JA, Metelko Z, Bogoev M, Dedov I. Twice daily biphasic insulin aspart improves postprandial glycaemic control more effectively than twice daily NPH insulin, with low risk of hypoglycaemia, in patients with type 2 diabetes. Diabetes Obes Metab. 2003;5:446–54. doi: 10.1046/j.1463-1326.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- (33).Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P. Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin. J Diabetes Complications. 2003;17:307–13. doi: 10.1016/s1056-8727(03)00076-x. [DOI] [PubMed] [Google Scholar]
- (34).Herz M, Sun B, Milicevic Z, Erickson P, Fovenyi J, Grzywa M, et al. Comparative efficacy of preprandial or postprandial Humalog Mix75/25 versus glyburide in patients 60 to 80 years of age with type 2 diabetes mellitus. Clin Ther. 2002;24:73–86. doi: 10.1016/s0149-2918(02)85006-8. [DOI] [PubMed] [Google Scholar]
- (35).Yamada S, Watanabe M, Kitaoka A, Shiono K, Atsuda K, Tsukamoto Y, et al. Switching from premixed human insulin to premixed insulin lispro: a prospective study comparing the effects on glucose control and quality of life. Intern Med. 2007;46:1513–7. doi: 10.2169/internalmedicine.46.0236. [DOI] [PubMed] [Google Scholar]
- (36).Ushakova O, Sokolovskaya V, Morozova A, Valeeva F, Zanozina O, Sazonova O, et al. Comparison of biphasic insulin aspart 30 given three times daily or twice daily in combination with metformin versus oral antidiabetic drugs alone in patients with poorly controlled type 2 diabetes: a 16-week, randomized, open-label, parallel-group trial conducted in russia. Clin Ther. 2007;29:2374–84. doi: 10.1016/j.clinthera.2007.11.017. [DOI] [PubMed] [Google Scholar]
- (37).Robbins DC, Beisswenger PJ, Ceriello A, Goldberg RB, Moses RG, Pagkalos EM, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther. 2007;29:2349–64. doi: 10.1016/j.clinthera.2007.11.016. [DOI] [PubMed] [Google Scholar]
- (38).Raskin P, Matfin G, Schwartz SL, Chaykin L, Chu PL, Braceras R, et al. Addition of biphasic insulin aspart 30 to optimized metformin and pioglitazone treatment of type 2 diabetes mellitus: The ACTION Study (Achieving Control Through Insulin plus Oral ageNts) Diabetes Obes Metab. 2007 doi: 10.1111/j.1463-1326.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- (39).Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care. 2008;31:20–5. doi: 10.2337/dc07-1122. [DOI] [PubMed] [Google Scholar]
- (40).Hirao K, Arai K, Yamauchi M, Takagi H, Kobayashi M. Six-month multicentric, open-label, randomized trial of twice-daily injections of biphasic insulin aspart 30 versus multiple daily injections of insulin aspart in Japanese type 2 diabetic patients (JDDM 11) Diabetes Res Clin Pract. 2008;79:171–6. doi: 10.1016/j.diabres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- (41).McNally PG, Dean JD, Morris AD, Wilkinson PD, Compion G, Heller SR. Using continuous glucose monitoring to measure the frequency of low glucose values when using biphasic insulin aspart 30 compared with biphasic human insulin 30: a double-blind crossover study in individuals with type 2 diabetes. Diabetes Care. 2007;30:1044–8. doi: 10.2337/dc06-1328. [DOI] [PubMed] [Google Scholar]
- (42).Roach P, Malone JK. Comparison of insulin lispro mixture 25/75 with insulin glargine during a 24-h standardized test-meal period in patients with Type 2 diabetes. Diabet Med. 2006;23:743–9. doi: 10.1111/j.1464-5491.2006.01895.x. [DOI] [PubMed] [Google Scholar]
- (43).Jacober SJ, Scism-Bacon JL, Zagar AJ. A comparison of intensive mixture therapy with basal insulin therapy in insulin-naive patients with type 2 diabetes receiving oral antidiabetes agents. Diabetes Obes Metab. 2006;8:448–55. doi: 10.1111/j.1463-1326.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- (44).Malone JK, Kerr LF, Campaigne BN, Sachson RA, Holcombe JH. Combined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapy. Clin Ther. 2004;26:2034–44. doi: 10.1016/j.clinthera.2004.12.015. [DOI] [PubMed] [Google Scholar]
- (45).Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with Type 2 diabetes. Diabet Med. 2005;22:374–81. doi: 10.1111/j.1464-5491.2005.01511.x. [DOI] [PubMed] [Google Scholar]
- (46).Niskanen L, Jensen LE, Rastam J, Nygaard-Pedersen L, Erichsen K, Vora JP. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther. 2004;26:531–40. doi: 10.1016/s0149-2918(04)90055-0. [DOI] [PubMed] [Google Scholar]
- (47).Schernthaner G, Kopp HP, Ristic S, Muzyka B, Peter L, Mitteregger G. Metabolic control in patients with type 2 diabetes using Humalog Mix50 injected three times daily: crossover comparison with human insulin 30/70. Horm Metab Res. 2004;36:188–93. doi: 10.1055/s-2004-814345. [DOI] [PubMed] [Google Scholar]
- (48).Coscelli C, Iacobellis G, Calderini C, Carleo R, Gobbo M, Di Mario U, et al. Importance of premeal injection time in insulin therapy: Humalog Mix25 is convenient for improved post-prandial glycemic control in type 2 diabetic patients with Italian dietary habits. Acta Diabetol. 2003;40:187–92. doi: 10.1007/s00592-003-0110-2. [DOI] [PubMed] [Google Scholar]
- (49).Roach P, Arora V, Campaigne BN, Mattoo V, Rangwala S. Humalog Mix50 before carbohydrate-rich meals in type 2 diabetes mellitus. Diabetes Obes Metab. 2003;5:311–6. doi: 10.1046/j.1463-1326.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- (50).Herz M, Arora V, Campaigne BN, Scholtz HE, Potgieter MA, Mollentze W. Humalog Mix25 improves 24-hour plasma glucose profiles compared with the human insulin mixture 30/70 in patients with type 2 diabetes mellitus. S Afr Med J. 2003;93:219–23. [PubMed] [Google Scholar]
- (51).Mattoo V, Milicevic Z, Malone JK, Schwarzenhofer M, Ekangaki A, Levitt LK, et al. A comparison of insulin lispro Mix25 and human insulin 30/70 in the treatment of type 2 diabetes during Ramadan. Diabetes Res Clin Pract. 2003;59:137–43. doi: 10.1016/s0168-8227(02)00202-4. [DOI] [PubMed] [Google Scholar]
- (52).Herz M, Profozic V, Arora V, Smircic-Duvnjak L, Kovacevic I, Boras J, et al. Effects of a fixed mixture of 25% insulin lispro and 75% NPL on plasma glucose during and after moderate physical exercise in patients with type 2 diabetes. Curr Med Res Opin. 2002;18:188–93. doi: 10.1185/030079902125000615. [DOI] [PubMed] [Google Scholar]
- (53).McSorley PT, Bell PM, Jacobsen LV, Kristensen A, Lindholm A. Twice-daily biphasic insulin aspart 30 versus biphasic human insulin 30: a double-blind crossover study in adults with type 2 diabetes mellitus. Clin Ther. 2002;24:530–9. doi: 10.1016/s0149-2918(02)85129-3. [DOI] [PubMed] [Google Scholar]
- (54).Roach P, Yue L, Arora V. Improved postprandial glycemic control during treatment with Humalog Mix25, a novel protamine-based insulin lispro formulation. Humalog Mix25 Study Group. Diabetes Care. 1999;22:1258–61. doi: 10.2337/diacare.22.8.1258. [DOI] [PubMed] [Google Scholar]
- (55).Roach P, Trautmann M, Arora V, Sun B, Anderson JH., Jr Improved postprandial blood glucose control and reduced nocturnal hypoglycemia during treatment with two novel insulin lispro-protamine formulations, insulin lispro mix25 and insulin lispro mix50. Mix50 Study Group. Clin Ther. 1999;21:523–34. doi: 10.1016/s0149-2918(00)88307-1. [DOI] [PubMed] [Google Scholar]
- (56).Cox DJ, McCall A, Kovatchev B, Sarwat S, Ilag LL, Tan MH. Effects of blood glucose rate of changes on perceived mood and cognitive symptoms in insulin-treated type 2 diabetes. Diabetes Care. 2007;30:2001–2. doi: 10.2337/dc06-2480. [DOI] [PubMed] [Google Scholar]
- (57).Schwartz S, Zagar AJ, Althouse SK, Pinaire JA, Holcombe JH. A single-center, randomized, double-blind, three-way crossover study examining postchallenge glucose responses to human insulin 70/30 and insulin lispro fixed mixtures 75/25 and 50/50 in patients with type 2 diabetes mellitus. Clin Ther. 2006;28:1649–57. doi: 10.1016/j.clinthera.2006.10.017. [DOI] [PubMed] [Google Scholar]
- (58).Kapitza C, Rave K, Ostrowski K, Heise T, Heinemann L. Reduced postprandial glycaemic excursion with biphasic insulin Aspart 30 injected immediately before a meal. Diabet Med. 2004;21:500–1. doi: 10.1111/j.1464-5491.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- (59).Hermansen K, Colombo M, Storgaard H, OStergaard A, Kolendorf K, Madsbad S. Improved postprandial glycemic control with biphasic insulin aspart relative to biphasic insulin lispro and biphasic human insulin in patients with type 2 diabetes. Diabetes Care. 2002;25:883–8. doi: 10.2337/diacare.25.5.883. [DOI] [PubMed] [Google Scholar]
- (60).Malone JK, Woodworth JR, Arora V, Yang H, Campaigne BN, Halle JP, et al. Improved postprandial glycemic control with Humalog Mix75/25 after a standard test meal in patients with type 2 diabetes mellitus. Clin Ther. 2000;22:222–30. doi: 10.1016/s0149-2918(00)88480-5. [DOI] [PubMed] [Google Scholar]
- (61).Monnier L, Colette C, Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12(Suppl 1):42–6. doi: 10.4158/EP.12.S1.42. [DOI] [PubMed] [Google Scholar]
- (62).Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- (63).Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- (64).Lowe LP, Liu K, Greenland P, Metzger BE, Dyer AR, Stamler J. Diabetes, asymptomatic hyperglycemia, and 22-year mortality in black and white men. The Chicago Heart Association Detection Project in Industry Study. Diabetes Care. 1997;20:163–9. doi: 10.2337/diacare.20.2.163. [DOI] [PubMed] [Google Scholar]
- (65).Orencia AJ, Daviglus ML, Dyer AR, Walsh M, Greenland P, Stamler J. One-hour postload plasma glucose and risks of fatal coronary heart disease and stroke among nondiabetic men and women: the Chicago Heart Association Detection Project in Industry (CHA) Study. J Clin Epidemiol. 1997;50:1369–76. doi: 10.1016/s0895-4356(97)00201-1. [DOI] [PubMed] [Google Scholar]
- (66).de Vegt F, Dekker JM, Ruhe HG, Stehouwer CD, Nijpels G, Bouter LM, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–31. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- (67).Curb JD, Rodriguez BL, Burchfiel CM, Abbott RD, Chiu D, Yano K. Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation. 1995;91:2591–5. doi: 10.1161/01.cir.91.10.2591. [DOI] [PubMed] [Google Scholar]
- (68).Boden-Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS) Diabetes Care. 2008;31:1132–7. doi: 10.2337/dc07-0797. [DOI] [PubMed] [Google Scholar]
- (69).Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Treggiari MM, Karir V, Yanez ND, Weiss NS, Daniel S, Deem SA. Intensive insulin therapy and mortality in critically ill patients. Crit Care. 2008;12:R29. doi: 10.1186/cc6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).White JR., Jr Insulin glargine clinical trials. Clin Ther. 2004;26:1179–81. doi: 10.1016/s0149-2918(04)90190-7. discussion 1182-3. [DOI] [PubMed] [Google Scholar]
- (72).Raskin PR, Hollander PA, Lewin A, Gabbay RA, Bode B, Garber AJ. Basal insulin or premix analogue therapy in type 2 diabetes patients. Eur J Intern Med. 2007;18:56–62. doi: 10.1016/j.ejim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- (73).Boehm BO, Home PD, Behrend C, Kamp NM, Lindholm A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med. 2002;19:393–9. doi: 10.1046/j.1464-5491.2002.00733.x. [DOI] [PubMed] [Google Scholar]
- (74).Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes. 2007;5:8. doi: 10.1186/1477-7525-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Roach, Koledova E, Metcalfe S, Hultman C, Milicevic Z. Glycemic control with Humalog Mix25 in type 2 diabetes inadequately controlled with glyburide. Clin Ther. 2001;23:1732–44. doi: 10.1016/s0149-2918(01)80140-5. [DOI] [PubMed] [Google Scholar]
- (76).Malone JK, Yang H, Woodworth JR, Huang J, Campaigne BN, Halle JP, et al. Humalog Mix25 offers better mealtime glycemic control in patients with type 1 or type 2 diabetes. Diabetes Metab. 2000;26:481–7. [PubMed] [Google Scholar]