Abstract
T lymphocytes are critical mediators of the adaptive immune system and have the capacity to serve as therapeutic agents in the areas of transplant and cancer immunotherapy. While T cells can be isolated and expanded from patients, T cells derived in vitro from both hematopoietic stem/progenitor cells (HSPCs) and human pluripotent stem cells (hPSCs) offer great potential advantages in generating a self-renewing source of T cells that can be readily genetically modified. T-cell differentiation in vivo is a complex process requiring tightly regulated signals; providing the correct signals in vitro to induce T-cell lineage commitment followed by their development into mature, functional, single positive T cells, is similarly complex. In this review, we discuss current methods for the in vitro derivation of T cells from murine and human HSPCs and hPSCs that use feeder-cell and feeder-cell-free systems. Furthermore, we explore their potential for adoption for use in T-cell-based therapies.
Keywords: Induced pluripotent stem cells, CD34 +, Cell culture, Cord blood, Differentiation, Embryonic stem cells, Hematopoietic stem cells, T cells
Introduction
Hematopoietic Stem/Progenitor Cell-Derived T Cells on OP9-DL Cells
The identification of Notch signaling as a crucial mediator of T-cell development led to dramatically improved methods for in vitro derivation of T cells [1, 2]. At the forefront of these has been a coculture system based on a mouse bone marrow (BM)-derived stromal cell line, called OP9, engineered to overexpress the Notch ligand, Delta-like ligand 1 (Dll-1), and hence termed OP9-DL1. Similar to thymic stromal cells, OP9 cells provide key factors that support T lymphocyte development, including interleukin 7 (IL-7), chemokine (C-X-C motif) ligand 12 (CXCL12), and stem cell factor (SCF). Furthermore, OP9 cells have a dysfunctional csf1 gene, which would normally support myelopoiesis [3].
Notch activation on hematopoietic stem/progenitor cells (HSPCs) by OP9-DL1 cells first drives their differentiation into T-lineage cells, then stimulates the cells to survive through the different stages of T-cell ontogeny, from CD4−CD8− double negative progenitor T cells to the CD4+CD8+ double positive (DP) stage [4]. Ultimately, differentiation achieved using the human pluripotent stem cell (hPSC)/OP9-DL1 coculture system results in a large number of phenotypically and functionally mature conventional single positive (SP) CD8+ T cells with a diverse T-cell receptor (TCR) repertoire. In many respects these CD8+ T cells are functionally equivalent to ex vivo thymus-derived CD8+ T cells in response to activating signals while maintaining tolerance for self [5].
The OP9-DL culture system permits the generation of HSPC-derived T cells in vitro, serving both as a means to facilitate the study of T-cell differentiation, as well as the potential to produce large numbers of cells for adoptive transfer, which is often a limiting factor [6, 7]. However, Delta-like molecules do not share equal expression, and therefore have varying potentials in terms of activation of the Notch family of proteins [8, 9]. Case in point, targeted deletion of Dll1 in thymic epithelial cells (TECs) has no effect on T-cell ontogeny [10], although its ortholog, Dll4, is the physiological, critical, nonredundant Notch1 ligand expressed in the thymus [11, 12]. Despite the functional differences of Dll1 and Dll4 in vivo, OP9-DL1 and OP9-DL4 cells yield similar results when driving T-cell differentiation in vitro [13]. The difference between the signaling capacities of Dll1 and Dll4 becomes apparent at limiting levels, where Dll4 appears to be more effective than Dll1 at activating Notch and inducing a T-lineage phenotype [14]. It has a higher capacity to bind Notch1, although unlike Dll1, Dll4 is unable to signal through Notch2 [13]. Hence, while Dll4 may be the preferred Dll to use for early stages of T-cell development, this may change depending on the expression of Notch molecules in the target hematopoietic cells.
While the OP9-DL system was originally created to support T-cell development in the mouse system, it was successfully adapted for use with human umbilical cord blood (UCB)- derived progenitor cells (UCB-HSPCs) [15]. In mice, fetal liver-derived hPSCs possess a higher capacity for in vitro T-cell development than BM HSPCs; similarly, UCB-derived HSPCs (CD34+CD38lo/−) cells are also found to generate greater numbers committing to the T-cell lineage, reaching developmental milestones in less time than BM HSPCs [16]. UCB-HSPCs undergo the expected program of human T-cell differentiation and give rise to CD34+CD7+ progenitor T cells (pro-T). When allowed to continue differentiating in vitro on OP9-DL cells, mature SP cells with a strong CD8 bias are generated, with the majority being CD3+TCRαβ+CD27+CD1a−. These CD8+ cells respond to CD3/CD28 stimulation in a manner similar to ex vivo CD8 SP cells as measured by surface marker modulation, proliferation, and production of proinflammatory cytokines [17].
Both human and allogeneic murine pro-T cells (murine pro-Ts are defined as CD4−CD8−CD25+) were able to engraft within the thymus of immune-deficient mice without instigating graft versus host disease (GVHD). While human pro-Ts mature in vivo at least through the DP stage, expressing high levels of CD3 and TCRαβ [18, 19], their murine counterparts undergo positive, and more importantly, negative selection. Thus, the host thymus selects T cells that can respond to antigen in the context of the host major histocompatibility complex (MHC), eliminating T cells that could mediate GVHD. Engrafted cells mature with a varied TCR-Vβ repertoire that can respond to stimulation, and do not require cytokine administration to persist in vivo. In preclinical studies, the descendants of the adoptively transferred pro-T cells have been shown to be present 60 days post-transfer, at which point they are not only tolerant but offer protection against infection and tumors [20]. Pro-T cells have the additional advantage of enhancing immune system reconstitution after total body irradiation [21, 22], lessening the duration and intensity of the resulting immunodeficiency. If this also proves true for human pro-T cells, it would be monumental for patients undergoing chemo/radio-therapy, after which the lost T cells could be replenished from an ex vivo source.
Alternative Methods to OP9-DL Cells in HSPC-to-T-Cell Differentiation
Murine HSPC Differentiation into T Cells
Apart from OP9 cells, other murine cells have been shown to have varying degrees of success (but not as much as OP9 cells) in inducing T-cell development when forced to express Dll molecules (Table 1). Murine primary stromal cells have also demonstrated a robust ability to support T-cell development, including fetal thymic stromal cells, either in a three-dimensional matrix or in a monolayer. Exposure of human BM-derived HSPC with irradiated murine fetal thymic stromal cells in a three-dimensional matrix in the presence of IL-12 and FMS-like tyrosine kinase 3 ligand (Flt3L) resulted in the generation of mature SP CD4 and CD8 cells [23]. When cultured as a monolayer, thymic stromal cells lose their ability to support T-cell development, as expression of Dll4 is rapidly downregulated. However, ectopic expression of Dll1 or Dll4 on these same primary thymic cultures is sufficient to restore their ability to support T-cell development [24]. Nevertheless, the required reagent, namely the fetal thymic stromal cells, is the limiting factor to broadly applying this technique.
Table 1.
Summary of culture conditions using mouse cells as a starting source
| Feeder cells | Supplements | Media | Duration | Functional SP? | Reference | ||
|---|---|---|---|---|---|---|---|
| 0P9-DL | |||||||
| BM-HSPC | 0P9-DL | IL-7 | FIt3L | α-MEM + 20% FBS | 5 weeks | Yes | Schmitt [6] |
| FL-HSPC | OP9-DL | IL-7 | Flt3L | α-MEM + 20% FBS | 5 weeks | Yes | Schmitt [6] |
| ESC | OP9 0P9-DL |
IL7 IL-15 |
FIt3L | α-MEM + 20% FBS | 3 weeks | Yes | Schmitt [39] |
| iPSC | OP9-DL | IL-7 | FIt3L | α-MEM + 20% FBS | 3 weeks | Yes | Lei [44] |
| pFib-DL | |||||||
| FL-HSPC | Primary fibroblast-DL |
IL-7 SCF |
Flt3L GW2580 |
α-MEM + 15% FBS | 2 weeks | Yes | Mohtashami [25] |
| Dll-Fc | |||||||
| FL-HSPC | – | IL-7 SCF |
FIt3L | RPMI + 10% FBS | 2 weeks | Yes | Ikawa [33] |
Abbreviations: BM-HSPC, bone marrow-derived hematopoietic stem progenitor cells; DL/Dll, delta-like ligand; ESC, embryonic stem cell; FBS, fetal bovine serum; FL-HSPC, fetal liver-derived hematopoietic stem progenitor cells; Flt3L, FMS-like tyrosine kinase 3 ligand; IL, interleukin; iPSC, induced pluripotent stem cell; α-MEM, minimum essential medium α; pFib, primary fibroblast; RPMI, Roswell Park Memorial Institute medium; SCF, stem cell factor; SP, single positive.
Conversely, primary fibroblasts (pFib) are highly abundant, and when modified to express Dll they can direct Notch-regulated T-cell differentiation [25, 26], despite being unable to produce endogenous cytokines to mediate lymphoid development. Interestingly, unlike OP9-DL cells, pFib-DL/HSPC cocultures do give rise to myeloid cells as well as T-lineage cells. In fact, the system that most closely approaches the efficiency of OP9-DLs appears to be pFib derived from a mouse model with a triple deletion of the myelogenic M-, G-, and GM-CSF genes, but with the addition of cytokines Flt3-L, IL-7, and SCF [26].
Generating such a system using human resources would have great clinical impact. Primary fibroblasts are accessible, and could be used to create an autologous system where both the hematopoietic and the feeder cells expressing Dll could be derived from the same individual, eliminating the possibility of rejection upon transplant. Moreover, they are able to satisfy two distinct interests in generating T cells: (a) they support the generation of pro-Ts that can give rise to mature T cells in vivo when adoptively transferred [25, 26]; and (b) the primary fibroblasts generate mature CD3+ SP CD8 and CD4 T cells in the context of self-MHC. While selection in the context of MHC for specific peptides does occur on OP9-DL/HSPC cocultures [27, 28], creating an individualized feeder system could potentially allow for selection of mature T cells that would specifically recognize and react against particular tumors.
Human HSPC Differentiation into T Cells In Vitro
In vitro differentiation of murine T cells has been a valuable tool for investigating T-cell development and has allowed preclinical testing of T-cell-based cellular therapies. Despite its many advantages, OP9-DL cells have not yet been used for clinical purposes because of their mouse origin. To circumvent the potential issue of clinical applicability, two approaches have been taken to develop an effective xenogeneic-free technique for in vitro T-cell differentiation. One is to create feeder cells of human origin that can support T-cell development similar to OP9-DL cells. The second is to create a feeder-cell-free culture system where all the molecular requirements to induce T-cell development are provided through addition of defined media, cytokines, and proteins with minimal animal or human products (Table 2).
Table 2.
Summary of culture conditions using human cells as a starting source
| Feeder cells | Supplements | Media | Duration | Functional SP? | Reference | ||
|---|---|---|---|---|---|---|---|
| 0P9-DL | |||||||
| UCB-HSPC | OP9-DL | IL-7 | FIt3L | α-MEM + 20% FBS | 9–10 Weeks | Yes | Awong [17] |
| ESC | OP9 0P9-DL |
Ascorbic acid MTG transferrin BMP4 bFGF Activin A SB VEGF |
Dkk | Stem Pro 34 | 4 weeks | Yes | Kennedy [46] |
| IL-6 | |||||||
| IGF-1 | |||||||
| IL-11 | |||||||
| SCF | |||||||
| EPO | |||||||
| TPO | |||||||
| IL-3 | |||||||
| Flt3L | |||||||
| iPSC | 0P9 OP9-DL |
Ascorbic acid MTG transferrin BMP4 bFGF Activin A SB VEGF |
Dkk | Stem Pro 34 | 4 weeks | No | Kennedy [46] |
| IL-6 | |||||||
| IRF-1 | |||||||
| IL-11 | |||||||
| SCF | |||||||
| EPO | |||||||
| TPO | |||||||
| IL-3 | |||||||
| Flt3L | |||||||
| FTSC | |||||||
| BM-HSPC | Fetal thymic stromal cells | IL-2 IL-7 IL-12 |
Flt3L SCF |
IMDM + 20% FBS | 5 weeks | Yes | Freedman [23] |
| pFib-DL | |||||||
| UCB-HSPC | Primary fibroblast-DL | IL-7 SCF |
FIt3L | α-MEM1 20% FBS | 5 weeks | Yes | Mohtashami [25] |
| pFib | |||||||
| UCB-HSPC primary fibroblast | Keratinocytes | IL-7 IL-15 |
FIt3L | DMEM + 10% FBS | 3–4 weeks | No | LaPenna [29] |
| DII-Fc | |||||||
| UCB-HSPC | - | IL-7 SCF TPO |
Flt3L ascoribic acid |
α-MEM + 20% FBS | 3–4 weeks | No | Huijskens [35] |
Abbreviations: bFGF, basic fibroblast growth factor; BM-HSPC, bone marrow-derived hematopoietic stem progenitor cells; BMP, bone morphogenic protein; Dkk, Dickkopf protein; DL/Dll, delta-like ligand; DMEM, Dulbecco’s modified Eagle’s medium; EPO, erythropoietin; ESC, embryonic stem cell; FBS, fetal bovine serum; Flt3L, FMS-like tyrosine kinase 3 ligand; FTSC, fetal thymic stromal cells; IGF, insulin-like growth factor; IL, interleukin; IMDM, Iscove’s Modified Dulbecco’s Medium; iPSC, induced pluripotent stem cell; α-MEM, minimum essential medium α; MTG, monothioglycerol; pFib, primary fibroblast; RPMI, Roswell Park Memorial Institute medium; SB, SB-431542; SCF, stem cell factor; SP, single positive; TPO, thrombopoietin; UCB-HSPC, umbilical cord blood-derived hematopoietic stem progenitor cells; VEGF, vascular endothelial growth factor.
While the results of murine pFib-DL/HSPC cocultures were very encouraging, it is not clear why the human pFib-DL failed to accommodate T-cell development, despite the addition of macrophage colony stimulating factor inhibitors [25]. In another strategy for the development of a human feeder-cell-based system, primary fibroblasts and human keratinocytes, both primary [28] and immortalized lines [29], were combined and seeded with UCB CD34+ progenitors. When in a three-dimensional matrix, keratinocytes and fibroblasts upregulate Dll4 and IL-7, and with additional IL-7, IL-15, and Flt3L, support T-cell development. However, the difficulty and the inefficiency of this method to generate pro-Ts or functional SPs have resulted in controversy regarding the authenticity and potential of this method [30]. A more consistent method has been the coculture of UCB CD34+ cells on a human TEC line overexpressing murine Dll1 or Dll4 in the presence of IL-7, SCF, and Flt3L. The TEC line supports T-cell expansion and differentiation up to the DP stage [31]. However, there is a decrease in viability after 2–3 weeks in culture, and T-lineage cells do not progress to CD4+ or CD8+ CD3+ SPs, necessitating a different approach to their generation.
Feeder-Free Systems of T-Cell Development
So far, attempts at creating human-derived feeder cells for T-cell development have been met with disappointing results and more research is required to discover the right source of cells. In contrast, there has been a steady improvement of the outcome of the feeder-free system, increasing the clinical translational potential of this method. The essential component of this system has been the Dll1 or Dll4 recombinant protein fused to the Fc portion of human IgG, and hence named Dll-Fc. When it was first attempted, Dll-Fc was bound to a matrix or plate supplemented with combinations of Flt3-L, IL-7, and SCF. The outcome was inefficient, with the generation of CD90+ cells from BM HSPC [4, 32]. It has since been determined that the IL-7 concentration must be adjusted throughout the hPSC differentiation to T-lineage cells, with high levels at the start of the culture diminishing to lower levels, to allow the DPs to emerge [33]. Other factors that appear to improve the outcome of T-cell differentiation in this system, either with murine or human HSPCs, are the addition of CXCL12 [34], ascorbic acid [35] or Wnt3a [36].
In order to expedite clinical use, one of the goals of the feeder-cell-free method is to use defined media that contain no animal products. Remarkably, this was achieved with the culture of CD34+CD38− UCB cells on plates coated with immobilized Dll-Fc fragments in serum-free Stemspan media with the addition of SCF, Flt3L, IL-6, IL-3, thrombopoietin, and low-density lipoprotein [37]. This led to a many-fold expansion of CD34+ cells within 2 weeks of culture and significant improvement in preclinical engraftment into immunodeficient mice. Based on its success, this methodology has been used to clinically investigate the efficacy in repopulating the hematopoietic cells in patients undergoing cord blood transplantation following chemo- and/or radiation therapy. However, clinical trials showed that using CD34+ cells preincubated with Dll1-Fc resulted in a more rapid myeloid reconstitution after adoptive transplant, as opposed to T-lineage [37]. This suggested that further improvements are required to enhance T-cell expansion. Nonetheless, the approval of this methodology for clinical trials reinforces the use of Dll-Fc as a viable path to expand and differentiate T cells for clinical applications. In addition, following this method of expansion, human CD8+ T cells were generated in vitro with a diverse TCR-Vβ repertoire and selected on antigen-specific peptide loaded tetramers, although only in small numbers [38].
Embryonic Stem Cell and Induced Pluripotent Stem Cell-Derived T Cells
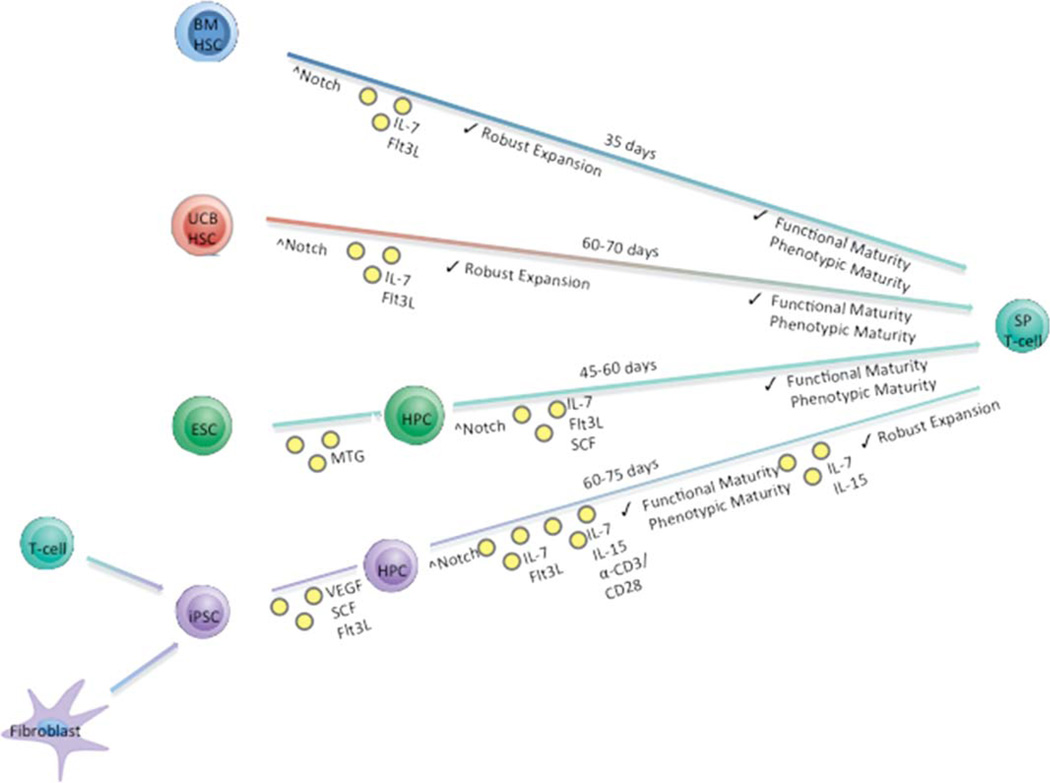
Despite the advantage of using human UCB over BM in its robustness to expand and differentiate in vitro, the relatively paucity of UCB and the difficulty of its maintenance in an undifferentiated form in culture are great obstacles to overcome. In contrast, embryonic stem cell (ESC) or induced pluripotent stem cell (iPSC), which are renewable sources of cells, can in theory be perpetually propagated and hence provide an unlimited source for generating hematopoietic cells. Investigations into using ESC or iPSC as an alternative source of stem cells for the in vitro generation of T cells have been ongoing in the mouse system with some recent successes in the human system (Figure 1).
Figure 1.
Simplified in vitro differentiation schema describing the approximate time needed to generate mature SP T cells from four starting progenitor cell populations. The relative timing of Notch signaling induction and the addition of various cytokines are also depicted, along with the proliferative potential induced by each method and general characterizations of the resulting SP T cells obtained from each culture condition. Abbreviations: BM, bone marrow; ESC, embryonic stem cell; Flt3L, FMS-like tyrosine kinase 3 ligand; IL, interleukin; iPSC, induced pluripotent stem cell; MTG, monothioglycerol; SCF, stem cell factor; SP, single positive; UCB, umbilical cord blood; VEGF, vascular endothelial growth factor.
Shortly after its advent, the OP9-DL1 coculture system was adapted to generate T cells from mouse ESCs in vitro. With modification of ESC passaging protocols and the timed addition of first Flt3L and then IL-7, cells cultured in this manner completed a normal course of T-cell differentiation, eventually resulting in mature, functional CD8+ SP cells by day 22. When implanted into immunodeficient hosts, pro-T cells were able to complete differentiation into mature CD4 and CD8 SP cells and provide protection against viral challenge [39]. Addition of tetramers to the coculture was able to induce generation of antigen-specific mature CD8+ T cells, which—despite being a small percentage of the total hematopoietic cells— were able to induce activated caspase 3 expression in target cells [40].
In vitro differentiation of human ESC to T cells could not be readily established in either OP9-DL1 coculture or fetal thymic organ cultures, despite the successes of these protocols when used with human UCB- or BM-derived HSPC and mouse ESCs. However, a combination of initial culture on OP9 cells followed by in vivo differentiation in human thymic tissue in an immunodeficient mouse model allowed ESCs to fully differentiate into mature T cells [41]. Comparison of human ESC with CD34+ UCB suggested that the human ESC gene expression program strongly favored the NK lineage at the expense of the T lineage [42]. Further investigation of human ESC cultured on OP9 cells led to the discovery of T-lineage potential in human ESC, specifically the CD34+CD43lo fraction, when allowed to form hematopoietic zones. When transferred to OP9-DL1 cultures, these cells gave rise to CD3+TCRαβ+ or TCRγδ+ T cells that were functionally mature [43].
With the discovery of induced pluripotency came renewed efforts in the development of methods for in vitro generation of T cells from iPSC. Mouse iPSCs were successfully differentiated to mature T cells in vitro, beginning with an embryonic fibroblast-derived iPSC line cultured on OP9-DL1 cells in the presence of IL-7 and Flt3L [44]. In contrast with other adult or ESCs, iPSCs derived from either fibroblast or lymphoid cells demonstrate a bias for the T lineage over the B lineage in this culture system [45]. This was followed by relatively well-defined and successful differentiation strategies that directed human iPSCs to generate definitive-like HSPC and subsequently T-cell differentiation on OP9-DL4 cells. Inhibition of the Activin/Nodal pathway and stimulation of the Wnt pathway during mesoderm specification greatly enhanced T-cell potential of this method as defined by CD34+ cells having the capacity to produce CD45+CD43+CD7+CD5+ pro-T cells [46].
The success of these methods to differentiate iPSCs into T cells provided the opportunity to exploit these techniques to potentially achieve two very important goals of immunotherapy: (a) the genetic manipulation of iPSCs to correct mutations that result in immunodeficiencies, as exemplified by the correction of a SCID-X1 mutation in a human iPSC line [32]; and (b) the ability to create iPSCs from T cells with Ag-specificity that could be clinically effective, virtually guaranteeing an unlimited supply of antigen-specific T cells. Several groups have reported deriving human iPSCs from mature peripheral blood T cells [47–49] with unique TCRs. iPSC-derived T cells displayed a broad T-cell repertoire [50] and retained antigen specificity at the DNA level, although this specificity was sometimes lost during differentiation as a result of RAG-mediated recombination. Prevention of undesired recombination events required stimulation of TCR signaling using anti-CD3/CD28 mAb-coated beads in the presence of exogenous cytokines [51]. Furthermore, these cells expanded up to 1,000-fold in response to cytokine signaling, maintained a memory T-cell surface antigen expression profile, and produced cytolytic molecules to lyse target cells in an antigen-specific manner [52].
At this point, the most viable autologous anti-cancer T-cell-based therapies are chimeric antigen receptor (CAR) and TCR gene transfer technology into mature, functional CD8+ T cells. Most often, mature T cells are harvested from peripheral blood or tumor infiltrating lymphocytes, activated and transduced with a CAR or tumor-specific TCR in vitro to confer antigen specificity, and adoptively transferred back to the patient. The autologous nature of this procedure ensures that there is no GVHD unless the antigen is present at extratumor sites. This attractive feature is offset by obstacles to the successful clinical implementation of this method, which necessitates long-term T-cell survival and function in vivo and potential need for additional autologous T-cell products over time for repetitive T-cell infusions, if needed to control tumor burden [53].
Clinical Possibilities for In Vitro-Derived T-Cell Precursors and Conclusion
Continued advancement in in vitro production of human T cells will have a great impact in ameliorating the prospects of patients with immunodeficiencies, congenital or otherwise, or for those undergoing chemo-/radiotherapy. It is conceivable that such systems could lend themselves to the production of T cells for the reversal of autoimmunity, but these possibilities have yet to be investigated. Much success has been achieved using progenitors purified from human UCB, which are already routinely used in hematopoietic stem cell transplantation [54], but represent a much more finite source of starting material. If a feeder-cell-free process of pro-T-cell generation from iPSCs can be actuated, refined, and scaled up to provide large quantities, the hope is that the advent of a curative method for T-cell adoptive transfer could be realized. Unlike CAR-based immunotherapy, pro-T cells do not require an MHC match between the donor and recipient or the disruption of the endogenous TCR, permitting universal clinical application. Upon maturation, an appropriate subset can become central memory T cells, which show the greatest potential for long-term in vivo persistence [55]. For immunotherapy, the expectation is that hPSCs derived from iPSC culture systems can lend themselves well to genetic manipulation, either to correct deficiencies, or to express exogenous designer TCRs to counter cancer. Creative approaches have been taken to improve the timing of CAR expression; for example, the restricted expression of a CAR reactive against hCD19 for anti-B cell-leukemia response until after negative selection, enabled complete and long-term protection against a hCD19-expressing syngeneic tumor [56]. The incorporation of CTLA-4 or PD-1-based inhibitory CARs, promoting self-regulation of cytokine secretion, proliferation, and cytotoxicity, are strategies that have been taken to address complications or inadequate specificity of engineered T cells [57]. However, iPSC-derived cells are just now entering the clinic, and their long-term safety is unknown. Methods to permit high levels of proliferation and robust differentiation to T-lineage cells are imperative since these immunotherapies must undergo rigorous clinical testing to reveal their safety and benefit. Although first-in-human trials of pro-Ts using OP9-DL1 cells are being considered for obtaining proof-of-principle that pro-Ts can speed immune recovery in patients, approval of clinical translation would be facilitated by the development of effective differentiation techniques independent of feeder cells or animal products. While such techniques are in their beginning stages [58, 59] and cannot yet support robust and efficient T-cell differentiation, they are being improved and will hopefully unlock the immense potential of immunotherapy.
Significance.
This concise review covers recent advances in the in vitro generation of T cells from adult, embryonic, and induced pluripotent stem cells. Today’s leading techniques are described and evaluated on the basis of cell yield, cell function, and ease of translation to the clinic. The potential use of in vitro-derived T cells as therapeutic agents is discussed. This review is an unbiased survey of current advances and obstacles in the field, and as such is a valuable resource for those conducting related research.
Acknowledgments
This work was supported in part by NIH Grants R01 AI081918, R01 CA 72669, and 2P01 CA065493 and T32 HL007062.
Footnotes
Author Contributions
M.J.S. and B.R.W.: conception and design and manuscript writing; M.M.: manuscript editing and manuscript writing; H.E.S.: manuscript editing; J.C.Z.-P.: manuscript writing and editing and final approval of manuscript; B.R.B.: financial support, manuscript writing and editing, and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 2.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 3.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 4.Ciofani M, Zúñiga-Pflücker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 5.Dervovic DD, Ciofani M, Kianizad K, et al. Comparative and functional evaluation of in vitro generated to ex vivo CD8 T cells. J Immunol. 2012;189:3411–3420. doi: 10.4049/jimmunol.1200979. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 7.Ciofani M, Knowles GC, Wiest DL, et al. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Radtke F, Wilson A, Mancini SJC, et al. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 9.Abe N, Hozumi K, Hirano K-I, et al. Notch ligands transduce different magnitudes of signaling critical for determination of T-cell fate. Eur J Immunol. 2010;40:2608–2617. doi: 10.1002/eji.200940006. [DOI] [PubMed] [Google Scholar]
- 10.Hozumi K, Negishi N, Suzuki D, et al. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 11.Hozumi K, Mailhos C, Negishi N, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 2008;205:2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch U, Fiorini E, Benedito R, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med. 2008;205:2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besseyrias V, Fiorini E, Strobl LJ, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med. 2007;204:331–343. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohtashami M, Shah DK, Nakase H, et al. Direct comparison of Dll1- and Dll4-mediated notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol. 2010;185:867–876. doi: 10.4049/jimmunol.1000782. [DOI] [PubMed] [Google Scholar]
- 15.Awong G, La Motte-Mohs RN, Zúñiga-Pflücker JC. Generation of pro-T cells in vitro: Potential for immune reconstitution. Semin Immunol. 2007;19:341–349. doi: 10.1016/j.smim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.De Smedt M, Leclercq G, Vandekerckhove B, et al. T-lymphoid differentiation potential measured in vitro is higher in CD34+CD38−/lo hematopoietic stem cells from umbilical cord blood than from bone marrow and is an intrinsic property of the cells. Haematologica. 2011;96:646–654. doi: 10.3324/haematol.2010.036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awong G, Herer E, La Motte-Mohs RN, et al. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC Immunol. 2011;12:22. doi: 10.1186/1471-2172-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awong G, Herer E, Surh CD, et al. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114:972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 19.Van Coppernolle S, Verstichel G, Timmermans F, et al. Functionally mature CD4 and CD8 TCRalphabeta cells are generated in OP9-DL1 cultures from human CD34 + hematopoietic cells. J Immunol. 2009;183:4859–4870. doi: 10.4049/jimmunol.0900714. [DOI] [PubMed] [Google Scholar]
- 20.Zakrzewski JL, Suh D, Markley JC, et al. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat Biotechnol. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awong G, Singh J, Mohtashami M, et al. Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood. 2013;122:4210–4219. doi: 10.1182/blood-2012-12-472803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 23.Freedman AR, Zhu H, Levine JD, et al. Generation of human T lymphocytes from bone marrow CD34 + cells in vitro. Nat Med. 1996;2:46–51. doi: 10.1038/nm0196-46. [DOI] [PubMed] [Google Scholar]
- 24.Mohtashami M, Zuniga-Pflucker JC. Cutting edge: Three-dimensional architecture of the thymus is required to maintain delta-like expression necessary for inducing T cell development. J Immunol. 2006;176:730–734. doi: 10.4049/jimmunol.176.2.730. [DOI] [PubMed] [Google Scholar]
- 25.Mohtashami M, Shah DK, Kianizad K, et al. Induction of T-cell development by Delta-like 4-expressing fibroblasts. Int Immunol. 2013;25:601–611. doi: 10.1093/intimm/dxt027. [DOI] [PubMed] [Google Scholar]
- 26.Smeets MFMA, Mackenzie-Kludas C, Mohtashami M, et al. Removal of myeloid cytokines from the cellular environment enhances T-cell development in vitro. Int Immunol. 2013;25:589–599. doi: 10.1093/intimm/dxt025. [DOI] [PubMed] [Google Scholar]
- 27.Dervovic DD, Liang HCY, Cannons JL, et al. Cellular and molecular requirements for the selection of in vitro-generated CD8 T cells reveal a role for notch. J Immunol. 2013;191:1704–1715. doi: 10.4049/jimmunol.1300417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark RA, Yamanaka K-I, Bai M, et al. Human skin cells support thymus-independent T cell development. J Clin Invest. 2005;115:3239–3249. doi: 10.1172/JCI24731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapenna A, B-Lynch C, Kapeni C, et al. A simple model system enabling human CD34 + cells to undertake differentiation towards T cells. PLoS One. 2013;8:e69572. doi: 10.1371/journal.pone.0069572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meek B, Van Elssen CHMJ, Huijskens MJAJ, et al. T cells fail to develop in the human skin-cell explants system; an inconvenient truth. BMC Immunol. 2011;12:17. doi: 10.1186/1471-2172-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehre N, Nusser A, Muenchow von L, et al. A stromal cell free culture system generates mouse pro-T cells that can reconstitute T-cell compartments in vivo. Eur J Immunol. 2014;45:932–942. doi: 10.1002/eji.201444681. [DOI] [PubMed] [Google Scholar]
- 32.Menon T, Firth AL, Scripture-Adams DD, et al. Lymphoid regeneration from gene-corrected SCID-X1 subject-derived iPSCs. Cell Stem Cell. 2015;16:367–372. doi: 10.1016/j.stem.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikawa T, Hirose S, Masuda K, et al. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 34.Janas ML, Varano G, Gudmundsson K, et al. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207:247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huijskens MJAJ, Walczak M, Koller N, et al. Technical advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J Leukoc Biol. 2014;96:1165–1175. doi: 10.1189/jlb.1TA0214-121RR. [DOI] [PubMed] [Google Scholar]
- 36.Aoyama K, Delaney C, Varnum-Finney B, et al. The interaction of the Wnt and Notch pathways modulates natural killer versus T cell differentiation. Stem Cells. 2007;25:2488–2497. doi: 10.1634/stemcells.2007-0102. [DOI] [PubMed] [Google Scholar]
- 37.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez I, Ooi TP, Roy K. Generation of functional, antigen-specific CD8 + human T cells from cord blood stem cells using exogenous notch and tetramer-TCR signaling. Stem Cells. 2014;32:93–104. doi: 10.1002/stem.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt TM, de Pooter RF, Gronski MA, et al. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 40.Lin J, Nie H, Tucker PW, et al. Controlled major histocompatibility complex-T cell receptor signaling allows efficient generation of functional, antigen-specific CD8 + T cells from embryonic stem cells and thymic progenitors. Tissue Eng Part A. 2010;16:2709–2720. doi: 10.1089/ten.tea.2009.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galic Z, Kitchen SG, Kacena A, et al. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin CH, Woll PS, Ni Z, et al. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112:2730–2737. doi: 10.1182/blood-2008-01-133801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timmermans F, Velghe I, Vanwalleghem L, et al. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 2009;182:6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 44.Lei F, Haque R, Weiler L, et al. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260:1–5. doi: 10.1016/j.cellimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Wada H, Kojo S, Kusama C, et al. Successful differentiation to T cells, but unsuccessful B-cell generation, from B-cell-derived induced pluripotent stem cells. Int Immunol. 2010;23:65–74. doi: 10.1093/intimm/dxq458. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Brown ME, Rondon E, Rajesh D, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 2010;5:e11373–e11379. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh Y-H, Hartung O, Li H, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Chang C-W, Lai Y-S, Lamb LS, et al. Broad T-cell receptor repertoire in T-lymphocytes derived from human induced pluripotent stem cells. PLoS One. 2014;9:e97335. doi: 10.1371/journal.pone.0097335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vizcardo R, Masuda K, Yamada D, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8 + T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother. 2002;25:82–87. doi: 10.1097/00002371-200201000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lund TC, Boitano AE, Delaney CS, et al. Advances in umbilical cord blood manipulation-from niche to bedside. Nat Rev Clin Oncol. 2015;12:163–174. doi: 10.1038/nrclinonc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sprent CDSJ. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Papapetrou EP, Kovalovsky D, Beloeil L, et al. Harnessing endogenous miR-181a to segregate transgenic antigen receptor expression in developing versus post-thymic T cells in murine hematopoietic chimeras. J Clin Invest. 2009;119:157–168. doi: 10.1172/JCI37216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006597. 215ra172–215ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chou B-K, Gu H, Gao Y, et al. A facile method to establish human induced pluripotent stem cells from adult blood cells under feeder-free and xeno-free culture conditions: A clinically compliant approach. Stem Cells Transl Med. 2015;4:320–332. doi: 10.5966/sctm.2014-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salvagiotto G, Burton S, Daigh CA, et al. A defined, feeder-free, serum-free system to generate in vitro hematopoietic progenitors and differentiated blood cells from hESCs and hiPSCs. PLoS One. 2011;6:e17829. doi: 10.1371/journal.pone.0017829. [DOI] [PMC free article] [PubMed] [Google Scholar]