Abstract
Mitochondrial fission and fusion play critical roles in maintaining functional mitochondria when cells experience metabolic or environmental stresses. Fusion helps mitigate stress by mixing the contents of partially damaged mitochondria as a form of complementation. Fission is needed to create new mitochondria, but it also contributes to quality control by enabling the removal of damaged mitochondria and can facilitate apoptosis during high levels of cellular stress. Disruptions in these processes affect normal development, and they have been implicated in neurodegenerative diseases, such as Parkinson’s.
Mitochondria are double-membrane–bound subcellular organelles that provide a host of metabolic functions, including energy production through oxidative phosphorylation. Mitochondrial morphologies vary widely among different cell types. Fibroblast mitochondria, for example, are usually long filaments (1 to 10 μm in length with a fairly constant diameter of ~700 nm), whereas hepatocyte mitochondria are more uniformly spheres or ovoids. When mitochondria are viewed in live cells, it becomes immediately apparent that their morphologies are far from static. Their shapes change continually through the combined actions of fission, fusion, and motility. Rapid fission and fusion of mitochondria in cultured fibroblasts allows for the complete redistribution of mitochondrial green fluorescent protein (GFP) from one mitochondrion to all the other mitochondria of a cell within an hour. The wide range of mitochondrial lengths observed in different cell types and under different conditions results from changes in the balance between the rates of mitochondrial fission and fusion. Here, we discuss how fission and fusion contribute to mitochondrial quality control and the responses of mammalian cells to stress.
Mitochondrial Fusion and Fission Proteins
Mitochondrial fission and fusion processes are both mediated by large guanosine triphosphatases (GTPases) in the dynamin family that are well conserved between yeast, flies, and mammals (1). Their combined actions divide and fuse the two lipid bilayers that surround mitochondria. The mitochondrial inner membrane, which encloses the matrix, is folded into cristae that contain membrane-bound oxidative phosphorylation enzyme complexes and the bulk of the soluble electron transport proteins such as cytochrome c, whereas the smooth mitochondrial outer membrane encapsulates the inner membrane and an intermembrane space.
Fission is mediated by a cytosolic dynamin family member (Drp1 in worms, flies, and mammals and Dnm1 in yeast). Drp1 is recruited from the cytosol to form spirals around mitochondria that constrict to sever both inner and outer membranes. Yeast share with mammals this core function of Drp1 but have distinct accessory proteins. Mdv1 recruits Dnm1 to mitochondrial fission sites in yeast, whereas Mid49, Mid51, and Mff recruit Drp1 to mitochondria in mammals (2), often at sites where mitochondria make contact with the endoplasmic reticulum (3). Fusion between mitochondrial outer membranes is mediated by membrane-anchored dynamin family members named Mfn1 and Mfn2 in mammals, whereas fusion between mitochondrial inner membranes is mediated by a single dynamin family member called Opa1 in mammals. Mitochondrial fission and fusion machineries are regulated by proteolysis and posttranslational modifications (1).
Mitochondrial fission is essential for growing and dividing cells to populate them with adequate numbers of mitochondria. It has been less clear why mitochondrial fission and fusion are also needed for nonproliferating cells, but the importance of these processes is evident from nonproliferating neurons, which cannot survive without mitochondrial fission, and from two human diseases, dominant optic atrophy and Charcot Marie Tooth disease type 2A, which are caused by fusion defects. The importance of mitochondrial fusion for embryogenesis was shown with Mfn1 and Mfn2 knock-out mice, which die in utero at midgestation because of a placental deficiency, whereas the Mfn1 Mfn2 double knockout mice die even earlier in development (4). Mouse embryo fibroblasts (MEFs) derived from the double knock-out mice do survive in culture, despite a complete absence of fusion, but some of their mitochondria display a reduced mitochondrial DNA (mtDNA) copy number and lose membrane potential, causing problems with adenosine triphosphate (ATP) synthesis (5). Mitochondrial fusion is therefore not absolutely essential for cell survival in vitro, but it is required for embryonic development and for cell survival at later stages in development (4). These differential requirements for fusion may stem from higher demands on oxidative metabolism in different cell types or on other functions that are indirectly affected by fusion, such as mitochondrial motility in neurons.
Fusion Promotes Complementation Between Damaged Mitochondria
Mitochondria have their own small circular genomes, encoding select subunits of ATP synthesis and electron transport proteins that form oxidative phosphorylation complexes with other subunits encoded by the nuclear genome, as well as transfer and ribosomal RNAs (tRNAs and rRNAs) needed for their translation. A single somatic cell can have thousands of copies of these genomes, which are grouped in protein-rich complexes called nucleoids, with between one and eight genome copies per nucleoid (6). Mutations and deletions that occasionally arise in mitochondrial DNA yield a heteroplasmic mixture of wild-type and mutant mitochondrial genomes within one cell. Maternal inheritance of these mutations can cause mitochondrial diseases, such as mitochondrial encephalomyopathy with lactic acidosis and strokelike episodes (MELAS) and myoclonus epilepsy with ragged-red fibers (MERRF). Fortunately, mitochondria with mutant DNA can still fuse with other mitochondria in the same cell, allowing mitochondria with wild-type DNA to compensate for defects in mitochondria with mutant DNA by sharing components as long as the mutation load remains below 80 to 90% per cell (7, 8). Because nucleoids do not appear to exchange DNA (6), mitochondria in heteroplasmic cells complement one another by sharing RNA or protein components. Fusion between mitochondria can also rescue two mitochondria with mutations in different genes by cross-complementation to one another, and it can mitigate the effects of environmental damage through the exchange of proteins and lipids with other mitochondria. Mitochondrial fusion can therefore maximize oxidative capacity in response to toxic stress, as long as the stress is below a critical threshold (Fig. 1).
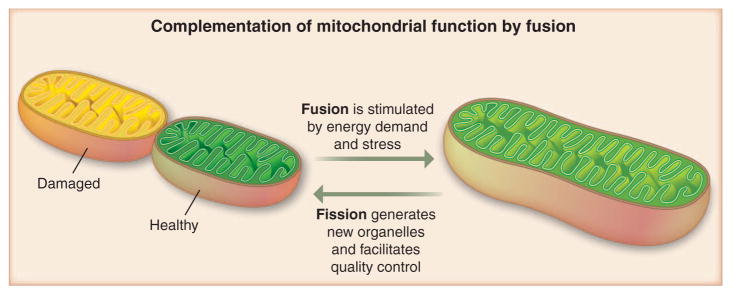
Fig. 1.
Fusion rescues stress by allowing functional mitochondria (green) to complement dysfunctional mitochondria (yellow) by diffusion and sharing of components between organelles. Stress-induced hyperfusion yields maximal potential (light green), whereas under relaxed conditions cells are able to segregate the damaged (yellow) ones.
Mitochondrial Morphology Is Controlled by Metabolism
Rates of mitochondrial fission and fusion respond to changes in metabolism. Mitochondria become more fused when they are forced to rely on oxidative phosphorylation by withdrawing glucose as a carbon source (9). Increased fusion may be necessary to maximize the fidelity for oxidative phosphorylation by stimulating complementation among mitochondria (Fig. 1). Fusion is also enhanced by treatments that directly or indirectly inhibit protein synthesis and by starvation and mTOR (mammalian target of rapamycin) inhibition–induced autophagy (10–12). Starvation-induced autophagy may enhance fusion by increasing the reliance on oxidative phosphorylation through the metabolism of lipids and proteins (9). Alternatively, starvation may evoke a specific stress response called stress-induced mitochondrial hyperfusion (10), or it may inhibit fission to protect mitochondria from autophagic catabolism when they are most needed (11, 12). Each of these effects is consistent with a model in which mitochondrial dynamics help maximize the capacity for oxidative phosphorylation under stressful conditions (Fig. 1).
Repairing Small Amounts of Mitochondrial Damage
Mitochondria continually produce highly reactive superoxide anions as a byproduct of electron transport during oxidative phosphorylation. These reactive oxygen species (ROS) damage proteins, lipids, and DNA (Box 1). Damage to proteins in the electron transport chain may worsen the situation by producing even more ROS (13). Mitochondria use quality-control proteases to eliminate damaged proteins (14) and respond to unfolded protein stress in the matrix through transcriptional induction of chaperone expression (15). Damaged mitochondrial outer membrane proteins also may be removed by the ubiquitin proteasome quality-control pathway (16). Mitochondria respond to genotoxic damage by some, but not all, of the DNA repair pathways found in the nucleus. These proteotoxic and genotoxic damage-response pathways target individual molecules for quality control, thereby rescuing mitochondria with minor damage without the need for altered fission or fusion rates (14). Another level of quality control entails the wholesale elimination of mitochondria by autophagy, a process that is linked to mitochondrial fission and fusion.
Box 1. Mitochondrial Stress.
Various insults can cause damage
Environmental (radiation, toxic chemicals)
Genetic (mutations in genes for metabolic processes or repair pathways)
Spontaneous (ROS generated as byproduct of electron transport)
Types of damage
DNA
Proteins
Lipids
Problems caused by damage
Loss of metabolic functions (ATP synthesis, etc.)
More ROS made by defective mitochondria
F1F0-ATPase may, instead of making ATP, consume ATP to generate membrane potential
Cellular responses to damage
DNA repair
Proteases
Lipases
Mitochondrial unfolded protein response
Mitophagy
Apoptosis
Scrapping Mitochondria That Are Beyond Repair
Autophagy is a well-established mechanism to compensate for nutrient depletion by degrading cellular components and to protect cells from deleterious protein aggregates by encapsulating and degrading them. Autophagy is also required for maintaining a healthy mitochondrial network, presumably by eliminating old and damaged mitochondria (17, 18). The importance of this process is shown by the accumulation of swollen and defective mitochondria in hepatocytes and MEFs from mice lacking the key autophagy gene Ulk1 (17) and the appearance of deformed mitochondria in hepatic cells in Atg7-deficient mice (18).
The autophagic elimination of mitochondria, mitophagy, appears to be intimately linked to mitochondrial fission and fusion processes. A study of fibroblast mitochondrial dynamics showed that one in five daughter mitochondria is depolarized and eliminated by mitophagy (19). In most fission events, one daughter mitochondrion is transiently hyperpolarized while the sister mitochondrion is hypopolarized, suggesting that fission embodies a “stress test” that could push a daughter mitochondrion to completely depolarize if it functions suboptimally. Mitophagy could be prevented with a dominant-negative mutant of Drp1, suggesting that fission is required for mitophagy (19). Photodamaged mitochondria undergo selective mitophagy (20), which is also consistent with the model that fission provides a form of quality control by segregating damaged parts of mitochondria and targeting them for elimination by autophagy (Fig. 2).
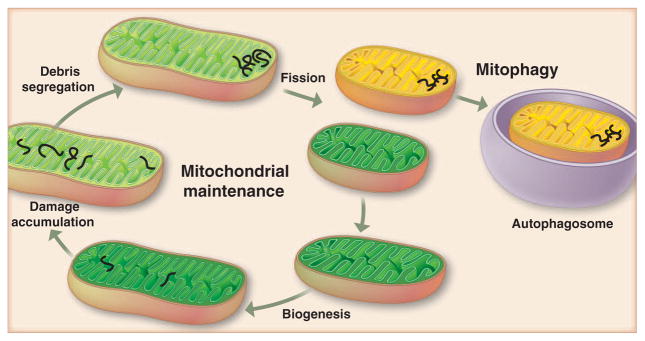
Fig. 2.
Autophagy could purify the cellular pool of mitochondria if debris is aggregated and segregated by fission in a subset of mitochondria. If deleterious components (black fibers) are asymmetrically distributed or aggregated, fission could lead to cleansing of daughter mitochondrion (green) by preventing fusion and inducing mitophagy of the impaired ones (yellow).
Recent work on two gene products mutated in familial Parkinson’s disease, PINK1 and Parkin, yields insight into a molecular mechanism of quality control via the elimination of damaged mitochondria (Fig. 3). The abundance of the kinase PINK1 is constitutively repressed in healthy mitochondria by import into the inner mitochondrial membrane and degradation by the rhomboid protease PARL. When a mitochondrion becomes uncoupled, protein import to the inner mitochondrial membrane is prevented so PINK1 is diverted from PARL and accumulates on the outer mitochondrial membrane. This yields a sensor of mitochondrial damage that can flag an individual impaired mitochondrion in a milieu of healthy ones. PINK1 on a damaged mitochondrion, through its kinase activity, recruits the E3 ligase Parkin from the cytosol specifically to that impaired mitochondrion (Fig. 3). Once there, Parkin ubiquitinates outer mitochondrial membrane proteins and induces autophagic elimination of the flagged mitochondrion (21).
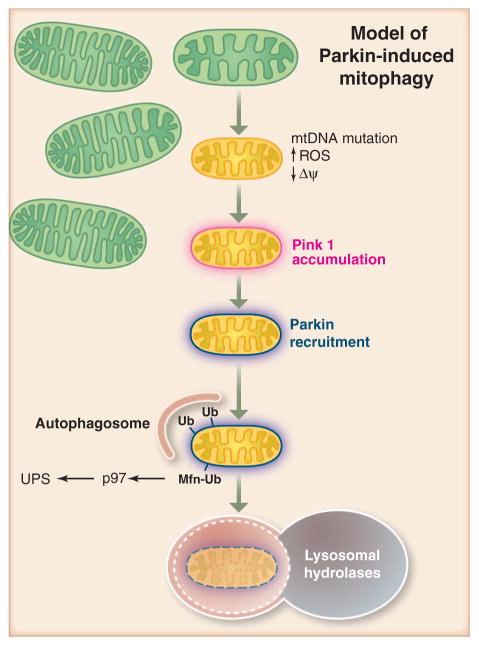
Fig. 3.
PINK1 is constitutively degraded by the inner mitochondrial membrane protease PARL and maintained at low levels on healthy mitochondria. When a mitochondrion becomes damaged to the point of depolarizing the membrane potential across the inner membrane, PINK1 import to the inner membrane is prevented, thereby sequestering it on the outer mitochondrial membrane and away from PARL. PINK1 accumulates there and recruits the E3 ligase Parkin from the cytosol via PINK1 kinase activity. Parkin conjugates ubiquitin (Ub) to a variety of proteins on the outer mitochondrial membrane and mediates the proteosomal elimination of mitofusins 1 and 2. Lastly, Parkin induces autophagic elimination of the dysfunctional mitochondria. This pathway may constitute a quality-control mechanism to eliminate damaged mitochondria. UPS, ubiquitin proteasome system.
This molecular pathway fits nicely with the fission model (19) (Fig. 2) to yield the mitochondrial quality-control model (Fig. 3). However, mitochondria have to be severely depolarized to accumulate PINK1, and the degree to which this happens physiologically is not clear. At least in cultured tumor cells that can maintain robust ATP levels by glycolysis, mitochondrial F1F0 ATPase can cleave ATP derived from glycolysis and reconstitute membrane potential despite the complete loss of membrane potential maintenance through respiration (22). Furthermore, mitochondrial fusion as discussed previously can lead to compensation for missing components, thereby rescuing impaired organelles. These forces would be expected to counteract damage-induced depolarization of mitochondria and mitigate PINK1-mediated mitophagy. The stress test on membrane potential during fission (Fig. 2), however, might overcome those forces to trigger complete depolarization.
Mutations in PINK1 (23) and Parkin (24) lead to early-onset autosomal recessive Parkinson’s disease, suggesting that defects in mitochondrial quality control could cause certain forms of parkinsonism and supporting more general models that mitochondrial dysfunction is an etiology of substantia nigral neuron degeneration. PINK1-and Parkin-deficient Drosophila display muscle and neuron degeneration that is associated with swollen and defective mitochondria (25–27). Consistent with the model that mitochondrial fission and fusion promotes mitochondrial quality control, inhibition of mitochondrial fusion or promotion of mitochondrial fission compensates for deficiencies of PINK1 and Parkin in flies. Furthermore, Parkin overexpression in flies rescues unfolded protein stress of mitochondria through autophagy (28), and stimulation of autophagy rescues depolarized mitochondria accumulation in dopaminergic neurons from Parkin-deficient Drosophila (29).
Banish Mitochondria That Truly Are Uncoupled
Defective mitochondria can be toxic by generating excessive amounts of ROS, by consuming ATP through reversal of ATP synthase, and by interfering with a host of other metabolic processes (Box 1). Low levels of damage might be corrected by complementation through mitochondrial fusion, but badly damaged mitochondria will contaminate other mitochondria if they are allowed to rejoin the mitochondrial network before their elimination by autophagy. Several mechanisms are at work to stop this from happening. A first line of defense is provided by a built-in requirement of the mitochondrial inner membrane fusion machinery for membrane potential (30). Vertebrates have elaborated on this mechanism by providing a second line of defense through proteolytic inactivation of the inner membrane fusion dynamin OPA1. Proteolysis is mediated by the mitochondrial inner membrane protease OMA1, which is rapidly activated by low membrane potential and low levels of ATP (31, 32). The outer membranes of these mitochondria can still fuse, even without functional OPA1 or membrane potential, but the inner membrane–bound matrix compartments do not fuse, resulting in several matrix compartments surrounded by a common outer membrane, like peas in a pod.
The last line of defense is provided by the Pink1 and Parkin pathway through the ubiquitination of the mitochondrial outer membrane fusion proteins Mfn1 and Mfn2. Ubiquitination of these proteins leads to their extraction from the membrane by p97 and their degradation by proteasomes (16). In addition, Pink1 and Parkin disrupt mitochondrial motility by degrading the small GTPase Miro, which serves as an adaptor for kinesin-dependent transport and is also needed for mitochondrial fusion (33). Ultimately, uncoupled mitochondria lose both their inner and outer membrane fusion machineries, thereby preventing them from fusing with and poisoning the healthy mitochondrial network. Purposeful segregation and disposal of damaged mitochondria through changes in fission and fusion pathways are therefore integral parts of mitochondrial quality-control mechanisms.
Is Debris Also Sorted Inside Mitochondria?
The gradual accumulation of damaged components poses a problem for the mitophagic disposal process. If damaged components were evenly distributed, then the simple act of fission through Drp1 would not generate the asymmetry needed for inducing mitophagy by selective loss of membrane potential. It seems that asymmetric sorting of debris would be needed to generate the differences in membrane potential between daughter mitochondria that have been observed immediately after fission (19). Accumulation of damaged components in a subset of daughter mitochondria would enable their selective disposal, thus helping to rejuvenate the remaining population of mitochondria (Fig. 2).
How might mitochondria achieve this type of asymmetric fission? The mechanism is not yet known, but it seems likely that damaged proteins form aggregates within the mitochondrial matrix. Perhaps there is a way to stow these aggregates at the tips of mitochondria, thus providing a starting point for polarized fission. A precedent for this was set by bacteria, which remove aggregates by asymmetric fission, thus enhancing the growth rates of those daughter cells that do not receive aggresomes (34). A similar asymmetry was observed during mammalian cell division, where aggregates accumulate at the centrosome and are selectively inherited by one of the two daughter cells (35). If mitochondria also have such a deliberate mechanism, then they might have a mechanism for inducing fission when too many aggregates are formed inside mitochondria.
Such an inducing mechanism is suggested by genetic studies showing that Pink1 and Parkin act upstream of the fission machinery in Drosophila. However, studies with mammalian cells have only shown effects of Pink1 and Parkin after fission is completed. Mammalian cells may have developed an additional, as yet undiscovered, mechanism to induce fission when mitochondrial aggregates accumulate, analogous to the rapid proteolytic inactivation of the fusion machinery through Oma1-mediated proteolysis when mitochondria lose membrane potential or ATP.
Aggregation of misfolded proteins in the cytosol is facilitated by p62 and NBR1, which can lead to their disposal by autophagy (36). Interestingly, p62 also accumulates on mitochondria after Pink1 and Parkin activation. Once there, p62 triggers mitochondrial aggregation through its oligomerization domain (36). Mitochondrial aggregation may be an indirect result of aggregating ubiquitinated proteins on the mitochondrial outer membrane to segregate debris before fission. When protein damage accumulates, small vesicles bud from the outer mitochondrial surface. The trafficking of these vesicles to lysosomes suggests another and surprisingly direct pathway of mitochondrial debris removal that is independent of Drp1, therefore independent of classic mitochondrial fission, and also independent of autophagy (37).
Selective Removal of Mutant mtDNA
Can mitophagy cleanse genotoxic stress in addition to proteotoxic stress? Mutations in mtDNA accumulate as mammals age and could accumulate generation after generation were it not for germline purification of mtDNA. Although the mechanisms are not yet known, mitochondrial genomes with strong deleterious mutations can be removed during oogenesis (38, 39). Models for this cleansing mechanism include selective expansion of less impaired mitochondria to populate oocytes, apoptosis of oocytes with excessive mutant mtDNAs, and removal of poorly functioning mitochondria by mitophagy. Whether mutated mtDNA is selectively removed from somatic cells is not known.
A requisite for elimination of deleterious mitochondrial DNA mutations by mitophagy, be it in the germ line or soma, is physical linkage between the mutated mtDNA and the mutated gene product (40). Might there be a mechanism to identify malfunctioning nucleoids through their defective protein products, for example, through physical association with protein aggregates? Integral inner membrane proteins diffuse much more slowly than soluble matrix or intermembrane space proteins and therefore are more likely to be retained with their parental nucleoid than soluble tRNAs after mitochondrial fusion and fission events. This physical proximity might link nucleoids with mutant gene products that affect protein coding sequences and facilitate their autophagic purification. Such differential diffusibility between integral membrane proteins and tRNAs might explain why mutations in tRNAs are much more common in human diseases than mutations in the integral protein components of the oxidative phosphorylation machinery. Also, protein aggregates may start to form immediately during protein synthesis, which is physically linked with nucleoids (41). A mechanism for purifying mtDNA, by retaining mutant proteins with their genome, may prove to be an unexpected bonus of coupled transcription and translation to mitochondrial nucleoids.
Mitochondrial Fission and Apoptosis
When all else fails, stressed cells undergo apoptosis. In the past decade, many connections have been discovered between apoptosis and mitochondrial dynamics, as discussed more fully in this issue by Hoppins and Nunnari. High levels of cell stress that lead to apoptosis also lead to excessive fission of mitochondria. This occurs almost simultaneously with two steps of apoptosis that involve mitochondria: translocation from the cytosol to mitochondria of the pro-apoptotic Bcl-2 family member Bax and cytochrome c release. When Bax translocates to mitochondria, it accumulates in concentrated foci that colocalize with Drp1 and mitofusins. Inhibition of mitochondrial fission by Drp1 knock-down delays cytochrome c release, indicating that mitochondrial fission participates in Bax-mediated permeabilization of the outer mitochondrial membrane (42). The link may be that Bax is activated to oligomerize and release cytochrome c by membrane hemifusion intermediates that are formed during mitochondrial fission (43). Intriguingly, Bcl-2 family members also participate in mitochondrial fission and fusion in nonapoptotic cells (44). Thus, mitochondrial dynamics are involved not only in regulating individual mitochondrial fidelity within cells but also at the whole-cell level by participating in apoptotic cell death.
Outlook
Fusion allows mitochondria to compensate for one another’s defects by sharing components and thereby helps maintain energy output in the face of stress. However, when a certain threshold of damage is reached, mitochondria are eliminated wholesale by autophagy. Fission segregates the most seriously damaged mitochondria to preserve the health of the mitochondrial network in addition to regulating morphology and facilitating mitochondrial trafficking. The highly dynamic mitochondrial fusion and fission cycle is proposed to balance two competing processes: compensation of damage by fusion and elimination of damage by fission. Failure of these stress responses may lead to neuron death and neurodegenerative disorders. In-depth understanding of mitophagic processes could aid the development of new treatments for mitochondrial and neurodegenerative diseases: It was recently shown that reactivation of autophagy can mitigate certain other diseases, such as muscular dystrophies associated with mitophagy (45).
Acknowledgments
We thank members of the Youle lab for thoughtful comments. This work was supported by Intramural Program of the National Institute of Neurological Disorders and Stroke and grants from the NIH (GM051866) and the NSF (0552271) to A.M.v.d.B.
References and Notes
- 1.Hoppins S, Lackner L, Nunnari J. Annu Rev Biochem. 2007;76:751. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 2.Elgass K, Pakay J, Ryan MT, Palmer CS. Biochim Biophys Acta. published online 10 May 2012. [Google Scholar]
- 3.Friedman JR, et al. Science. 2011;334:358. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Chan DC. Ann N Y Acad Sci. 2010;1201:21. doi: 10.1111/j.1749-6632.2010.05615.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Chomyn A, Chan DC. J Biol Chem. 2005;280:26185. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 6.Schon EA, Gilkerson RW. Biochim Biophys Acta. 2010;1800:245. doi: 10.1016/j.bbagen.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Yoneda M, Miyatake T, Attardi G. Mol Cell Biol. 1994;14:2699. doi: 10.1128/mcb.14.4.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakada K, et al. Nat Med. 2001;7:934. doi: 10.1038/90976. [DOI] [PubMed] [Google Scholar]
- 9.Rossignol R, et al. Cancer Res. 2004;64:985. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- 10.Tondera D, et al. EMBO J. 2009;28:1589. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Proc Natl Acad Sci USA. 2011;108:10190. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes LC, Di Benedetto G, Scorrano L. Nat Cell Biol. 2011;13:589. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaban RS, Nemoto S, Finkel T. Cell. 2005;120:483. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Baker MJ, Tatsuta T, Langer T. Cold Spring Harbor Perspect Biol. 2011;3:a007559. doi: 10.1101/cshperspect.a007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Science. 2012;337:587. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, et al. J Cell Biol. 2010;191:1367. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan DF, et al. Science. 2011;331:456. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komatsu M, et al. J Cell Biol. 2005;169:425. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Twig G, et al. EMBO J. 2008;27:433. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim I, Lemasters JJ. Antioxid Redox Signal. 2011;14:1919. doi: 10.1089/ars.2010.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narendra D, Tanaka A, Suen DF, Youle RJ. J Cell Biol. 2008;183:795. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchet K, Godinot C. J Biol Chem. 1998;273:22983. doi: 10.1074/jbc.273.36.22983. [DOI] [PubMed] [Google Scholar]
- 23.Valente EM, et al. Science. 2004;304:1158. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 24.Kitada T, et al. Nature. 1998;392:605. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 25.Greene JC, et al. Proc Natl Acad Sci USA. 2003;100:4078. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J, et al. Nature. 2006;441:1157. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 27.Clark IE, et al. Nature. 2006;441:1162. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 28.Pimenta de Castro I, et al. Cell Death Differ. 2012;19:1308. doi: 10.1038/cdd.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burman JL, Yu S, Poole AC, Decal RB, Pallanck L. Proc Natl Acad Sci USA. 2012;109:10438. doi: 10.1073/pnas.1120688109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meeusen S, et al. Cell. 2006;127:383. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Ehses S, et al. J Cell Biol. 2009;187:1023. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. J Cell Biol. 2009;187:959. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, et al. Cell. 2011;147:893. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner AB, Madden R, Demarez A, Stewart EJ, Taddei F. Proc Natl Acad Sci USA. 2008;105:3076. doi: 10.1073/pnas.0708931105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuentealba LC, Eivers E, Geissert D, Taelman V, De Robertis EM. Proc Natl Acad Sci USA. 2008;105:7732. doi: 10.1073/pnas.0803027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansen T, Lamark T. Autophagy. 2011;7:279. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soubannier V, et al. Curr Biol. 2012;22:135. doi: 10.1016/j.cub.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 38.Stewart JB, et al. PLoS Biol. 2008;6:e10. doi: 10.1371/journal.pbio.0060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan W, et al. Science. 2008;319:958. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowald A, Kirkwood TB. Commun Integr Biol. 2011;4:627. doi: 10.4161/cib.4.5.17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J, et al. Nucleic Acids Res. 2012;40:6109. doi: 10.1093/nar/gks266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suen DF, Norris KL, Youle RJ. Genes Dev. 2008;22:1577. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montessuit S, et al. Cell. 2010;142:889. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Autret A, Martin SJ. Mol Cell. 2009;36:355. doi: 10.1016/j.molcel.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Grumati P, et al. Nat Med. 2010;16:1313. doi: 10.1038/nm.2247. [DOI] [PubMed] [Google Scholar]