Abstract
OBJECTIVE
At present, there is no consensus on the optimal monitoring method for cerebral blood flow (CBF) in neurointensive care patients. The aim of the present study was to investigate whether continuous transcranial Doppler (TCD) monitoring with modulation of partial pressure of CO2 reflects CBF changes. This hypothesis was tested in 2 pathological settings in which cerebral ischemia can be imminent: after an episode of cerebral ischemia and during vasospasm after subarachnoid hemorrhage.
METHODS
Sixteen cynomolgus monkeys were divided into 3 groups: 1) chemoregulation in control animals to assess the physiological range of CBF regulation, 2) chemoregulation during vasospasm after subarachnoid hemorrhage, and 3) chemoregulation after transient cerebral ischemia. We surgically placed a thermal CBF probe over the cortex perfused by the right middle cerebral artery. Corresponding TCD values were acquired simultaneously while partial pressure of CO2 was changed within a range of 25 to 65 mm Hg (chemoregulation). A correlation coefficient of CBF with TCD values of greater than r equals 0.8 was considered clinically relevant.
RESULTS
CBF and CBF velocity correlated strongly after cerebral ischemia (r = 0.83, P < 0.001). Correlations were poor in chemoregulation controls (r = 0.2) and in the vasospasm group (r = 0.55).
CONCLUSION
The present study provides experimental support that, in clearly defined conditions, continuous TCD monitoring combined with chemoregulation testing may provide an estimate of CBF in the early postischemic period.
Keywords: Cerebral blood flow, Cerebral ischemia, Chemoregulation, Primates, Subarachnoid hemorrhage, Transcranial Doppler, Vasospasm
To date, there is no consensus on the optimal tool for cerebral blood flow (CBF) monitoring of neurointensive care patients at risk of developing cerebral ischemia. After it was first used in patients by Aaslid et al. (1), transcranial Doppler (TCD) was proposed as a noninvasive, inexpensive, and mobile method of measuring CBF. The rationale was a theoretical direct relationship of flow and flow velocity according to the following equation: Q = V × A, where Q is CBF, V is CBF velocity (CBF-V), and A is the surface of the vascular lumen. Dahl et al. (9) acknowledged that validation of the correlation between TCD and CBF ideally requires their simultaneous assessment. However, for technical reasons, most studies conducted CBF and TCD measurements either at different times in the same patient (2, 9) or evaluated the usefulness of TCD based on different patient populations (18) and found poor correlations between absolute CBF and CBF-V values (2). Factors attributed to such poor correlation included unknown vascular diameter, interobserver variation, and, foremost, dynamic changes in CBF that occur in healthy subjects. With this plethora of inaccuracies, TCD has long been rejected as the “universal” monitoring tool for CBF. However, it has been suggested that in some clearly defined pathological conditions, the regulatory mechanisms of CBF are abolished in a manner that would enable TCD to accurately reflect a decline in CBF (23). In the present study, we focus on chemoregulation as one “subtype” of cerebral vessel tone regulation.
Autoregulation is vasomotor activity in response to changes in perfusion pressure. Thus, dysautoregulation refers to a dysfunction of pressure-dependent vessel tone changes. This does not necessarily imply an impaired chemosensitivity.
The goal of this study was to assess whether TCD values reflect CBF during chemoregulation in two clinical situations particularly relevant to patients in the neurointensive care unit: delayed cerebral vasospasm after subarachnoid hemorrhage (SAH) and after global cerebral ischemia.
MATERIALS AND METHODS
Experimental Groups
CBF and CBF-V were measured simultaneously in 3 groups with a total of 16 cynomolgus monkeys weighing 2.7 to 5.8 kg. The first group served to confirm the validity of the model by showing that chemoregulation is intact in healthy primates (group 1). Then, we tested the working hypothesis of this study to determine whether chemoregulation combined with continuous TCD recordings could reflect CBF during vasospasm (group 2) or ischemia (group 3).
The correlation of CBF and CBF-V was first assessed in healthy primates during continuous changes of partial pressure of CO2 (PCO2) in control animals (chemoregulation control group; n = 9). Groups 2 and 3 were dedicated to the investigation of chemoregulation during cerebral vasospasm (n = 4) and chemoregulation after global cerebral ischemia (n = 3).
The protocol was reviewed by the National Institute of Neurological Disorders and Stroke Animal Care and Use Committee and met the National Institutes of Health guidelines for animal care. The animal data were collected from a single study within a timeframe of approximately 2 years after approval of the National Institutes of Health Animal Care and Use Committee. The study was performed for the purpose of measuring cerebral hemodynamic parameters of primates.
CBF, CBF-V, and Mean Arterial Pressure
A thermal probe developed by Brawley (4) and modified by Carter et al. (6) was used to continuously assess CBF. The probe consists of a cold pad and a warm pad, which are positioned on the cortex. Local blood circulation underneath the 2 thermal probes dissipates the heat, producing a temperature difference. These changes in temperature between the 2 probes are transformed into voltage (“Peltier stack”) and have been shown to reflect CBF (7). To measure CBF, all animals underwent a small right parietal craniectomy under general anesthesia. After opening the dura, a CBF probe (Saber thermomonitoring device; Flow tronics, Phoenix, AZ) was slipped between the dura and brain to lie over a region perfused by the right middle cerebral artery (MCA) as confirmed by cerebral arteriography. The position of the probe was confirmed by lateral skull radiography. After calibration of the instrument, regional CBF was measured continuously.
TCD measurements of flow velocities in the right MCA were obtained through an anterior temporal window at a depth of 25 to 35 mm. A 2-MHz pulsed bidirectional Doppler probe (Transpect TCD; MedaSonics, Mountain View, CA) was used to measure CBF-V in the right MCA by the same observer (RMP or BGT). Systolic CBF-V values were used in the analysis.
The right femoral and left brachial arteries were cannulated for cerebral arteriography and for continuous measurement of mean arterial pressure (MAP). Electrocardiography, end-tidal CO2, and rectal temperature were monitored continuously.
Anesthesia
The monkeys received intramuscular atropine sulfate (0.05 mg/kg), sodium thiopental (25 mg/kg), and ketamine (10 mg/kg). They were intubated and ventilated with N2O:O2 (1:1); 0.5% isoflurane was used as the anesthetic agent. In this study, PCO2 values are assumed to correlate with end-tidal PCO2. The mean difference of arterial to end-tidal PCO2 is variable around 2 to 5 mm Hg (26, 28). It is directly proportional to the ventilatory dead space. The cynomolgus monkeys in the present experiment have a small dead space compared with humans and no respiratory impairment. O2 saturation was kept constant at 100%.
Chemoregulation in Control Animals
PCO2 changes were evoked in 9 control animals. Hypercapnia was induced by 3 episodes of apnea, each lasting 75 seconds, until a maximum PCO2 of 65 mm Hg was achieved. Simultaneous recording of CBF and CBF-V was initiated during a graded stepwise decrease in PCO2 to 25 mm Hg via controlled ventilation, as described elsewhere (40).
Vasospasm after SAH
Four animals underwent right frontotemporal craniectomy with dissection of the arachnoid layer of the sylvian fissure, as described previously (30). In short, after the right MCA was exposed, a clot of arterial blood was placed around the artery. Surgery was performed 7 days before CBF and CBF-V monitoring. These animals underwent arteriography twice, once 2 days before SAH and again on day 7 after surgery. To assess the degree of vasospasm, the proximal 14 mm of the right MCA were measured and compared on pre-and postoperative anteroposterior arteriography using a computerized image analysis system (National Institutes of Health Image J1.25). All 4 animals had moderate vasospasm, defined as a decrease of the anteroposterior MCA area by 38%–50% (31). After measuring CBF and CBF-V in steady state, the chemoregulation protocol described above was carried out.
Transient Global Ischemia Protocol
Global cerebral ischemia was produced in 3 animals by extreme hypotension induced by a continuous infusion of trimetaphan camsylate (500 mg/500 mL D5W; 3 mg/min; Arfonad ; Roche Laboratories, Basel, Switzerland) to lower CBF to the range of 20 mL/l00 g/min for 20 minutes (3). Arfonad infusion was discontinued after 20 minutes of ischemia, and MAP was permitted to return to its normal level (dopamine or phenylephrine was used transiently when necessary). Measurements of CBF and CBF-V were performed 30 minutes after discontinuation of Arfonad using the chemoregulation protocol described above.
Statistical Analysis
Values are presented as mean ± standard deviation. StatView (SAS Institute, Inc., Cary, NC) was used for statistical analysis. Analysis of variance, correlation analysis, and paired t test were used for comparison of the data. Statistical significance was defined as a P value of less than 0.05. A strong correlation was defined as r greater than 0.8 (2). This value was chosen arbitrarily to ensure that more than two-thirds (r2 = 0.64) of the variance of CBF is associated with CBF-V (36).
RESULTS
Chemoregulation and MAP
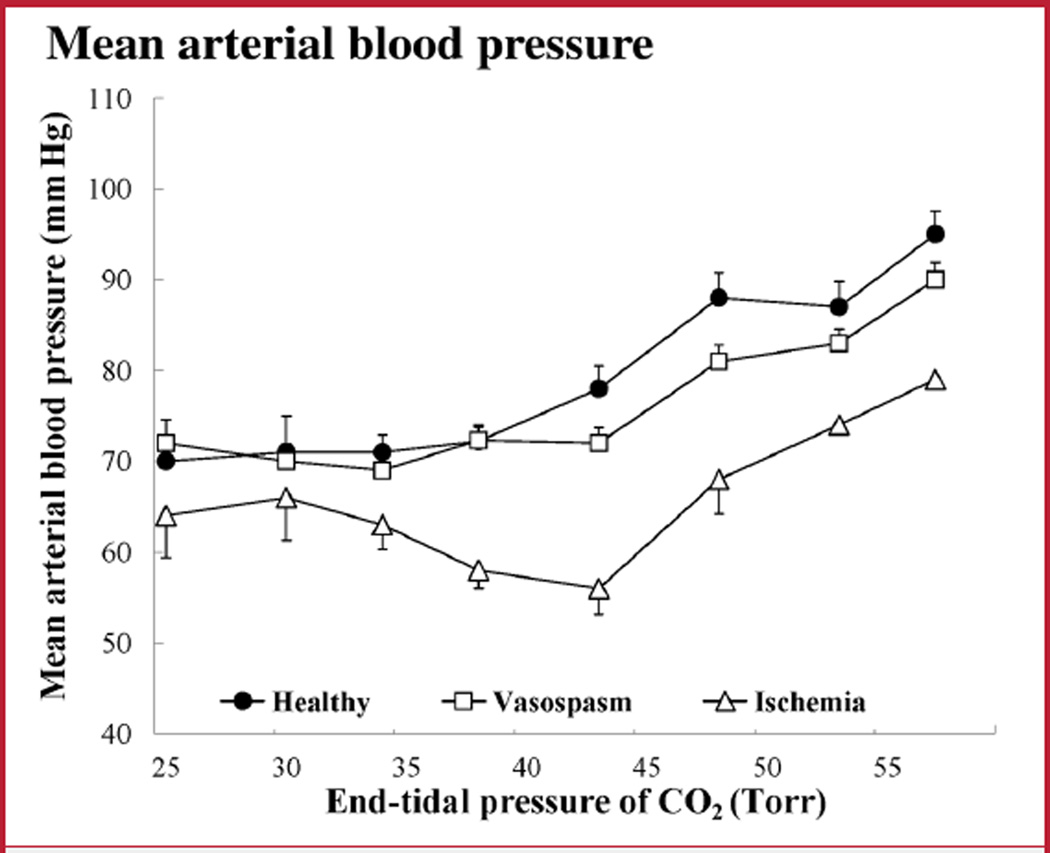
MAP was recorded during changes in PCO2. There was a parallel and similar increase of MAP in response to hypercapnia in all groups (Fig. 1). Pearson’s correlation coefficient for MAP was r equals 0.96 between the control and vasospasm groups and r equals 0.76 between controls and the ischemia group (P < 0.05 for both). MAP remained within the range of autoregulation during PCO2 changes in all groups.
FIGURE 1.
Graph showing mean arterial pressure (MAP) values during changes in partial pressure of carbon dioxide (PCO2). The ischemia group had a significantly lower MAP (P < 0.001). However, MAP remained within the autoregulation range in all groups, and the general pattern of MAP changes attributable to changes in PCO2 was similar. Bars indicate mean ± standard deviation (SD).
Chemoregulation in Control Animals
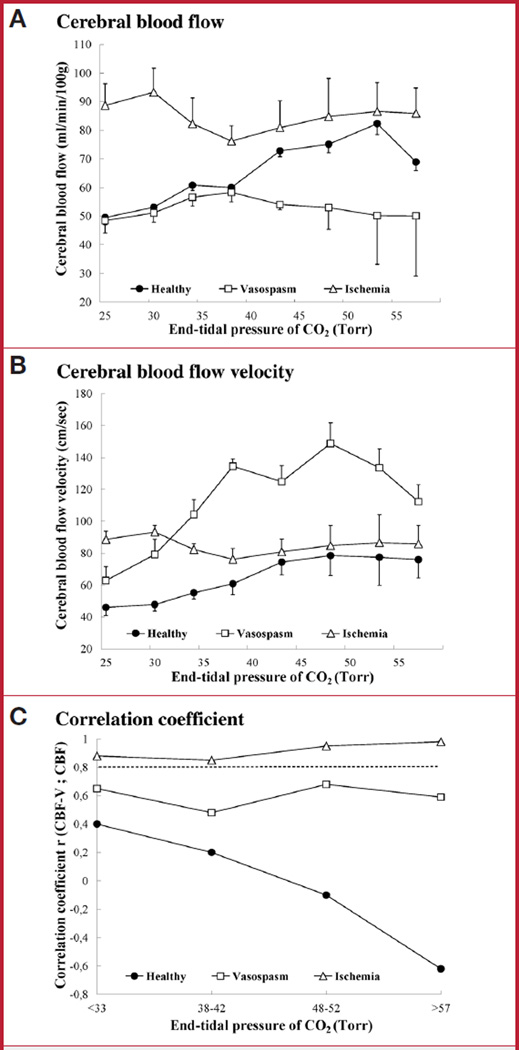
In control animals, the highest CBF value was reached during hypercapnia, and the lowest value occurred during deep hypocapnia (Fig. 2A). CBF-V followed a similar pattern (Fig. 2B). Under normocapnia (PCO2 = 38–42 mm Hg), the correlation between absolute values of CBF and CBF-V was weak (n = 153; r = 0.2; P < 0.05) (Fig. 2C) and did not improve significantly during either hyper-or hypocapnia.
FIGURE 2.
Changes in cerebral blood flow (CBF), CBF velocity (CBF-V), and their correlation during chemoregulation. A, CBF during changes in PCO2 in control animals (n = 9, filled circles), animals with vasospasm (n = 4, open squares), and after ischemia (n = 3, open triangles). B, CBF-V during changes in PCO2. C, correlation between CBF and CBF-V during changes in PCO2. Correlations above 0.8 are considered strong (dashed line). Only postischemic animals maintained a strong correlation of CBF-V and CBF throughout the wide range of PCO2 changes. Number of measurements: control animals, n = 153; vasospasm, n = 106; global ischemia, n = 43. Bars indicate mean ± SD.
Vasospasm after SAH
In 4 monkeys with vasospasm of the right MCA (the mean decrease of the anteroposterior area of the right MCA ranged from 38%–50% [mean, 40%]), baseline CBF was similar to controls (Fig. 2A). During hypocapnia, CBF decreased as in control animals. However, CBF did not change in response to hypercapnia. CBF-V in the physiological range of PCO2 (38–42 mm Hg) was significantly higher than in control animals and exceeded 120 cm/s (P < 0.001) (Fig. 2B). During hypocapnia, it dropped, but it did not change in hypercapnia (P = 0.11). The correlation between CBF and CBF-V during normocapnia was 0.55 (n = 106; P < 0.05) and did not achieve the threshold for strong correlation in either hypo-or hypercapnia (Fig. 2C).
Transient Global Ischemia
In normocapnia, baseline CBF was higher than in control animals (P < 0.05), despite significantly lower MAP (Fig. 2A). Paradoxically, CBF increased when hypocapnia was induced (P < 0.05), instead of decreasing as in control animals. In hypercapnia, CBF remained unchanged and did not differ significantly from controls. CBF-V was higher in postischemic animals than in controls during normocapnia and hypocapnia (Fig. 2B) (P < 0.01), but did not differ from controls during hypercapnia. The correlation between CBF and CBF-V values after ischemia was strong throughout the range of PCO2 changes (n = 43; 0.83 < r < 0.97; P < 0.001) (Fig. 2C).
DISCUSSION
The goal of this study to define the precise circumstances under which assessment of CBF using TCD is possible during CO2 challenge was achieved in animals that underwent global cerebral ischemia.
Conditions for Measurement of CBF Based on CBF-V Values
The following formula describes the relationship between velocity and flow (37):
where CBF is regional CBF measured in mL/l00 g/min, α is the cosine of the insonation angle, K is a constant, A is the cross sectional area of the vessel in cm2, T is the territory of vessel supply in l00 g of brain tissue, and CBF-V represents CBF velocity in cm/s. Therefore, a linear relationship of CBF and CBF-V is expected when the vessel diameter remains constant, the artery’s supply territory remains constant, and the angle of insonation is less than 10 degrees (cosine = 0.985) (18). Under our experimental conditions, the MCA should have a constant diameter because autoregulation and chemoregulation affect the diameters of cerebral arterioles, but not conductive arteries (14). The supply territory is the “amount of brain tissue” supplied by the insonated vessel and is expressed in multiples of 100 g. A change in the supply territory would imply that variations in CBF-V occur through collateral flow. Because variations in CBF-V occur mainly through diameter changes in resistance vessels (14), the territory supplied by the MCA remained stable throughout the experiment. Moreover, Newell et al. (25) demonstrated that the change in MCA diameter is so small during changes in blood pressure that it is unlikely to contribute significantly to cerebral autoregulation. TCD recordings were obtained by the same observer (BGT), and variations in the angle of insonation were minimized by using a stable experimental setup.
Chemoregulation in Control Animals
Chemoregulation is known as the vasomotor reaction of the resistance vessels to changes in PCO2. An increase in PCO2 is a potent stimulus for vasodilation, expressed by an increase in both CBF-V and CBF. As expected, we observed preserved chemoregulation in the control group of our study. CBF-V increased by 3.4% per 1-mm Hg increase in PCO2 in the physiological range of PCO2. This reflects the range of experimental and clinical reports of 2.5% to 5.0% in CBF-V for every 1-mm Hg change in PCO2 (18, 21, 24, 32). CO2 challenge produced the physiological curve of rising CBF as a response to hypercapnia and CBF reached a plateau at maximal cerebral perfusion pressure. At high values of PCO2, CBF did not increase further.
Previous studies found that the percentage changes of CBF-V and CBF correlate (18, 21, 29). However, the correlation between the absolute values remained poor. Our results confirmed that, even under experimental conditions, TCD values do not reflect absolute CBF during chemoregulation testing in control animals.
Vasospasm after SAH
A recent prospective series of TCD measurements in SAH patients revealed that a decline in CO2 vasoreactivity was a highly sensitive indicator of early vasospasm (13). In our study, CBF decreased during hypocapnia but did not rise in hypercapnia, suggesting that the moderate vasospasm in our model partially reduces CO2-mediated vasoreactivity. This phenomenon warrants further study, particularly because an abolished vasodilatory property may play a role in the pathogenesis of cerebral ischemia after SAH (e.g., when the hypercapnic stimulus cannot provoke a metabolically necessary increase in CBF). In contrast to studies based on TCD alone, the described experimental setup allowed for a description of large-vessel behavior as reflected by TCD values and a description of local microcirculation resulting from the use of a cortical CBF probe. During vasospasm, we observed that hypercapnia induced a change in neither TCD nor cortical CBF. Thus, we concluded that neither large nor small vessels in our model respond to hypercapnic stimuli during delayed cerebral vasospasm. Voldby et al. (41) observed that PCO2 reactivity can be preserved in mild vasospasm but will gradually deteriorate with the degree of vasospasm. In a study of patients with mild vasospasm, the authors termed the phenomenon of maintained CO2 reactivity despite abolished autoregulation dissociated vasomotor paralysis (19). Development of cerebral ischemia caused by vasospasm with dissociative vasoparalysis may progress to complete vasoparalysis. In turn, complete vasoparalysis could lead to pressure-passive CBF, which could be reflected by TCD measurement. However, TCD values need to be interpreted with particular care, because a decreased CBF-V may not necessarily indicate resolved vasospasm but could be secondary to reduced CBF. It seems that patients often have impaired autoregulation paralleling their clinical grade (17). Experimental data with simultaneous measurement of CBF and TCD in rabbits suggests a trend toward impaired autoregulation in SAH (24). CBF in these animals fell gradually as MAP dropped below 80 mm Hg. Although pressure-passive CBF indicating vasoparalysis is believed to be one of the few situations where CBF-V is directly proportional to CBF, no strong correlation was found between CBF reactivity and TCD values in the study by Nelson et al. (24). Our findings enhance this concept, showing that even changes in PCO2 cannot positively influence the correlation between CBF and CBF-V after SAH. Supporting findings are reported in a small study of anesthetized patients with SAH (33). Other studies, however, find that many patients with delayed neurological deficits show no increase, and some even show a decrease in CBF-V. Clearly, this undermines the use of TCD velocities alone to predict outcome (20). The effort to achieve both a better early detection of clinical deterioration and of angiographic vasospasm has culminated in the recent development of the vasospasm probability index (15). This index combines clinical (Hunt and Hess grade) and imaging (Fisher grade) findings with CBF-V measurements (CBF-V and Lindegaard ratio) and shows promise as a clinical decision-making tool (15). Although TCD is useful for detecting vasospasm, it is unlikely that a single CBF-V measurement can help to assess the risk of clinical deterioration or even sufficiency of CBF during vasospasm (16, 35).
Transient Global Ischemia
In cerebral ischemic events, such as stroke or temporary cardiac arrest, TCD is used to screen large cerebral arteries for CBF-V changes caused by severe stenosis or occlusion. Although TCD is used to detect microemboli and large artery stenoses and to monitor recanalization (35), its role in assessing the adequacy of CBF after transient cerebral ischemia, such as after resuscitation, is less well defined (39). As cerebral ischemia develops, affected vessels dilate maximally to increase flow. After the onset of ischemia, autoregulatory and chemoregulatory mechanisms are impaired or absent. Decreased CO2 reactivity was observed after a 12-minute transient ischemia episode in a resuscitation model in dogs (8), whereas longer lasting ischemia produced complete vasoparalysis in primates (10). Sundgreen et al. (39) found impaired cerebrovascular reactivity in 13 of 18 patients after cardiac arrest. Decreased CBF-V reflects a state of hypoperfusion, whereas an increase may point toward a postischemic luxury perfusion syndrome, with significance for cerebral vasculature after ischemic insults. Hyperperfusion in areas of previously low perfusion can occur after resection of arteriovenous malformations (38) and carotid endarterectomy (34). In our study, postischemic CBF was elevated despite decreased MAP, suggesting global cerebral vasodilation (i.e., vasoparalysis). When PCO2 was decreased, we observed increased CBF. The correlation between CBF and CBF-V was consistently high, suggesting the absence of active blood flow regulation throughout the range of PCO2 changes. Although the question of CBF and CBF-V correlation was previously addressed in ischemic brains, the comparison was based on autoradiography. In a study comparing TCD and CBF in rabbit ischemia, the correlation was r = 0.94 (12). Unfortunately, the study only compared a single mean value of CBF with an averaged TCD value per animal. Here, we show that TCD measurements after ischemia can reflect CBF quantitatively throughout a wide range of CO2 changes in primates with postischemic vasoparalysis.
Effect of Anesthesia
Because the present study is based on CO2 dependence of blood flow, an agent was needed that preserves cerebrovascular chemoregulation. Isoflurane is well characterized and widely used for studies of CBF (40). It decreases baseline CBF at low concentrations, as well as increasing blood flow and altering metabolic coupling at higher concentrations (5). Compared with other agents, however, isoflurane permits a relatively physiological vascular reactivity to CO2 (42). Simi larly, autoregulation response during low-to medium-level isoflurane anesthesia is preserved and is affected only with alveolar concentrations beyond the concentration used in the present study (27).
Confirming our approach, a pilot experiment with isoflurane anesthesia showed that the range of autoregulation and vasomotoricity are similar to those of healthy humans (see Figure, Supplemental Digital Content 1, http://links.lww.com/A818). In this figure, CBF (squares) and CBF-V (circles) are shown during changes in MAP in the autoregulation control group (n = 8) under isoflurane anesthesia. The lines represent third-order polynomes fitted to the data points. Note the plateau of CBF within the autoregulation range (40–140 Torr of MAP). Only a weak correlation was found between CBF-V and CBF during changes in MAP within the autoregulation range (n = 215; r = 0.51; P < 0.001). However, this correlation increased significantly at MAP values less than 40 and greater than 140 Torr (MAP < 40 Torr: r = 0.81, P < 0.02; MAP > 140 Torr: r = 0.91, P < 0.001). Bars indicate means ± standard deviation.
As for propofol sedation, there is indirect TCD-based evidence that autoregulation is partially preserved (22). However, propofol can reduce CBF-V by approximately one-third, which in itself may have represented an obstacle to our experimental paradigm (11).
Clinical Value of the Presented Findings
Our ischemia model produced global cerebral ischemia most closely resembling syndromes with generalized cerebral hypoperfusion. By demonstrating a strong correlation of TCD with CBF in ischemia, our findings suggest the possibility of continuous or serial TCD to guide blood pressure management in these patients (i.e., triple-H therapy) to maintain optimal cerebral perfusion in an intensive care setting. Using TCD probes attached to a head frame may facilitate continuous and reliable measurement over an extended period. Furthermore, after global ischemia, a patient has attenuated vasomotor responses, which will lead to an MAP-passive flow pattern on TCD reflecting CBF. MAP and CBF management could be performed, depending on mean flow velocities, until signs of vasomotor recovery occur (e.g., improved CO2 response, autoregulation index).
Experimental Considerations
Primates in the present study underwent neuromonitoring 30 minutes after global cerebral ischemia. During this timeframe, it appears that neuromonitoring using TCD reflects CBF. Because of animal care and use limitations, it was not possible to record measurements at multiple time points, and data from clinical studies are needed to test the validity of this monitoring paradigm in the days after ischemia.
CONCLUSION
Continuous TCD recordings during changes in PCO2 cannot provide an estimate of CBF when cerebrovascular regulatory mechanisms are present, such as in healthy primates. Chemoregulation appeared to be partly preserved in our primate model of vasospasm, comparable to the dissociative vasoparalysis described in humans. In consequence, correlations between continuous TCD measurements and CBF were poor, even during modifications in PCO2.
In cerebral ischemia, in which vessel reactivity is known to be abolished, modification of PCO2 during chemoregulation produced a strong correlation of TCD with CBF. Thus, the present study provides experimental support that, in clearly defined conditions, continuous TCD monitoring combined with chemoregulation testing may provide an estimate of CBF in the early postischemic period. An important limitation of this study was that we were unable to perform recordings in the days after ischemia. This limitation arose as a result of ethical issues, because the global ischemic insult in our model is likely to leave the primates moribund. We believe that clinical studies are now needed to decide whether TCD is useful and beneficial for follow-up neuromonitoring after ischemic insult.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. Bawarjan Schatlo, M.D., was supported by a scholarship of the Boehringer Ingelheim Foundation and the International Academy of Life Sciences. Sven Gläsker, M.D., was supported by a grant from the German Research Society.
ABBREVIATIONS
- CBF
cerebral blood flow
- CBF-V
cerebral blood flow velocity
- MAP
mean arterial pressure
- MCA
middle cerebral artery
- Pco2
partial pressure of CO2
- SAH
subarachnoid hemorrhage
- TCD
transcranial Doppler
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.neurosurgery-online.com).
Disclosures
The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Bawarjan Schatlo, Surgical Neurology Branch, National Institutes of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland Department of Experimental Neurology, Charité University Medicine, Berlin, Germany; Department of Neurosurgery, University Hospital Geneva, Geneva, Switzerland.
Sven Gläsker, Surgical Neurology Branch, National Institutes of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland
Alois Zauner, Department of Neurosurgery, Santa Barbara Cottage Hospital, Santa Barbara, California
B. Gregory Thompson, Department of Neurosurgery, University of Michigan, Ann Arbor, Michigan
Edward H. Oldfield, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia
Ryszard M. Pluta, Surgical Neurology Branch, National Institutes of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland
REFERENCES
- 1.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 2.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: A validation study. Stroke. 1986;17:913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 3.Boysen G, Engell HC, Pistolese GR, Fiorani P, Agnoli A, Lassen NA. Editorial: On the critical lower level of cerebral blood flow in man with particular reference to carotid surgery. Circulation. 1974;49:1023–1025. doi: 10.1161/01.cir.49.6.1023. [DOI] [PubMed] [Google Scholar]
- 4.Brawley BW. Construction of a regional cerebral blood flow probe using a Peltier device. J Surg Res. 1969;9:395–398. doi: 10.1016/0022-4804(69)90110-3. [DOI] [PubMed] [Google Scholar]
- 5.Brüssel T, Fitch W, Brodner G, Arendt I, Van Aken H. Effects of halothane in low concentrations on cerebral blood flow, cerebral metabolism, and cerebrovascular autoregulation in the baboon. Anesth Analg. 1991;73:758–764. [PubMed] [Google Scholar]
- 6.Carter LP, Erspamer R, Bro WJ. Cortical blood flow: Thermal diffusion vs isotope clearance. Stroke. 1981;12:513–518. doi: 10.1161/01.str.12.4.513. [DOI] [PubMed] [Google Scholar]
- 7.Carter LP, Weinand ME, Oommen KJ. Cerebral blood flow (CBF) monitoring in intensive care by thermal diffusion. Acta Neurochir Suppl (Wien) 1993;59:43–46. doi: 10.1007/978-3-7091-9302-0_7. [DOI] [PubMed] [Google Scholar]
- 8.Christopherson TJ, Milde JH, Michenfelder JD. Cerebral vascular autoregulation and CO2 reactivity following onset of the delayed postischemic hypoperfusion state in dogs. J Cereb Blood Flow Metab. 1993;13:260–268. doi: 10.1038/jcbfm.1993.32. [DOI] [PubMed] [Google Scholar]
- 9.Dahl A, Lindegaard KF, Russell D, Nyberg-Hansen R, Rootwelt K, Sorteberg W, Nornes H. A comparison of transcranial Doppler and cerebral blood flow studies to assess cerebral vasoreactivity. Stroke. 1992;23:15–19. doi: 10.1161/01.str.23.1.15. [DOI] [PubMed] [Google Scholar]
- 10.Dettmers C, Young A, Rommel T, Hartmann A, Weingart O, Baron JC. CO2 reactivity in the ischaemic core, penumbra, and normal tissue 6 hours after acute MCA-occlusion in primates. Acta Neurochir (Wien) 1993;125:150–155. doi: 10.1007/BF01401843. [DOI] [PubMed] [Google Scholar]
- 11.Ederberg S, Westerlind A, Houltz E, Svensson SE, Elam M, Ricksten SE. The effects of propofol on cerebral blood flow velocity and cerebral oxygen extraction during cardiopulmonary bypass. Anesth Analg. 1998;86:1201–1206. doi: 10.1097/00000539-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Els T, Daffertshofer M, Schroeck H, Kuschinsky W, Hennerici M. Comparison of transcranial Doppler flow velocity and cerebral blood flow during focal ischemia in rabbits. Ultrasound Med Biol. 1999;25:933–938. doi: 10.1016/s0301-5629(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 13.Frontera JA, Rundek T, Schmidt JM, Claassen J, Parra A, Wartenberg KE, Temes RE, Mayer SA, Mohr JP, Marshall RS. Cerebrovascular reactivity and vasospasm after subarachnoid hemorrhage: A pilot study. Neurology. 2006;66:727–729. doi: 10.1212/01.wnl.0000200777.96896.3d. [DOI] [PubMed] [Google Scholar]
- 14.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–742. [PubMed] [Google Scholar]
- 15.Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: A combination of transcranial Doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:1101–1112. doi: 10.3171/JNS-07/12/1101. [DOI] [PubMed] [Google Scholar]
- 16.Grosset DG, Straiton J, McDonald I, Cockburn M, Bullock R. Use of transcranial Doppler sonography to predict development of a delayed ischemic deficit after subarachnoid hemorrhage. J Neurosurg. 1993;78:183–187. doi: 10.3171/jns.1993.78.2.0183. [DOI] [PubMed] [Google Scholar]
- 17.Hassler W, Chioffi F. CO2 reactivity of cerebral vasospasm after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1989;98:167–175. doi: 10.1007/BF01407344. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham FJ, Padayachee TS, Parsons S, Seargeant LS, House FR, Gosling RG. Transcranial measurement of blood velocities in the basal cerebral arteries using pulsed Doppler ultrasound: Velocity as an index of flow. Ultrasound Med Biol. 1986;12:15–21. doi: 10.1016/0301-5629(86)90139-0. [DOI] [PubMed] [Google Scholar]
- 19.Larsen FS, Adel Hansen B, Pott F, Ejlersen E, Secher NH, Paulson OB, Knudsen GM. Dissociated cerebral vasoparalysis in acute liver failure. A hypothesis of gradual cerebral hyperaemia. J Hepatol. 1996;25:145–151. doi: 10.1016/s0168-8278(96)80066-3. [DOI] [PubMed] [Google Scholar]
- 20.Laumer R, Steinmeier R, Gönner F, Vogtmann T, Priem R, Fahlbusch R. Cerebral hemodynamics in subarachnoid hemorrhage evaluated by transcranial Doppler sonography: Part 1—Reliability of flow velocities in clinical management. Neurosurgery. 1993;33:1–9. doi: 10.1227/00006123-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Markwalder TM, Grolimund P, Seiler RW, Roth F, Aaslid R. Dependency of blood flow velocity in the middle cerebral artery on end-tidal carbon dioxide partial pressure: A transcranial ultrasound Doppler study. J Cereb Blood Flow Metab. 1984;4:368–372. doi: 10.1038/jcbfm.1984.54. [DOI] [PubMed] [Google Scholar]
- 22.Matta BF, Lam AM, Strebel S, Mayberg TS. Cerebral pressure autoregulation and carbon dioxide reactivity during propofol-induced EEG suppression. Br J Anaesth. 1995;74:159–163. doi: 10.1093/bja/74.2.159. [DOI] [PubMed] [Google Scholar]
- 23.Moppett IK, Mahajan RP. Transcranial Doppler ultrasonography in anaesthesia and intensive care. Br J Anaesth. 2004;93:710–724. doi: 10.1093/bja/aeh205. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RJ, Perry S, Hames TK, Pickard JD. Transcranial Doppler ultrasound studies of cerebral autoregulation and subarachnoid hemorrhage in the rabbit. J Neurosurg. 1990;73:601–610. doi: 10.3171/jns.1990.73.4.0601. [DOI] [PubMed] [Google Scholar]
- 25.Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- 26.Nunn JF, Hill DW. Respiratory dead space and arterial to end-tidal carbon dioxide tension difference in anesthetized man. J Appl Physiol. 1960;15:383–389. doi: 10.1152/jappl.1960.15.3.383. [DOI] [PubMed] [Google Scholar]
- 27.Olsen KS, Henriksen L, Owen-Falkenberg A, Dige-Petersen H, Rosenørn J, Chraemmer-Jørgensen B. Effect of 1 or 2 MAC isoflurane with or without ketanserin on cerebral blood flow autoregulation in man. Br J Anaesth. 1994;72:66–71. doi: 10.1093/bja/72.1.66. [DOI] [PubMed] [Google Scholar]
- 28.Phan CQ, Tremper KK, Lee SE, Barker SJ. Noninvasive monitoring of carbon dioxide: A comparison of the partial pressure of transcutaneous and end-tidal carbon dioxide with the partial pressure of arterial carbon dioxide. J Clin Monit. 1987;3:149–154. doi: 10.1007/BF01695936. [DOI] [PubMed] [Google Scholar]
- 29.Piepgras A, Schmiedek P, Leinsinger G, Haberl RL, Kirsch CM, Einhäupl KM. A simple test to assess cerebrovascular reserve capacity using transcranial Doppler sonography and acetazolamide. Stroke. 1990;21:1306–1311. doi: 10.1161/01.str.21.9.1306. [DOI] [PubMed] [Google Scholar]
- 30.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 31.Pluta RM, Zauner A, Morgan JK, Muraszko KM, Oldfield EH. Is vasospasm related to proliferative arteriopathy? J Neurosurg. 1992;77:740–748. doi: 10.3171/jns.1992.77.5.0740. [DOI] [PubMed] [Google Scholar]
- 32.Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke. 1988;19:963–969. doi: 10.1161/01.str.19.8.963. [DOI] [PubMed] [Google Scholar]
- 33.Romner B, Brandt L, Berntman L, Algotsson L, Ljunggren B, Messeter K. Simultaneous transcranial Doppler sonography and cerebral blood flow measurements of cerebrovascular CO2-reactivity in patients with aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 1991;5:31–37. doi: 10.3109/02688699108998444. [DOI] [PubMed] [Google Scholar]
- 34.Schaafsma A, Veen L, Vos JP. Three cases of hyperperfusion syndrome identified by daily transcranial Doppler investigation after carotid surgery. Eur J Vasc Endovasc Surg. 2002;23:17–22. doi: 10.1053/ejvs.2001.1545. [DOI] [PubMed] [Google Scholar]
- 35.Schatlo B, Pluta RM. Clinical applications of transcranial Doppler sonography. Rev Recent Clin Trials. 2007;2:49–57. doi: 10.2174/157488707779318125. [DOI] [PubMed] [Google Scholar]
- 36.Snedecor GW, Cochran WG. Statistical Methods. Ames: Iowa State University Press; 1986. pp. 183–190. [Google Scholar]
- 37.Sorteberg W. Cerebral Artery Blood Velocity and Cerebral Blood Flow. In: Newell DW, Aaslid R, editors. Transcranial Doppler. New York: Raven Press; 1992. pp. 57–66. [Google Scholar]
- 38.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 39.Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 40.Thompson BG, Pluta RM, Girton ME, Oldfield EH. Nitric oxide mediation of chemoregulation but not autoregulation of cerebral blood flow in primates. J Neurosurg. 1996;84:71–78. doi: 10.3171/jns.1996.84.1.0071. [DOI] [PubMed] [Google Scholar]
- 41.Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg. 1985;62:59–67. doi: 10.3171/jns.1985.62.1.0059. [DOI] [PubMed] [Google Scholar]
- 42.Werner C, Standl T, Thiel H, Kochs E, Schulte am Esch J. Propofol-alfentanil reduced cerebrovascular CO2 reactivity in comparison with isoflurane [in German] Anaesthesist. 1995;44:417–422. doi: 10.1007/s001010050170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.