Abstract
Object
Tumor-initiating cells are uniquely resilient to current treatment modalities and play an important role in tumor resistance and recurrence. The lack of specific tumor-initiating cell markers to identify and target these cells presents a major obstacle to effective directed therapy.
Methods
To identify tumor-initiating cell markers in primary brain tumors, the authors compared the proteomes of glioma tumor-initiating cells to their differentiated progeny using a novel, nongel/shotgun-based, multidimensional liquid-chromatography protein separation technique. An in vivo xenograft model was used to demonstrate the tumorigenic and stem cell properties of these cells. Western blot and immunofluorescence analyses were used to confirm findings of upregulated ciliary neurotrophic factor receptor subunit–α (CNTFRα) in undifferentiated tumor-initiating cells and gliomas of increasing tumor grade. Sequencing of the CNTFRα coding regions was performed for mutation analysis. Finally, antibody-dependent cell-mediated cytotoxicity was used to establish the role of CNTFRα as a potential immunotherapeutic target.
Results
Ciliary neurotrophic factor receptor subunit–α expression was increased in tumor-initiating cells and was decreased in the cells' differentiated progeny, and expression levels increased with glioma grade. Mutations of CNTFRα are not common in gliomas. Functional studies using CNTF treatment in glioma tumor-initiating cells showed induction of differentiation through the CNTFRα pathway. Treatment with anti-CNTFRα antibody resulted in increased antibody-dependent cell-mediated cytotoxicity in CNTFRα expressing DAOY cells but not in cell lines that lack CNTFRα.
Conclusions
These data indicate that CNTFRα plays a role in the formation or maintenance of tumor-initiating cells in gliomas, is a marker that correlates with histological grade, may underlie treatment resistance in some cases, and is a potential therapeutic target.
Keywords: ciliary neurotrophic factor receptor subunit–α, glioma, tumor-initiating cell, oncology, mouse
OVER the last decade, cells shown to initiate tumors have been identified and have been speculated to be cancer stem cells.18,39 These tumor-initiating cells are thought to share the same genetic changes of the tumors they populate while retaining stem cell characteristics.18,35 Controversy exists within the literature regarding the most accurate definition of these tumor-initiating cells. Several stem cell-like properties of this cell population have been identified, which include tumorigenesis2,18,35 and expression of the markers Nestin, Sox2,22 and cytoplasmic nCoR.32 This cell's infiltrative nature and ability to invade brain parenchyma is thought to reflect the underlying malignant nature of tumors1 and the progression of disease. Tumor-initiating cells were first discovered in leukemia but have since been shown to be present in solid tumors including glioma.6,10,16,17,39 In vivo glioma studies have demonstrated that these cells are uniquely resilient to current treatment regimens, contributing to both cancer resistance and recurrence.4 To achieve remission or cure in resistant tumors, including primary brain tumors, it will be necessary to eradicate this tumor-initiating cell population, as well as their differentiated progeny. However, due to the lack of specific markers, in vitro identification of these cells has presented a major challenge to their targeting and achieving cure. To identify new biomarkers and potential therapeutic targets we sought to find unique proteins from glioma-derived tumor-initiating cells.
To identify novel proteins specific to tumor-initiating cells, we compared the proteomes of glioma tumor-initiating cells with their differentiated progeny. We developed a concurrent population of tumor-initiating cells and their specific progeny for analysis following incubation of isolated glioma tumor-initiating cells with RA.32 Proteomic analysis of the glioma tumor-initiating cells and their progeny was performed using a newly developed nongel/shotgun-based multidimensional liquid-chromatography protein separation, followed by MS/MS sequencing (CIEF-nRPLC-MS/MS).43 Compared with conventional 2D polyacrylamide gel electrophoresis (2D PAGE), this multidimensional liquid-chromatography protein separation and MS/MS sequencing approach allows for a 15-fold increase in sensitivity in identifying proteins.43 Using this technique, we were able to isolate proteins in undifferentiated tumor-initiating cells with a difference in relative expression compared with their differentiated progeny. These proteins included CNTFRα.
Ciliary neurotrophic factor is a member of the neuropoietic cytokine family and is structurally and functionally homologous to interleukin-6.7,8,23 Ciliary neurotrophic factor binds to CNTFRα and forms a complex with glycoprotein 130 and leukemia inhibitory factor receptor β to act on the downstream Jak/Stat signaling pathway.40 This pathway has been implicated in mechanisms of differentiation, proliferation, migration, and apoptosis in several cell lines34 and has a prosurvival effect in neuronal populations.14,28 Ciliary neurotrophic factor has also been shown to induce astrocytic differentiation in neural stem cells and may suppress the growth of glioma cell lines in vitro.11,12,15,22,31,32 The CNTF receptor is expressed in neuronal cell populations and has been demonstrated in mature astrocytes after mechanical injury.13 Moreover, CNTFRα mRNA has been detected in human gliomas,44 as well as in developing embryonic rat and mouse brains.13,25 Taken together, these prior studies indicate a potential role for CNTFRα in tumor-initiating cells, glioma pathogenesis, and as a therapeutically targetable membrane protein.
Methods
Glioblastoma Tumor-Initiating Cell Isolation and Differentiation
Tumor-initiating cells were isolated and treated as previously described.32 Briefly, a freshly resected glioblastoma specimen was procured and confirmed as glioblastoma by histopathological analysis. Cells from this tumor were dissociated by enzymatic digestion and automated mechanical disaggregation (MediMachine, Becton Dickinson). Cells were grown on poly-d-lysine/laminin coated dishes in DMEM/F12 medium (Invitrogen) with N-2 supplement (0.5 Invitrogen), B-27 supplement without vitamin A (0.5×, Invitrogen), basic fibroblast growth factor (20 ng/ml, Invitrogen), and epidermal growth factor (20 ng/ml, R&D Systems). Media were changed every other day, and growth factors were added at 24-hour intervals. Nestin- and Sox2-expressing cells were selected and amplified. The glioblastoma origin of amplified cells was confirmed through karyotype analysis as described previously.32 Tumor-initiating cell differentiation was carried out in the aforementioned medium modified with the addition of RA (1 μM) and the removal of growth factors.
In Vivo Analysis of Tumor-Initiating Cell Properties
To confirm the tumorigenic characteristics of the tumor-initiating cell culture, cells cultured in the aforementioned media were resuspended in 5 μl PBS (40,000 cells/μl) and were stereotactically injected into 10 nude athymic mice. The mice were euthanized by carbon dioxide asphyxiation and cervical dislocation at the onset of neurological symptoms (5 weeks). The brain was embedded in paraffin and cut at 5-μm sections. These sections were stained using H & E, and immunofluorescence was performed as described below. Fluorescence in situ hybridization, using probes directed against the human X chromosome (CEP X Spectrum Orange, Abbott Laboratories) or mouse X (Empire Genomics), was performed on the paraffin-embedded tissue sections using the manufacturer's protocol to confirm the human origin of infiltrating cells within the mouse brain. The mice were used in this study with the approval from the National Institute of Neurological Disorders and Stroke Office of Animal Care and Use.
Proteomic Profile Generation and Comparison
Samples of tumor-initiating cells before and after RA treatment were isolated for proteomic profiling. Tissues were analyzed using an online combination of CIEF with nRPLC followed by MS/MS, developed by Calibrant Biosystems, as previously described.43 This method separates peptides based on differences in isoelectric point and hydrophobicity. Briefly, prepared cells were digested with trypsin and filled into a CIEF capillary with ampholytes. The focused peptides were sampled into a total of 12 unique fractions. These fractions were analyzed in sequence, and the elutants from nRPLC were monitored by a quadrupole time-of-flight micro mass spectrometer (Waters). Peptide and protein identifications were performed using MASCOT 2.0 (Matrix Science) utilizing a reverse database search approach to determine a false-positive rate. Comparison of proteomic profiles between control tumor-initiating cells and their differentiated progeny was performed using Excel software (Microsoft Corp.).
Clinical Materials
Tissues were collected from the Surgical Neurology Branch at the National Institute of Neurological Disorders and Stroke and from the Cleveland Clinic Foundation. All tissues and clinical information were obtained as part of an institutional review board–approved study at the respective institutions.
Western Blot Analysis
Western blot analysis was performed as previously described.32 Briefly, frozen tissues were dissected and dissociated in T-PER lysis solution (Thermo Scientific). Thirty micrograms of total protein was resolved on Nu-PAGE Novex Bis-Tris Gel (Invitrogen). Polyclonal CNTFRα antibody (Santa Cruz Biotechnology) was applied to Western blotting. Anti–β-actin monoclonal antibody (Sigma-Aldrich, Inc.) was used as an internal loading control.
Immunofluorescence Analysis
For double immunofluorescence staining, tumor sections or the tumor-initiating cell monolayer and floating spheres were fixed in Histochoice (AMRESCO) with 0.1% Triton X-100 for 12 minutes. The cells were washed in PBS and blocked in 5% normal serum matching the host of the secondary antibody and were incubated with primary antibodies overnight at 4°C. Spheres, sections, or monolayer cells were incubated in a rhodamine TRITC-conjugated secondary antibody (Jackson ImmunoResearch) for 1 hour at 4°C. The second primary antibody was applied overnight at 4°C after incubation with normal serum matching the host of that secondary antibody. Sections were incubated in a fluorescein isothiocyanate–conjugated secondary antibody (Jackson ImmunoResearch). The cells were treated with Hoechst 33342 (Invitrogen) at room temperature for nuclear staining. After washing in PBS, the sections were mounted with Vectashield mounting medium (Vector Laboratories), and coverslips were applied.
CNTFRα Sequencing
Genomic DNA was extracted from human glioblastoma tissue using a Wizard Genomic DNA Purification Kit (Promega Corp.). The entire coding sequence of the CNTFRα gene was amplified through polymerase chain reaction and was directly sequenced by DyeTerminator Sequencing Protocol (Perkin-Elmer) on an automated sequencer (ABI 373A). Sequence analysis and mutation identification were performed using GCG software package (GCG Lite, National Institutes of Health).
Antibody-Dependent Cell-Mediated Cytotoxicity
DAOY, U87, or HeLa cell lines were placed into culture and incubated with either IgG or anti-CNTFRα antibody (Santa Cruz) for 30 minutes. Following antibody incubation, the cells were washed with PBS and were incubated for 6 or 24 hours with complement serum (Calbiochem). Cell death was visualized using trypan blue staining.
Results
Stem Cell and Tumorigenic Characteristics of Tumor-Initiating Cells
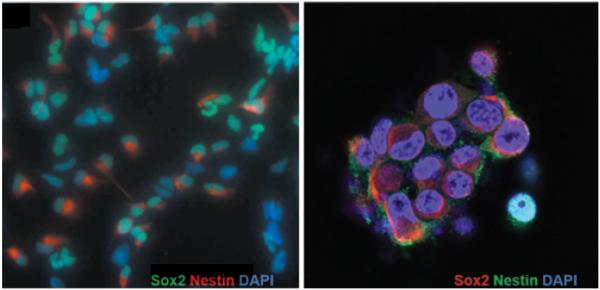
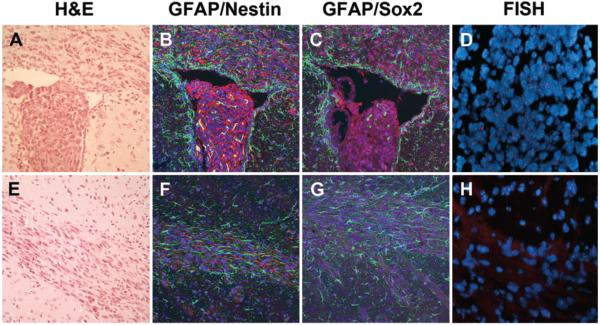
Tumor-initiating cells were established from freshly resected human glioblastoma tissue. Cultured cells were maintained in an undifferentiated state as confirmed by the neurosphere phenotype in culture (Fig. 1 left), as well as by immunostaining for the neural stem cell markers Nestin and Sox2 (Fig. 1). Tumorigenic characteristics of these cells were confirmed using a xenograft mouse model (Fig. 2). Intracranial implantation of undifferentiated tumor-initiating cells demonstrated diffuse infiltration into surrounding parenchyma, a key feature of human glioblastoma.26 These cells were identified within white matter tracts such as the corpus callosum and anterior commissure, and several nests of tumor-initiating cells were identified in the parenchyma distant from the injection site. Interestingly, tumor cells growing within the ventricle exhibited a neurosphere morphology and stained positively for Sox2 and Nestin but not GFAP. Tumor-initiating cells located near the ventricular border had a radial morphology and expressed higher levels of GFAP, while stem cell marker expression was weaker. Within the corpus callosum, migrating tumor cells stained positively for GFAP and showed decreased Nestin and Sox2 expression. Fluorescence in situ hybridization analysis showed human X-chromosome staining in Nestin/Sox2-positive cells, confirming the human origin of the diffusely infiltrating tumor-initiating cells (Fig. 2D and H).
Fig. 1.
Stem cell phenotype marker expression in glioblastoma-derived tumor-initiating cells. Left: Low-magnification immunofluorescence staining for Sox2 (green) and Nestin (red) in cultured tumor-initiating cells. Right: High-magnification immunofluorescence staining for Sox2 (red) and Nestin (green) showing the neurosphere morphology of glioblastoma-derived tumor-initiating cells in culture. Nuclei of cells are counterstained with DAPI (blue). Original magnification ×5 (left) and ×40 (right).
Fig. 2.
Xenograft tumor-initiating cells showing an infiltrative phenotype and stem cell markers in mouse brain parenchyma. A–D: Intraventricular injection of tumor-initiating cells. H & E staining of a brain section showing that the intraventricular cells maintain a neurosphere morphology. A portion of cells demonstrate a radial-glial morphology at the ependymal surface, suggesting invasion (A). Immunofluorescence staining for GFAP (green) and Nestin (red) showing Nestin-positive staining within the intraventricular tumor-initiating component as well as cells within the corpus callosum. The intraventricular cell component is negative for GFAP while cells along the ependymal surface and within the corpus callosum stain intensely for GFAP (B). Immunofluorescence staining for Sox-2 (purple) and GFAP (green) showing a similar pattern (C). Fluorescence in situ hybridization (FISH) analysis of the intraventricular cell mass confirming the human origin of the tumor-initiating cells (red staining for human X chromosome) (D). E–H: Tumor cells within the corpus callosum. H & E staining of a brain section showing high cellularity within the corpus callosum, away from the site of injection, suggesting the presence of tumor cells (E). Immunofluorescence staining for GFAP (green) and Nestin (red) showing the presence of Nestin- and GFAP-positive tumor cells within the corpus callosum (F). Immunofluorescence staining for Sox-2 (purple) and Nestin (green) showing a similar pattern (G). Fluorescence in situ hybridization analysis of the tumor cells confirming their human origin (red staining for human X chromosome) (H). Original magnification ×10 (A–C), ×20 (D), ×10 (E–G) and ×20 (H).
CIEF-nRPLC-MS/MS Identifies Proteome Differences in Undifferentiated and Differentiated Tumor-Initiating Cells
As previously described, differentiation of tumor-initiating cells was induced by treatment with RA and confirmed by loss of Nestin and Sox2 expression.32,45 Furthermore, differentiated astrocyte phenotypes were confirmed with increased GFAP expression and nuclear translocation of N-CoR.32 Both undifferentiated tumor-initiating cells and their RA-treated differentiated progeny were analyzed using CIEF-nRPLC-MS/MS. Peptide identification was based on 3 runs of a single tissue sample and was limited by high-mass-accuracy (60 ppm) and high-confidence (5% false-positive) hits to fully tryptic proteins. This allowed for detection of 19,904 peptides, leading to the identification of more than 3700 distinct proteins. Expression differences between the undifferentiated and the differentiated tumor-initiating cell proteomes yielded approximately 175 proteins with a difference in relative expression.
CNTFRα Expression Is Upregulated in Human Glioma Tumor-Initiating Cells and Correlates With Glioma Tumor Grade
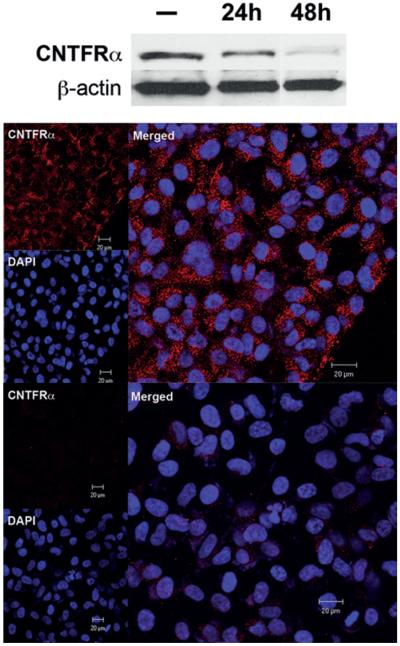
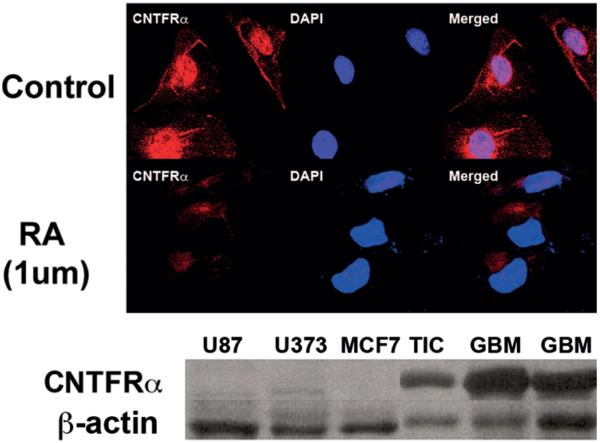
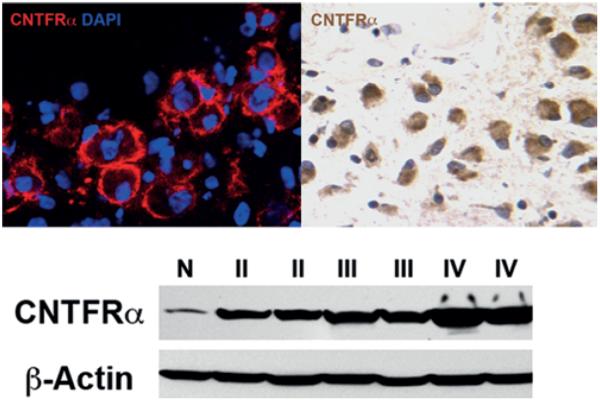
Expression of CNTFRα by CIEF-nRPLC-MS/MS was minimally identified in differentiated tumor-initiating cells but was 3.7 times greater in their undifferentiated counterparts. This was confirmed by both Western blot (Fig. 3 upper) and immunostaining (Fig. 3 lower), which showed decreased CNTFRα expression in RA-treated (1 μM), differentiated tumor-initiating cells. Using Western blot, immunohistochemical, and immunofluorescence analyses, CNTFRα expression was also seen to be evaluated in cancer cell lines including the U87 glioma cell line, the U373 glioma cell line, HeLa cervical cancer cells, MCF-7 breast cancer cells, and the DAOY medulloblastoma cell line. CNTFRα was expressed by DAOY cells (Fig. 4 upper) but not in U87, U373, or MCF-7 cell lines (Fig. 4 lower). Furthermore, CNTFRα in DAOY cells was lost after RA treatment, indicating the potential existence of a tumor-initiating cell component in these cells (Fig. 4 upper). CNTFRα expression was then tested in 35 human primary brain tumors, including 7 low-grade astrocytomas (Grade II), 10 anaplastic astrocytomas (Grade III), and 18 glioblastomas (Grade IV). CNTFRα protein was present in all 35 gliomas. CNTFRα expression in malignant glioma tissue (Fig. 5 upper) was associated with increasing glioma pathological grade (Fig. 5 lower).
Fig. 3.
Ciliary neurotrophic factor receptor subunit–α is selectively expressed in undifferentiated tumor-initiating cells. Upper: Western blot analysis showing decreased CNTRFα (molecular weight 27 kD) expression in glioma-derived tumor-initiating cells with 1 μM RA treatment over 24 and 48 hours. The CNTRFα expression decreased with RA treatment over time. The β-actin was used as internal positive quantitative controls. Lower: Immunofluorescence staining for CNTRFα in 1-μM RA-treated tumor-initiating cells showing a marked decrease in CNTFRα expression. Cell nuclei are counterstained with DAPI, showing the cytoplasmic location of CNTRFα.
Fig. 4.
Ciliary neurotrophic factor receptor subunit–α is selectively expressed in glioblastoma and DAOY cells. Upper: Immunofluorescence staining for CNTFRα in control and 1 μM RA-treated DAOY cells, a medulloblastoma cell line. The DAOY cells incubated in control media show robust staining of CNTFRα, which is down-regulated with RA treatment. Nuclei are counterstained with DAPI. Lower: Western blot for CNTFRα from glioma cell lines U87 and U373, breast cancer cell line MCF7, cultured tumor-initiating cells, and patient glioblastoma tissue. Lanes 4–6 show CNTFRα expression in glioblastoma tissue and glioma-derived tumor-initiating cells (TIC). Lanes 1–3 show lack of CNTFRα expression in the other cell lines.
Fig. 5.
Ciliary neurotrophic factor receptor subunit–α expression is confirmed in a patient's glioblastoma tissue and increases with glioma grade. Upper: Immunofluorescence staining (left) and immunohisto-chemical analysis (right) for CNTFRα from a patient's glioblastoma tissue. Lower: Western blot for CNTFRα from normal brain tissue (N) and whole lysate from Grade II, Grade III and Grade IV glioma tissue. Ciliary neurotrophic factor receptor subunit–α expression increases with glioma tumor grade. Original magnification ×40.
CNTFRα Is Infrequently Mutated in Human Glioblastomas and Remains Functional in Cultured Tumor-Initiating Cells
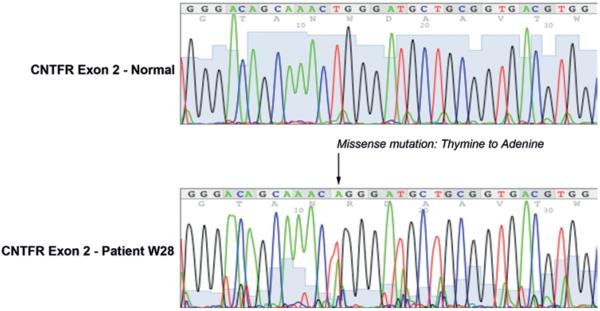
The CNTF pathway's role in astroglial differentiation led us to consider CNTFRα genetic mutations as a potential explanation of aberrant expression in less differentiated highly malignant tumors of the CNS. To further elucidate the role that CNTF plays in the differentiation of malignant gliomas, we genetically sequenced the malignant gliomas from the study patients. Using primers for all 7 exons (Table 1), we tested the CNTFRα gene in 32 human malignant glioma samples by sequencing the entire coding region. Thirty-one tumors (97% of tumors analyzed) did not harbor a genetic defect in the CNTFRα coding sequence. Only 1 tumor (3%) contained a genetic missense mutation (T151A) in exon 2 (Fig. 6).
TABLE 1.
Primer sets used for CNTFRα exon sequencing
| Exon | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| 1 | 5′-CAGTCTTGCCTGAGAACAG(G)-3′ | 5′-GAGTCAGGATGCAGCTATG(C)-3′ |
| 2 | 5′-CTGTGACCTGTGACTTTTG(C)-3′ | 5′-GTCTGGGTCTCAAGGAAG-3′ |
| 3a | 5′-GCTCCAAGAATGCCCTGAC-3′ | 5′-GTATGGACAGAGGGCATGG-3′ |
| 3b | 5′-(G)CTCCAAGAATGCCCTGAC-3′ | 5′-GCACTGTTACGAACATCAAG-3′ |
| 4 | 5′-CTTGATGTTCGTAACAGTGC-3′ | 5′-CACACATCCACTTACATTCC-3′ |
| 5 | 5′-GGAGTTGACAAAGTGGGTTG-3′ | 5′-CCACTCACTGCACATGATTC-3′ |
| 6 | 5′-CATGCTCACTTCCTCTGG(AG)-3′ | 5′-(CA)GCAAAGCCAGGAGGTAG-3′ |
| 7 | 5′-GAATGGTATGTCTCATGAGC-3′ | 5′-CACTGTAGAGACAGGCAG-3′ |
Fig. 6.
Mutation analysis in CNTFRα coding sequence from a patient with glioblastoma. Mutation analysis showing a missense mutation (T151A) in the only patient (W28) of 32 sampled (lower). This mutation occurred in exon 2 and coded for a tryptophan to arginine (W→R) change. Representative sequencing profile for normal CNTFRα exon 2 coding region (upper).
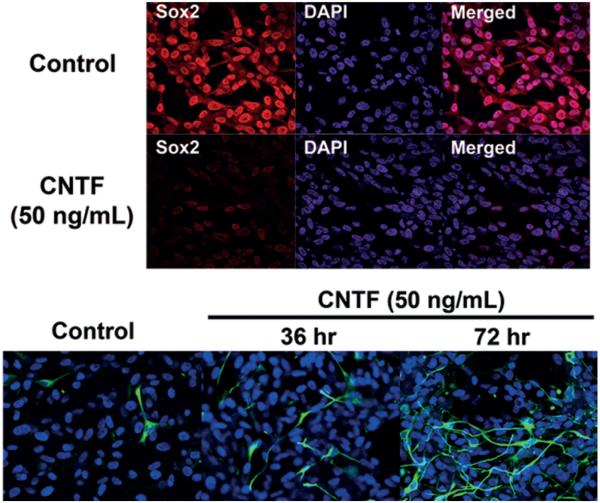
To determine the effect of CNTF pathway induction in primary brain tumors, we evaluated its function in resected malignant glioma tumor-initiating cells. We treated cultured tumor-initiating cells with CNTF (50 mM) while withholding other growth factors and examined GFAP expression and proliferation using immunofluorescence. Following treatment, glioma tumor-initiating cells lost Sox2 expression (Fig. 7 upper) and began to express GFAP (Fig. 7 lower), which increased with prolonged exposure from 36 to 96 hours. Compared with tumor-initiating cells grown in control media, CNTF signaling was shown to induce differentiation while control media maintained the cells in an undifferentiated state.
Fig. 7.
Ciliary neurotrophic factor ligand treatment of glioma tumor-initiating cells. Upper: Immunofluorescence staining of tumor-initiating cells treated with CNTF ligand (50 ng/ml) after removal of control growth factors demonstrating Sox2 loss. This suggests differentiation of tumor-initiating cells through an intact CNTF pathway. Nuclei are counterstained with DAPI. Lower: Immunofluorescence staining showing GFAP expression in 50 ng/ml CNTF ligand-treated tumor-initiating cells. Compared with tumor-initiating cells treated with control media, GFAP expression is markedly increased in tumor-initiating cells after 36 and 72 hours of treatment. Original magnification ×20.
CNTFRα as a Potential Immunotherapeutic Target for Glioblastoma
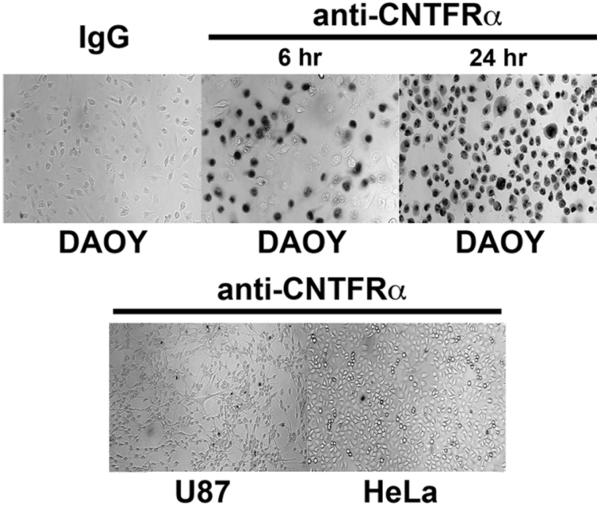
To assess the potential of CNTFRα as a therapeutic target, ADCC was developed and tested using a polyclonal antibody against the extracellular C-terminal27 of CNTFRα. Given its abundant CNTFRα expression, the DAOY cell line was used as a model for targeted therapy. Because HeLa and U87 cells were found to lack CNTFRα expression, these cells were compared with CNTFRα-expressing DAOY cells in their response to ADCC using the CNTFRα polyclonal antibody. Antibody-dependent cell-mediated cytotoxicity had no effect on U87 or HeLa cells but induced lysis in DAOY cells, which increased with prolonged exposure to the antibody and complement (Fig. 8). The DAOY cells treated with IgG and complement did not induce lysis, showing the specificity of this assay for CNTFRα.
Fig. 8.
Antibody-dependent cell-mediated cytotoxicity induces cytotoxic effect in DAOY cells. Trypan blue staining showed a cytotoxic effect by treatment with a CNTFRα specific antibody and complement serum. The DAOY cells were lysed after 6 hours of treatment, which increased with 24 hours. Immunoglobulin G served as a negative control, underlining the necessity of the anti–CNTFRα antibody in DAOY ADCC. The U87 and HeLa cells (both lacking CNTFRα) were not affected by treatment.
Discussion
We sought to identify novel glioma tumor-initiating cell markers using a newly developed proteomic technique, CIEF-nRPLC-MS/MS. Compared with traditional methods, this technique allows identification of a wider spectrum of proteins, including membrane proteins, that may have the greatest potential for diagnostic and therapeutic targeting.43
The stem cell nature of these tumor-initiating cells was suggested in vitro by their characteristic neurosphere morphology and by positive staining for stem cell markers Nestin and Sox2 (Fig. 1). An in vivo xenograft mouse model further confirmed the tumorigenic and stem cell–like characteristics of these cells. After implantation into mouse brain, the undifferentiated human (confirmed by fluorescence in situ hybridization; Fig. 2D and H) tumor-initiating cells infiltrated diffusely throughout the mouse brain parenchyma and were found in large numbers within white matter tracts, mimicking some of the phenotypic characteristics of diffuse human gliomas. The injected cells stained positively for markers Nestin and Sox2, further suggesting their potential stem cell–like nature. Interestingly, cells injected directly into the mouse ventricle maintained, after 5 weeks, a morphology reminiscent of neurospheres seen typically in vitro and stained more strongly for Sox2 and Nestin than tumor cells seen elsewhere in the brain. Also, many cells at the interface between the ventricular mass and the adjacent parenchyma strongly expressed GFAP and displayed a radial glia-like morphology. Although we were unable to demonstrate definitively that the radial glia-like cells were human in origin, the presence of human cells within white matter tracts and parenchymal rests in the hemisphere contralateral to the ventricular injection site strongly suggests that tumor-initiating cells begin to differentiate and migrate in response to attachment to a substrate and chemotactic factors present within the parenchyma. Many similar properties of neurospheres have been previously demonstrated in vitro.46 Taken together, these in vitro immunofluorescence and in vivo xenograft data strongly suggest the tumorigenicity of the cell line used. Although technically challenging due to the toxic nature of RA, future intracranial models utilizing completely differentiated cell cultures should be pursued to confirm the tumorigenicity of these cells.
From the membrane proteins that had a relative difference in expression, we found elevated levels of CNTFRα in tumor-initiating cells with stem cell–like properties. Because CNTFRα plays a critical role in glial differentiation,11,12,15,32,37 we explored its impact on tumor-initiating cell differentiation pathways. Specifically, we examined the differences in CNTFRα expression in glioma tumor-initiating cells compared with their progeny, potential correlations between CNTFRα expression and histological grade, the differentiation potency of the CNTFRα pathway in glioma tumor-initiating cells, as well as CNTFRα as a potential therapeutic target of treatment of CNTFRα-expressing primary brain neoplasms.
Consistent with previous studies showing that CNTFRα mRNA is upregulated in human gliomas,44 the current study strongly implies that the upregulation of CNTFRα is derived from tumor-initiating cells (Figs. 2 and 3). Studies have demonstrated that in gliomas and other brain tumors, the tumor-initiating cell component increases with tumor grade.3 In the current study, immunofluorescence and immunohistochemical analysis showed significant staining for CNTFRα, suggesting an abundance of tumor-initiating cells within glioblastoma (Fig. 5 upper). Moreover, Western blot analysis of 32 different glial tumors revealed a direct correlation between CNTFRα expression and tumor pathological grade (Fig. 5 lower). These data provide evidence for the prognostic use of CNTFRα and suggest that tumor-initiating cells play a role in glioma tumorigenicity.
Because CNTF signaling plays an important role in cell survival and differentiation,11,12,15,32 we postulated that the genetic loss of proper regulation could contribute to aberrant inhibition of differentiation and sustained survival of the glioma tumor-initiating cell population. Genetic analysis identified a mutation in only 1 (3%) of 32 gliomas tested. However, in the mutant, both wild type (T) and mutant (A) nucleic acids were present (Fig. 6). Thus, it appears that a genetic mutation in CNTFRα is unlikely to contribute to malignant glioma tumorigenesis, and the findings of a single mutation in one patient could be interpreted as a “passenger” mutation that has no effect on the fitness of the cell. Additionally, we found that CNTF was still able to drive the differentiation (Fig. 7 upper and lower) of glioma tumor-initiating cells, indicating a properly functioning CNTF receptor. Nonetheless, lack of a direct genetic mutation affecting CNTF expression does not preclude a genetic cause. Mutations in upstream and downstream regulators of CNTFRα may still contribute to pathogenesis in glioma by affecting receptor/ligand balance and may warrant future analysis.
Based on the present data suggesting CNTFRα as a putative tumor-initiating cell marker, we considered the therapeutic potential of CNTFRα modulators. Previous reports have shown that direct treatment with CNTF ligand in combination with the CNTFRα subunit suppressed growth in C6 glioma cells,31 suggesting the potential role for CNTF therapy. Also, the use of drugs to potentiate chemotherapy or radiotherapy by inducing cancer cell proliferation or differentiation is a developing treatment paradigm.24 As we have shown, CNTF ligand causes the stem cell–like tumor-initiating cell component in gliomas to differentiate (Fig. 7 upper and lower). While these findings indicate that CNTF could play a role as an adjuvant therapeutic agent, clinical use of recombinant human CNTF in amyotrophic lateral sclerosis30 and direct use of the ligand have been associated with systemic toxic effects.29 To avoid systemic toxicity, recombinant human CNTF may need to be delivered locally or reformulated. Alternatively, downstream effectors in the CNTF signaling pathway might be targeted using small molecule analogs. Currently, downstream effectors including inhibitors for Stat-3 signaling and N-CoR are under investigation for therapeutic application.32
Recently, clinical trials using monoclonal antibodies for targeted radiotherapy5,36 and recombinant toxin therapy21 have been attempted. While these trials have demonstrated tumor reduction, toxicity due to nonspecific antibody binding remains a problem, thus restricting therapeutic potential. Furthermore, trials using targeted antibody-mediated immunotherapy have been attempted in gliomas with some early success.38 However, these trials are limited to patients harboring a specific genotype (that is, epidermal growth factor receptor–expressing tumors). The current in vitro results demonstrate that immunological targeting of CNTFRα induces cell death in the CNTFRα-expressing DAOY cells but not in U87 or HeLa cells, which lack the receptor (Fig. 8), suggesting the potential for targeting cells overexpressing CNTFRα.
As previously shown, specificity of an overexpressed marker is based on relative levels of target protein in tumor versus normal tissue.9,19,33,41,42 Even though our results show slight CNTFRα expression in differentiated tumor-initiating cells, these levels are minimal compared with their undifferentiated counterparts (Fig. 3 upper and lower). Similarly, there has been evidence of CNTFRα expression in normal CNS tissue.20 Immunofluorescence, immunohistochemical, and Western blot analyses of glioblastoma and surrounding tissue in the current study (Fig. 5 upper and lower) showed relatively insignificant CNTFRα expression in surrounding tissue when compared with the strong staining found in glioblastoma tumor-initiating cells. These results suggest that the immunological targeting of tumor-initiating cells using CNTFRα should be considered despite minimal expression of CNTFRα in differentiated cells and normal tissue. Future investigation should assess the quantitative difference in CNTFRα expression in tumor-initiating cells compared with various other human tissues. Future immunotherapeutic studies may also include the use of a humanized monoclonal antibody in ADCC, the use of tumor-initiating cells in ADCC and evaluation of treatment in in vivo models.
These results suggest an important role for CNTFRα in primary brain tumors, based on robust expression levels in both whole tumor extracts and purified tumor-initiating cells. The clinical implications of CNTFRα as a tumor-initiating cell marker include insight into the understanding of the pathogenesis of these tumors, a tool in the histological diagnosis of high-grade gliomas and the potential to be used for targeted imaging, radiotherapy, or chemotherapy. Furthermore, CNTFRα may be a promising therapeutic target, both through receptor-directed immunotherapy and receptor-mediated prodifferentiation effects.
Conclusions
Ciliary neurotrophic factor receptor subunit–α is up-regulated in tumor-initiating cells, and its expression directly correlates with pathological grade. Thus, CNTFRα is a potential marker for tumor-initiating cells and can aid in the diagnosis and prognosis of glioblastoma. Furthermore, CNTFRα can be used as a target for imaging, radiotherapy, chemotherapy, and immunotherapy in the treatment of malignant glioma.
Acknowledgments
The authors thank James W. Neagle of the DNA-sequencing facility and Paul E. Gallant of the Light Microscopy Facility, National Institute of Neurological Disorders and Stroke, National Institutes of Health.
The Melvin Burkhardt Chair in Neurosurgical Oncology and the Karen Colina Wilson research endowment within the Burkhardt Brain Tumor and Neuro-Oncology Center at the Cleveland Clinic Foundation provided partial support and funding. Dr. Bagley has ownership in Bioproximity, LLC.
Abbreviations used in this paper
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CIEF
capillary isoelectric focusing
- CNTF
ciliary neurotrophic factor
- CNTFRα
CNTF receptor subunit–α
- IgG
immunoglobulin G
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- N-COR
nuclear receptor corepressor
- nRPLC
nano–reversed-phase liquid chromatography
- PBS
phosphate-buffered saline
- RA
retinoic acid
Footnotes
Disclosure Author contributions to the study and manuscript preparation include the following. Conception and design: Lonser, Lu, Ksendzovsky, Zhuang. Acquisition of data: Lu, Ksendzovsky, Yang, Mehta, Yong, Park, Mushlin, Fang, Balgley, Lee, Zhuang. Analysis and interpretation of data: Lonser, Lu, Ksendzovsky, Yang, Mehta, Yong, Weil, Mushlin, Fang, Balgley, Lee, Zhuang. Drafting the article: Lonser, Lu, Ksendzovsky, Mehta, Zhuang. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Lonser. Administrative/technical/material support: Lu, Ksendzovsky, Yang, Weil, Park, Mushlin, Fang, Balgley, Lee. Study supervision: Lonser, Lu, Zhuang
References
- 1.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 5.Bigner DD, Brown MT, Friedman AH, Coleman RE, Akabani G, Friedman HS, et al. Iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with recurrent malignant gliomas: phase I trial results. J Clin Oncol. 1998;16:2202–2212. doi: 10.1200/JCO.1998.16.6.2202. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 7.Davis GE, Manthorpe M, Varon S. Parameters of neuritic growth from ciliary ganglion neurons in vitro: influence of laminin, schwannoma polyornithine-binding neurite promoting factor and ciliary neuronotrophic factor. Brain Res. 1985;349:75–84. doi: 10.1016/0165-3806(85)90133-6. [DOI] [PubMed] [Google Scholar]
- 8.Davis S, Aldrich TH, Valenzuela DM, Wong VV, Furth ME, Squinto SP, et al. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- 9.Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzman ML, Jordan CT. Considerations for targeting malignant stem cells in leukemia. Cancer Contr. 2004;11:97–104. doi: 10.1177/107327480401100216. [DOI] [PubMed] [Google Scholar]
- 11.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419:934–939. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- 12.Hughes SM, Lillien LE, Raff MC, Rohrer H, Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988;335:70–73. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- 13.Ip NY, McClain J, Barrezueta NX, Aldrich TH, Pan L, Li Y, et al. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- 14.Ip NY, Nye SH, Boulton TG, Davis S, Taga T, Li Y, et al. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992;69:1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- 15.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 16.Jordan CT. The potential of targeting malignant stem cells as a treatment for leukemia. Future Oncol. 2005;1:205–207. doi: 10.1517/14796694.1.2.205. [DOI] [PubMed] [Google Scholar]
- 17.Jordan CT. Targeting the most critical cells: approaching leukemia therapy as a problem in stem cell biology. Nat Clin Pract Oncol. 2005;2:224–225. doi: 10.1038/ncponc0164. [DOI] [PubMed] [Google Scholar]
- 18.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 19.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 20.Kordower JH, Yaping-Chu, Maclennan AJ. Ciliary neurotrophic factor receptor alpha-immunoreactivity in the monkey central nervous system. J Comp Neurol. 1997;377:365–380. doi: 10.1002/(sici)1096-9861(19970120)377:3<365::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Lin LF, Mismer D, Lile JD, Armes LG, Butler ET, III, Vannice JL, et al. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF) Science. 1989;246:1023–1025. doi: 10.1126/science.2587985. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Kovach JS, Johnson F, Chiang J, Hodes R, Lonser R, et al. Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc Natl Acad Sci U S A. 2009;106:11697–11702. doi: 10.1073/pnas.0905930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLennan AJ, Gaskin AA, Lado DC. CNTF receptor alpha mRNA expression in rodent cell lines and developing rat. Brain Res Mol Brain Res. 1994;25:251–256. doi: 10.1016/0169-328x(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 26.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 27.Man D, He W, Sze KH, Gong K, Smith DK, Zhu G, et al. Solution structure of the C-terminal domain of the ciliary neurotrophic factor (CNTF) receptor and ligand free associations among components of the CNTF receptor complex. J Biol Chem. 2003;278:23285–23294. doi: 10.1074/jbc.M301976200. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda T, Hirano T. Association of p72 tyrosine kinase with Stat factors and its activation by interleukin-3, interleukin-6, and granulocyte colony-stimulating factor. Blood. 1994;83:3457–3461. [PubMed] [Google Scholar]
- 29.Miller RG, Bryan WW, Dietz MA, Munsat TL, Petajan JH, Smith SA, et al. Toxicity and tolerability of recombinant human ciliary neurotrophic factor in patients with amyotrophic lateral sclerosis. Neurology. 1996;47:1329–1331. doi: 10.1212/wnl.47.5.1329. [DOI] [PubMed] [Google Scholar]
- 30.Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, Goodpasture JC, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol. 1996;39:256–260. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- 31.Ozog MA, Bechberger JF, Naus CC. Ciliary neurotrophic factor (CNTF) in combination with its soluble receptor (CNTFRalpha) increases connexin43 expression and suppresses growth of C6 glioma cells. Cancer Res. 2002;62:3544–3548. [PubMed] [Google Scholar]
- 32.Park DM, Li J, Okamoto H, Akeju O, Kim SH, Lubensky I, et al. N-CoR pathway targeting induces glioblastoma derived cancer stem cell differentiation. Cell Cycle. 2007;6:467–470. doi: 10.4161/cc.6.4.3856. [DOI] [PubMed] [Google Scholar]
- 33.Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, et al. von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4:e60. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 35.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 36.Riva P, Franceschi G, Arista A, Frattarelli M, Riva N, Cremonini AM, et al. Local application of radiolabeled monoclonal antibodies in the treatment of high grade malignant gliomas: a six-year clinical experience. Cancer. 1997;80(12 Suppl):2733–2742. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2733::aid-cncr53>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.Rudge JS, Li Y, Pasnikowski EM, Mattsson K, Pan L, Yancopoulos GD, et al. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci. 1994;6:693–705. doi: 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 38.Sampson JH, Crotty LE, Lee S, Archer GE, Ashley DM, Wikstrand CJ, et al. Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A. 2000;97:7503–7508. doi: 10.1073/pnas.130166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 40.Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, Witthuhn BA, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 41.Vogel TW, Brouwers FM, Lubensky IA, Vortmeyer AO, Weil RJ, Walther MM, et al. Differential expression of erythropoietin and its receptor in von hippel-lindau-associated and multiple endocrine neoplasia type 2-associated pheochromocytomas. J Clin Endocrinol Metab. 2005;90:3747–3751. doi: 10.1210/jc.2004-1899. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Li HG, Xia ZS, Wen JM, Lv J. Prognostic significance of erythropoietin and erythropoietin receptor in gastric adenocarcinoma. World J Gastroenterol. 2011;17:3933–3940. doi: 10.3748/wjg.v17.i34.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Rudnick PA, Evans EL, Li J, Zhuang Z, Devoe DL, et al. Proteome analysis of microdissected tumor tissue using a capillary isoelectric focusing-based multidimensional separation platform coupled with ESI-tandem MS. Anal Chem. 2005;77:6549–6556. doi: 10.1021/ac050491b. [DOI] [PubMed] [Google Scholar]
- 44.Weis J, Schönrock LM, Züchner SL, Lie DC, Sure U, Schul C, et al. CNTF and its receptor subunits in human gliomas. J Neurooncol. 1999;44:243–253. doi: 10.1023/a:1006303221064. [DOI] [PubMed] [Google Scholar]
- 45.Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, et al. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene. 2011;30:3454–3467. doi: 10.1038/onc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]