Abstract
Object
Central nervous system hemangioblastomas are the most common manifestation of von Hippel-Lindau (VHL) disease, an autosomal dominant tumor suppressor syndrome that results in loss of VHL protein function and continuous upregulation of hypoxia-inducible factors. These tumors are composed of neoplastic stromal cells and abundant vasculature. Stromal cells express markers consistent with multipotent embryonically arrested hemangioblasts, which are precursors for hematopoietic and vascular lineages. Notch receptors are transmembrane signaling molecules that regulate multiple developmental processes including hematopoiesis and vasculogenesis. To investigate the importance of notch signaling in the development of VHL disease–associated CNS hemangioblastomas, the authors examined the presence of the four notch receptors and downstream notch effectors in this setting.
Methods
The authors used surgical specimens obtained from confirmed VHL-associated hemangioblastomas. Immunohistochemical analysis for the four notch receptors and the downstream effectors was performed on formalin-fixed paraffin-embedded sections. Western blot analysis for HES1 was performed on frozen specimens.
Results
All four notch receptors are present in hemangioblastomas. NOTCH1 and NOTCH4 receptors were widely and prominently expressed in both the stromal and vascular cells, NOTCH2 receptor expression was limited to primarily stromal cells, and NOTCH3 receptor expression was limited to vascular cells. All 4 receptors displayed a nuclear presence. Immunohistochemical analysis also demonstrated that downstream notch effectors, HES1 and HES5, were uniformly expressed in tumor stromal and vascular cells, but HES3, HEY1, and HEY2 were not. Strong HES1 expression was confirmed by Western blot analysis.
Conclusions
The presence of all four notch receptors and downstream effector molecules suggests that the notch signaling pathway plays a critical role in the maintenance of the undifferentiated pluripotent phenotype of these tumors and in the associated vascular response. Moreover, the prominent expression of notch receptors in VHL-associated CNS hemangioblastomas reveals a new and possibly potent therapeutic target.
Keywords: notch, von Hippel-Lindau disease, hemangioblastoma, HES, vasculature, oncology
Von Hippel-Lindau (VHL) disease is a tumor suppressor gene syndrome resulting from an inherited mutation in the VHL gene. Tumorigenesis follows after somatic inactivation of the wild-type allele in specific organ systems.2,15,30 In patients with VHL disease, multiple histologically similar and highly vascular visceral and nervous system tumors will often develop over a patient's lifetime. Recently, the molecular changes associated with loss of the VHL protein have been elucidated.16 The VHL protein functions as the recognition subunit of the E3 Ligase complex that targets specific proteins for “ubiquitylation” and subsequent proteasome degradation. The best studied of these VHL-targeted proteins are the hypoxia-inducible factors (HIFs). The loss of functional VHL protein results in continuous elevation of HIF-1 and -2, with subsequent upregulation of downstream HIF-regulated proteins including vascular endothelial growth factor (VEGF) and erythropoietin.
The most common manifestation of VHL disease (occurring in 60%–80% of patients with VHL disease) is the CNS hemangioblastoma. Patients with VHL disease frequently present with multiple CNS hemangioblastomas and will develop multiple additional tumors during their lifetime. Hemangioblastomas of the CNS occur in a highly conserved anatomical pattern that is almost exclusively limited to the cerebellum, brainstem, and spinal cord.15 Although CNS hemangioblastomas are classified as benign, they are a significant potential cause of morbidity and death in patients with VHL disease. Currently, effective treatment includes resection of clinically significant tumors in eloquent areas of the CNS. Histologically, hemangioblastomas are composed of 2 predominant cell populations, stromal and vascular, identifiable by immunohistochemical staining for neuron-specific enolase and CD31, respectively.1
Notch receptors are a family of transmembrane signaling molecules that undergo an intracellular cleavage following interaction with one of the notch ligands.7 This cleaved ICD of the receptor contains the C-terminus of the molecule and is translocated to the nucleus where it acts as a transcription factor to activate the downstream notch effector molecules such as HES and HEY. Notch receptors are particularly important during development, as well as in the maintenance of undifferentiated pools of pluripotent progenitor cells including hematopoietic and vascular cells.14
Previous studies have demonstrated that VHL protein–deficient hemangioblastoma cells express multiple markers associated with pluripotent embryonic hemangioblasts and are capable of differentiating into hematopoietic and vascular lineages.5,25,37 The pluripotency of VHL disease–associated hemangioblastoma cells and the expression of markers consistent with embryological hemangioblasts led us to hypothesize that the notch family of developmental signaling molecules is expressed in these tumors. The purpose of this study was to characterize notch receptor expression and downstream effector status in VHL disease–associated CNS hemangioblastomas. All four notch receptors were expressed along with downstream effectors HES1 and HES5. These results suggest the importance of notch signaling in the development of hemangioblastomas, and they identify the notch signaling cascade as a potential new therapeutic target in the management of VHL disease–associated hemangioblastomas.
Methods
Specimens
Formalin-fixed, paraffin-embedded surgical specimens of confirmed hemangioblastomas from patients with VHL disease were obtained according to National Institutes of Health tissue procurement guidelines. Staining was performed on 7–10 tumors.
Immunohistochemical Analysis
Five-micrometer sections were deparaffinized and rehydrated by standard techniques. Staining was performed as described29 using the ABC/DAB method (anti–rabbit IgG and DAB kits, Vector Lab). Primary rabbit antibodies (1 μg/ml each) were as follows: NOTCH1 (ab27526), NOTCH1 ICD (ab8925), NOTCH3 (ab23426), NOTCH4 (ab23427) (all from Abcam); NOTCH2 (LS-C40783; Lifespan Biosciences); and normal rabbit IgG (011–000–003, Jackson Immunoresearch). Primary rabbit antibodies (2 μg/ml each) were as follows: HES1 (AB5702), HES5 (AB5708), and HEY2 (AB5716) (all from Millipore); HES3 (LS-C7390, Lifespan Biosciences); and HEY1 (ab22614, Abcam). Images were obtained using a Leica DM LB microscope with a Spot Imaging camera and software (Sterling Heights). In Adobe Photoshop, the background was adjusted using the “Set White Point” feature. No other manipulations were performed.
Western Blot Analysis
Frozen hemangioblastoma specimens were lysed in T-PER solution plus HALT (Thermo Scientific) and sonicated for 2 minutes. Ten micrograms of protein/lane were separated on 4%–12% gradient gel and transferred, and membranes were blocked using Superblock with phosphate-buffered saline (Thermo Scientific) with 0.1% Tween 20. Primary antibodies were anti-HES1 (ab71559, diluted 1:3000; Abcam) and antiactin (A3853, diluted 1:2500; Sigma-Aldrich). Following incubation with horseradish peroxidase–conjugated secondary antibodies, bands were detected using SuperSignal chemiluminescent substrate (Thermo Scientific) and HyBlot CL film (Denville Scientific).
Results
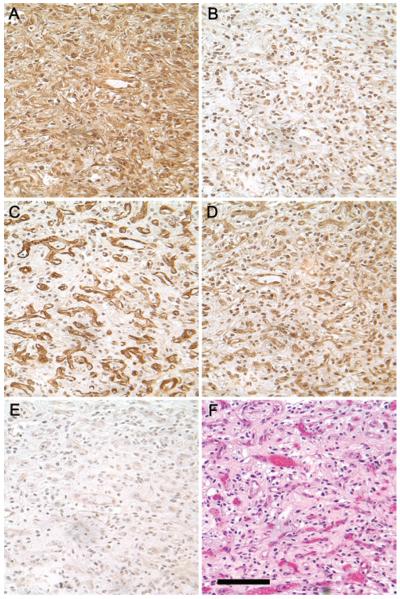
Von Hippel-Lindau disease–associated CNS hemangioblastomas were screened by immunohistochemistry for the presence of the four notch receptors. A representative hemangioblastoma, displaying expression of all four notch receptors in the same tumor, is shown (Fig. 1). NOTCH1 staining displayed robust cytoplasmic and nuclear immunoreactivity in both stromal and vascular cells (Fig. 1A); NOTCH2 immunoreactivity exhibited a more discreet nuclear pattern (Fig. 1B); NOTCH3 staining displayed a distinctly vascular pattern, with the absence of reactivity in the stromal cells (Fig. 1C); and NOTCH4 expression was similar to NOTCH1 in that there was widespread staining in both vascular and stromal cells (Fig. 1D). These antibodies recognize both the membrane-bound and cleaved forms of notch, which explains why both cytoplasmic and nuclear staining features are observed. Although the patterns of expression differ among the notch receptor subtypes, the intra- and intertumoral patterns for each subtype were consistent. Previous studies have demonstrated an absence of notch receptors in normal adult brain.40,43
Fig. 1.
Immunohistochemical analysis of notch receptors 1 through 4 in a representative CNS hemangioblastoma. A: NOTCH1 immunoreactivity is distributed uniformly, with cytoplasmic and nuclear staining observed in both the stromal and vascular cells. B: NOTCH2 immunoreactivity is confined to the nucleus. C: NOTCH3 immunoreactivity is strongly expressed in the vascular component of the tumor. D: NOTCH4 immunoreactivity is similar to NOTCH1, with cytoplasmic and nuclear staining observed in both the stromal and vascular cells. E: Nonimmune rabbit IgG control. F: Hematoxylin & eosin staining demonstrates the typical hemangioblastoma appearance with “foamy” stromal cells and abundant vasculature. All antibodies were raised against the C-terminus of the molecule and do not distinguish between the full-length and the cleaved forms. Staining for all four Notch receptors was performed at the same time in the same tumor. Photomicrographs were taken from the same area of the tumor with the same exposure settings, original magnification × 40. Bar = 100 μm.
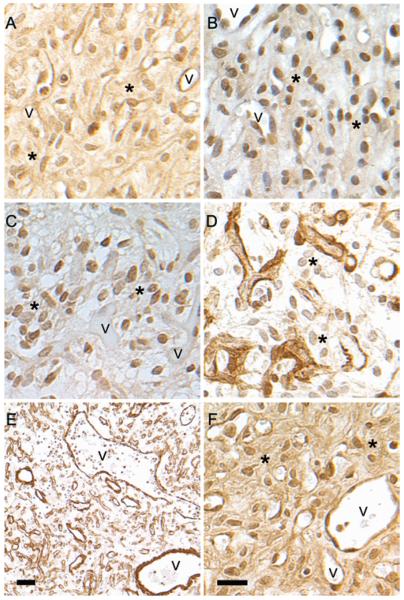
Additional analysis was performed to more carefully delineate the localization of each member of the notch family. Both nuclear and cytoplasmic immunoreactivity for NOTCH1 were observed throughout the hemangioblastoma when an antibody recognizing both the full-length transmembrane and the cleaved intracellular forms of NOTCH1 was used. (Fig. 2A). However, using an antibody raised against the internal cleavage site of the NOTCH1 ICD revealed exclusive localization of the NOTCH1 ICD to the nuclei of both stromal and vascular cells (Fig. 2B). Nuclear localization of NOTCH2 was primarily observed in the stromal cells, with minimal reactivity in the vasculature (Fig. 2C). In contrast, NOTCH3 expression was limited to the vascular cells (Fig. 2D). The presence of NOTCH3 in vessels of different morphologies was readily apparent; both small capillary structures and large thick-walled vessels expressed NOTCH3 (Fig. 2E). NOTCH4 immunoreactivity localized to the cytoplasm and nuclei of both stromal and vascular cells (Fig. 2F).
Fig. 2.
Cellular localization of notch receptors. A: NOTCH1 antibody to the C-terminus recognizes both full-length and cleaved NOTCH1 and reacts with both cytoplasmic and nuclear Notch. B: Antibody against NOTCH ICD recognizes only the signaling portion of notch and localizes to the nucleus. The same area of the same tumor is presented in A and B. C: NOTCH2 displays a nuclear localization in stromal cells with only occasional reactivity in vascular cells. D: NOTCH3 staining from the same area presented in panel C reveals an exclusively vascular localization. E: Low-magnification image of NOTCH3 immunoreactivity displaying abundant vasculature. NOTCH3-positive vasculature of multiple morphologies, including large thick- and thin-walled vessels as well as microvessels, is observed. F: NOTCH4 reactivity is seen in the cytoplasm and nuclei of both stromal and vascular cells. Asterisks indicate stromal cells. v = blood vessel. Bar = 20 μm (A–D, and F); bar = 100 μm (E).
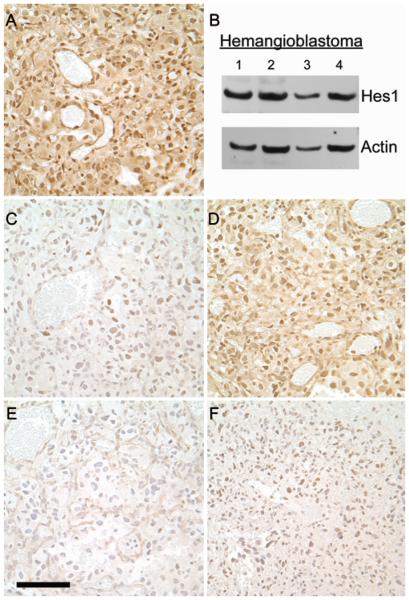
The presence of notch immunoreactivity in the nucleus is strongly suggestive of active notch signaling. To confirm the observation, an analysis of downstream effector molecules was performed (Fig. 3). HES1 was strongly detected in the nuclei of both stromal and vascular cells and was readily apparent by Western blot analysis; HES5 was similar to HES1 but not as strong; HES3 and HEY2 staining revealed minimal nuclear reactivity in some stromal cells; HEY1 was not notable under these conditions. HES1 and HES5 are considered key notch-dependent downstream effectors, and both are important in nervous system development,23 whereas HES3 is not necessarily notch dependent,10 which may explain its relative absence in this setting.
Fig. 3.
Immunohistochemical staining of Notch downstream effectors in a representative CNS hemangioblastoma. A: HES1 was observed in the nuclei of both stromal cells and blood vessels. B: Western blot analysis of 4 hemangioblastomas also demonstrating the presence of HES1. C: Scattered, faint HES3 immunoreactivity observed in the nuclei of some cells. D: HES5 displaying a pattern similar to HES1 although not as intense. E: HEY1 staining is not distinguishable from the negative IgG control (not shown). F: Scattered, faint HEY2 immunoreactivity observed in the nuclei of some cells. Staining for all 5 effector molecules was performed in the same tumor. Original magnification × 40. Bar = 100 μM.
Discussion
The presence of notch receptors in hemangioblastomas is consistent with the embryonic markers and pluripotency observed in this setting. Von Hippel-Lindau disease–associated hemangioblastoma stromal cells simultaneously express markers associated with multiple developmental lineages including hematopoiesis/erythropoiesis, angiogenesis/vasculogenesis, and neurogenesis. The notch family of signaling molecules plays critical functions in all of these processes.7,22,36 In addition, NOTCH1 and NOTCH3 have been associated with renal cell carcinoma27,34 and NOTCH2 with pancreatic cancer;18 tumors also associated with VHL disease. The presence of NOTCH2 in the stromal compartment of CNS hemangioblastomas is interesting because NOTCH2 also acts to maintain a pool of neuronal precursors35 and to regulate erythropoiesis.42 Both neural antigens and tumor-derived erythrocytes have been detected in hemangioblastomas.1,37
The presence of notch in the vasculature of hemangioblastomas is not surprising because notch receptors are induced by VEGF,12 and VEGF is continuously overexpressed in hemangioblastomas. Notch receptors are key players in both vasculogenesis and angiogenesis.8,9,26,31 In addition, NOTCH1 and NOTCH4 induce a proangiogenic state and are associated with development of brain arteriovenous malformations and thick-walled vessels,20,43 similar in morphology to those observed in this study (Fig. 2E). The exclusive expression of NOTCH3 in the vascular compartment is of interest since NOTCH3 is critical for proper angiogenesis and is associated with the mural cells of developing vasculature.13 The NOTCH3-positive blood vessels may represent the component of the vasculature that is reactive to VEGF stimulus, as this same pattern of NOTCH3 expression has been observed in angiogenic settings not associated with VHL disease.
The expression of all four notch receptors in VHL disease–associated CNS hemangioblastomas is noteworthy because during normal development the individual notch receptor types often have tissue-specific functions or act in a specific temporal sequence.7 Although a source of considerable morbidity with some phenotypic features resembling a more malignant phenotype (for example, HIF overexpression), CNS hemangioblastomas are benign tumors. Thus, a picture of the VHL disease–associated hemangioblastoma as a developmental, rather than oncological, phenomenon has begun to emerge. Referred to as “developmentally arrested,” the implication is that hemangioblastomas originate from a subset of pluripotent embryonic cells that are unable to complete their normal developmental program in the absence of functional VHL protein.25,37 This hypothesis is supported by the observation that certain cells/tissues require a transient period of hypoxia and HIF upregulation to progress along their normal developmental path.17,28 As development progresses and tissue oxygen rises, HIF decreases and subsequent signaling pathways associated with normoxia emerge.38 In VHL disease, HIF remains elevated, affected cells are unable to differentiate normally, and these cells may subsequently develop into a tumor resembling (but not identical to) the embryonic pluripotent cells from which they arose. The continuous presence of HIF may interfere with the normal differentiation process by perpetuating notch signaling.6 The presence of HES1 and HES5 in hemangioblastoma stromal cells is consistent with continued notch signaling and the perpetuation of an undifferentiated state.23,32
The presence of all four notch receptors in CNS hemangioblastomas suggests a novel therapeutic option in this setting. Inhibition of notch signaling has gained prominence recently with the development of notch inhibitors and success in model systems.11,24,34 Several types of notch signaling inhibitors are currently available or in development.3,33,41 Specifically, gamma secretase inhibitors prevent cleavage of the membrane-associated full-length notch, thereby preventing release of the intracellular signaling portion of the molecule.33 This class of inhibitor has a well-tolerated clinical safety profile in other settings.4 Other inhibitors interfere with notch ligand binding or with notch ICD transcription complex formation.19
Notch inhibitors may have an advantage over existing therapies in that they can target both the tumor cell and the reactive vasculature.39 Previous results that involved anti-notch strategies in experimental systems have shown efficacy and prevention of the development of functional vasculature.31 The presence of notch receptors and the downstream effector HES in the nuclei of both the stromal and vascular compartments of the hemangioblastoma is important because it indicates that both cell populations may be susceptible to inhibitors of notch. Anti-notch therapies have also been useful as a way to specifically target VEGF-induced vasculature,21 which is a prominent feature of hemangioblastomas.
Conclusions
This study demonstrates the expression and nuclear localization of notch receptors 1–4 in VHL disease–associated hemangioblastomas. In addition, the presence of the downstream nuclear effectors HES1 and HES5 indicates active notch signaling in this setting. These findings lend support to the hypothesis that the notch family of signaling molecules contributes to the development and maintenance of the pluripotent phenotype of these tumors. The notch pathway may provide a novel therapeutic target for a disease currently manageable only by surgery.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke.
Abbreviations used in this paper
- ABC/DAB
avidin-biotin complex/3,3′-diaminobenzidine
- HIF
hypoxia-inducible factor
- ICD
intracellular domain
- VEGF
vascular endothelial growth factor
- VHL
von Hippel-Lindau.
Footnotes
Disclosure The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Merrill, Lonser. Acquisition of data: Merrill, Edwards. Analysis and interpretation of data: all authors. Drafting the article: Merrill, Edwards. Critically revising the article: Merrill, Lonser. Administrative/technical/material support: Edwards. Study supervision: Merrill, Lonser.
References
- 1.Böhling T, Plate KH, Haltia MJ, Alitalo K, Neumann HPH. Von Hippel-Lindau disease and capillary haemangioblastoma. In: Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumors of the Nervous System. World Health Organization classification of tumors; IARC Press; Lyon: 2000. pp. 223–226. [Google Scholar]
- 2.Calzada MJ. Von Hippel-Lindau syndrome: molecular mechanisms of the disease. Clin Transl Oncol. 2010;12:160–165. doi: 10.1007/s12094-010-0485-9. [DOI] [PubMed] [Google Scholar]
- 3.Dikic I, Schmidt MH. Notch: implications of endogenous inhibitors for therapy. Bioessays. 2010;32:481–487. doi: 10.1002/bies.200900140. [DOI] [PubMed] [Google Scholar]
- 4.Fleisher AS, Raman R, Siemers ER, Becerra L, Clark CM, Dean RA, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer dis ease. Arch Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gläsker S, Li J, Xia JB, Okamoto H, Zeng W, Lonser RR, et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 2006;66:4167–4172. doi: 10.1158/0008-5472.CAN-05-3505. [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ. Notch signaling in development and disease. Clin Genet. 2003;64:461–472. doi: 10.1046/j.1399-0004.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 9.Karsan A. The role of notch in modeling and maintaining the vasculature. Can J Physiol Pharmacol. 2005;83:14–23. doi: 10.1139/y04-125. [DOI] [PubMed] [Google Scholar]
- 10.Katoh M, Katoh M. Integrative genomic analyses on HES/HEY family: Notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. Int J Oncol. 2007;31:461–466. [PubMed] [Google Scholar]
- 11.Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, et al. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 12.Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010;107:860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 15.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 17.Mazumdar J, Dondeti V, Simon MC. Hypoxia-inducible factors in stem cells and cancer. J Cell Mol Med. 2009;13:4319–4328. doi: 10.1111/j.1582-4934.2009.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazur PK, Einwächter H, Lee M, Sipos B, Nakhai H, Rad R, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2010;107:13438–13443. doi: 10.1073/pnas.1002423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci U S A. 2008;105:10901–10906. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 22.Ohishi K, Katayama N, Shiku H, Varnum-Finney B, Bernstein ID. Notch signalling in hematopoiesis. Semin Cell Dev Biol. 2003;14:143–150. doi: 10.1016/s1084-9521(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pannuti A, Foreman K, Rizzo P, Osipo C, Golde T, Osborne B, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, et al. von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4:e60. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Rae FK, Stephenson SA, Nicol DL, Clements JA. Novel association of a diverse range of genes with renal cell carcinoma as identified by differential display. Int J Cancer. 2000;88:726–732. doi: 10.1002/1097-0215(20001201)88:5<726::aid-ijc7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez-Bergeron DL, Runge A, Dahl KD, Fehling HJ, Keller G, Simon MC. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–4634. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- 29.Renshaw S. Immunochemical staining techniques. In: Renshaw S, editor. Immunohistochemistry. Scion; Oxfordshire: 2007. pp. 45–79. [Google Scholar]
- 30.Roberts AM, Ohh M. Beyond the hypoxia-inducible factor-centric tumour suppressor model of von Hippel-Lindau. Curr Opin Oncol. 2008;20:83–89. doi: 10.1097/CCO.0b013e3282f310de. [DOI] [PubMed] [Google Scholar]
- 31.Sainson RC, Harris AL. Regulation of angiogenesis by homo-typic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angio-genesis. 2008;11:41–51. doi: 10.1007/s10456-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 32.Sang L, Roberts JM, Coller HA. Hijacking HES1: how tumors co-opt the anti-differentiation strategies of quiescent cells. Trends Mol Med. 2010;16:17–26. doi: 10.1016/j.molmed.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih IeM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 34.Sjölund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, et al. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest. 2008;118:217–228. doi: 10.1172/JCI32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 36.Varnum-Finney B, Purton LE, Yu M, Brashem-Stein C, Flowers D, Staats S, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–4091. [PubMed] [Google Scholar]
- 37.Vortmeyer AO, Frank S, Jeong SY, Yuan K, Ikejiri B, Lee YS, et al. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res. 2003;63:7051–7055. [PubMed] [Google Scholar]
- 38.Westfall SD, Sachdev S, Das P, Hearne LB, Hannink M, Roberts RM, et al. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. 2008;17:869–881. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 40.Xu P, Yu S, Jiang R, Kang C, Wang G, Jiang H, et al. Differential expression of Notch family members in astrocytomas and medulloblastomas. Pathol Oncol Res. 2009;15:703–710. doi: 10.1007/s12253-009-9173-x. [DOI] [PubMed] [Google Scholar]
- 41.Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80:690–701. doi: 10.1016/j.bcp.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Zeuner A, Francescangeli F, Signore M, Venneri MA, Pedini F, Felli N, et al. The Notch2-Jagged1 interaction mediates stem cell factor signaling in erythropoiesis. Cell Death Differ. 2011;18:371–380. doi: 10.1038/cdd.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ZhuGe Q, Zhong M, Zheng W, Yang GY, Mao X, Xie L, et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain. 2009;132:3231–3241. doi: 10.1093/brain/awp246. [DOI] [PMC free article] [PubMed] [Google Scholar]