Abstract
Object
The tumors most frequently associated with von Hippel-Lindau (VHL) disease are hemangioblastomas. While they are associated with significant neurological impairment and mortality, their natural history and optimal management have not been fully defined.
Methods
Patients with VHL were enrolled in a prospective study designed to define the natural history of CNS hemangioblastomas. In the present analysis, serial imaging, laboratory, genetic, and clinical data were evaluated in those with at least 2 years of follow-up data.
Results
At study entrance 225 patients (111 males, 114 females) harbored 1921 CNS hemangioblastomas in the supratentorial compartment (21 tumors [1%]), cerebellum (865 [45%]), brainstem (129 [7%]), spinal cord (689 [36%]), cauda equina (212 [11%]), and nerve roots (5 [0.3%]; follow-up 15,819 hemangioblastoma-years). Increased tumor burden was associated with partial deletions in the VHL gene (p = 0.005) and male sex (p = 0.002). Hemangioblastoma development (median 0.3 new tumors/year) was associated with younger age (p < 0.0001) and more tumors at study entrance (p < 0.0001). While 1278 hemangioblastomas (51%) did not grow, 1227 hemangioblastomas (49%) grew in a saltatory (886 [72%]), linear (76 [6%]), or exponential (264 [22%]) pattern. Faster tumor growth was associated with male sex (p = 0.001), symptomatic tumors (p < 0.0001), and tumors associated with cysts (p < 0.0001). Location-dependent tumor size was the primary predictor of eventual symptom formation (159 symptomatic tumors [6.3%]; area under the curve > 0.9).
Conclusions
Central nervous system hemangioblastoma burden in VHL is associated with partial germline deletions and male sex. Unpredictable growth of hemangioblastomas compromises assessment of nonsurgical therapies. The judicious treatment of symptom-producing hemangioblastomas, while avoiding unnecessary treatment of asymptomatic tumors that may not progress, can provide clinical stability. Clinical trial registration no.: NCT00005902 (ClinicalTrials.gov).
Keywords: central nervous system, hemangioblastoma, natural history, von Hippel-Lindau disease, oncology
Germline mutations of the von Hippel-Lindau (VHL) disease gene on chromosome 3 underlie the pathogenesis of this neoplastic disorder. Transmitted in an autosomal dominant manner, VHL disease can result in the development of tumors and cysts of the viscera and nervous system.16 The most common neoplastic manifestations of VHL are CNS hemangioblastomas. 7,22 Prior reports estimate that multiple hemangioblastomas will develop in 60%–90% of patients with VHL disease.5,6,10,15,17,20,21,23 While CNS hemangioblastomas are benign, they are associated with significant neurological morbidity and mortality based on their location and multiplicity.4 Despite the frequency and devastating effects of CNS hemangioblastomas in VHL, their natural history and optimal management have not been defined.
To determine the natural history of CNS hemangioblastomas, to gain insight into factors affecting tumor development and/or progression, and to improve tumor management, patients with VHL disease and associated hemangioblastomas were prospectively evaluated using clinical, imaging, and genetic analyses. Specifically, analyses were performed to determine the biological properties of CNS hemangioblastomas, as well as to identify factors associated with tumor burden, new tumor development, and the production of symptoms requiring treatment.
Methods
Patients
This study was registered with the ClinicalTrials.gov database (http://clinicaltrials.gov), and its registration no. is NCT00005902. Patients with at least one hemangioblastoma visible on MRI provided written informed consent and were enrolled in an institutional review board–approved protocol to determine the natural history of VHL-associated hemangioblastomas (accrual ceiling 250 participants). Patients were enrolled from November 2001 to October 2005. All patients had genetic and/or clinical confirmation of VHL.12,19
Germline Genotype Analysis
Analysis of VHL mutation was performed on samples of peripheral blood from the study participant or at least one family member to determine germline genotype, as described previously.26
Study Evaluation
Clinical Evaluation
Patients were evaluated at 6-month intervals. At each visit, detailed neurological examinations were performed and Karnofsky Performance Scale (KPS) scores were determined. Abdominal imaging and patient records were used to document the presence of visceral lesions.
Imaging Evaluation
Craniospinal high-resolution (1-mm slice thickness) FLAIR and T1- and T2-weighted MRI (with and without contrast) was performed at each clinical evaluation.
Hemangioblastoma Characteristics
To accurately assess tumor distribution, development, and growth over time, patients and/or tumors with less than 2 years of follow-up were excluded from analysis. Data analysis for patients and/or tumors treated with systemic chemotherapy, stereotactic radiosurgery, or craniospinal radiation was terminated on initiation of those therapies.2,25
Postcontrast T1-weighted, spoiled gradient recalled, and T2-weighted MRI sequences were acquired at clinic visits and analyzed. Hemangioblastoma and associated cyst (if present) volumes were calculated using a modified ellipsoid formula at each visit.14 Peritumoral cysts were assessed using T2-weighted (hyperintensity) MRI. Intratumoral cysts were evaluated using T1-weighted (hypointensity within enhancing tumor) postcontrast MRI. Volume assessment values were initially assessed serially by a single observer and confirmed by at least one other observer. Patterns of tumor growth were classified as saltatory (exhibiting periods of growth and growth quiescence), linear, or exponential. Tumors that did not progress in size were classified as stable.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics and hemangioblastoma features. To examine the effect of factors, such as age and sex, on tumor growth rate, a linear mixed model (MIXED procedure, SAS Institute Inc.) with the patient as random effect was used since most patients had more than 1 tumor. All tumors in the study had at least 2 years of follow-up and 4 data points.
Tumor growth pattern was determined with mathematical characterization. “No growth” was defined as the difference in tumor size ≤7.5 mm3/year between baseline and the last visit divided by the years of follow-up. “Saltatory growth” was defined as the total number of days at zero-growing intervals divided by the total number of days of follow-up for the tumor (≥25%), where the zerogrowing interval was defined as the interval with 0 difference between tumor size at adjacent time points. A tumor was defined as having “linear growth” if the R2 (R12) from the regression of tumor size (dependent variable) on the visit date (independent variable) was ≥0.85 and greater than or equal to the R2 (R22) from the regression of log-transformed tumor size (dependent variable) on the visit date. A tumor was defined as having “exponential growth” if R22 was ≥0.85 and > R12.
A general linear model (GLM procedure, SAS Institute Inc.) was applied to examine the association of patient total number of tumors with age (categorized as 12–20, 21–30, 31–40, 41–50, or > 50 years), sex, years of follow-up, and mutation genotype. A general linear model was also applied to determine the association of newly developed tumor per year with age (categorized), sex, number of tumors at study entrance, and mutation genotype. Pedigree effect was examined using a mixed model (MIXED procedure). Since most patients had more than 1 tumor and the tumors from the same patient were not independent, a general linear mixed model with subject as the random effect (MIXED procedure) was used to assess the association of tumor growth rate with age, sex, tumor location, cyst-linked tumor type (4 levels, including linked to intratumoral cyst, linked to peritumoral cyst, linked to both cysts, and linked to none), and tumor symptom type (that is, asymptomatic or symptomatic tumor). A p value of 0.1 was used as a model selection criterion for the above association analysis.
To examine the effect of factors on a patient’s total number of tumors and number of newly developed tumors per year, a general linear model was performed since pedigree effect was not significant. Receiver operating characteristic (ROC) curve analysis using a generalized mixed model (GLIMMIX procedure) and a logistic regression model (LOGISTIC procedure, both SAS Institute Inc.) was applied to evaluate tumor volume for the prediction of symptomatic tumors. Discrimination was assessed using the area under the ROC curve (AUC). Survival analysis was performed using growth as the event of interest, defined as the difference in tumor volume > 7.5 mm3 between the last and first evaluation, and not growing as censored. The timing of events was determined using the time the tumor was detectable on imaging (12 mm3) to a time in which growth was > 7.5 mm3.
Quantitative outcome measures were logarithmically transformed because their distributions had a long right tail. For any fixed factor with more than 2 levels in which significance was found, Tukey-Kramer multiple pairwise comparisons were performed. A p value of 0.01 was used as the significance level to adjust for multiple tests, and a p value of 0.1 was used as the covariate selection. For statistical analyses, SAS software version 9.2 was used (SAS Institute Inc.).
Results
Patient Characteristics
Two hundred fifty patients with VHL disease (119 males, 131 females) were enrolled in the study. The mean age at study entrance was 38.5 ± 12.3 years (range 12.3–66.1 years). Two hundred twenty-five patients (90%) had tumors as well as more than 2 years of follow-up and were used for analysis (Table 1). The mean follow-up was 6.9 ± 1.6 years (range 2.1–9.0 years). During the study, 16 patients died (mean age 41.9 ± 10.9 years, range 17.7–56.3 years) from renal cell carcinoma (2 patients), CNS hemangioblastoma progression (4 patients), stroke (1 patient), pneumonia (1 patient), and unknown causes (8 patients). Twenty-five patients (10%) had less than 2 years of evaluation as a result of death (7 patients), study withdrawal (5 patients), or loss to follow-up (13 patients).
TABLE 1.
Lesions associated with VHL disease in 225 patients at study entrance
| Lesion | No. of Patients (%) |
|---|---|
| nervous system | |
| craniospinal hemangioblastoma | |
| supratentorial | 23 (10.2) |
| cerebellum | 197 (87.6) |
| brainstem | 94 (41.8) |
| spinal cord | 193 (85.8) |
| cauda equina | 139 (61.8) |
| nerve root | 5 (2.2) |
| retinal hemangioblastoma | 118 (52.4) |
| endolymphatic sac tumor | 29 (12.9) |
| visceral lesions | |
| renal cell carcinoma | 131 (58.2) |
| renal cyst | 144 (64.0) |
| pancreatic neuroendocrine tumor | 75 (33.3) |
| pancreatic cyst | 130 (57.8) |
| pheochromocytoma | 47 (20.9) |
| reproductive adnexal organ cystadenoma | 71 (31.6) |
Hemangioblastoma Features
Hemangioblastoma Burden
At study entrance, the 225 patients harbored 1921 craniospinal hemangioblastomas (mean 8.5 ± 7.0 tumors/patient, median 7 tumors/patient, range 1–33 tumors/patient) located in the supratentorial compartment (21 tumors [1%]), brainstem (129 [7%]), cerebellum (865 [45%]), spinal cord (689 [36%]), cauda equina (212 [11%]), and nerve roots (5 [0.3%]; Table 1). The mean total tumor volume per patient upon study entrance was 562.8 ± 1306.6 mm3 (median 182.5 mm3, range 12–11,919.9 mm3).
At the last evaluation, patients harbored 2336 craniospinal hemangioblastomas (mean 10.4 ± 7.8 tumors/patient, median 8 tumors/patient). Hemangioblastomas were found in the supratentorial compartment (21 tumors [1%]), brainstem (132 tumors [6%]), cerebellum (1049 tumors [45%]), spinal cord (867 tumors [37%]), cauda equina (261 tumors [11%]), and nerve roots (6 tumors [0.3%]). The mean total craniospinal hemangioblastoma volume per patient was 1618.4 ± 3734.7 mm3 (median 369.1 mm3, range 0–28,304.5 mm3). Total tumor follow-up was 15,819 hemangioblastoma-years.
New Hemangioblastoma Development
One hundred sixty-two patients (72%) exhibited new CNS hemangioblastomas during the study. Consistent with the overall anatomical distribution of tumors, new hemangioblastomas developed in the supratentorial compartment (4 [0.7%]), cerebellum (291 [50%]), brainstem (16 [3%]), spinal cord (217 [37%]), cauda equina (54 [9%]), or nerve roots (2 [0.3%]). Over the study duration, a mean of 0.4 ± 0.4 new tumors/year/patient developed (median 0.3 tumors).
Hemangioblastoma Growth
Twelve hundred seventy-eight hemangioblastomas (51% of tumors evaluated) remained stable and 1227 (49%) grew during the study. Progression was observed in 25% of tumors followed up for 1.5–5.0 years and in 50% of tumors followed up for 5.6–8.9 years, depending on tumor location (Fig. 1). Patterns of growth varied and were categorized as saltatory (886 tumors [72%]), linear (76 [6%]), or exponential (264 [22%]; Fig. 2). The median growth rate was 3.7 mm3/year (range 0.8–2331.9 mm3/year) for tumors growing in a saltatory pattern, 23.8 mm3/year (range 0.8–1245.4 mm3/year) in a linear pattern, and 79.2 mm3/year (range 1.8– 6216.6 mm3/year) in an exponential pattern (p < 0.0001 for all pairwise comparisons).
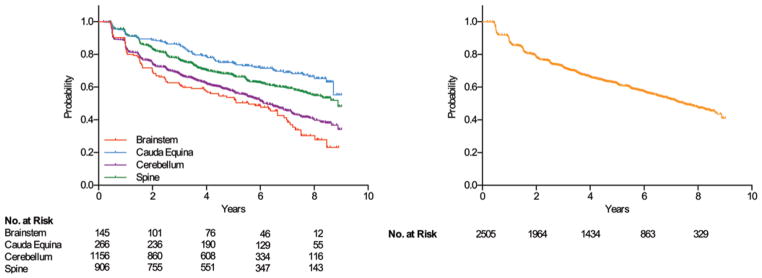
Fig. 1.
Kaplan-Meier analysis of hemangioblastoma progression over an 8-year period in patients with VHL disease. Left: Fifty percent of (nongrowing) hemangioblastomas located in the brainstem progressed in 5.6 years. Twenty-five percent of hemangioblastomas located in the cauda equina progressed in 5.0 years. Fifty percent of hemangioblastomas located in the cerebellum progressed in 6.2 years. Fifty percent of hemangioblastomas located in the spine progressed in 8.9 years. Right: Twenty-five percent of hemangioblastomas at all tumor locations progressed in 2.5 years and 50% in 7.5 years. No. at risk = tumors at risk for growing. The numbers of tumors at risk refer to the years on the x-axis under which they align.
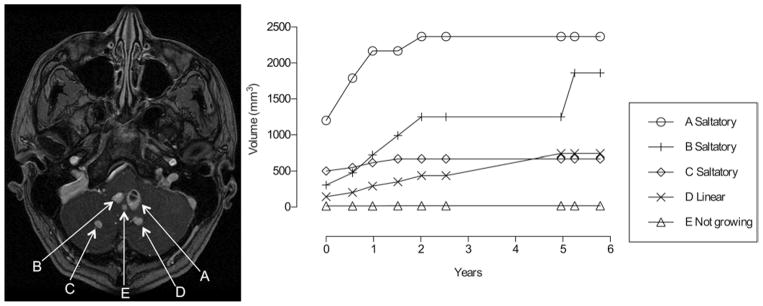
Fig. 2.
Characteristic patterns of growth associated with CNS hemangioblastomas in patients with VHL disease. Left: Axial contrast-enhanced MR image of the cerebellum in a patient showing 5 tumors (A–E) that demonstrated 3 growth patterns. Right: Tumor growth patterns, including saltatory (A–C), linear (D), and stable (E).
While the various patterns of growth were represented among the different anatomical regions, hemangioblastomas grew at significantly different rates depending on their anatomical region. Hemangioblastomas located in the brainstem (median 3.1 mm3/year) and cerebellum (median 1.2 mm3/year) grew significantly faster than those arising in the spinal cord (median 0.3 mm3/per year) or cauda equina (median 0 mm3/year; p < 0.0001).
Hemangioblastoma-Associated Cysts
During the study, 295 hemangioblastomas developed cysts. Two hundred forty-seven tumors (9.9% of total 2505) developed peritumoral cysts, 37 (1.5%) developed intratumoral cysts, and 11 (0.4%) developed both types of cyst. Five cysts arose in the supratentorial compartment (1.7%), 190 in the cerebellum (64.4%), 32 in the brainstem (10.8%), 67 in the spinal cord (22.7%), and 1 in a nerve root (0.3%).
Tumors associated with cysts demonstrated increased lesion volume, growth rate, and production of symptoms. For instance, there were 100 symptomatic cerebellar tumors (8.6%) among 1156 cerebellar hemangioblastomas, and 60 were associated with peritumoral cysts, as compared with 89 (8.4%) of the 1056 cerebellar hemangioblastomas without symptoms. The ratio of the median growth rate of peritumoral cysts to the tumor growth rate for symptomatic cerebellar hemangioblastomas was 22:1, and the ratio of the median volume of the peritumoral cyst compared with tumor was 10:1.
Hemangioblastoma Symptom Association
While 2346 hemangioblastomas were asymptomatic (93.6%) at the final evaluation, 159 hemangioblastomas (6.3%) in 75 patients produced symptoms and required treatment. Neurological symptoms and signs were caused by mass effect related to hemangioblastoma and/or cyst size in their specific anatomical location (Table 2). Fourteen of the hemangioblastomas (8.8%) that became symptomatic were not present on imaging at study entrance.
TABLE 2.
Symptoms based on anatomical location of 159 tumors*
| Lesion Location | Frequency of Symptomatic Lesions (%) |
|---|---|
| brainstem | |
| headache | 10 (83.3) |
| singultus | 8 (66.7) |
| nausea/vomiting | 6 (50.0) |
| dysphagia | 5 (41.7) |
| cough | 3 (25.0) |
| paresthesia | 3 (25.0) |
| cauda equina | |
| urinary/bowel abnormalities | 5 (100.0) |
| pain | 5 (100.0) |
| paresthesia | 4 (80.0) |
| headache | 2 (40.0) |
| cerebellum | |
| headache | 77 (77.0) |
| gait ataxia | 57 (57.0) |
| nausea/vomiting | 19 (19.0) |
| vertigo | 18 (18.0) |
| speech difficulties | 15 (15.0) |
| dysmetria | 11 (11.0) |
| nerve root | |
| cruciate paralysis | 1 (3.3) |
| paresthesia | 28 (73.7) |
| pain | 24 (63.2) |
| gait ataxia | 13 (34.2) |
| dysesthesias | 9 (23.7) |
| urinary/bowel abnormalities | 7 (18.4) |
| supratentorial | |
| vision disturbance/loss | 2 (50.0) |
| weakness | 2 (50.0) |
The symptoms listed here are the major symptoms recorded in patients who underwent resection of symptomatic tumors. Patients who underwent resections at outside institutions are not included in this analysis because of limited records.
Clinical Progression
The KPS score remained unchanged in 136 patients (60%), improved in 32 (14%; all associated with resection of symptom-producing hemangioblastomas), and worsened in 57 (25%). Declines in KPS scores during the study were a result of disease progression (45 patients [20% of all patients]), postoperative morbidity (10 patients [4%]), or unknown reasons (2 patients [1%]).
Analysis of Factors Associated With Tumor Behavior
Tumor Burden
Factors associated with an increased tumor burden included male sex (p = 0.002; mean 11.5 tumors/male patient vs 9.2 tumors/female patient; median 9 vs 7, respectively), longer follow-up (p = 0.007), and genotype (p = 0.002; Table 3). Specifically, patients harboring partial germline deletions (60 patients [27%]) had more tumors than patients with missense mutations (86 patients [38%]; p = 0.005).
TABLE 3.
Analysis of factors associated with tumor behavior*
| Outcome Measure & Factor | p Value |
|---|---|
| total tumor burden (no. of tumors; 225 patients) | |
| age | NS |
| yrs of follow-up | 0.0070 |
| sex | 0.002 |
| mutation† | 0.0021 |
| partial deletion | reference |
| deletion | 0.0667 |
| frameshift | 0.0450 |
| missense | 0.0046 |
| nonsense | 0.2508 |
| new tumor development (no. of tumors; 225 patients) | |
| sex | NS |
| mutation† | NS |
| no. of tumors at study entrance | <0.0001 |
| age in yrs | <0.0001 |
| 12–20 | reference |
| 21–30 | 0.0224 |
| 31–40 | 0.0005 |
| 41–50 | <0.0001 |
| >50 | <0.0001 |
| tumor growth rate (mm3/yr; 2505 tumors) | |
| age | NS |
| mutation† | NS |
| sex | 0.0014 |
| tumor location | <0.0001 |
| tumor symptom type | <0.0001 |
| tumor location × tumor symptom type | 0.0001 |
| cerebellum tumor growth rate (mm3/yr; 1156 tumors) | |
| age | NS |
| mutation† | NS |
| sex | NS |
| cyst-linked tumor type | <0.0001 |
| tumor symptom type | <0.0001 |
| cyst-linked tumor type × tumor symptom type | <0.0001 |
| cerebellum cyst growth rate (mm3/yr; 175 cysts) | |
| sex | NS |
| mutation† | NS |
| age (continuous) | 0.0079 |
| tumor symptom type | <0.0001 |
| cyst type | NS |
| tumor symptom type × cyst type | 0.0342 |
Linear or linear mixed models were performed to evaluate the association of factors with outcome measures. Age, sex, and mutation would be dropped from the model if they were not significant based on a significance level of 0.1.
NS = not significant.
Genotype was available for 197 of the 225 patients.
New Tumor Development
Sex and genotype had no effect on the development of new tumors (Table 3). Patients with a greater tumor burden at study entrance demonstrated more tumors over the course of the study (p < 0.0001). Age had a significant (p < 0.0001) effect on tumor development. Patients 12–20 years of age developed more tumors per year than older groups.
Tumor Growth
Tumors grew faster in males than in females (p = 0.001; Table 3). The effects of tumor growth rate on the presence and type of symptoms varied with location (p < 0.0001). For each anatomical compartment, the symptom-producing tumors grew faster than the asymptomatic ones (p < 0.0001). Symptom-producing tumors in the spine grew significantly slower than those in the cerebellum (p = 0.0007). Asymptomatic tumors in the spine and cauda equina (no significant difference between these 2 regions, p = 1.0) grew significantly (p < 0.0001) slower than those in the brainstem and cerebellum (no significant difference between these 2 regions, p = 0.7). For each anatomical compartment, tumors associated with a cystic component grew substantially faster than tumors not associated with cysts (p < 0.0001). For the cerebellar compartment, symptomatic tumors associated with intratumoral cysts grew faster than those associated with peritumoral cysts (p < 0.0001).
Cyst Growth
All types of cerebellar cysts (peritumoral, intratumoral, or combination of the two) grew at greater rates in younger patients (p = 0.01; Table 3). Peritumoral cysts grew faster in symptomatic (p < 0.0001) than in asymptomatic hemangioblastomas.
Factors That Predict Symptom Formation
Receiver operating characteristic analysis demonstrated that thresholds of location-dependent tumor size predict symptom formation (AUC of 0.99, 0.94, and 0.97 for brainstem, cerebellum, and spine, respectively). When specificity was set to at least 90%, cerebellar hemangioblastomas reaching a diameter of 5.6 mm, brainstem hemangioblastomas reaching a diameter of 4.0 mm, and spinal cord hemangioblastomas reaching a diameter of 4.5 mm would be diagnosed as symptomatic with a sensitivity of 78% (specificity 96%), 53% (specificity 92%), and 78% (specificity 95%), respectively.
Discussion
Previous Reports
Clinical studies have been used to develop successful paradigms for the treatment of VHL-associated visceral tumors (renal cell carcinoma, pancreatic neuroendocrine tumors, and pheochromocytomas)11,27,28 and endolymphatic sac tumors.3,18 Previous reports defining the natural history of VHL-associated hemangioblastomas have been inconclusive because of their retrospective analyses, inadequate numbers of patients, and limited follow-up.1,17,24,29 Given these limitations, the natural history of CNS hemangioblastomas, the timing for removing VHL-associated CNS hemangioblastomas, and the role of radiosurgery and/or chemotherapy are unknown. To better understand features that can be used to guide management of CNS hemangioblastomas in VHL, we performed a long-term, prospective natural history study in a large cohort of patients.
Biological and Clinical Implications
Tumor Burden and New Tumor Development
Male sex was associated with increased CNS hemangioblastoma burden and growth rate, which may indicate that male hormonal influences affect the development and growth of CNS hemangioblastomas. This effect of sex on tumor development is consistent with previous findings that pregnancy did not impact hemangioblastoma development or progression in VHL patients of childbearing years.34
New hemangioblastomas developed in most patients (72%). An increased rate of tumor development was associated with tumor burden at study entrance and younger age. These findings suggest that patients with more tumors at any point in time may be predisposed to additional tumors over a lifetime. Moreover, tumors developed in younger patients at a faster rate than in older patients. This finding indicates that CNS hemangioblastoma development in VHL may be affected by biological features related to developmental processes, hormonal factors, other systemic factors, and/or proteasomal processing.
Patients with partial germline deletions had an increased tumor burden as compared with the patients with missense mutations. Recent observations indicate that protein produced by missense germline alterations in neoplasia and metabolic syndromes, including VHL,33 can maintain intrinsic function.13,32 Further, the severity of disease burden and tumor development in patients with familial neoplasia syndromes may be the result of the rapid degradation of functional mutant protein via proteasome control pathways32 and/or an age-related decline in proteasome function of mutated tumor suppressor protein.9 These findings suggest that residual VHL protein (tumor suppressor) function associated with a germline missense mutation may underlie reduced disease severity.
Growth of Tumors
Consistent with prior retrospective studies,1,29 variable patterns of tumor growth were observed. While approximately half (51%) of all CNS hemangioblastomas remained stable in size during long-term follow-up, 49% grew. The most common pattern of growth was saltatory (72% of growing tumors), followed by exponential (22%) and linear (6%). Because most tumors remain stable or grow in a saltatory pattern (characterized by prolonged periods, often years, of quiescence), extended periods of follow-up (probably 5 or more years) are necessary to accurately assess the efficacy of nonsurgical therapies, such as chemotherapy and radiation therapy, and tumor stability. Previously, we analyzed CNS hemangioblastomas treated with stereotactic radiosurgery in a cohort of patients with VHL (40 hemangioblastomas in 20 patients).2 At nearly 6 years after stereotactic radiosurgery, no progression occurred in 33% of tumors, similar to the natural history of (untreated) CNS hemangioblastoma progression in the current study (25%).
Clinical Progression
Previous reports have indicated that patients with VHL have a shortened lifespan because of complications related to renal cell carcinoma and CNS hemangioblastomas.30 Similarly, our study indicates that 25% of mortality was the result of VHL-associated tumor progression. Specifically, progression of CNS hemangioblastomas was the leading cause of VHL-associated mortality, followed by renal cell carcinoma–related disease. These findings differ from those in a number of previous reports that describe renal cell carcinoma as the leading cause of VHL-associated mortality4,17 and may reflect the universal occurrence of VHL-associated hemangioblastomas in the current study population.
The mainstay of therapy for sporadic and VHL-associated CNS hemangioblastomas is resection. Surgery can be safely performed in most patients. Better patient function in the current study was the result of resection of symptomatic CNS hemangioblastomas. These findings are similar to those in previous studies demonstrating symptom stability or improvement in most patients after resection of hemangioblastomas.8,31 Together, these results underscore the lasting clinical stability provided in most patients by the judicious treatment of symptom-producing hemangioblastomas and the avoidance of unnecessary therapies for asymptomatic tumors that may not progress.
Conclusions
Consistent with prior retrospective studies,1,29 signs and symptoms of hemangioblastomas are dictated by tumor location, associated edema, and cyst formation and propagation (Table 2).8 However, absolute size of the lesion did not dictate the symptoms for tumors in all locations, and neither did the rate of growth. Although a threshold size was used as the primary factor in the ROC modeling to predict eventual symptom formation, the likely time to symptom formation for individual lesions remains undefined because of the saltatory growth pattern exhibited by many tumors. These findings support the onset of symptoms associated with a particular CNS hemangioblastoma as the indication for surgery.
Abbreviations used in this paper
- AUC
area under the ROC curve
- KPS
Karnofsky Performance Scale
- ROC
receiver operating characteristic
- VHL
von Hippel-Lindau
Footnotes
Disclosure
This research was supported by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke, the Clinical Center, National Eye Institute and the National Cancer Institute at the National Institutes of Health. The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Lonser, Oldfield. Acquisition of data: Lonser, Butman, Huntoon, Asthagiri, Bakhtian, Chew, Zhuang, Linehan, Oldfield. Analysis and interpretation of data: Lonser, Butman, Huntoon, Bakhtian, Chew, Zhuang, Oldfield. Drafting the article: Lonser, Huntoon, Bakhtian, Zhuang, Oldfield. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Lonser. Statistical analysis: Huntoon, Wu. Administrative/technical/material support: Lonser, Bakhtian. Study supervision: Lonser, Oldfield.
References
- 1.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: implications for treatment. J Neurosurg. 2006;105:248–255. doi: 10.3171/jns.2006.105.2.248. [DOI] [PubMed] [Google Scholar]
- 2.Asthagiri AR, Mehta GU, Zach L, Li X, Butman JA, Camphausen KA, et al. Prospective evaluation of radiosurgery for hemangioblastomas in von Hippel-Lindau disease. Neuro Oncol. 2010;12:80–86. doi: 10.1093/neuonc/nop018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butman JA, Kim HJ, Baggenstos M, Ammerman JM, Dambrosia J, Patsalides A, et al. Mechanisms of morbid hearing loss associated with tumors of the endolymphatic sac in von Hippel-Lindau disease. JAMA. 2007;298:41–48. doi: 10.1001/jama.298.1.41. [DOI] [PubMed] [Google Scholar]
- 4.Butman JA, Linehan WM, Lonser RR. Neurologic manifestations of von Hippel-Lindau disease. JAMA. 2008;300:1334–1342. doi: 10.1001/jama.300.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 6.Filling-Katz MR, Choyke PL, Oldfield E, Charnas L, Patronas NJ, Glenn GM, et al. Central nervous system involvement in Von Hippel-Lindau disease. Neurology. 1991;41:41–46. doi: 10.1212/wnl.41.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Gläsker S. Central nervous system manifestations in VHL: genetics, pathology and clinical phenotypic features. Fam Cancer. 2005;4:37–42. doi: 10.1007/s10689-004-5347-6. [DOI] [PubMed] [Google Scholar]
- 8.Jagannathan J, Lonser RR, Smith R, DeVroom HL, Oldfield EH. Surgical management of cerebellar hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2008;108:210–222. doi: 10.3171/JNS/2008/108/2/0210. [DOI] [PubMed] [Google Scholar]
- 9.Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamiell JM, Salazar FG, Hsia YE. von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore) 1989;68:1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Libutti SK, Choyke PL, Bartlett DL, Vargas H, Walther M, Lubensky I, et al. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124:1153–1159. doi: 10.1067/msy.1998.91823. [DOI] [PubMed] [Google Scholar]
- 12.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Yang C, Chen M, Ye DY, Lonser RR, Brady RO, et al. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc Natl Acad Sci U S A. 2011;108:21200–21205. doi: 10.1073/pnas.1119181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundin P, Pedersen F. Volume of pituitary macroadenomas: assessment by MRI. J Comput Assist Tomogr. 1992;16:519–528. doi: 10.1097/00004728-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Maddock IR, Moran A, Maher ER, Teare MD, Norman A, Payne SJ, et al. A genetic register for von Hippel-Lindau disease. J Med Genet. 1996;33:120–127. doi: 10.1136/jmg.33.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maher ER, Iselius L, Yates JR, Littler M, Benjamin C, Harris R, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28:443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel- Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 18.Manski TJ, Heffner DK, Glenn GM, Patronas NJ, Pikus AT, Katz D, et al. Endolymphatic sac tumors. A source of morbid hearing loss in von Hippel-Lindau disease. JAMA. 1997;277:1461– 1466. doi: 10.1001/jama.277.18.1461. [DOI] [PubMed] [Google Scholar]
- 19.Melmon KL, Rosen SW. Lindau’s disease. Review of the literature and study of a large kindred. Am J Med. 1964;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- 20.Neumann HP, Eggert HR, Scheremet R, Schumacher M, Mohadjer M, Wakhloo AK, et al. Central nervous system lesions in von Hippel-Lindau syndrome. J Neurol Neurosurg Psychiatry. 1992;55:898–901. doi: 10.1136/jnnp.55.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann HP, Lips CJ, Hsia YE, Zbar B. Von Hippel-Lindau syndrome. Brain Pathol. 1995;5:181–193. doi: 10.1111/j.1750-3639.1995.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 22.Richard S, Campello C, Taillandier L, Parker F, Resche F. Haemangioblastoma of the central nervous system in von Hippel-Lindau disease. J Intern Med. 1998;243:547–553. doi: 10.1046/j.1365-2796.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 23.Richard S, Chauveau D, Chrétien Y, Beigelman C, Denys A, Fendler JP, et al. Renal lesions and pheochromocytoma in von Hippel-Lindau disease. Adv Nephrol Necker Hosp. 1994;23:1–27. [PubMed] [Google Scholar]
- 24.Richard S, David P, Marsot-Dupuch K, Giraud S, Béroud C, Resche F. Central nervous system hemangioblastomas, endolymphatic sac tumors, and von Hippel-Lindau disease. Neurosurg Rev. 2000;23:1–24. doi: 10.1007/s101430050024. [DOI] [PubMed] [Google Scholar]
- 25.Simone CB, II, Lonser RR, Ondos J, Oldfield EH, Camphausen K, Simone NL. Infratentorial craniospinal irradiation for von Hippel-Lindau: a retrospective study supporting a new treatment for patients with CNS hemangioblastomas. Neuro Oncol. 2011;13:1030–1036. doi: 10.1093/neuonc/nor085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stolle C, Glenn G, Zbar B, Humphrey JS, Choyke P, Walther M, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12:417–423. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Walther MM, Choyke PL, Glenn G, Lyne JC, Rayford W, Venzon D, et al. Renal cancer in families with hereditary renal cancer: prospective analysis of a tumor size threshold for renal parenchymal sparing surgery. J Urol. 1999;161:1475–1479. doi: 10.1016/s0022-5347(05)68930-6. [DOI] [PubMed] [Google Scholar]
- 28.Walther MM, Reiter R, Keiser HR, Choyke PL, Venzon D, Hurley K, et al. Clinical and genetic characterization of pheochromocytoma in von Hippel-Lindau families: comparison with sporadic pheochromocytoma gives insight into natural history of pheochromocytoma. J Urol. 1999;162:659–664. doi: 10.1097/00005392-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82–94. doi: 10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 30.Wilding A, Ingham SL, Lalloo F, Clancy T, Huson SM, Moran A, et al. Life expectancy in hereditary cancer predisposing diseases: an observational study. J Med Genet. 2012;49:264–269. doi: 10.1136/jmedgenet-2011-100562. [DOI] [PubMed] [Google Scholar]
- 31.Wind JJ, Bakhtian KD, Sweet JA, Mehta GU, Thawani JP, Asthagiri AR, et al. Long-term outcome after resection of brainstem hemangioblastomas in von Hippel-Lindau disease. Clinical article. J Neurosurg. 2011;114:1312–1318. doi: 10.3171/2010.9.JNS10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C, Asthagiri AR, Iyer RR, Lu J, Xu DS, Ksendzovsky A, et al. Missense mutations in the NF2 gene result in the quantitative loss of merlin protein and minimally affect protein intrinsic function. Proc Natl Acad Sci U S A. 2011;108:4980–4985. doi: 10.1073/pnas.1102198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C, Huntoon K, Ksendzovsky A, Zhuang Z, Lonser RR. Proteostasis modulators prolong missense VHL protein activity and halt tumor progression. Cell Rep. 2013;3:52–59. doi: 10.1016/j.celrep.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye DY, Bakhtian KD, Asthagiri AR, Lonser RR. Effect of pregnancy on hemangioblastoma development and progression in von Hippel-Lindau disease. Clinical article. J Neurosurg. 2012;117:818–824. doi: 10.3171/2012.7.JNS12367. [DOI] [PubMed] [Google Scholar]