Abstract
Object
Investigators in experimental and clinical studies have used the intrathecal route to deliver drugs to prevent or treat vasospasm. However, a clot near an artery or arteries after subarachnoid hemorrhage (SAH) may hamper distribution and limit the effects of intrathecally delivered compounds. In a primate model of right middle cerebral artery (MCA) SAH, the authors examined the distribution of Isovue-M 300 and 3% Evans blue after infusion into the cisterna magna CSF.
Methods
Ten cynomolgus monkeys were assigned to SAH and sham SAH surgery groups (5 in each group). Monkeys received CSF injections as long as 28 days after SAH and were killed 3 hours after the contrast/Evans blue injection. The authors assessed the distribution of contrast material on serial CT within 2 hours after contrast injection and during autopsy within 3 hours after Evans blue staining.
Results
Computed tomography cisternographies showed no contrast in the vicinity of the right MCA (p < 0.05 compared with left); the distribution of contrast surrounding the entire right cerebral hemisphere was substantially reduced. Postmortem analysis demonstrated much less Evans blue staining of the right hemisphere surface compared with the left. Furthermore, the Evans blue dye did not penetrate into the right sylvian fissure, which occurred surrounding the left MCA. The authors observed the same pattern of changes and differences in contrast distribution between SAH and sham SAH animals and between the right and the left hemispheres on Days 1, 3, 7, 14, 21, and 28 after SAH.
Conclusions
Intrathecal drug distribution is substantially limited by SAH. Thus, when using intrathecal drug delivery after SAH, vasoactive drugs are unlikely to reach the arteries that are at the highest risk of delayed cerebral vasospasm.
Keywords: subarachnoid hemorrhage, vasospasm, thrombolysis, intrathecal, cerebrospinal fluid
DRUG distribution after intrathecal administration has been extensively studied in clinical and experimental settings.34,37 This route is an important conduit for treating CNS infections, leptomeningeal involvement in certain neoplasms, spasticity, and intractable pain.5,8,13,20,29 Almost 40 years ago, to increase local drug concentrations and avoid complications associated with systemic administration,6,18,32 intrathecal administration was explored to treat delayed cerebral vasospasm after aneurysmal SAH. Recently, intrathecal delivery has gained renewed interest among vasospasm researchers because of new drug development (recombinant tPA and urokinase), new drug packaging techniques (slow-releasing polymers and tablets), new technologies,14 and new biological therapeutic approaches (viral gene delivery, liposomes for drug and gene therapies, and antisense oligonucleotides) that must be delivered locally or into the CSF to produce an anti-vasospastic effect (Appendix, available online). However, increased use of the intrathecal route to treat vasospasm has not been associated with studies designed to better understand drug distribution in the subarachnoid space after SAH. Studies of drug distribution in the CSF after SAH are scant and have been performed only with small animal models of SAH.33,36 Furthermore, when a primate model of SAH has been investigated in treatment studies, inconsistent effects are reported using the same drug.1,25,27,38 These discrepancies were attributed to the drug or drugs and usually resulted in abandoning the preclinical therapy or discontinuing clinical trials.42,43
Both the presence of a clot encompassing a conductive artery or arteries and inflammatory changes in the vicinity of the clot after SAH may preclude an intrathecally delivered drug from reaching the artery or arteries in spasm. We used CT and macroimaging (photography) to examine the distribution of different compounds after intrathecal administration using a primate model of SAH. We sought to establish whether a subarachnoid clot affects drug distribution in the presence of SAH. We chose a primate model because this model mimics the relationship between development of vasospasm and the presence of blood in the subarachnoid space in humans9,28,35 and because the amount of blood placed around the right MCA9,35 corresponds to Fisher Grade 3 after aneurysmal bleeding.12
Methods
Experimental Design
Ten cynomolgus monkeys were randomly assigned to SAH or sham SAH groups (5 in each group). Animals underwent a right frontotemporal craniectomy with transsylvian exposure of the proximal portion of the right MCA and bifurcation of the ICA. Four animals each in the SAH and sham SAH groups comprised the “acute” group and 2 animals constituted the “subacute” group. The monkeys in the sham SAH group underwent the same surgery as those in the SAH group, but saline instead of blood was placed in the subarachnoid space.
Serial CT contrast (Isovue-M 300, Bracco Diagnostics, Inc.) cisternography was used for in vivo assessment and quantification of contrast distribution in the CSF, whereas Evans blue (Sigma) cisternography was used to detect CSF distribution with sensitivity. Evans blue distribution was assessed during autopsy.
Four monkeys experienced SAH followed by CT contrast cisternography performed on Day 1 after SAH (2 animals), and CT contrast with 3% Evans blue cisternography on Day 3 (1 animal) or 7 (1 animal). Four monkeys in the sham SAH group underwent cisternographies on postoperative Day 3 (1 animal) or 7 (3 animals). These 8 animals constituted the acute group and were killed within 1 hour after the last CT scan. Two additional animals, which formed the subacute group (1 SAH animal and 1 sham SAH animal), underwent repeated CT cisternographies performed on postoperative Days 7, 14, 21 (SAH only), and 28. These animals were killed on postoperative Day 28 after the last cisternal injection of CT contrast material and 3% Evans blue. Comparison of the presence of contrast material and dye was performed between the right and left hemispheres, sylvian fissures, and MCA walls. The animal protocol was approved by the Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke and met the National Institutes of Health guidelines for animal care.
Subarachnoid Hemorrhage Model
As described in previous studies,9,35 under 1.0% isoflurane general anesthesia and aseptic conditions, mon-keys underwent a right frontotemporal craniectomy (2 × 1.5 cm) and opening of the arachnoid over the proximal portion of the MCA, bifurcation of the ICA, and sharply opening of the Liliequist membrane. Five milliliters of arterial blood was collected from the left femoral artery, allowed to clot, and placed around the exposed right MCA. The dura was closed to form a watertight seal and the wound was closed in layers; the monkey was extubated on regaining a gag reflex. In the sham SAH group, the same procedures were performed except that 5 ml of warm saline was injected around the right MCA.
Computed Tomography Cisternography and Data Acquisition
On postoperative Days 1, 3, 7, 14, 21, and 28, animals were sedated (using intramuscular injection of 10–15 mg/kg ketamine and 2 mg/kg xylazine), intubated, and transported to the CT facility to undergo CT cisternography. The general and neurological status of all animals was closely monitored during the experimental period, and respiration, pulse, O2 saturation, and body temperature (maintained using hot water blankets) were monitored during the procedures by the investigators and the veterinary technical support staff. Each animal was placed in a prone position with the head slightly below the level of the chest in the CT gantry.
Computed tomography was performed on a slice helical scanner (Mx8000 IDT 16, Philips). Computed tomography scan parameters were 90 kV, 400 mAs, field of view 98 mm, collimation 4 × 0.75, pitch 0.35, thickness 1.0 mm, and overlap 0.5 mm. Computed tomography was acquired in helical mode using 1-mm slice thickness and reconstructed to a field of view of 9.8 cm, 90 kV, 400 mAs, and rotation time of 1 second.
Baseline axial CT images of the brain were acquired. Axial CT was used to identify the space between the foramen magnum and C-1, and the skin surface was marked. Using sterile technique, the back of the monkey’s head was then shaved and prepared with 3 alternative applications of Betadine solution and alcohol. A 22-gauge needle was carefully introduced into the cisterna magna under CT guidance. To minimize the infusion effect on intracranial pressure, free drainage of 1 ml of CSF was allowed; 1 ml of contrast material (Isovue-M 300) or contrast mixed with 3% Evans blue (0.5 ml) was then delivered over the course of 3 minutes. Cranial CT was performed immediately following the injection. Subsequent CT scans were performed every 15 minutes for as long as 2 hours following injection. For quantification, the CT cisternogram showing a maximal concentration of contrast on the unoperated side was used; typically this was the scan performed 30 minutes after intrathecal injection. Quantification was performed by placing small (~ 50 voxel) regions of interest in the region of the M1 segment of the MCAs and on the brain surface above the sylvian fissure in the parietal lobe on both sides using a picture archiving and communications system (PACS) workstation (DirectView, Kodak Health Systems) or the Analysis of Functional NeuroImages program (AFNI; http://afni.nimh.nih.gov/afni).
For assessment of Evans blue distribution after the last of the serial CT cisternographies, monkeys were killed using 90 mg/kg intravenous pentobarbital followed by immediate fixation via intracardiac perfusion of 3% glutaraldehyde (Electron Microscopy Sciences) and 1% formaldehyde (Electron Microscopy Sciences) in 0.1 M phosphate-buffered saline (pH 7.4). The brain was quickly removed and photographed, and the arteries of the circle of Willis were dissected and photographed.
Statistical Analysis
Statistical tests included a 1-way ANOVA and the Student-Newman-Keuls test for pairwise comparisons. Statistical analysis of logarithmic data was performed using MedCalc software. A probability value < 0.05 was considered statistically significant.
Results
Acute Group
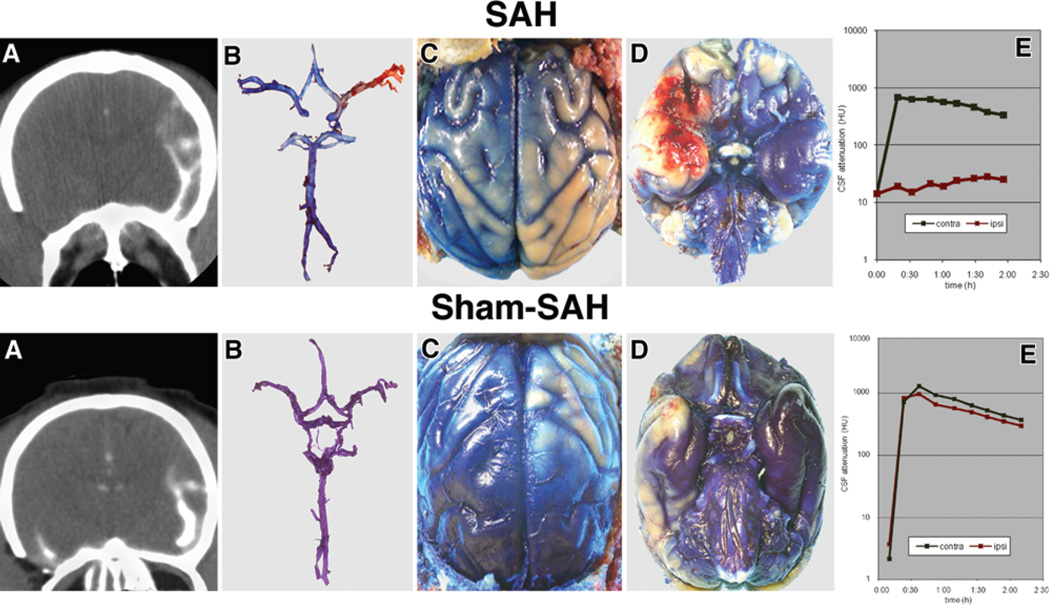
In the acute group, CT cisternography performed within 1 week of surgery consistently showed the distribution of contrast material. On the side contralateral to the surgery, contrast material freely distributed around the MCA, the sylvian fissure, and the sulci over the convexities for animals in both the SAH and sham SAH groups (Fig. 1A, upper and lower). On the side ipsilateral to the SAH (clot placed around the MCA), contrast material did not penetrate the cisterns around the MCA, did not enter the sylvian fissure, and only weakly distributed over the convexities in 4 of the 5 animals (p < 0.05). In 1 animal, contrast material did penetrate the region of the SAH around the MCA, but the contrast distribution was delayed and weak compared with the contralateral side. Evans blue distribution recorded photographically (performed in 4 animals) showed a distribution equivalent to that observed on CT (Fig. 1B–D, upper).
Fig. 1.
Images from the animals in the SAH (top row) and sham SAH (bottom row) groups. SAH Group: On Day 7 after SAH, CT cisternography (A) revealed contrast material entering the sylvian fissure and distributed over the surface of the cerebral hemisphere on the side opposite the SAH, but no contrast present in the vicinity of the right MCA. There is significantly less contrast on the surface of the right hemisphere. Evans blue dye injected 3 hours before death (B) stains blue the proximal (M1) portion of the right MCA, the segment close to the ICA, leaving the M2 branches unstained. The convexity of the right hemisphere (C) and the base of the frontal and temporal lobes near the right Sylvian fissure (D) are also less stained than the corresponding areas of the left cerebral hemisphere. The graph (E) depicts the CSF attenuation in the sylvian fissure on the CT cisternographies performed every 30 minutes for 2 hours on postoperative Day 7 after SAH in the same animal. There was a significant difference (p < 0.05) in the CSF attenuation between the right (ipsilateral) and left (contralateral) sides. Sham SAH Group: On Day 7 after sham SAH, CT cisternography (A) revealed contrast material entering the sylvian fissure along the left MCA and distribution of the material over the convexity of the cerebral hemisphere on the side opposite surgery. Contrast is also present in the vicinity of the right MCA in the right sylvian fissure. There is less contrast on the surface of the right cerebral hemisphere. Evans blue dye injected 3 hours before death (B) evenly stained blue the right and left MCAs as well as the rest of the cerebral arteries, although there is an appreciable difference in dye distribution over the convexity of the right and left hemispheres (C) and at the base on the brain (D). The graph (E) depicts the CSF attenuation in the sylvian fissure on the CT cisternographies performed every 30 minutes for 2 hours on postoperative day 7 after sham SAH surgery in the same animal. There was no difference (p > 0.05) in the CSF attenuation between the right (ipsilateral) and left (contralateral) sides but there was diminished contrast in the parietal region on the ipsilateral compared with contralateral side (p < 0.02).
On the side ipsilateral to the sham surgery, contrast material rapidly accumulated in the CSF spaces surrounding the MCA, as well as in the sylvian fissure and sulci of the convexities in 5 animals. There was no delay of contrast accumulation, although the density of the maximal contrast accumulation was less than that in sulci contralateral to the surgery (p < 0.02; Fig. 1A, lower). Evans blue data (recorded in 4 animals) showed a distribution equivalent to that observed on CT (Fig. 1B–D, lower).
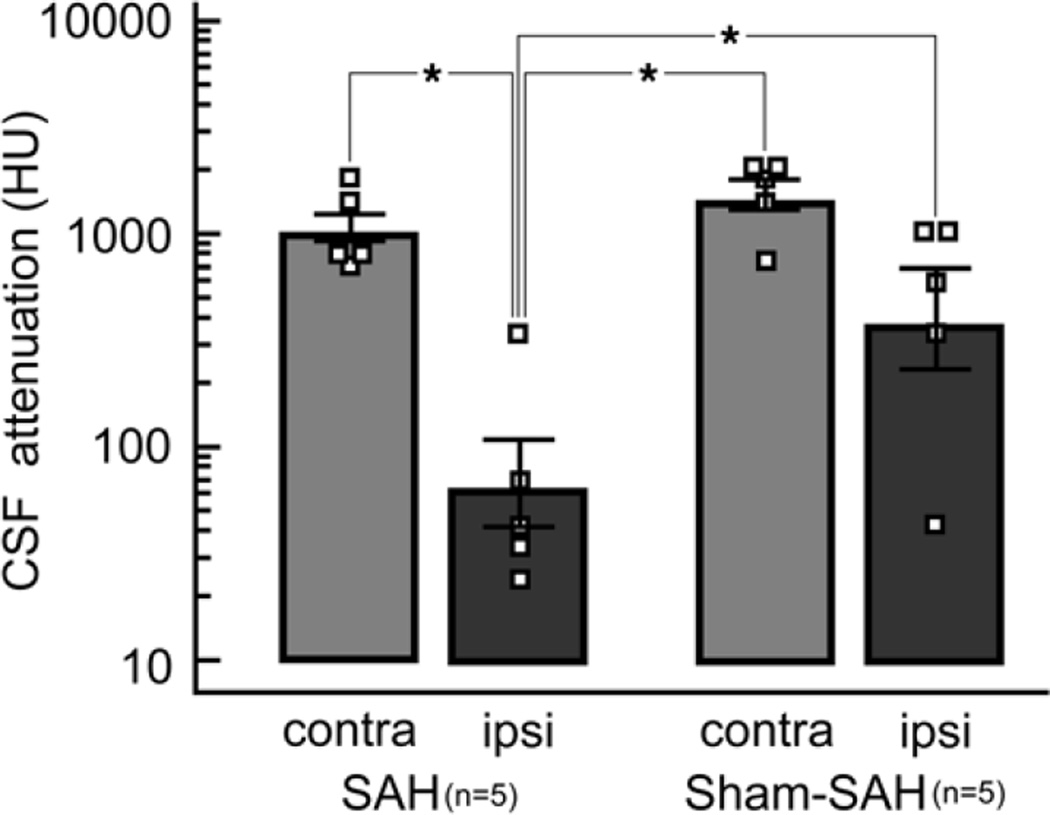
Contrast enhancement in the sylvian fissure on the side ipsilateral to clot placement in the SAH was signifi-cantly less than that in the sylvian fissure of the sham-operated animals as well as the contralateral sylvian fissures (p < 0.05; Fig. 2).
Fig. 2.
Bar graph of the peak CSF attenuation adjacent to the M1 segment of the MCA contralateral (contra) and ipsilateral (ipsi) to surgery in which either a clot was placed to mimic SAH or in which identical surgery was performed, but no clot was placed (sham-SAH). Contrast distribution in the region of the clot is limited compared with the contralateral side and compared with both sides after sham SAH (*p < 0.05, Student-Newman-Keuls test for all pairwise comparisons, 1-way ANOVA).
Subacute Group
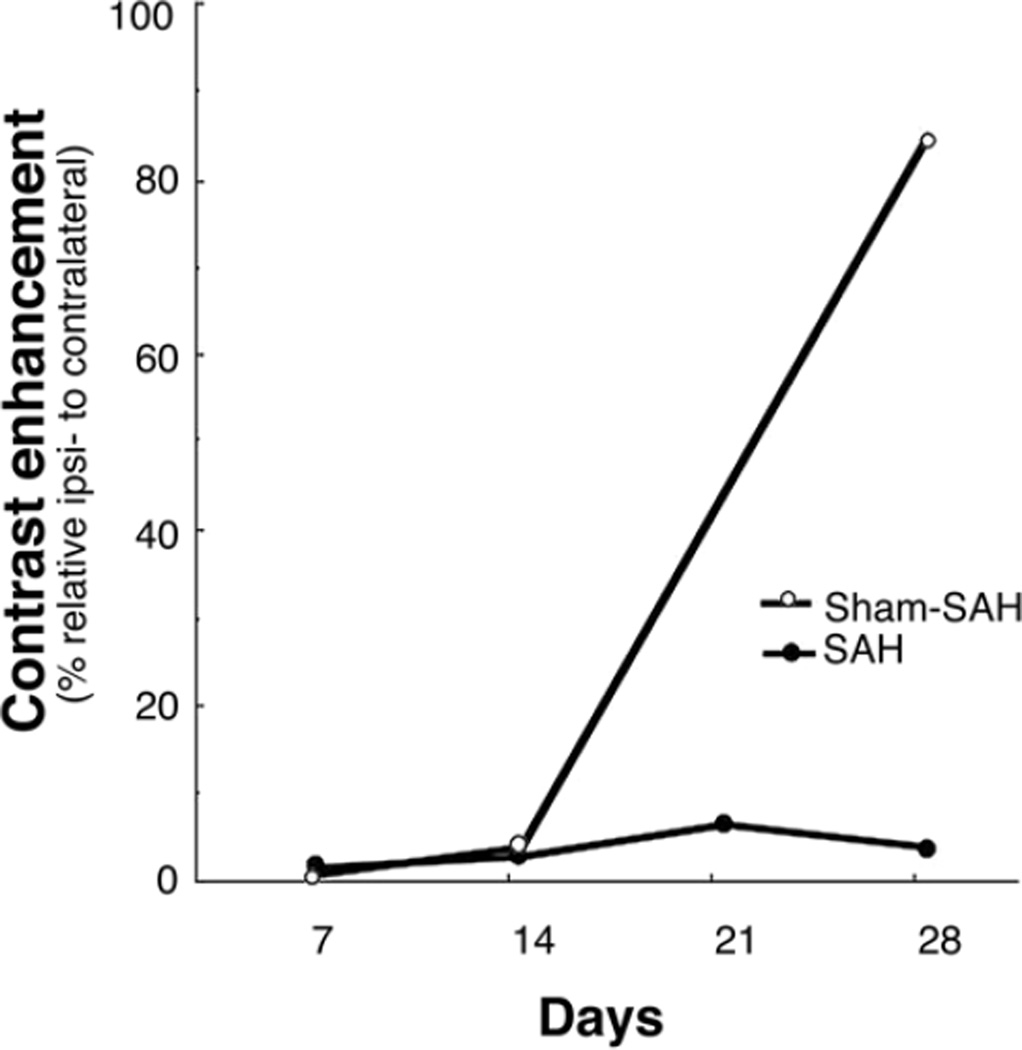
In the subacute group, CT cisternography revealed persistence of the diminished contrast distribution on the right side of the brain and within the right sylvian fissure on Days 7, 14, 21, and 28 after SAH and on Days 7 and 14 after sham SAH surgery (Fig. 3). On Day 28 after sham surgery, the contrast material was distributed almost evenly between the 2 sides.
Fig. 3.
Line graph depicting the relative density (%) of the contrast in the sylvian fissure on the side ipsilateral to surgery compared with the maximum density on the side contralateral to surgery after injection of contrast into the cisterna magna on CT cisternography on Days 7, 14, 21, and 28 after SAH and on Days 7, 14, and 28 after sham SAH surgery. The graph demonstrates a persistent decrease of contrast penetration for 2 weeks after sham SAH surgery and for 4 weeks (the end of study) after SAH. Note the undisturbed distribution of contrast on the unoperated side and initial limitation, but recovery, of CSF distribution on the side of sham surgery (Sham-SAH) between 2 and 4 weeks after surgery.
Discussion
The results of this study confirm our hypothesis that, in the presence of a clot, the distribution of contrast and dye from the CSF into the subarachnoid space is substantially restricted in the vicinity of arteries covered by the clot and at risk for spasm and that this limited distribution persists for a prolonged interval. The authors recognize the limitation of this experimental study. However, because similar imaging procedures (such as serial CT cisternography) and the interventions that they require would be extremely difficult after severe aneurysmal SAH in patients, and because this primate model of SAH is widely recognized as the best model of vasospasm, we believe that the conclusions of this study are valid and that its applicability to the clinical setting is strongly supported by our findings.
Vasospasm Treatment Strategy Using Intrathecal Drug Delivery
Easy access to the CSF has prompted numerous researchers to use intrathecal delivery of drugs in an attempt to treat or prevent delayed cerebral vasospasm following aneurysmal SAH in preclinical and clinical trials (Appendix, available online). In the 1980s, intrathecally administered nimodipine was reported to increase efficacy without evoking a deleterious reduction of blood pressure and cerebral perfusion.4,15,45 Intrathecal delivery of other drugs also was reported to prevent or reverse delayed cerebral vasospasm after SAH, while avoiding the complications of systemic administration.3,45 However, Lewis and colleagues,25 using a primate model, found that intrathecally administered nimodipine dilated the MCA in only 1 of 8 animals. In a clinical study of intrathecal nicardipine,39 vasospasm was not reversed except in arteries in the proximity of the catheter tip. Additionally, although intrathecally administered endothelin-1 antagonists showed potent antivasospastic effects in rat and rabbit SAH models,26,48 this effect did not occur in canine or primate models.7, 19
Regardless of these observations, the discovery of new classes of potentially effective drugs with a variety of mechanisms of action led to renewed interest in intrathecal delivery of many drugs. Despite limited knowledge about drug distribution in the subarachnoid cisterns after SAH, researchers and clinicians describing negative results often concluded that the failure was due to ineffective drugs.1,38 The possibility that the presence of a blood clot in the subarachnoid space limited drug distribution was never addressed. This error in interpretation persists despite knowledge that clot thickness is related to development of vasospasm,12,24 and its thickness differs greatly from patient to patient24 and among models of disease.28
Clot Effect on Drug Distribution in the Subarachnoid Space
Physical removal and irrigation of the clot during surgery as well as during recovery (with a head-shaking device)21 have been advocated and were found to lower the risk of vasospasm in experimental47 and clinical trials. 22,23,31 However, a complete removal of blood from a clot in the subarachnoid space remains impossible. Nevertheless, few studies quantify drug distribution in the subarachnoid space after SAH.25,33,36 In a rabbit model of SAH, Pradilla et al.36 tested the rate and degree of Evans blue distribution after intrathecal placement (in the cisterna magna or subfrontal cisterns) of a polymer impregnated with Evans blue for controlled release. The peak concentration at 3 and 14 days was at least 4–6 times lower in the region of interest (frontal cisterns) than in the cervical region, where peak concentrations were detected. Curiously, despite this observation, the authors concluded that “…SAH did not interfere with Evans blue diffusion.”36 Furthermore, in a canine model of SAH, Ohta et al.33 observed that intracisternally administered horseradish peroxidase entered the adventitia and media of the cerebral arteries, but the effect was diminished compared with control animals. Nevertheless, the authors concluded that their findings support intrathecal drug administration after SAH.33
Intrathecal Thrombolysis
The thickness of the subarachnoid blood clot after aneurysmal bleeding is directly related to the risk of vasospasm and clinical outcome.12,24 The risk of vasospasm development, which is zero during the first 2 days after SAH, 46 gradually increases by almost 11% a day through Day 7 and then decreases through the next week.40,46 The dissolution of the clot in the subarachnoid space has a half-life of 5.4 days and is independent of initial clot volume.30 Because resolution of vasospasm is related to diminishing clot volume,40,47 many investigators attempt to accelerate clot resolution using intrathecal or intraventricular thrombolysis with heparin,41 urokinase,16,17 or recombinant tPA.11,44 However, the only double-blind, placebo-controlled, randomized trial using tPA showed no beneficial effect on vasospasm.10 Additionally, a meta-analysis of accessible data found no strong support for the use of thrombolysis to prevent vasospasm.2 It appears likely that the presence of a clot in the subarachnoid space restricts not only the distribution of antivasospastic drugs distributed via the CSF, but also that thrombolysis using recombinant tPA has a limited effect on the thickness of the clot enveloping cerebral arteries because of limited distribution,30 explaining its limited effect on arterial spasm.2,10
Surgical Effect on Intrathecal Drug Distribution
Another issue that has not been investigated previously is the influence of surgical manipulation in the region of an aneurysm on CSF distribution in the subarachnoid space. In our surgery group without SAH (sham SAH group), we examined the CSF distribution of 2 different compounds and observed long-lasting impairment of regional distribution of CT contrast and Evans blue dye following precise and bloodless microsurgical dissection of the sylvian fissure and membrane of Liliequist. Thus, surgical intervention alone limits the distribution of intrathecally delivered therapeutics to the region of surgical manipulation.
Conclusions
The pattern of changes and differences in contrast distribution between animals in the SAH and sham SAH groups and between the right and left hemispheres after producing SAH confirmed our hypothesis that intrathecal drug distribution is substantially limited by SAH. Thus, with intrathecal drug delivery after SAH, vasoactive drugs are unlikely to reach the arteries that are at the highest risk of delayed cerebral vasospasm.
Supplementary Material
Acknowledgments
Disclosure
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke. Bawarjan Schatlo, M.D., was supported by a scholarship from the Boehringer Ingelheim Foundation.
Abbreviations used in this paper
- ANOVA
analysis of variance
- ICA
internal carotid artery
- MCA
middle cerebral artery
- SAH
subarachnoid hemorrhage
- tPA
tissue plasminogen activator
Footnotes
Supplemental online information: Appendix: http://thejns.org/doi/full/10.3171/2009.2.JNS081256.
References
- 1.Aihara Y, Jahromi BS, Yassari R, Sayama T, Macdonald RL. Effects of a nitric oxide donor on and correlation of changes in cyclic nucleotide levels with experimental vasospasm. Neurosurgery. 2003;52:661–667. doi: 10.1227/01.neu.0000048188.88980.86. [DOI] [PubMed] [Google Scholar]
- 2.Amin-Hanjani S, Ogilvy C, Barker FII. Does intracisternal thrombolysis prevent vasospasm after aneurysmal subarachnoid hemorrhage? A meta-analysis. Neurosurgery. 2004;54:326–335. doi: 10.1227/01.neu.0000103488.94855.4f. [DOI] [PubMed] [Google Scholar]
- 3.Asano T, Sasaki T, Koide T, Takakura K, Sano K. Experimental evaluation of the beneficial effect of an antioxidant on cerebral vasospasm. Neurol Res. 1984;6:49–53. doi: 10.1080/01616412.1984.11739663. [DOI] [PubMed] [Google Scholar]
- 4.Auer LM. Preventive nimodipine and acute aneurysm surgery. Heading for the control of complications after aneurysmal subarachnoid hemorrhage. Neurochirurgia (Stuttg) 1985;28:87–92. doi: 10.1055/s-2008-1054110. [DOI] [PubMed] [Google Scholar]
- 5.Berg SL, Chamberlain MC. Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy and symptom management. Cancer Treat Res. 2005;125:121–146. doi: 10.1007/0-387-24199-x_8. [DOI] [PubMed] [Google Scholar]
- 6.Boullin DJ, Adams CB, Mohan J, Green AR, Hunt TM, du Boulay GH, et al. Effects of intracranial dopamine perfusion: behavioural arousal and reversal of cerebral arterial spasm following surgery for clipping of ruptured cerebral aneurysms. Proc R Soc Med. 1977;70(Suppl 2):55–70. doi: 10.1177/00359157770700S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosentino F, McMahon EG, Carter JS, Katusic ZS. Effect of endothelinA-receptor antagonist BQ-123 and phosphorami-don on cerebral vasospasm. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S332–S335. doi: 10.1097/00005344-199322008-00087. [DOI] [PubMed] [Google Scholar]
- 8.Elkersh MA, Simopoulos TT, Bajwa ZH. Fundamentals of interventional pain medicine. Neurologist. 2005;11:285–293. doi: 10.1097/01.nrl.0000162955.44879.8b. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa F, Weir B, Overton T, Castor W, Grace M, Boisvert O. A randomized placebo-controlled double-blind trial of nimodipine after SAH in monkeys. Part I: clinical and radiological findings. J Neurosurg. 1984;60:1167–1175. doi: 10.3171/jns.1984.60.6.1167. [DOI] [PubMed] [Google Scholar]
- 10.Findlay JM, Kassell NF, Weir BK, Haley EC, Jr, Kongable G, Germanson T, et al. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery. 1995;37:168–178. [PubMed] [Google Scholar]
- 11.Findlay JM, Weir BK, Gordon P, Grace M, Baughman R. Safety and efficacy of intrathecal thrombolytic therapy in a primate model of cerebral vasospasm. Neurosurgery. 1989;24:491–498. doi: 10.1227/00006123-198904000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Follett KA, Boortz-Marx RL, Drake JM, DuPen S, Schneider SJ, Turner MS, et al. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004;100:1582–1594. doi: 10.1097/00000542-200406000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara K, Mikawa S, Ebina T. Continuous intrathecal administration of nicardipine using a portable infusion pump system for management of vasospasm after subarachnoid hemorrhage. No Shinkei Geka. 2001;29:23–30. [PubMed] [Google Scholar]
- 15.Gioia AE, White RP, Bakhtian B, Robertson JT. Evaluation of the efficacy of intrathecal nimodipine in canine models of chronic cerebral vasospasm. J Neurosurg. 1985;62:721–728. doi: 10.3171/jns.1985.62.5.0721. [DOI] [PubMed] [Google Scholar]
- 16.Hamada J, Mizuno T, Kai Y, Morioka M, Ushio Y. Microcatheter intrathecal urokinase infusion into cisterna magna for prevention of cerebral vasospasm: preliminary report. Stroke. 2000;31:2141–2148. doi: 10.1161/01.str.31.9.2141. [DOI] [PubMed] [Google Scholar]
- 17.Hariton GB, Findlay JM, Weir BK, Kasuya H, Grace MG, Mielke BW. Comparison of intrathecal administration of urokinase and tissue plasminogen activator on subarachnoid clot and chronic vasospasm in a primate model. Neurosurgery. 1993;33:691–697. doi: 10.1227/00006123-199310000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Heidrich R, Markwardt F, Endler S, Hindersin P. Antifibrinolytic therapy of subarachnoid hemorrhage by intrathecal administration of p-aminomethylbenzoic acid. J Neurol. 1978;219:83–85. doi: 10.1007/BF00313371. [DOI] [PubMed] [Google Scholar]
- 19.Hino A, Weir BK, Macdonald RL, Thisted RA, Kim CJ, Johns LM. Prospective, randomized, double-blind trial of BQ-123 and bosentan for prevention of vasospasm following subarachnoid hemorrhage in monkeys. J Neurosurg. 1995;83:503–509. doi: 10.3171/jns.1995.83.3.0503. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan KM, Brose WG. Intrathecal methods. Neurosurg Clin N Am. 2004;15:289–296. doi: 10.1016/j.nec.2004.02.011. vi. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto S, Tsutsumi K, Yoshikawa G, Shinozaki MH, Yako K, Nagata K, et al. Effectiveness of the head-shaking method combined with cisternal irrigation with urokinase in preventing cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 2004;100:236–243. doi: 10.3171/jns.2004.100.2.0236. [DOI] [PubMed] [Google Scholar]
- 22.Kırış T, Erden T, Sahinbas M, Omay B, Esen F. CSF drainage for prevention and reversal of cerebral vasospasm after surgical treatment of intracranial aneurysms. In: Macdonald RL, editor. Cerebral Vasospasm: Advances in Research and Treatment. New York: Thieme; 2005. pp. 255–258. [Google Scholar]
- 23.Klimo P, Kestle J, MacDonald J, Schmidt R. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004;100:215–224. doi: 10.3171/jns.2004.100.2.0215. [DOI] [PubMed] [Google Scholar]
- 24.Kramer AH, Hehir M, Nathan B, Gress D, Dumont AS, Kassell NF, et al. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg. 2008;109:199–207. doi: 10.3171/JNS/2008/109/8/0199. [DOI] [PubMed] [Google Scholar]
- 25.Lewis PJ, Weir BK, Nosko MG, Tanabe T, Grace MG. Intrathecal nimodipine therapy in a primate model of chronic cerebral vasospasm. Neurosurgery. 1988;22:492–500. doi: 10.1227/00006123-198803000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Lin CL, Jeng AY, Howng SL, Kwan AL. Endothelin and subarachnoid hemorrhage-induced cerebral vasospasm: pathogenesis and treatment. Curr Med Chem. 2004;11:1779–1791. doi: 10.2174/0929867043364919. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald RL, Zhang Z-D, Curry D, Elas M, Aihara Y, Halp-ern H, et al. Intracisternal sodium nitroprusside fails to prevent vasospasm in nonhuman primates. Neurosurgery. 2002;51:761–770. [PubMed] [Google Scholar]
- 28.Megyesi JF, Vollrath B, Cook DA, Findlay JM. In vivo animal models of cerebral vasospasm: a review. Neurosurgery. 2000;46:448–461. [PubMed] [Google Scholar]
- 29.Miles J. Intrathecal treatment for spasticity. Stereotact Funct Neurosurg. 2001;76:246–248. doi: 10.1159/000066726. [DOI] [PubMed] [Google Scholar]
- 30.Naff NJ, Williams MA, Rigamonti D, Keyl PM, Hanley DF. Blood clot resolution in human cerebrospinal fuid: evidence of first-order kinetics. Neurosurgery. 2001;49:614–621. doi: 10.1097/00006123-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Nakagomi T, Takagi K, Narita K, Nagashima H, Tamura A. Cisternal washing therapy for the prevention of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2001;(Suppl 77):161–165. doi: 10.1007/978-3-7091-6232-3_34. [DOI] [PubMed] [Google Scholar]
- 32.Ogata M, Marshall BM, Lougheed WM. Observations on the effects of intrathecal papaverine in experimental vasospasm. J Neurosurg. 1973;38:20–25. doi: 10.3171/jns.1973.38.1.0020. [DOI] [PubMed] [Google Scholar]
- 33.Ohta T, Satoh G, Kuroiwa T. The permeability change of major cerebral arteries in experimental vasospasm. Neurosurgery. 1992;30:331–336. doi: 10.1227/00006123-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Penn RD. Intrathecal medication delivery. Neurosurg Clin N Am. 2003;14:381–387. doi: 10.1016/s1042-3680(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 35.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions prevent cerebral artery vasospasm in a primate model of subarachnoid aneurismal hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 36.Pradilla G, Wang P, Legnani F, Frazier J, Tamargo R. Pharmacokinetics of controlled-release polymers in the subarachnoid space after subarachnoid hemorrhage in rabbits. J Neurosurg. 2004;101:99–103. doi: 10.3171/jns.2004.101.1.0099. [DOI] [PubMed] [Google Scholar]
- 37.Proescholdt MG, Hutto B, Brady L, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C] inulin. Neuroscience. 2000;95:577–592. doi: 10.1016/s0306-4522(99)00417-0. [DOI] [PubMed] [Google Scholar]
- 38.Raabe A, Zimmermann M, Setzer M, Vatter H, Berkefeld J, Seifert V. Effect of intraventricular sodium nitroprusside on cerebral hemodynamics and oxygenation in poor-grade aneurysm patients with severe, medically refractory vasospasm. Neurosurgery. 2002;50:1006–1014. doi: 10.1097/00006123-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Shibuya M, Suzuki Y, Enomoto H, Okada T, Ogura K, Sugita K. Effects of prophylactic intrathecal administrations of nicardipine on vasospasm in patients with severe aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1994;131:19–25. doi: 10.1007/BF01401450. [DOI] [PubMed] [Google Scholar]
- 40.Stoodley M, MacDonald RL, Weir B, Marton LS, Johns L, Du Zhang Z, et al. Subarachnoid hemorrhage as a cause of an adaptive response in cerebral arteries. J Neurosurg. 2000;93:463–470. doi: 10.3171/jns.2000.93.3.0463. [DOI] [PubMed] [Google Scholar]
- 41.Tekkok IH, Tekkok S, Ozcan OE, Erbengi T, Erbengi A. Preventive effect of intracisternal heparin for proliferative angiopathy after experimental subarachnoid haemorrhage in rats. Acta Neurochir (Wien) 1994;127:112–117. doi: 10.1007/BF01808557. [DOI] [PubMed] [Google Scholar]
- 42.Thomas JE, Rosenwasser RH. Reversal of severe cerebral vasospasm in three patients after aneurysmal subarachnoid hemorrhage: initial observations regarding the use of intraventricular sodium nitroprusside in humans. Neurosurgery. 1999;44:48–58. doi: 10.1097/00006123-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Vajkoczy P, Hubner U, Horn P, Bauhuf C, Thome C, Schilling L, et al. Intrathecal sodium nitroprusside improves cerebral blood flow and oxygenation in refractory cerebral vasospasm and ischemia in humans. Stroke. 2000;31:1195–1197. doi: 10.1161/01.str.31.5.1194-b. [DOI] [PubMed] [Google Scholar]
- 44.Varelas PN, Rickert KL, Cusick J, Hacein-Bey L, Sinson G, Torbey M, et al. Intraventricular hemorrhage after aneurysmal subarachnoid hemorrhage: pilot study of treatment with intraventricular tissue plasminogen activator. Neurosurgery. 2005;56:205–213. doi: 10.1227/01.neu.0000147973.83688.d8. [DOI] [PubMed] [Google Scholar]
- 45.Voldby B, Petersen OF, Buhl M, Jakobsen P, Ostergaard R. Reversal of cerebral arterial spasm by intrathecal administration of a calcium antagonist (nimodipine) Acta Neurochir (Wien) 1984;70:243–254. doi: 10.1007/BF01406653. [DOI] [PubMed] [Google Scholar]
- 46.Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48:173–178. doi: 10.3171/jns.1978.48.2.0173. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZD, Yamini B, Komuro T, Ono S, Johns L, Marton LS, et al. Vasospasm in monkeys resolves because of loss of and encasement of subarachnoid blood clot. Stroke. 2001;32:1868–1874. doi: 10.1161/01.str.32.8.1868. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann M, Jung C, Raabe A, Spanehl O, Fach K, Seifert V. Inhibition of endothelin-converting enzyme activity in the rabbit basilar artery. Neurosurgery. 2001;48:902–910. doi: 10.1097/00006123-200104000-00043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.