Abstract
HIV+ individuals with and without substance use disorders make significantly poorer decisions when information about the probability and magnitude of wins and losses is not available. We administered the Game of Dice Task, a measure of decision making under risk that provides this information explicitly, to 92 HIV+ and 134 HIV− substance dependent men and women. HIV+ participants made significantly poorer decisions compared with HIV− participants, but this deficit appeared more prominent among HIV+ women. These data indicate that decision making under risk is impaired among HIV+ SDIs. Potential factors for the HIV+ women’s relatively greater impairment are discussed.
Keywords: HIV, Drug Abuse, Executive Function, Sex Differences, Decision Making, Cocaine, Addiction
The construct of “decision making” can be broadly defined as the process of selecting an option with a more favorable expected value (Bechara, Damasio, Tranel, & Damasio, 1997; Weller, Levin, Shiv, & Bechara, 2007). Recent findings from neuroeconomics, cognitive and clinical neuroscience studies of normal and clinical populations have characterized decision making as a dynamic process (Fellows, 2004; Loewenstein, Rick, & Cohen, 2008; Sanfey, 2007); the timing, magnitude, expected value, and certainty of reward exert significant influence on one’s willingness to take risks (Ernst et al., 2004; Lejuez et al., 2002; Parker & Weller, 2015), both in the laboratory and the real world (Lejuez, Simmons, Aklin, Daughters, & Dvir, 2004). Similarly, patterns of normal brain activity in medial prefrontal cortex, striatum and amygdala vary depending on certainty, expected value, and size of reward (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005; Xue et al., 2009).
Poor decision making is common among substance dependent individuals (SDIs) (Bechara & Damasio, 2002). Vulnerability to impaired decision making is further increased among SDIs infected with HIV (Gonzalez et al., 2005; E Martin et al., 2013). Studies by our group have demonstrated impaired decision making among HIV+ compared with HIV− SDIs using the well-studied Iowa Gambling Task (IGT) introduced by Bechara and his colleagues (Bechara, Damasio, Damasio, & Anderson, 1994; Bechara et al., 1997). Briefly, the IGT requires the participant to make a series of selections from four virtual card decks over a total of 100 trials; each card selection results in a win or loss of some money. The participant is instructed to win as much money as possible but receives no additional directions. Unbeknownst to the participants the four card decks are associated with different probabilities and magnitude of wins and losses. On the early task trials (e.g., the first 20-40 trials), the IGT engages decision making under ambiguity: no explicit information about the likelihood or magnitude of wins or losses is provided to the participant, who must rely on self-generated strategies to deduce the optimal pattern of card choices. However, as the task progresses, normal participants typically shift their card selections to decks with smaller wins but infrequent losses; this strategy results in a winning score. Studies of decision making have proposed that the behavioral shift in IGT performance to choices from safer decks with smaller wins reflects the gradual engagement of decision making under risk (Brand, Recknor, Grabenhorst, & Bechara, 2007). Measures of decision making under risk provide explicit information about the likelihood of each outcome, which can guide the subject’s choices (Weller, Levin, & Bechara, 2010). By contrast SDIs often fail to shift their card selections, continuing to choose cards with large potential wins but larger or more frequent losses.
In a recent study of drug using men who have sex with men (MSMs) enrolled in the Multicenter AIDS Cohort Study (E Martin et al., 2013), we found that HIV+ SDIs performed the IGT more poorly compared with HIV− SDIs, and this performance deficit was most evident on the early trial blocks; however, HIV+ and HIV− SDIs performed comparably on the Cups Task, a two choice measure of decision making under specified risk (Weller et al., 2007). These findings raised the question if impaired decision making among HIV+ SDIs was most apparent when they had to rely primarily on self-generated or internal strategies with insufficient external information available to guide their choices.
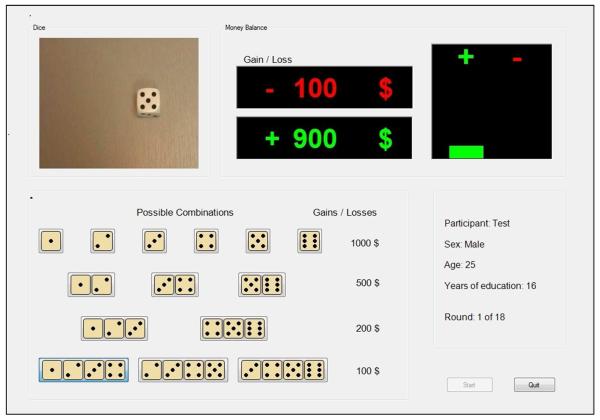
In the current study, we investigated decision making under specified risk among a large group of HIV+ and HIV− SDIs using the Game of Dice Task (GDT) (Brand et al., 2005). The GDT consists of 18 virtual dice throws with varying amounts of potential wins or losses. The GDT resembles the IGT in that participants are instructed to win as much money as possible, but only the GDT provides specific information about the likelihood and magnitude of wins or losses, which is continuously displayed throughout the task (See Figure 1). Brand and his colleagues designed the GDT with the goal of engaging decision making while minimizing task demands on learning (Brand et al., 2005). Functional brain imaging studies have shown activation in anterior cingulate, lateral prefrontal and parietal cortex during GDT performance (Labudda et al., 2010); by contrast, IGT performance is associated with activation in dorsolateral and ventromedial prefrontal cortex, insula, and ventral striatum (Li, Lu, D'Argembeau, Ng, & Bechara, 2010) , indicating that performance of these tasks engages nonidentical cognitive and neural mechanisms.
Figure 1.
Stimulus display for the Game of Dice Task.
The GDT has been administered to a range of normal and clinical populations, including individuals with Korsakoff syndrome (Brand et al., 2005), opiate dependence (Brand, Roth-Bauer, Driessen, & Markowitsch, 2008), and Parkinson disease (Brand et al., 2004). Fujiwara and colleagues recently administered the GDT to a small sample of primarily male HIV+ individuals with no history of substance use (Fujiwara, Tomlinson, Purdon, Gill, & Power, 2015); they reported that HIV+ group made significantly fewer advantageous decisions and employed less systematic strategies over task trials compared with healthy HIV− controls.
To our knowledge GDT performance has not been investigated among HIV+ SDIs. In the current study, we administered the GDT to a newly recruited sample of 226 HIV+ and HIV− SDIs.
The primary goal was to build on our previous studies by characterizing decision making under specified risk among a larger and more diverse sample of HIV+ and HIV− SDIs. Over 50% of the current study sample was female, which enabled us to explore potential sex differences or interactive effects of sex and HIV serostatus on GDT performance. Decision making among HIV+ men and women has not been compared directly, and the available literature on decision making among normal participants has yielded mixed results: males typically outperform females on the IGT (van den Bos, Homberg, & de Visser, 2013) and on the Cups Task (Weller et al., 2010), but there is no evidence of sex differences in GDT performance (Brand & Schiebener, 2013).
METHOD
Participants
We tested a group of 110 men and 116 women enrolled in a larger study of sex differences in the neurocognitive effects of HIV serostatus and cocaine dependence. The study was approved by the IRBs for the Rush University Medical Center, the University of Illinois, and the Rothstein Core Center at Stroger (formerly Cook County) Hospital. The sample included 92 HIV+ and 134 EIA-verified HIV− participants recruited from infectious disease and substance abuse programs at RUMC, the University of Illinois at Chicago, the Core Center, and from the community. Potential study subjects who met DSM-IV criteria for current or previous opioid dependence; dependence on alcohol but no other substances; or with AIDS defining or any other CNS illness or injury, including stroke, closed head injury with greater than 30 minutes’ loss of consciousness, open head injury of any kind, seizure disorder, schizophrenia, or current neuroleptic treatment were excluded from participation. The overall sample was 87% African American. 94% of the HIV+ participants were prescribed combination antiretroviral therapy (cART). 81% of participants’ HIV RNA levels (viral loads) were undetectable with a lower limit of 40. Median CD4 lymphocyte counts at testing were 452 (Interquartile range (IQR) = 305,696) and median nadir CD4 counts were 191 (IQR = 71,342).
Procedure
Tests administered were part of a larger study protocol administered over two 120-150 minute visits to the Outpatient Psychiatry Clinic at Rush University Medical Center. Testing was conducted by bachelor’s level research assistants under the supervision of the PI (EMM), a board certified clinical neuropsychologist. Written informed consent was obtained on arrival for the first study visit. On each study visit the participants provided a urine sample for on-site rapid toxicology screen for cocaine, cannabis, opioids, benzodiazepines and methamphetamines using DrugCheck NxStep kits, and underwent a breathalyzer test to ensure abstinence from drugs and alcohol at the time of testing. If a potential participant tested positive, the visit was terminated, the participant received no payment, and the visit was rescheduled1. All participants were informed of these contingencies prior to the testing visit. They received $75 cash compensation for their time and transportation costs at the completion of each study visit.
Measures
Clinical and personality measures
Subjects were administered the Wechsler Test of Adult Reading (WTAR) (Wechsler, 2001) as an index of educational quality (Maki et al., 2014; Manly et al., 2011), and a series of paper and pencil measures of potentially confounding conditions comorbid with substance use disorders (SUDs). Measures of comorbid conditions included the PTSD Check List-Civilian Version (PCLC) (Weathers, Keane, & Davidson, 2001); the Wender Utah Rating Scale (WURS) for symptoms of Attention Deficit Disorder (Stein et al., 1995); the Affective Disorder Module from the Structured Clinical Interview for DSM-IV (SCID-IV) (First, Spitzer, Gibbon, & Williams, 1995); and the Levenson Self-Report Psychopathy Scale (SRPS) to index antisociality (Levenson, Kiehl, & Fitzpatrick, 1995). These measures were administered to determine comparability of study groups and as potential covariates.
Substance use
All participants were administered the Substance Abuse Module from the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 1995) to determine if they met criteria for current or previous SUDs; and the Kreek-McHugh-Schluger-Kellogg Scale (Kellogg et al., 2003), employed as a proxy of severity of alcohol, cocaine, and opioid dependence based on the participant’s estimate of the amount of money spent, time duration, and frequency of use during the period of their maximum lifetime use of each substance. A subset of 192 participants (84% of the total sample) also completed the Addictions Severity Index (McLellan, Luborsky, Woody, & O'Brien, 1980), a standardized measure of severity of recent drug and alcohol use.
Game of Dice Task
All subjects completed the English version of the original Game of Dice Task (Brand et al., 2005; Brand et al., 2007), an 18-trial computerized measure of decision making under specified risk. The GDT stimuli consist of a continuous visual display of 14 separate combinations of 1 to 4 dice [see Figure 1]. The participant is informed that each dice combination is associated with a win or loss of a specific amount of money. The participants are instructed that on each trial a single die will be thrown and they must choose one of the 14 possible dice combinations. Explicit information about the amount of wins or losses associated with each combination is continuously displayed throughout the task. The size of each combination (i.e., the number of dice) indexes the probability of a win or loss. Thus, information about the degree of risk – a joint function of the probability and amount of wins and losses – is available to the participant. Higher risk choices (e.g. selecting a single or two-dice combination) are associated with higher wins but the probability of a loss is higher. Conversely, lower-risk choices (3- and 4-dice combinations) are associated with a higher probability of winning but a smaller amount of money. The dependent variable is a net score (number of low-risk decisions minus number of high-risk decisions) that indexes “advantageous” choices based on the degree of risk and amount of money won or lost after 18 trials.
Statistical analyses
Demographic, substance use, and comorbidity data were compared using one-way analyses of variance (ANOVA) for parametric data, Kruskal-Wallis and Mann-Whitney U tests with the z approximation for non-normally distributed data, and chi-square tests for categorical data. A p value of .05 was employed for all group comparisons. Bonferroni-corrected t tests were employed for post-hoc comparisons.
RESULTS
Demographics
Tables 1-2 show demographic, substance dependence and comorbid characteristics for the four participant groups. Nonsignificant trends toward group differences were noted for mean age, F(3,222) = 2.46, p = .06, with HIV+ women slightly younger than HIV− men; mean estimated WTAR IQ scores, F(3,214) = 2.46, p = .06, with slightly lower mean scores for HIV+ compared with HIV− women; and a higher percentage of African American participants among the HIV− men compared with the other three groups, χ2 (3) = 6.96, p = .07.
Table 1.
Demographic, HIV disease severity and comorbidity characteristics for all participants
| HIV−Men | HIV+Men | HIV− Women | HIV+Women | Statistic | p | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| n | 50 | 60 | 84 | 32 | ||
| Age | 50.4 (5.2) | 47.8 (7.8) | 47.9 (8.8) | 45.8 (8.7) | 2.46 | .06 |
| WTAR | 89.5 (11.2) | 89.4 (10.9) | 89.9 (10.4) | 84.2 (7.8) | 2.46 | .06 |
| %African American | 96 | 83 | 80 | 88 | 8.95 | .07 |
| % HCV+ | 6 | 21 | 14 | 14 | 4.6 | .20 |
| HIV Disease | ||||||
| Mean CPE scores | 7.8 (2.5) | 7.2 (2.5) | 1.26 | .26 | ||
| Md Current CD4 | 449 | 491 | −.33 | .74 | ||
| Md Nadir CD4 | 188 | 199 | −.84 | .40 | ||
| % Undetectable VL | 76 | 90 | 2.34 | .13 | ||
| % cART | 95 | 94 | .08 | .77 | ||
| Comorbidities | ||||||
| % MDD | 22 | 32 | 36 | 38 | 3.3 | .35 |
| PCLC | 35.3 (14.0) | 34.4 (13.5) | 38.8 (14.3) | 39.3 (14.9) | 1.68 | .17 |
| WURS | 25.3 (18.5) | 30.8 (20.8) | 29.8 (22.1) | 34.1 (21.5) | 1.24 | .30 |
| SRPS | 49.7 (20.2) | 50.1 (10.5) | 49.6 (10.2) | 51.0 (10.2) | .16 | .92 |
Note. All values represent mean scores unless otherwise indicated.
Note. WTAR = Wechsler Test of Adult Reading; HCV = hepatitis C virus; CPE = CNS Penetration Effectiveness; PCLC = PTSD Check List – Civilian version; WURS = Wender Utah Rating Scale; SRPS = Self Report Psychopathy Scale.
Table 2.
Substance Use Characteristics
| HIV−Men | HIV+Men | HIV− Women | HIV+ Women | Statistic | p | |
|---|---|---|---|---|---|---|
| Mean ASI Scores (n=190) | ||||||
| Alcohol | .12 (.20) | .06 (.11) | .04 (.10) | .01 (.06) | 5.47 | .001 |
| Drug | .02 (.06) | .02 (.06) | .03 (.05) | .02 (.06) | .09 | .97 |
| Mean KMSK Scores | ||||||
| Alcohol | 11.1 (2.8) | 11.0 (2.5) | 10.1 (3.5) | 11.1 (2.8) | 1.77 | .15 |
| Cocaine | 12.7(5.1) | 13.4 (4.3) | 12.6 (5.0) | 12.5 (5.5) | 0.30 | .82 |
| Heroin | 2.2 (3.8) | 1.4 (2.8) | 4.5 (5.2) | 2.1 (3.6) | 8.0 | .0001 |
| % DSM-IV Dependence | ||||||
| Alcohol | 54 | 63 | 50 | 72 | 5.73 | .13 |
| Cannabis | 38 | 44 | 37 | 28 | 3.26 | .35 |
| Cocaine | 72 | 70 | 70 | 75 | .32 | .96 |
| Md Days Since Last Use | ||||||
| Alcohol | 14 | 68 | 331 | 220 | 6.36 | .10 |
| Cannabis | 4018 | 1096 | 1292 | 1461 | 8.0 | .05 |
| Cocaine | 447 | 476 | 1461 | 1826 | 8.4 | .04 |
| Heroin | 444 | 1461 | 608 | 1194 | 1.23 | .75 |
| % Use Past 6 Months | ||||||
| Alcohol | 63 | 63 | 44 | 43 | 5.95 | .11 |
| Cannabis | 11 | 26 | 15 | 15 | 2.97 | .40 |
| Cocaine | 30 | 38 | 18 | 10 | 7.15 | .07 |
| Heroin | 22 | 17 | 14 | 0 | .88 | .83 |
| % IDU | 4 | 18 | 14 | 9 | 5.7 | .12 |
| % OD | 10 | 10 | 16 | 19 | 2.2 | .53 |
Note. ASI = Addiction Severity Index; KMSK = Kreek-McHugh-Schluger-Kellogg scale; IDU = injection drug use.
Substance use
There were no significant group differences in prevalence of lifetime DSM-IV diagnoses of alcohol, cannabis or cocaine dependence (Alcohol: χ2 (3) = 5.73, p = .13; Cannabis: χ2 (3) = 3.78, p = .29; Cocaine: χ2 (3) = .32, p = .96). Although opioid dependent individuals were excluded from study, we found a significant group difference in mean KMSK Opioid scores, omnibus F(3,222) = 8.04, p < .001; post hoc comparisons revealed that mean self-reported amount of past opioid use was significantly higher among the HIV− women compared with the HIV− men, p < .008, the HIV+ men, p < .001, and the HIV+ women, p = .03. There were no significant group differences in mean KMSK scores for severity of peak alcohol, F(3,222) = 1.78, p = .159, or cocaine use, F(3,222) = .30, p = .82.
There were no significant group differences in prevalence of a positive hepatitis C virus (HCV) serostatus, χ2 (3) = 4.63, p = .20; history of injection drug use (IDU), χ2 (3) = 5.74, p = .12; or history of overdose, χ2 (3) = 2.21, p = .53. A significantly higher percentage of HIV+ participants tested positive for THC metabolites on rapid toxicology screening (4% vs 29%; χ2 (1) = 30.2, p < .0001).
There were significant group differences in mean ASI-Alcohol scores, omnibus F(3,186) = 5.64, p = .001. Post hoc comparisons revealed that HIV− males scored significantly higher compared with HIV− women and HIV+ women (p = .003 for each comparison) with a nonsignificant trend toward higher scores compared with HIV+ males (p = .08). There were no significant group differences in mean ASI-Drug scores, F(3,186) = .054 p = .98.
Data on the number of days since last use were available for a subset of 162 participants (72% of the sample). There were no significant group differences in the median number of days since last use of alcohol or opioids, (Alcohol: χ2 (3) = 6.36, p = .10; Opioids, χ2 (3) = 1.23, p = .75), but there were significant group differences in the number of days since last use of cannabis, χ2 (3) = 7.97, p = .05, and cocaine, χ2 (3) = 8.40, p = .04. HIV− men’s last use of alcohol was significantly more recent compared with HIV− women, z = −1.95, p = .05; and HIV+ men had used cocaine significantly more recently compared with HIV− women, z = −2.18, p = .03, and with HIV+ women, z = −2.17, p = .05.
Comorbid conditions
Table 2 shows mean scores for each group on measures of comorbid conditions with potentially confounding effects. There were no significant group differences in mean scores on the SRPS, F(1,225) = .16, p = .92; PCLC, F(3,222) = 1.68, p =.17; or WURS, F(3,220) = 1.24, p = .30. There were no significant group differences in prevalence of history of Major Depressive Disorder, χ2 (3) = 3.28, p = .35.
HIV disease severity
There were no significant group differences between HIV+ men and women in median current and nadir CD4 counts (Current: z = − .33, p = .74; Nadir: z = − .84, p = .40); undetectable HIV RNA levels, χ2 (1) = 2.34, p = .13; % on cART, χ2 (1) =.09, p = .76; or mean scores on the 2010 version of the CNS Penetrance Effectiveness ranking system (Letendre et al., 2010), which indexes how effectively each antiretroviral combination crosses the blood-brain barrier, F(1,89) = 1.41, p = .24.
Results from these initial comparisons of demographic, substance use, and comorbid variables indicated generally satisfactory group matching and no evidence of sex differences in HIV disease status.
Game of Dice Task
Net Scores
The GDT computer program provides percentile values for raw GDT net scores. We converted percentile scores to z scores in order to employ parametric tests, then transformed z values to T scores so that all values were non-negative. We computed a series of Pearson correlations between GDT T scores and age, ethnicity, WTAR IQ estimate, and THC+ tox screen for use as potential covariates; none of the correlations was significant (p ≥ .09 for all tests).
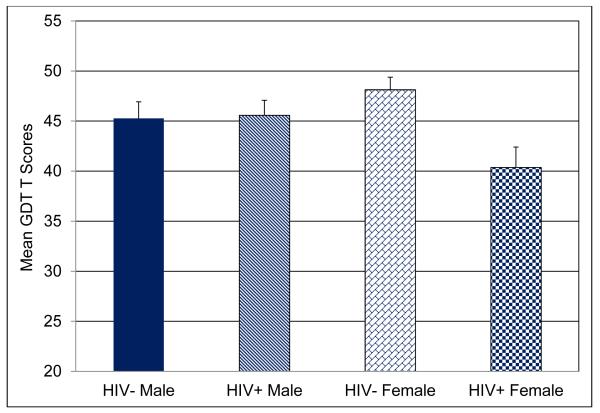
Figure 2 shows the mean T scores for the four participant groups. A Sex × HIV Serostatus factorial analysis of variance (ANOVA) revealed a significant main effect for HIV Serostatus, F(1, 221) = 6.54, p = .01, Cohen’s d = .35, and inspection of the means showed that HIV+ participants scored significantly lower compared with HIV− groups. Additionally, we found a significant Sex × HIV Serostatus interaction, F(1,221) = 5.30, p = .02, partial η2 =.023. Follow up tests of the interaction revealed that the HIV+ women scored significantly lower compared with the HIV− women, p = .01, Cohen’s d = −.74, and showed a nonsignificant trend toward lower mean scores compared with HIV+ men, p = .06, Cohen’s d = −.33.
Figure 2.
Mean T scores for overall GDT net scores for each group.
Degree of Risk
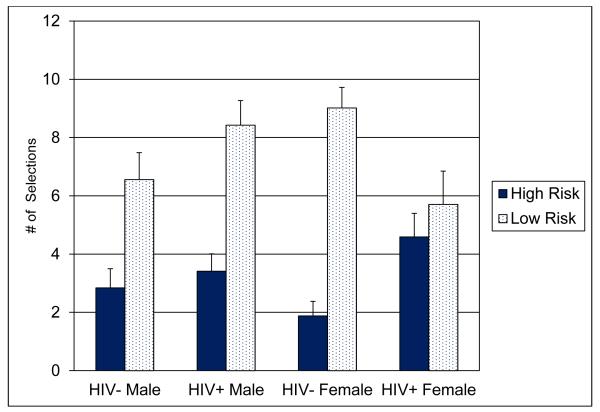
In order to obtain a finer grained measure of risk taking among the groups we compared each participant’s total number of single-die (highest risk) and four-dice (lowest risk) selections using a Risk × Sex × HIV Serostatus mixed design ANOVA. The analysis revealed a significant Risk × Sex × HIV Serostatus interaction, F(1,220) = 5.19, p = .02, partial η2 = .023. Tests of the interaction revealed that HIV+ participants selected significantly more high risk combinations compared with HIV− participants, p = .02, Cohen’s d = .33; the HIV+ women also selected significantly fewer low risk combinations compared with HIV− women, p = .01, Cohen’s d = −.56, and HIV+ men, p = .05, Cohen’s d = −.39.
Substance use and HIV Disease
There were no significant group differences in mean GDT net scores among individuals with and without a history of alcohol or cannabis dependence, urine toxicology screen positive for THC metabolites, or positive hepatitis C virus (HCV) serostatus, p ≥.39 for each test.
A recent study from the Women’s Interagency HIV Study (WIHS) reported that recent cocaine use was associated with poorer cognitive performance among HIV+ women but not HIV− women (V Meyer et al., 2013). We compared GDT T scores of HIV+ and HIV− SDIs who had used cocaine within the previous six months with those who reported using more than six months prior to testing2. We found a nonsignificant trend for the HIV Serostatus × 6 Months Cocaine Use interaction, F(1,142) = 2.98, p = .09, partial η2 = .02, with the lowest mean GDT scores obtained by the HIV+ SDIs who had used cocaine within 6 months, Cohen’s d = .36.
There were no significant differences in mean GDT T scores between HIV+ individuals with and without a current or lifetime immunologic AIDS diagnosis (i.e. CD4 < 200), or undetectable viral load, p ≥ .26 for all tests. However, we found a significant inverse correlation between mean net GDT T-scores and mean CPE total scores, Spearman’s ρ = −.24, p = .03.
DISCUSSION
Individuals infected with HIV typically make significantly fewer optimal choices compared with HIV− individuals when they are given minimal or no information about the degree of risk, e.g., the probability and magnitude of a potential win or loss associated with each choice (E Martin et al., 2013). This effect can be detected among HIV+ individuals with and without a history of substance dependence (Hardy, Hinkin, Levine, Castellon, & Lam, 2006; Iudicello et al., 2013; E Martin et al., 2004; Thames et al., 2012). In a recent study of HIV effects on decision making among substance-using men who have sex with men (MSMs) (E Martin et al., 2013) we found that HIV+ SDIs showed significantly impaired performance on the IGT compared to HIV− SDIs, particularly on the early trials when decision making under ambiguity is most prominently engaged; but performed virtually identically to HIV− SDIs on the Cups Task, a measure of decision making under risk that provides explicit information about the likelihood and magnitude of potential wins and losses. We speculated that the process of making an optimal choice by HIV+ SDIs might be critically dependent on their access to explicit information regarding degree of risk associated with each choice. In the current study we tested this hypothesis with a larger group of HIV+ and HIV− SDIs using the Game of Dice Task (GDT), which provides information sufficient for the participant to infer the probability and magnitude of a win or loss associated with each potential selection.
We found that HIV+ SDIs obtained significantly lower GDT net scores, indicating they selected more high risk choices overall compared with HIV− SDIs. However, the HIV+ participants’ GDT impairment appeared driven primarily by the poorer performance of the HIV+ women, as indexed by a statistically significant Sex × HIV Serostatus interaction. Similarly, a breakout of GDT scores by the degree of risk associated with each choice showed that HIV+ men and women made significantly more single-die (highest risk) selections; but only the HIV+ women made significantly fewer four-dice (lowest risk) choices compared with HIV− SDIs. The groups had comparable demographic characteristics and substance abuse-related comorbidities. Self-reported heroin use was significantly greater among HIV− women and alcohol use was significantly higher among HIV− men, suggesting that the HIV+ participants’ GDT deficits could not be attributed to nonspecific group differences in addiction severity. This suggestion is consistent with Fujiwara and colleagues’ report (Fujiwara et al., 2015) that GDT performance was poorer among a sample of primarily white male HIV+ individuals with no history of substance use or other comorbid disorders compared with healthy controls.
Our finding of a significant Sex × HIV Serostatus interaction is atypical compared with studies of GDT performance among non-clinical populations, which have shown no evidence of sex differences (Brand & Schiebener, 2013; Schiebener & Brand, 2015); however, the finding that GDT performance appears less impaired among HIV+ men than HIV+ women is consistent with previous reports by our group (E. Martin, Gonzalez, Vassileva, & Maki, 2011) that HIV+ women performed measures of probability and motor skill learning significantly more poorly than HIV− women, but HIV+ and HIV− men showed no differences in performance. Our findings are also compatible with Weller et al.’s report that normal men outperformed women on the Cups Task (Weller et al., 2010)
Additional cognitive mechanisms may have contributed to the HIV+ groups’ poorer performance on the GDT. Recent studies by Brand and his colleagues (Brand, Schiebener, Pertl, & Delazer, 2014) reported significant correlations between mathematical skills and GDT performance. Additionally, although the GDT provides explicit information that the participant can employ to deduce the exact probability that a specific dice combination will occur, this operation requires online mental processing, which will engage working memory mechanisms. HIV+ SDIs have shown reliable working memory deficits across a range of studies (Bartok et al., 1997; Farinpour et al., 2000). This interpretation is consistent with recent reports (Schiebener et al., 2014) of strong correlations between executive functions (executive control, in particular) and the GDT. Studies of working memory are currently in progress with this cohort, so data will be available to investigate the potential contribution of working memory to GDT performance. In this regard the addictions treatment literature has shown that working memory can be modified (Bechara, 2004; Wesley & Bickel, 2014), raising the question if newly designed cognitive rehabilitation strategies might confer additional benefit on HIV+ SDIs.
Approximately 75-80% of our sample met criteria for cocaine abuse or dependence. Studies from the addictions literature have shown that women are more vulnerable to development of stimulant addiction compared to men (Wetherington, 2007); further, data from the Women’s Interagency HIV Study (WIHS) have shown that disease progression is faster and neurocognitive risk is increased specifically by crack use among HIV+ women (Cook et al., 2008; V Meyer et al., 2013; V. Meyer et al., 2014). We observed a trend toward poorer GDT performance among HIV+ SDIs who had used cocaine within the last six months compared with other participant groups: this finding is compatible with Meyer and colleagues’ (V Meyer et al., 2013) report that recent (but not lifetime) crack use was associated with significantly greater neurocognitive impairment among HIV+ but not HIV− women. However, our sample size did not permit analysis of potential sex differences in the interaction of HIV Serostatus with Recent Cocaine Use. Increased sample sizes will provide greater power for tests of this interaction, which would also permit us to investigate if the effect is sex-specific.
We previously speculated that decision making under risk among HIV+ SDIs might be relatively less impaired compared with decision making under ambiguity. The current data provide partial support for this hypothesis, although the effect was more apparent among HIV+ men. This study did not include the IGT or other measures of decision making with an ambiguity component, thus administration of the IGT with the GDT to a new group of male and female HIV+ and HIV− SDIs is required for a direct test of this hypothesis. We also note that this speculation was generated on the basis of an all-male study of decision making by HIV+ and HIV− substance using MSMs and may not generalize to HIV+ women. This possibility is consistent with a recent report by Vassileva and colleagues (Vassileva et al., 2013), who administered the IGT to HIV+ and HIV− women with and without a substance abuse history. They found no significant main effects for either HIV serostatus or substance use on behavioral performance of the IGT; additionally, both HIV− and HIV+ groups failed to show consistent improvement in performance over trial blocks3, which is commonly observed among HIV+ and HIV− men. A recent study by Sutterer and his colleagues (Sutterer, Koscik, & Tranel, 2015) provides additional support for the critical importance of detailed characterization of both men and women’s decision capacity. Sutterer reported that compared with normal controls, greater risk aversion was shown by men with lesions of the right ventromedial prefrontal cortex (vmPFC) and women with left vmPFC lesions. This pattern was not observed among men with left vmPFC or women with right vmPFC lesions.
Results of the current study are limited by our use of the original GDT, which automatically assigns a negative score to higher risk choices. Brand and his colleagues have since introduced an updated version of the GDT, the Game of Dice Task-Double (GDT-Double) , which has been modified to reflect that some risky decisions result in positive outcomes under real world conditions (cf (Weller et al., 2007). Additionally, potential mechanisms of male and female HIV+ SDIs’ impairment in decision making under risk must be characterized more precisely with careful attention to specific task parameters. As an example, unlike the GDT, the Cups Task tests win- and loss-related decisions on separate trials, activates a dissimilar neural network (Xue et al., 2009) and shows a male advantage among normal subjects, suggesting that these tasks engage different mechanisms of decision making under risk.
The current findings are also limited by the relatively small number of HIV+ women (n = 32): however, neurocognitive studies with a larger group of HIV+ women are currently in progress in our lab, which will permit investigation of more detailed neurocognitive mechanisms, as well as possible risk factors for decision making deficits. Larger sample sizes will also permit more specific investigation of the association of recent substance use (particularly cocaine) with decision making among HIV+ and HIV− men and women.
We found a significant inverse correlation between GDT and CPE scores, indicating that individuals treated with highly CNS-penetrant antiretroviral compounds performed the GDT more poorly. The significance of this incidental finding is unclear in the absence of longitudinal data, although compatible with reports of neurotoxic effects among highly CNS penetrant antiretroviral compounds (Robertson, Liner, & Meeker, 2012; Wilson, Martin-Engel, Vassileva, Gonzalez, & Martin, 2013).
The HIV literature has suggested that impaired decision making may influence both high risk sexual practices and level of adherence with antiretroviral therapy (Gonzalez et al., 2005; Iudicello et al., 2013; Wardle, Gonzalez, Bechara, & Martin-Thormeyer, 2010). Additionally, as individuals live longer with HIV/AIDS the capacity to participate effectively in health care decisions and live independently will be critically dependent on the integrity of decision making processes. Potential sex-specific characteristics of decision making will also require more detailed investigation.
Figure 3.
Mean number of high-risk and low-risk dice combinations for each group.
Acknowledgements
We thank Christine Franco, Sida Chen and Leslie Ladd for data collection, and Mike Keutmann for data management and analyses.
Footnotes
These procedures were followed with the single exception that participants who tested positive for cannabis were not excluded if testing was negative for all other substances. The presence of THC metabolites in the urine did not necessarily indicate cannabis use within 1-2 days prior to testing due to its much longer elimination.
The n for one cell was too small for a full Sex × HIV Serostatus × 6 Month Cocaine Use analysis
However, more detailed computational modeling indicated that different cognitive mechanisms contributed to IGT performance by HIV− and HIV+ groups.
REFERENCES
- Bartok JA, Martin EM, Pitrak DL, Novak RM, Pursell KJ, Mullane KM, Harrow M. Working memory deficits in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 1997;3:451–456. Retrieved from PM:9322404. [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. Int Rev Neurobiol. 2004;62:159–193. doi: 10.1016/S0074-7742(04)620065X. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to the human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;39(4):376–389. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Brand M, Fujiwara E, Borsutzky S, Kalbe E, Kessler J, Markowitsch HJ. Decision-making deficits of Korsakoff patients in a new gambling task with explicit rules: Associations with executive functions. Neuropsychology. 2005;19(3):267–277. doi: 10.1037/0894-4105.19.3.267. [DOI] [PubMed] [Google Scholar]
- Brand M, Labudda K, Kalbe E, Hilker R, Emmans D, Fuchs G, Markowitsch HJ. Decision-making impairments in patients with Parkinson's disease. Behavioural Neurology. 2004;15(3-4):77–85. doi: 10.1155/2004/578354. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15706051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical and Experimental Neuropsychology. 2007;29(1):86–99. doi: 10.1080/13803390500507196. Retrieved from PM:17162725. [DOI] [PubMed] [Google Scholar]
- Brand M, Roth-Bauer M, Driessen M, Markowitsch HJ. Executive functions and risky decision-making in patients with opiate dependence. Drug and Alcohol Dependence. 2008;97(1-2):64–72. doi: 10.1016/j.drugalcdep.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Brand M, Schiebener J. Interactions of age and cognitive functions in predicting decision making under risky conditions over the life span. Journal of Clinical and Experimental Neuropsychology. 2013;35(1):9–23. doi: 10.1080/13803395.2012.740000. [DOI] [PubMed] [Google Scholar]
- Brand M, Schiebener J, Pertl MT, Delazer M. Know the risk, take the win: how executive functions and probability processing influence advantageous decision making under risk conditions. Journal of Clinical and Experimental Neuropsychology. 2014;36(9):914–929. doi: 10.1080/13803395.2014.955783. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22(11):1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Pine DS. Choice selection and reward anticipation: An fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. Retrieved from PM:15327927. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Harrow M. Verbal working memory in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 2000;6(5):548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- Fellows LK. The cognitive neuroscience of human decision making: A review and conceptual framework. Behavior and Cognitive Neuroscience Review. 2004;3(3):159–172. doi: 10.1177/1534582304273251. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM5-IV. Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. (Reprinted from: NOT IN FILE) [Google Scholar]
- Fujiwara E, Tomlinson SE, Purdon SE, Gill MJ, Power C. Decision making under explicit risk is impaired in individuals with human immunodeficiency virus (HIV) Journal of Clinical and Experimental Neuropsychology. 2015;37:733–750. doi: 10.1080/13803395.2015.1057481. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11(2):121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20(3):355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. Retrieved from PM:16339445. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Cattie JE, Doyle K, Grant I, Group, HIV Neurobehavioral Research Program Risky decision-making in HIV-associated neurocognitive disorders (HAND) The Clinical Neuropsychologist. 2013;27(2):256–275. doi: 10.1080/13854046.2012.740077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3. Retrieved from PM:12609695. [DOI] [PubMed] [Google Scholar]
- Labudda K, Brand M, Mertens M, Ollech I, Markowitsch HJ, Woermann FG. Decision making under risk condition in patients with Parkinson's disease: A behavioural and fMRI study. Behavioural Neurology. 2010;23(3):131–143. doi: 10.3233/BEN-2010-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):7–584. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Simmons BL, Aklin WM, Daughters SB, Dvir S. Risk-taking propensity and risky sexual behavior of individuals in residential substance use treatment. Addictive Behaviors. 2004;29(8):1643–1647. doi: 10.1016/j.addbeh.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Letendre SL, FitzSimons C, Ellis RJ, Clifford D, Collier AC, Gelman B, Group, CHARTER Correlates of CSF viral loads in 1,221 volunteers of the CHARTER cohort. Paper presented at the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology. 1995;68(1):151–158. doi: 10.1037//0022-3514.68.1.151. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7861311. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D'Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Hum Brain Mapp. 2010;31(3):410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G, Rick S, Cohen JD. Neuroeconomics. Annu Rev Psychol. 2008;59:647–672. doi: 10.1146/annurev.psych.59.103006.093710. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Anastos K. Cognitive function in women with HIV: Findings from the Women's Interagency HIV Study. Neurology. 2014;84:231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Young M. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV− women: The Women's Interagency HIV Study (WIHS) Neurocognitive Substudy. Journal of Clinical and Experimental Neuropsychology. 2011;33(8):853–863. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, DeHaan S, Vassileva J, Gonzalez R, Weller J, Bechara A. Decision making among HIV+ drug using men who have sex with men: A preliminary report from the Chicago Multicenter AIDS Cohort Study. Journal of Clinical and Experimental Neuropsychology. 2013;35:573–583. doi: 10.1080/13803395.2013.799122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. Journal of Clinical & Experimental Neuropsychology. 2011;33:112–120. doi: 10.1080/13803395.2010.493150. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20694870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meyer V, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ElT, Anastos K. HIV and recent illicit drug use interact to affect verbal memory in women. Journal of Acquired Immune Deficiency Syndromes. 2013;63(1):67. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer V, Little DM, Fitzgerald DA, Sundermann EE, Rubin LH, Martin EM, Maki PM. Crack cocaine use impairs anterior cingulate and prefrontal cortex function in women with HIV infection. J Neurovirol. 2014;20(4):352–361. doi: 10.1007/s13365-014-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AM, Weller JA. Greater decision-making competence is associated with greater expected-value sensitivity, but not overall risk taking: An examination of concurrent validity. Front Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. Journal of Neurovirology. 2012;18(5):388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG. Social decision-making: insights from game theory and neuroscience. Science. 2007;318(5850):598–602. doi: 10.1126/science.1142996. Retrieved from PM:17962552. [DOI] [PubMed] [Google Scholar]
- Schiebener J, Brand M. Decision making under objective risk conditions- A review of cognitive and emotional correlates, strategies, feedback processing, and external influences. Neuropsychology Review. 2015;25(2):171–198. doi: 10.1007/s11065-015-9285-x. [DOI] [PubMed] [Google Scholar]
- Schiebener J, Wegmann E, Gathmann B, Laier C, Pawlikowski M, Brand M. Among three different executive functions, general executive control ability is a key predictor of decision making under objective risk. Front Psychol. 2014;5:1386. doi: 10.3389/fpsyg.2014.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z. Psychometric characteristics of the Wender Utah Rating Scale (WURS): reliability and factor structure for men and women. Psychopharmacology Bulletin. 1995;31:425–433. [PubMed] [Google Scholar]
- Sutterer MJ, Koscik TR, Tranel D. Sex-related functional asymmetry of the ventromedial prefrontal cortex in regard to decision-making under risk and ambiguity. Neuropsychologia. 2015;75:265–273. doi: 10.1016/j.neuropsychologia.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Streiff V, Patel SM, Panos SE, Castellon SA, Hinkin CH. The role of HIV infection, cognition, and depression in risky decision-making. Journal of Neuropsychiatry and Clinical Neuroscience. 2012;24(3):340–348. doi: 10.1176/appi.neuropsych.11110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Homberg J, de Visser L. A critical review of sex differences in decision-making tasks: focus on the Iowa Gambling Task. Behavioural Brain Research. 2013;238:95–108. doi: 10.1016/j.bbr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Vassileva J, Ahn WY, Weber KM, Busemeyer JR, Stout JC, Gonzalez R, Cohen MH. Computational modeling reveals distinct effects of HIV and history of drug use on decision-making processes in women. PLoS One. 2013;8(8):e68962. doi: 10.1371/journal.pone.0068962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Gonzalez R, Bechara A, Martin-Thormeyer EM. Iowa Gambling Task performance and emotional distress interact to predict risky sexual behavior in individuals with dual substance and HIV diagnoses. Journal of Clinical and Experimental Neuropsychology. 2010;32(10):1110–1121. doi: 10.1080/13803391003757833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Weller JA, Levin IP, Bechara A. Do individual differences in Iowa Gambling Task performance predict adaptive decision making for risky gains and losses? Journal of Clinical and Experimental Neuropsychology. 2010;32(2):141–150. doi: 10.1080/13803390902881926. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychological Science. 2007;18(11):958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biological Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: a shift in the burden of proof? Exp Clin Psychopharmacol. 2007;15(5):411. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Martin-Engel L, Vassileva J, Gonzalez R, Martin EM. An investigation of the effects of antiretroviral central nervous system penetration effectiveness on procedural learning in HIV+ drug users. J Clin Exp Neuropsychol. 2013;35(9):915–925. doi: 10.1080/13803395.2013.838939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cerebral Cortex. 2009;19(5):1019–1027. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]