Abstract
System xc− is a cystine/glutamate antiporter that exchanges extracellular cystine for intracellular glutamate. Cystine is intracellularly reduced to cysteine, a building block of GSH. As such, system xc− can regulate the antioxidant capacity of cells. Moreover, in several brain regions, system xc− is the major source of extracellular glutamate. As such this antiporter is able to fulfill key physiological functions in the CNS, while evidence indicates it also plays a role in certain brain pathologies. Since the transcription of xCT, the specific subunit of system xc−, is enhanced by the presence of reactive oxygen species and inflammatory cytokines, system xc− could be involved in toxic extracellular glutamate release in neurological disorders that are associated with increased oxidative stress and neuroinflammation. System xc− has also been reported to contribute to the invasiveness of brain tumors and, as a source of extracellular glutamate, could participate in the induction of peritumoral seizures.
Two independent reviews (Lewerenz et al. 2013, Bridges et al. 2012), approached from a different perspective, have recently been published on the functions of system xc− in the central nervous system. In this review, we highlight novel achievements and insights covering the regulation of system xc− as well as its involvement in emotional behavior, cognition, addiction, neurological disorders and glioblastomas, acquired in the past few years.
Keywords: System xc−, Glutamate, Emotional and cognitive behavior, Addiction, Neurological disorders, Glioblastoma
Graphical abstract
Introduction
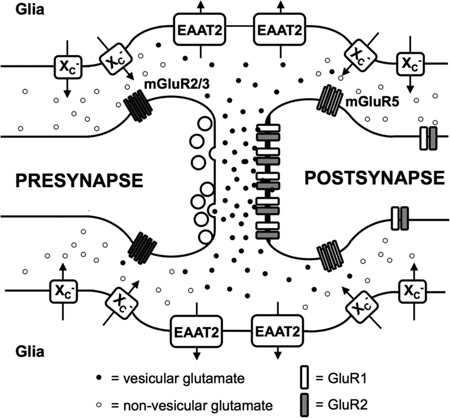
System xc− or the cystine/glutamate antiporter, composed of a heavy chain subunit 4F2hc (encoded by the slc3a2 gene) and a light chain specific subunit xCT (encoded by the slc7a11 gene), exchanges glutamate for cystine in a 1:1 ratio and according to the respective concentration gradients (figure 1). Under physiological conditions, cystine is imported and intracellularly reduced to cysteine, a building block of the antioxidant GSH. In vitro, cystine supply via system xc− is crucial for survival of certain cell types as they can only survive in the absence of system xc− when the medium is supplemented with reducing agents (Sato et al. 2005). In vivo, however, it has been shown that genetic deletion of system xc− does not necessarily lead to any gross abnormality in the CNS nor are there signs of increased oxidative stress since other sources of GSH can be supplied by different cell-types to sensitive cells (Massie et al. 2011, De Bundel et al. 2011, Sato et al. 2005). While cystine is imported, glutamate is obligatorily exported and system xc− has been identified as the major source of extracellular glutamate in several rodent brain regions (De Bundel et al. 2011, Massie et al. 2011, Baker et al. 2002b). Glutamate released via system xc− physiologically modulates synaptic transmission via activation of pre- and postsynaptic metabotropic glutamate receptors located in the vicinity of the synaptic cleft (Baker et al. 2002b). Moreover, it was recently shown that glutamate released via system xc− regulates glutamatergic synapse strength by reducing the number of postsynaptic alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptors (Williams & Featherstone 2014). Additionally, this glutamate could also activate extrasynaptic NMDA receptors, and as such in high concentrations may induce excitotoxicity (Hardingham & Bading 2010).
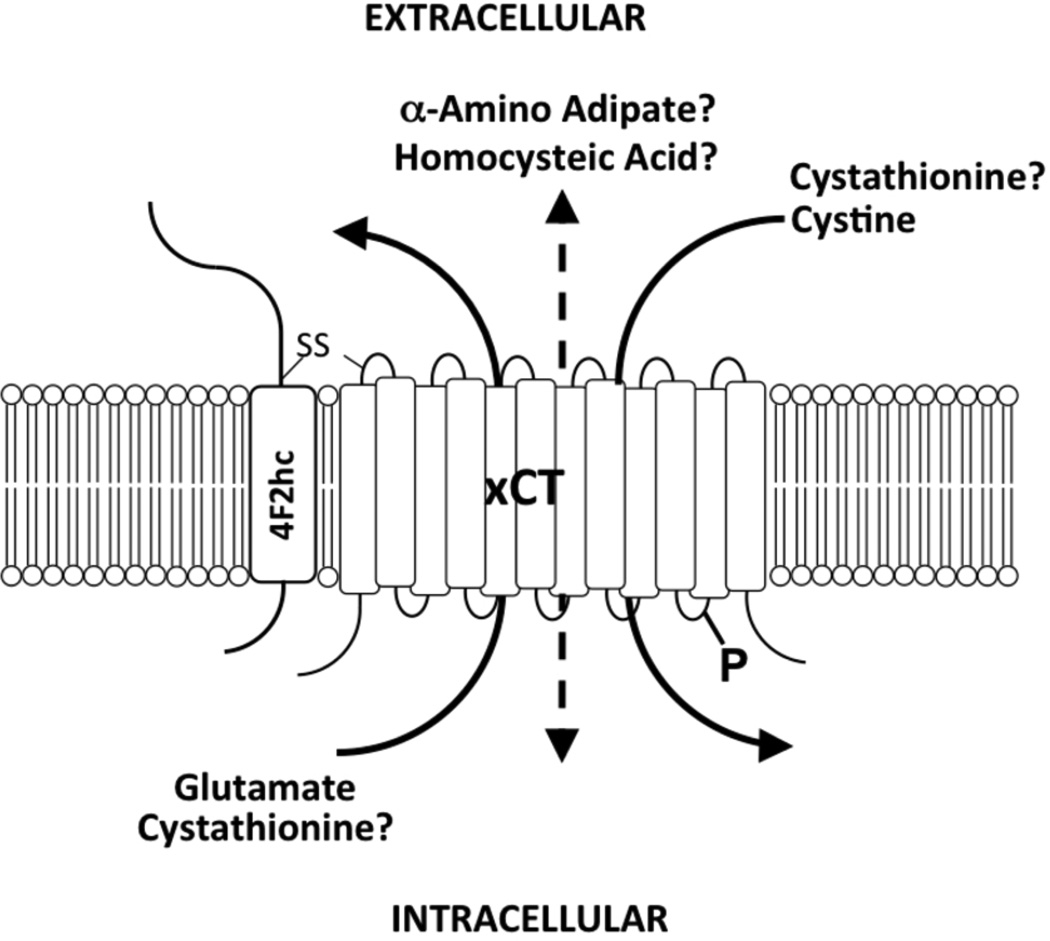
Figure 1. System xc−.
System xc− is composed of the 4F2 heavy chain (4F2hc) and the light chain, xCT, which are linked by a disulfide bond (-S-S-). System xc− imports cystine in exchange for glutamate. Cystathionine represents another transported substrate for system xc− of which it is not known whether it is preferentially imported or exported from neuronal cells. Homocysteic acid and α-amino adipate are other potentially relevant substrates in vivo. System xc− activity can be modulated by phosphorylation of xCT (-P).
System xc− is expressed in the brain parenchyma and at high levels in the meninges and the ependyma (Shih et al. 2006, Sato et al. 2002). The CNS cell types that contribute most to system xc− activity (measured by cystine uptake in acute brain slices (Xi et al. 2002) or by microdialysis in living animals (Massie et al. 2011, De Bundel et al. 2011, Baker et al. 2002a) and as xCT protein levels assessed by western blotting (Shih et al. 2006)) is not known in detail as high-sensitivity in situ hybridization data and reliable antibodies for immunohistochemistry are missing. Importantly, using antibodies that are supposed to bind xCT often leads to confusing and contradicting data. Whereas in western blot analyses many antibodies label a band of 50kDa, corresponding to the theoretical molecular weight of xCT (Sato et al. 1999), it has been shown that xCT – when proper controls are applied and depending on the source and the gel system used - migrates at a considerable lower molecular weight ranging from 35 to 45kDa (Massie et al. 2008, Shih et al. 2006, Lewerenz et al. 2012). Moreover, xCT antibodies are often used for immunohistochemistry. Of note, with most antibodies the staining is merely identical in xCT wildtype compared to knock-out tissues, indicating prominent unspecific binding of these antibodies (Mesci et al. 2015, Massie et al. 2008). In a recent study, only a single xCT antibody of a total of 53 antibodies tested, resulted in specific xCT staining and exclusively on acetone-fixed sections. The poor morphology of acetone-fixed sections did not allow analyzing the detailed cellular distribution of xCT-specific immunoreactivity. Yet, in certain brain regions neurons could be clearly distinguished. In addition, in line with previous findings obtained by non-radioactive in situ hybridization (Sato et al. 2002), strong labeling was observed in meninges and the ependymal cells of the choroid plexus (Van Liefferinge et al., in revision at J. Comp. Neurol.).
Also, the use of primary cell cultures to test for the relative expression of xCT and system xc− activity is inherently prone to give results that are likely to be very different from the in vivo situation as xCT is heavily induced under regular cell culture conditions (reviewed in (Lewerenz et al. 2013)). Still, an increased activity of system xc− in meningeal cells compared to astrocytes in vitro has been reported, suggesting that some fundamental differences in xCT expression, as described above, are preserved ex vivo (Shih et al. 2006). Whereas Jackman et al. observed highest system xc−-dependent cystine uptake in microglia, compared to astrocytes and neurons (i.e. microglia>astrocytes>neurons) (Jackman et al. 2010), Resch et al. compared either cortical astrocyte- or neuron-enriched to microglial primary cell cultures and concluded that system xc− activity in astrocytes is higher relative to neurons and microglia (Resch et al. 2014). Recent evidence using RT-PCR from laser-microdissected motor neurons in comparison to whole spinal cord indicated that at least this type of neuron does not express (or only traces of) xCT mRNA in vivo (Mesci et al. 2015). In contrast, microglia purified from spinal cord showed much higher xCT mRNA levels than whole spinal cord, indicating that an important contribution of the system xc− activity or xCT protein detected in the brain could be of microglial origin.
Given the widespread distribution of system xc− in the CNS and the contribution of glutamate released via system xc− to glutamatergic neurotransmission, it is evident that manipulation of this antiporter can significantly affect brain function under physiological as well as pathological conditions. Due to the absence of specific, blood-brain barrier-permeable inhibitors of system xc−, most of the information about the function of system xc− is obtained from xCT knock-out (xCT−/−) mice with a C57BL/6J background, descendants of the strain originally described by Sato and co-workers (Sato et al. 2005), or ‘subtle grey (sut)’ mice with a C3H/HeSnJ background that carry a spontaneous mutation resulting in a large 480kb deletion, including the last exon of slc7a11 (Chintala et al. 2005). Of note, conflicting data are often reported by using both system xc−-deficient mouse strains. Whereas in the xCT−/− mice, no anatomical or neurochemical changes are seen in the brain, besides the strong decrease in extracellular glutamate levels (De Bundel et al. 2011), sut/sut mice have been reported to develop brain atrophy after 15 weeks of age (Shih et al. 2006). Of note, Hewett and colleagues – who obtain sut/sut mice via heterozygous breeding - do not replicate these latter findings (SJH, unpublished observations). Whether the differences between xCT−/− mice and sut mice are thus background-specific, mutation-specific or due to genetic drift, has not been clarified yet.
For an extensive review on system xc− in health and disease, we refer to our previous review (Lewerenz et al. 2013). In the current review, we will discuss recent breakthroughs and novel findings concerning the involvement of system xc− in normal and pathological brain functioning with a focus on the transcriptional regulation of xCT expression, the role of system xc− in mood disorders and cognition, the potential of system xc− as a target in the development of new treatments for neurological disorders, the role of system in drug addiction and the link between system xc−, brain tumor development and peritumoral seizures.
New insights in the regulation of xCT expression and system xc− activity
As reviewed in detail previously (Lewerenz et al. 2013), system xc− is regulated on the levels of transcription (figure 2), protein insertion into the membrane and via the availability of counter-transported substrates (figure 1). Despite the fact that in cell culture, system xc− is prominently induced (probably because the ambient oxygen pressure is much higher in cell culture than in vivo) cell culture systems including primary cells as well as neuronal cell lines have been successfully employed to elucidate the pathways that regulate xCT expression and system xc− function. The two transcription factors that have a predominant role in the regulation of xCT expression are nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and activating transcription factor 4 (ATF4). Both of these explain some, maybe most, of the inducibility of system xc− activity by a plethora of cellular insults (figure 2).
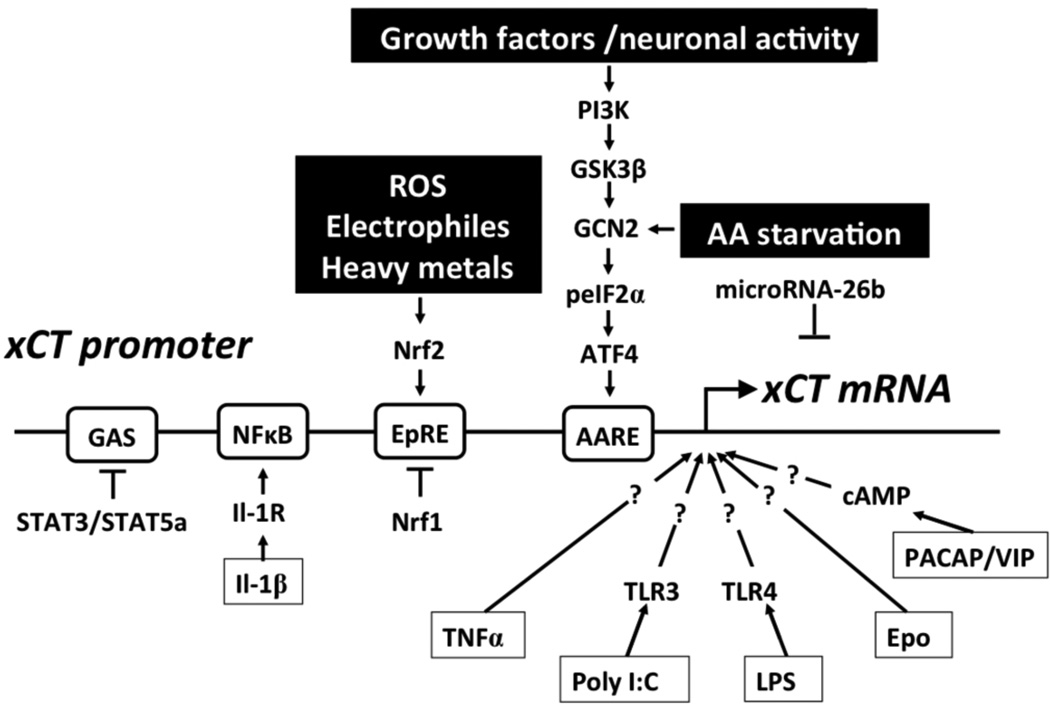
Figure 2. Transcriptional regulation of xCT expression.
A variety of stimuli, e.g. electrophiles, heavy metals, and reactive oxygen species (ROS), lead to activation of the nuclear factor NF-E2-related factor 2 (Nrf2), which binds to the electrophile response element (EpRE) within the xCT promoter region and activates transcription. Nrf1 inhibits the action of Nrf2. Amino acid (AA) starvation leads to phosphorylation of eIF2a (peIF2α), which via GCN2 leads to the translational up-regulation of the transcription factor activating transcription factor 4 (ATF4). ATF4 activates the transcription of xCT by binding to the amino acid response element (AARE) contained in the xCT promoter. Alternatively, growth factors and neuronal activity can induce xCT promoter activity via phosphoinositol-3 kinase (PI3K), glycogen synthase-3β (GSK-3β) and the GCN2/eIF2α/ATF4 module. STAT3/STAT5A negatively regulate the xCT promoter activity via a GAS element. Interleukin-1β (IL-1β) activates the xCT promoter by binding to the interleukin 1 receptor (Il-1R) and NF-κB. Tumor necrosis factor α (TNFα) and erythropoietin (EPO) increase the transcription of xCT through unknown signaling pathways. Bacterial lipopolysaccharides (LPS) and polyinosinic-polycytidylic acid (Poly I:C) activate the xCT promoter presumably via TLR4 and TLR3, respectively. PACAP and VIP increase cAMP and thereby activate xCT transcription. MicroRNA-26b directly targets xCT mRNA.
Induction of xCT through Nrf2
Nrf2 is induced by oxidative stress and other stimuli (reviewed in (Kensler et al. 2007)) and binds to an antioxidant response element (ARE) in the xCT promoter region (Sasaki et al. 2002). It has been documented that Nrf2 regulates system xc− activity in astrocytes (Shih et al. 2003). In addition, there is evidence that the neuroprotective β-lactam antibiotic ceftriaxone induces xCT expression via Nrf2 in astrocytes and motor neurons (Lewerenz et al. 2009). Before discovery of the effect of ceftriaxone on xCT, neuroprotective properties of this compound were ascribed to the enhancement of glutamate reuptake by the excitatory amino acid transporter 2 (EAAT2/GLT-1) (Rothstein et al. 2005). In murine retina, the sigma receptor 1 regulates xCT protein expression via Nrf2 in Müller cells, the radial glia of the retina (Wang et al. 2015, Ha et al. 2014). In contrast, Nrf1 constitutively binds to the ARE in the xCT promoter acting as a transcriptional suppressor of Nrf2 action, at least in the liver and in fibroblasts (Tsujita et al. 2014). It seems possible that Nrf1 is also involved in the regulation of xCT expression in the brain although experimental evidence is lacking.
Induction of xCT through ATF4
Via the four different kinases of the eukaryotic initiation factor 2α (eIF2α), i.e. protein kinase R (PKR), heme-regulated eIF2α kinase (HRI), PKR-like endoplasmic reticulum kinase (PERK) and general control non-depressible-2 (GCN2), signals as diverse as endoplasmic reticulum stress, amino acid starvation, infection and radiation are relayed via eIF2α phosphorylation and subsequent translational upregulation of ATF4 (reviewed in (Wek et al. 2006)). ATF4 plays a major role in the resistance of neuronal cell lines against oxidative stress via direct transcriptional upregulation of xCT (Lewerenz et al. 2012, Lewerenz & Maher 2009), ATF4 being able to bind an amino acid response element (AARE) in the xCT promotor (Sato et al. 2004). However, xCT expression and system xc− activity can also be upregulated by growth factor signaling. Recently, Lewerenz et al. could demonstrate that growth factor signaling induces xCT expression and system xc− activity via glycogen synthase kinase 3β, GCN2 and subsequently ATF4 (figure 2). This pathway upregulates system xc− in response to robust neuronal activity in vitro. In addition, markers for activation of this pathway suggested that it is involved in the upregulation of xCT in epileptic hippocampi in humans with temporal lobe epilepsy (Lewerenz et al. 2014). In breast cancer cells, insulin-like growth factor 1 (IGF-1) upregulates xCT in an IGF-1 receptor substrate 1 dependent manner (Yang & Yee 2014). In astrocytes, fibroblast growth factor 2 upregulates system xc− by a transcriptional mechanism whereas IGF-1 is ineffective (Liu et al. 2012, Liu et al. 2014). Thus, some of the responses to growth factors might be cell type-specific. To make the role of eIF2α regarding xCT expression even more complex, it was recently reported that in cancer cells and fibroblasts, eIF2α phosphorylation might directly regulate the stability of xCT mRNA via inhibition of the nonsense-mediated RNA decay (Martin & Gardner 2014). Whether this pathway contributes to the regulation of xCT expression in neuronal or glial cells remains to be demonstrated.
Regulation of system xc− activity and xCT expression by kinases, specifically cAMP-dependent pathways
Upon treatment with the NMDA receptor antagonist phencyclidine, McClatchy et al. observed that a phosphorylated peptide of xCT is highly upregulated in the prefrontal cortex in rats by an unknown kinase (McClatchy et al. 2015). In vitro, mutated xCT mimicking dephosphorylated xCT showed a lower activity than wildtype and mutated xCT mimicking a constitutively phosphorylated xCT. In acute rat striatal slices, the activation of metabotropic glutamate receptors 2/3 (mGluR 2/3), which lowers cellular cAMP levels, rapidly decreases system xc− activity (Baker et al. 2002b). However, Resch et al. could not detect any rapid upregulation of system xc− in astrocyte-enriched primary mixed cortical cell cultures when treated with the cAMP-increasing neuropeptide PACAP (Resch et al. 2014). Thus, system xc− activity might be rapidly regulated by direct phosphorylation, including cAMP-dependent pathways, under some circumstances but not others. In contrast to the lack of effect of short-term PACAP and VIP exposure on system xc− activity, Resch et al. observed that both neuropeptides induced system xc− in astrocytes via a pathway that includes the activation of the VPAC1 receptor and cAMP upon prolonged activation times of six hours and more. As xCT mRNA was increased, this pathway presumably upregulates system xc− via transcriptional mechanisms (Resch et al. 2014). This is in line with an early report by Gochenauer and Robinson, showing that prolonged treatment with the cAMP analogue dibutyryl-cAMP for 10 days induced system xc− in astrocytes (Gochenauer & Robinson 2001).
Inflammatory stimuli
An early report regarding the role of inflammatory stimuli in the regulation of xCT and system xc− indicated that cytokines like tumor necrosis factor α (TNFα) and the toll-like receptor 4 (TLR4) ligand bacterial lipopolysaccharide (LPS) upregulate system xc− activity in peritoneal macrophages (Sato et al. 1995). The mechanism for this upregulation remained elusive. Although a putative binding site for nuclear factor-κB (NF-κB) was identified in the murine xCT gene 5’ flanking region, LPS concentrations too low to activate NF-κB strongly stimulated xCT expression in macrophages (Sato et al. 2001), indicating that TLR4 activation acts independently of NF-κB. The LPS-induced upregulation of system xc− activity is preserved in macrophages derived from Nrf2 knock-out mice (Ishii et al. 2000). Thus, both Nrf2 and NF-κB can be excluded as responsible for the upregulation of system xc− activity via TLR4 activation, at least in macrophages.
Microglia are the resident macrophage population of the brain. It could be demonstrated that both LPS and TNFα strongly induce glutamate release via system xc− in primary microglia (Piani & Fontana 1994, Figuera-Losada et al. 2014) and by using xCT−/− microglia it was confirmed that system xc− was the major source of microglial glutamate (Mesci et al. 2015). Not only TLR4 activation but also TLR3 activation via the viral double-stranded RNA mimetic polyinosinic–polycytidylic acid (poly I:C) induced system xc− activity in mixed microglial/astrocytic cultures (Scumpia et al. 2014). In vivo, intraspinal injection of LPS robustly induced xCT mRNA levels, most possibly, as indicated by immunofluorescence, in microglia and macrophages (Kigerl et al. 2012). The authors observed that co-injection of cystine along with LPS amplified LPS-induced neurotoxicity (Kigerl et al. 2012). This effect is compatible with the idea that glutamate released by system xc− is, at least in part, responsible for the observed neurotoxicity of neuroinflammation in vivo. However, the exact mechanism through which inflammatory stimuli upregulate system xc− in microglia remains enigmatic. Interestingly, compounds like fisetin, minocycline and apigenine that inhibit microglial activation, also inhibit LPS-induced glutamate release via system xc− in this cell type (Figuera-Losada et al. 2014). The effect of the TLR3 agonist poly I:C on system xc− activity was found to be partially inhibited by the antioxidant α-lipoic acid (Scumpia et al. 2014). The role of microglia and neuroinflammation in the regulation of system xc− activity in the brain does not only rely on the expression of xCT in microglia. Upon inflammatory activation, these cells produce many compounds including the pro-inflammatory cytokine interleukin-1β (IL-1β). IL-1β specifically upregulated system xc− in astrocytes but not in neurons or microglia (Jackman et al. 2010). In contrast to the data that exclude NF-κB as a mediator of LPS-induced system xc− upregulation in macrophages (Sato et al. 2001), the effect of IL-1β on system xc− expression was found to be mediated via NF-κB (He et al. 2015). Most recently, it was shown that the janus kinase (JAK)/ signal transducer and activator of transcription (STAT) pathway negatively regulates xCT transcription by binding of STAT3 and/or STAT5A to a gamma-activated site (GAS) motif in the xCT promoter region in breast cancer cells (Linher-Melville et al. 2015). STATs are activated in response to cytokine and growth factor signaling (O'Shea et al. 2013). Thus, the JAK/STAT pathway might alleviate the upregulation of xCT via these pathways (figure 2). Interestingly, deficiency in xCT reduced the release of TNFα and, in part, of IL-1β in microglia, in vitro and in vivo, respectively, indicating a positive feedback loop from cytokine-induced xCT expression to cytokine release (Mesci et al. 2015).
In summary, multiple stressors induce system xc− in brain-derived cells, some of those aimed at neutralizing oxidative stress or amino acid deficiency. In addition, neuronal activity, second messengers like cAMP, growth factor and cytokine signaling regulate system xc−. Which of these pathways importantly regulates system xc− in the brain in vivo, remains to be determined.
System xc− substrates
As system xc− is a mandatory antiporter (Bannai 1986), its activity is not only regulated on the level of transcription and protein expression but also by the availability of the counter-transported substrates (figure 1). Intracellular glutamate, as a driving force for cystine import, is most probably not a rate-limiting substrate in the brain as the mean intracellular concentration is in the millimolar range (Kvamme et al. 1985) whilst the extracellular glutamate concentration is about 2–20 µM (Baker et al. 2002b, De Bundel et al. 2011). Using microdialysis, Baker et al. could demonstrate that extracellular cystine concentrations in the brain are extremely low (130–190 nM) (Baker et al. 2003). In general, system xc− is assumed to act as a cystine/glutamate antiporter. However, as deletion of xCT massively decreases cerebral extracellular glutamate concentration by >50% (De Bundel et al. 2011, Massie et al. 2011), other imported substrates more abundant than cystine have to be assumed. Candidates include the system xc− substrates L-α-aminoadipate (AA) and L-homocysteate (HCA) (figure 1). HCA was identified in astrocytes and is released from these cells (Do et al. 1997). As glutamate, HCA is an endogenous agonist of ionotropic glutamate receptors, specifically the NMDA receptor (Do et al. 1988). AA is a product of lysine metabolism and is also present in the brain (Chang 1982). In addition, cystathionine has been recently identified as a substrate of system xc− (Kobayashi et al. 2015) (figure 1). Interestingly, although cystathionine was absent in thymus and spleen in xCT knock-out mice, indicating that system xc− plays an essential role in importing cystathionine into these tissues, cystathionine levels were increased in the CNS in the absence of system xc−. Thus, it can be hypothesized that system xc− mediates a net efflux of cystathionine from the brain. In addition, cystathionine can replace cystine for GSH synthesis (Kobayashi et al. 2015). In summary, the action of system xc− in the brain might be much more complex than assumed previously. Further research is needed taking into account all possible transported substrates, either glutamate and glutamate receptor agonists like HCA, or GSH precursors like cystine and cystathionine or other substrates like AA.
The effect of inhibition or loss of system xc− on emotional and cognitive features of behavior
Depressive- and anxiety-like behavior
Changes in glutamatergic signaling may be involved in the development of mood disorders. It has been shown in animal models that interference with glutamate reuptake as well as glutamate receptor signaling can lead to changes in depressive – and/or anxiety-like behavior. Antagonists of NMDA receptors (Sanacora et al. 2008) as well as agonists of mGluRs 2/3 (Fell et al. 2011, Matrisciano et al. 2008) exert anxiolytic and anti-depressive-like effects. Anti-depressive-like effects of ceftriaxone have been attributed to enhancement of glutamate reuptake (Mineur et al. 2007). At that time however, it was not known yet that ceftriaxone is also an inducer of xCT expression (Lewerenz et al. 2009, Knackstedt et al. 2010a) and as such the potential involvement of system xc− in inducing this effect had not been considered. N-acetylcysteine, a cysteine prodrug and known activator of system xc−, has also been reported to possess anti-depressive-like activity in rodents. Although the authors do not exclude the possible involvement of glutamate released via system xc− and acting on mGluR 2/3, the effects of N-acetylcysteine were shown to be mediated through reduction of oxidative stress (Smaga et al. 2012).
Recently, the possible involvement of system xc− in depression and anxiety was further investigated by submitting xCT−/− mice (McCullagh & Featherstone 2014, Bentea et al. 2015a), sut/sut mice or a heteroallelic xCT+/−/sut mutant to a battery of behavioral paradigms commonly used in rodents as measures of such (McCullagh & Featherstone 2014). Moreover, the effect of inhibiting system xc− using sulfasalazine (SSZ; systemically and acutely) in male rats was investigated by Lutgen and colleagues (Lutgen et al. 2014). Of note, xCT−/− and sut/sut mice as well as rats treated with SSZ do not show any motor deficits in a plethora of different tests (Bentea et al. 2015a, McCullagh & Featherstone 2014, Lutgen et al. 2014). Also visual deficits have been excluded in xCT−/− mice (Bentea et al. 2015a) as they might, similar to motor deficits, represent important confounders for behavioral testing.
When it comes to emotional behavior, contradicting findings were reported. Male xCT−/− mice clearly demonstrated reduced anxiety (manifested as increased time-in-the-center of the open field during a 60-min recording period, increased time-outside-the-shelter in the light/dark paradigm and decreased latency-to-feed in the novelty suppressed feeding) as well as reduced depressive-like behavior (manifested as decreased immobility in both the tail suspension and forced swim test), compared to their wildtype littermates (Bentea et al. 2015a). Similar to the observations of Bentea et al. in xCT−/− mice after a five-min recording period, no effects were seen by McCullagh and Featherstone in the time-in-the-center of the open field during a eight-min recording period for the xCT−/− mice, sut/sut mice or xCT+/−/sut mice (McCullagh & Featherstone 2014). In contrast, an anxiogenic effect of SSZ was reported in rats as they spent less time in the open arm of the elevated plus-maze and less time-in-the-center of the open field (15-min recording) after SSZ administration. Moreover, SSZ had no effect on immobility in the forced swim test, a measure for depressive-like behavior (Lutgen et al. 2014).
The differences described above can of course result from the fact that inhibition of system xc− by SSZ is an acute effect whereas system xc−-deficient mice have a chronic loss of system xc−. Moreover, it is tempting to speculate that the effects of SSZ might be mediated via a mechanism independent of system xc−. SSZ has been demonstrated to inhibit NF-κB activation (Wahl et al. 1998). In addition, NF-κB-independent actions of SSZ were shown to be mediated by stimulation of adenosine release (Cronstein et al. 1999). Moreover, it is unknown how much of SSZ crosses the blood-brain barrier (for a critical review on the potential of SSZ to be used as a drug to inhibit system xc− in CNS see: (Sontheimer & Bridges 2012)). SSZ has been shown to decrease brain tumor growth and tumor-associated seizures in animal models of glioblastoma (GBM) and as such it has to reach the brain (Buckingham et al. 2011, Chung et al. 2005). Yet, in the case of brain tumors, the blood-brain barrier might be compromised. Finally, the half-life of SSZ in rodents is very short (80–180 minutes) (Zheng et al. 1993) and anticonvulsive effects were reported to last for only two-three hours (Buckingham et al. 2011), meaning that a behavioral characterization two hours after SSZ administration might be misleading. However, in the study of Lutgen and colleagues SSZ did affect extracellular glutamate levels in the brain and the effects of SSZ could be reversed by N-acetylcysteine (Lutgen et al. 2014). As such, the changes in behavior observed in rats after SSZ indeed are compatible with the hypothesized action of SSZ via inhibition of system xc−.
Cognition
As the major excitatory neurotransmitter in the mammalian CNS, glutamate is undeniably involved in most aspects of normal brain functioning, including higher brain functions such as cognition, memory and learning. A total loss or partial inhibition of system xc− can as such affect these functions. Although McCullagh and Featherstone describe deficits in spontaneous alternation tasks for the sut/sut mice when compared to wildtype C3H/HeSnJ mice, consistent with the impaired spatial working memory reported before in the xCT−/− mice (De Bundel et al. 2011), they could not confirm these effects in xCT−/− mice or xCT+/−/sut mutants when compared to C57BL/6J or C3H/HeSnJ/C57BL/6 first generation offspring, respectively (McCullagh & Featherstone 2014). SSZ was reported to induce some cognitive impairment as it negatively affected attentional set shifting in rats (Lutgen et al. 2014). Moreover, Li and co-workers described reduced long-term memory in sut/sut mice as evaluated by investigation of their fear memory in a fear conditioning as well as a passive avoidance paradigm (Li et al. 2012). It should be mentioned however, that fear memory might be affected by anxiolytic effects that have been observed in the xCT−/− animals (Bentea et al. 2015a). Still, adult xCT−/− mice do show impaired spatial working memory (De Bundel et al. 2011) and rodents receiving pharmacological drugs now known to inhibit system xc−, have impaired long term memory consolidation (Rickard & Ng 1995, Bianchin et al. 2000) as well as deficits in spatial working memory (Bordi et al. 1996, Wetzel et al. 1995), indicating that reductions in the physiological function of system xc− basally result in negative effects on cognition. Notably, enhanced levels of system xc− could also be ultimately deleterious to cognitive function via its propensity to contribute to excitotoxic neuronal injury, possibly explaining why xCT−/− mice are protected against age-dependent decline in spatial working memory (De Bundel et al. 2011).
It is clear that available data on cognition and memory are very diverse and covering many different aspects of this complex behavior. Therefore, more experimental evidence is certainly needed to provide a clear-cut view on the possible consequences of inhibiting system xc− (which might be an attractive new strategy for the development of new treatments for several neurological disorders – see below) on these brain functions.
System xc− and drug addiction
The nucleus accumbens (NAc) is a key region mediating the long-term behavioral pathologies produced by the self-administration of addictive drugs. In the NAc core, system xc− is a significant contributor to basal extracellular glutamate levels which are decreased by 60% when applying inhibitors of this system (Baker et al. 2002b). Basal levels of glutamate in this brain region were found to be altered by cocaine (Baker et al. 2003), methamphetamine (Parsegian & See 2014, Lominac et al. 2012) and ethanol (Griffin et al. 2014, Griffin et al. 2015). In the case of cocaine, these changes in basal glutamate have been shown to produce alterations in the function of both pre- and post-synaptic glutamate receptors and to contribute directly to the risk of relapse to cocaine-seeking in rodents (figure 3).
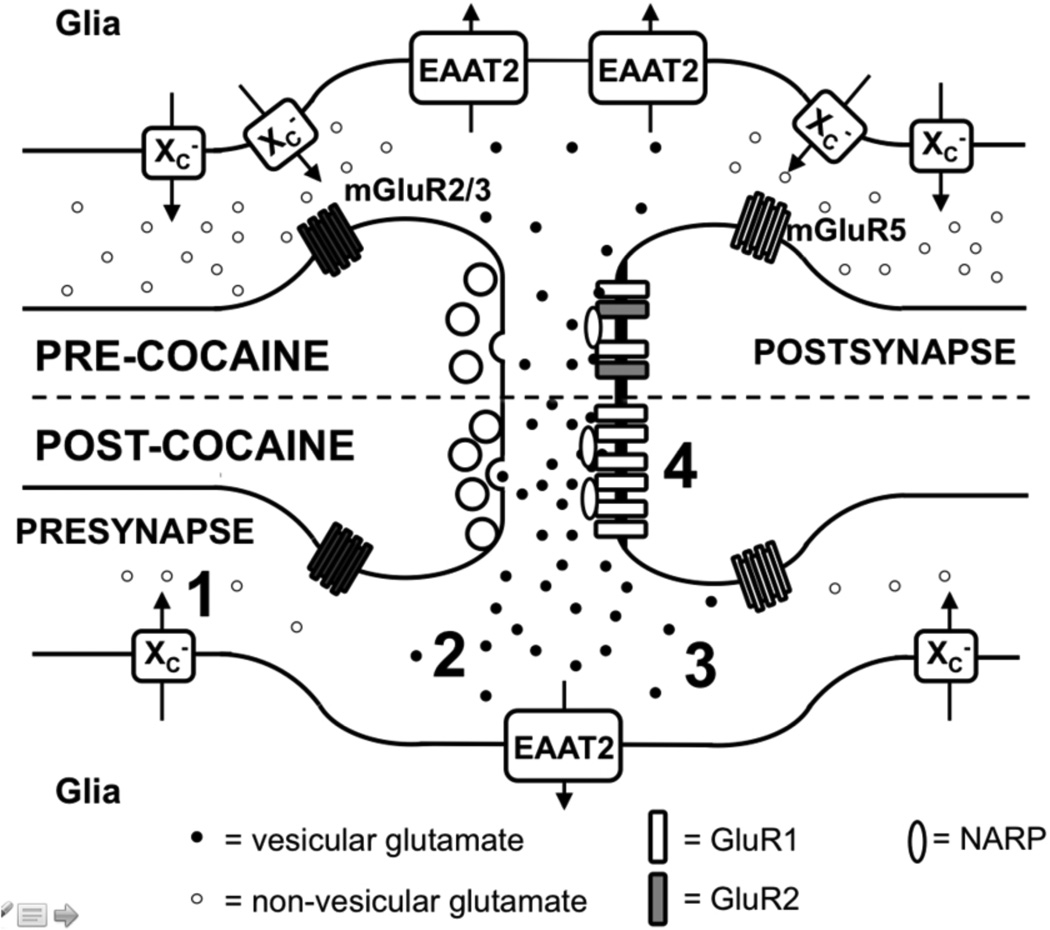
Figure 3. Four neuroadaptations in the NAc core promote the reinstatement of cocaine seeking.
The top panel depicts a NAc core synapse of a drug-naïve rat while the bottom panel depicts that of a rat post-cocaine. Relative to drug naïve animals, cocaine animals display: 1) reduced expression and function of xCT/system xc−, leading to reduced basal non-vesicular glutamate; 2) reduced expression and function of EAAT2, leading to increased glutamate overflow during reinstatement (3); and 4) increased expression of GluR2-lacking AMPA receptors and the AMPA-receptor clustering protein Narp. As discussed below, compounds which restore both xCT and GLT-1 restore basal levels of glutamate and attenuate glutamate spillover during reinstatement.
Drug seeking is studied in rodents using the extinction-reinstatement model of relapse, in which animals self-administer drug in the operant chamber and then undergo extinction training for one-three weeks. During extinction, the operant response that previously delivered drug no longer does so and responding typically declines. The drug-seeking response is reinstated with stimuli known to induce relapse in humans, namely stress, cues and the drug itself (Epstein et al. 2006). Relapse to cocaine seeking in the extinction-reinstatement model is associated with decreased basal extrasynaptic glutamate in the NAc core. The decrease in basal glutamate at this time stems from a decrease in NAc core expression and function of xCT/system xc− (Baker et al. 2003, Knackstedt et al. 2010a). Decreased basal glutamate in turn causes a loss of tone on release-regulating glutamate autoreceptors, namely mGluR 2/3 (Moran et al. 2005). This adaptation is likely a major contributing factor to the enhanced synaptically released (vesicular) glutamate that drives the drug-primed reinstatement of cocaine seeking (McFarland et al. 2003), in addition to the observed reduction in glutamate re-uptake (Knackstedt et al. 2010a). Synaptically-released glutamate promotes relapse via actions at post-synaptic AMPA and mGluR5 receptors, as the intra-NAc infusion of AMPA and mGluR5 antagonists attenuates cocaine relapse (Cornish & Kalivas 2000, Wang et al. 2013).
Chronic reductions in basal extracellular glutamate contribute to the development of post-synaptic adaptations. Following cocaine self-administration and extinction, we have observed increased expression of the protein Narp, responsible for clustering AMPA receptors and promoting long-term potentiation (Knackstedt et al. 2010b). Cocaine self-administration followed by both extinction (Moussawi et al. 2011) and withdrawal without extinction training (Conrad et al. 2008) produces enduring synaptic potentiation in the NAc core, accompanied by a change in AMPA receptor subunit composition towards the promotion of higher conductance GluR2-lacking AMPA receptors (Conrad et al. 2008). Taken together, these results suggest that cocaine self-administration induces a potentiation of glutamatergic transmission from the prefrontal cortex to the NAc core. It has been proposed that the decrease in basal glutamate, via system xc− downregulation, is the causative factor in the post-synaptic adaptations observed after cocaine (Wolf 2010) and restoring basal glutamate levels normalizes some of these observations (Trantham-Davidson et al. 2012). Figure 3 depicts these alterations and the two pools of glutamate impacted by cocaine: extrasynaptic (non-vesicular) and synaptic (vesicular).
Basal extracellular glutamate levels in the NAc are also reduced two weeks after methamphetamine self-administration but only when animals undergo extinction training (Parsegian & See 2014). Interestingly, following three weeks of abstinence without extinction training, methamphetamine self-administration produces an increase in basal glutamate in the NAc (Lominac et al. 2012). Differences in glutamatergic adapations following extinction vs. abstinence have been reported following cocaine as well (e.g.(Knackstedt et al. 2010b)). The contribution of system xc− to these changes in basal glutamate has not yet been investigated.
Non-contingent ethanol alone or in combination with ethanol self-administration has been repeatedly shown to increase basal glutamate in the NAc core and shell (e.g. (Griffin et al. 2014, Griffin et al. 2015). It was recently demonstrated that this increase in basal glutamate does not stem from increased release via system xc−, as its function was not altered in animals exposed to a non-contingent ethanol administration paradigm that increased basal glutamate (Griffin et al. 2015).
While ceftriaxone treatment (200 mg/kg for five-seven days) restores EAAT2/GLT-1 expression following cocaine self-administration, it also restores expression of xCT in the NAc (Knackstedt et al. 2010a). As discussed above, ceftriaxone induces a known transcriptional regulator of xCT, Nrf2, and thereby increases xCT expression and system xc− activity (Lewerenz et al. 2009). Later, it was demonstrated that the ceftriaxone-mediated increase in xCT expression leads to enhanced system xc− activity and increased basal glutamate (Trantham-Davidson et al. 2012). The chronic administration of ceftriaxone prevents the reinstatement of cocaine seeking in response to drug-associated cues and cocaine itself (Knackstedt et al. 2010a). Reinstatement is attenuated as late as two weeks after the cessation of ceftriaxone administration (Sondheimer & Knackstedt 2011), indicating a long-lasting reversal of cocaine-induced changes in brain physiology. Ceftriaxone reduces ethanol consumption in ethanol-preferring rats, while increasing xCT expression in the NAc and prefrontal cortex (Alhaddad et al. 2014). This finding is interesting in light of the consistently observed increases in basal glutamate in the NAc after ethanol and in theory increasing xCT may increase these levels even further.
In conclusion, the importance of xCT in addiction has been clearly demonstrated for cocaine and it remains a strong treatment target for reducing cocaine relapse. More work is needed to clarify the role of xCT in mediating the decrease in basal glutamate observed following methamphetamine self-administration and the reduction in ethanol consumption following ceftriaxone administration.
Contribution of system xc− to neurological disease
The bimodal actions (sustaining GSH synthesis and releasing glutamate) of system xc− intriguingly suggest that this antiporter can either protect from or contribute to neural injury in different affections of the CNS. Its expression and function have indeed been studied in different in vitro and in vivo models pertinent to CNS diseases/disorders, as described below.
Alzheimer’s disease
Alzheimer’s disease (AD) is the leading cause of dementia in the population and is caused by neurodegeneration predominantly of brain regions involved in mediating memory and cognition, including the hippocampus and cerebral cortex. Neurofibrillary tangles of intraneuronal phosphorylated Tau accumulation and extracellular amyloid-β (Aβ) plaques consisting of aggregated Aβ1–40/42 peptides proteolytically derived from the amyloid precursor protein (APP), are the hallmarks of AD brain pathology. An important field of research on AD focuses on the role of microglial cells, the macrophages of the CNS, as potent players in plaque removal as well as in the production of neurotoxic and/or neurotrophic factors. Microglial cells — depending on the origin, strength and length of stimuli —are able to release different cytokines, chemokines, reactive oxygen species (ROS) and amino acids that could positively or negatively influence AD brain pathology (Tang & Le 2015).
Initial studies that potentially link system xc− to AD pathology were performed in cell culture. Primary rodent microglial cell cultures treated with soluble APP (sAPP) or aggregated Aβ1–40 peptides increased cellular release of glutamate through system xc− (Qin et al. 2006, Barger & Basile 2001). Such activation of system xc− in microglia with sAPP led to compromised synaptic density in hippocampal neurons in co-culture (Barger & Basile 2001) whereas activation with Aβ1–40 caused frank excitotoxic cortical neuronal death (Qin et al. 2006). In addition to sAPP and Aβ, several inflammatory factors that are either associated with AD or recapitulate cellular responses in AD, including LPS, oxidative stress, and TNFα can also activate or increase the expression of macrophage/microglial system xc−, as discussed above (Piani & Fontana 1994, Sato et al. 2001, Mesci et al. 2015, Sato et al. 1995, Albano et al. 2013). Therefore, the possibility that several factors linked to chronic AD could together cooperate to increase glutamate release through system xc− and as such adding to ongoing neurodegeneration cannot be excluded.
In vivo, increased xCT expression has been demonstrated in the cerebral cortex of an animal model of AD, aged AβPP23 (18 month-old) mice, when gliosis and Aβ plaques are prominent (Schallier et al. 2011). Due to the non-specificity of antibodies against xCT for immunostaining on mouse tissues (Massie et al. 2008, Mesci et al. 2015, Van Liefferinge et al., in revision at J. Comp. Neurol.), the cell-types overexpressing system xc− protein in AD mouse brains have been difficult to identify. However, microglial cells activated by Aβ prominently express xCT mRNA as visualized by in situ hybridization in two different mouse models of AD: wildtype mice injected with Aβ into the hippocampus and in the vicinity of Aβ plaques in Thy1-APP751 mice (Qin et al. 2006). Interestingly, the glutamate transporter EAAT2/GLT1, which takes up glutamate from the extracellular space, was found to be downregulated in AβPP23 mice (Schallier et al. 2011). Transgenic overexpression of EAAT2 ameliorates AD pathology and memory loss in AD mice (Takahashi et al. 2015), suggesting that glutamate dysregulation is pathophysiologically important in AD (figure 4). However, studies involving inhibition of system xc− or its genetic deletion (Sato et al. 2005) in AD mouse models are needed to fully analyze the role of xCT in AD brain. Of note, when considering system xc− as a possible target in the treatment of AD, the effects of inhibition of system xc− on cognition should be taken into account (see above).
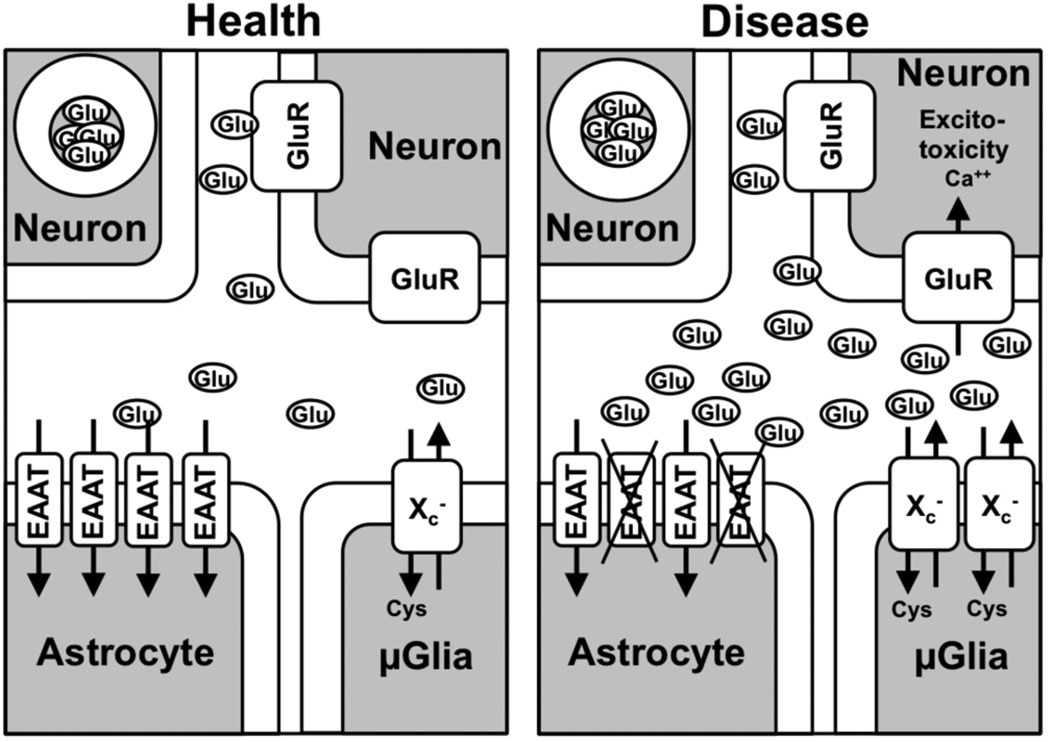
Figure 4. The hypothetical role of system xc− in excitotoxicity in neurodegenerative diseases.
(Left panel) In the healthy brain, glutamate release by system xc− (here only depicted in microglia but also present on astrocytes; conflicting data about neuronal expression have been published) is balanced by glutamate uptake by EAAT2, which leads to negligible activation of extrasynaptic ionotropic glutamate receptors (GluR). (Right panel) In many disease states of the brain, system xc− is upregulated, in ALS and AD especially in microglia and subsequently glutamate release is increased. Astrocytes may also contribute to the increase in glutamate release via system xc− (not shown). Simultaneously, EAAT2 is down-regulated. The increased extrasynaptic glutamate concentration activates extrasynaptic ionotropic glutamate receptors and induces excitotoxicity.
Parkinson’s disease
Parkinson’s disease (PD) is a slowly progressive neurodegenerative disorder affecting 2% of individuals over 65 years of age. The clinical motor symptoms are linked to a profound loss of striatal dopaminergic innervation caused by a progressive degeneration of dopaminergic neurons within the midbrain's substantia nigra pars compacta. To model the dopaminergic neurodegeneration seen in PD, toxins, including 6-hydroxydopamine (6-OHDA) and MPTP can be injected either into the rodent nigrostriatal pathway directly (6-OHDA) or systemically (MPTP) (Dauer & Przedborski 2003).
Of note, an ipsilateral increase in striatal xCT protein levels occurred in hemi-Parkinson rats three weeks after 6-OHDA injection into the medial forebrain bundle (Massie et al. 2008). A follow-up study in xCT−/− mice demonstrated that expression of system xc− can impact dopamine neuron survival. Specifically, dopamine neurons in the substantia nigra pars compacta of xCT−/− mice striatally injected with 6-OHDA were significantly protected as compared to those from 6-OHDA-injected wildtype mice (Massie et al. 2011). Reduced extracellular glutamate levels in xCT−/− mice, coupled with an increased survival of dopaminergic neurons in the 6-OHDA model, suggest that system xc− might be a promising pharmacological target for PD therapy. However, a more complex response is seen using the MPTP model (Bentea et al. 2015b). Mice lacking xCT are equally susceptible to MPTP-induced parkinsonism, both pathologically and behaviorally, as wildtype littermates, despite the latter having an increased expression of xCT in the striatum. Interestingly, the same treatment resulted in a down-regulation of xCT in the substantia nigra pars compacta (Bentea et al. 2015b). Regional control of xCT changes might represent a plausible reason for the differences seen between models, as do obvious differences in the experimental paradigms employed. Hence, additional studies are necessary to better understand the involvement of system xc− in the chronic neurodegenerative processes associated with PD.
Amyotrophic Lateral Sclerosis
Amyotrophic Lateral Sclerosis (ALS) is the most common motor neuron disease of the adult leading to progressive paralysis and death of the patients within two-five years after disease onset, which commonly occurs in the fifth decade of life (Johnston et al. 2006). While mainly sporadic, 10% of ALS cases have a familial history of which in two thirds of these cases the genetic cause has been discovered. Besides in the C9ORF72 gene (DeJesus-Hernandez et al. 2011), mutations occur in Cu/Zn superoxide dismutase (SOD1) —the first ALS gene discovered — which led to the design of ALS mouse models that express SOD1 with different mutations (Joyce et al. 2011). Studies in mutant SOD1 ALS mice have shown that ALS is a non-cell autonomous disease with glial cells participating in motor neuron degeneration (Boillée et al. 2006a, Boillée et al. 2006b, Beers et al. 2006).
Recently, the regulation of system xc− in acute spinal cord slices was assessed through measurement of cystine uptake in the rapidly-progressing ALS hSOD1G93A mice at different ages (Albano et al. 2013). In presymptomatic 70-day-old ALS hSOD1G93A mice (but not 55-day-old mice), cystine uptake was significantly increased as compared to age-matched non-transgenic controls. This change in system xc− function was, in this experimental context, transitory, since at later ages including at symptomatic stages, no difference was measured (Albano et al. 2013). The authors speculate that this increased expression might represent a compensatory change precipitated by oxidative stress that is measureable by 60 days in this mouse model (Liu et al. 1998).
In the more slowly progressing ALS hSOD1G37R mouse model, however, expression of xCT mRNA was demonstrated to be increased over the course of the disease. Cellular analysis determined that this increase occurred in microglia, but not in motor neurons (see above) (Mesci et al. 2015). Relevantly, post-mortem spinal cord tissue from ALS patients also demonstrated expression of xCT in spinal cord where levels are correlated with a macrophage marker of inflammation (CD68) (Mesci et al. 2015). In line with the view that system xc− is involved in the antioxidant defense, deleting xCT in ALS hSOD1G37R mice led to earlier symptom appearance. However, these mice demonstrated a prolonged symptomatic phase where xCT expression was found to be upregulated. Grip strength was also preserved at late symptomatic stages and overall motor neuron survival was increased at disease end stage (Mesci et al. 2015). Hence, a reduction in glutamate release from system xc− could be enough to slow the rise of extracellular glutamate levels that result from a reduction in its astrocytic clearance, which is also pathologically affected in ALS patients (Rothstein et al. 1995, Rothstein et al. 1992) and tardively in ALS mutant mice (Canton et al. 1998, Warita et al. 2002) due to loss of EAAT2/GLT-1 expression (figure 4).
Finally, another interesting link between system xc− and ALS stems from the finding that the excitotoxin β-N-methylamino-L-alanine is a transportable inhibitor of system xc− (Warren et al. 2004), which simultaneously competes with cystine uptake whilst eliciting glutamate release (Liu et al. 2009). β-N-methylamino-L-alanine has been described as the putative causative agent underlying the motor neuron degeneration and spastic paraparesis that affected the inhabitants of Guam in the late 1950s. In this context, enhanced oxidative stress as well as an increase in glutamate excitotoxicity affected neuronal survival (Liu et al. 2009).
All together, these data indicate that the contribution of enhanced system xc− function to ALS progression and pathology may be complex. It is intriguing to speculate that in an attempt to mitigate oxidative stress, a presymptomatic upregulation provides some protection initially, but eventually contributes to injury during the later stages of the disease via increased glutamate release and modulation of microglial functions (figure 4).
Huntington’s disease
Huntington’s disease (HD) is an autosomal dominant fatal neurodegenerative disease caused by polyglutamine expansion in the gene huntingtin (htt) that leads to the death of GABAergic medium-sized spiny neurons in the striatum, although other brain regions are affected as the disease progresses. HD presents as a movement disorder, with co-morbid psychiatric and cognitive symptomology (Nance 1997). Mechanistically, it has been proposed that transcriptional impairment resulting from the mutation of htt leads to mitochondrial dysfunction, ultimately rendering neurons more sensitive to excitotoxicity and oxidative stress (Johri & Beal 2012).
Recent studies demonstrate a dysregulation of system xc− in R6/2 mutant htt exon1 transgenic mice, a model for HD, and in immortalized striatal cells derived from htt Q111 knock-in mice (STHdhQ111/Q111) (Trettel et al. 2000). Specifically, xCT mRNA and protein levels were demonstrated to be decreased in the striatum of six-week-old R6/2 mice as well as in the STHdhQ111/Q111 cells. The activity of system xc− (only assessed in the striatal cell line) was also significantly decreased (Frederick et al. 2014). This change in the STHdhQ111/Q111 cells was accompanied by a decrease in cellular GSH levels and a corresponding increase in basal ROS expression (Ribeiro et al. 2012). Accordingly, cells showed enhanced sensitivity to oxidative stressors (Frederick et al. 2014). Interestingly, it has been discovered recently that cystathionine is an alternative substrate for system xc− and cystathionine γ-lyase, the enzyme that catalyzes the break-down of cystathionine into α-ketobutyrate, ammonia and the GSH precursor cysteine, is also downregulated in cellular and animal models of HD (Paul et al. 2014). Both cystine and cystathionine imported via system xc− can sustain intracellular GSH levels (Kobayashi et al. 2015). Of note, cysteine supplementation prolongs life span in R6/2 mice (Paul et al. 2014). Thus, the oxidative stress that has been found in HD (Browne et al. 1999) might be in part explained by the dysregulation of the cystine/cysteine metabolism. Whether a decrease in system xc− expression in HD mice and in STHdhQ111/Q111 striatal cells is due to its transcriptional dysregulation (as has been proposed, (Frederick et al. 2014)) remains to be determined. Interestingly, glutamate uptake via EAAT2 is also impaired in HD (reviewed in (Sheldon & Robinson 2007) and thus enhancing endogenous system xc− levels might lead to the risk of excitotoxocity. As such, dual EAAT2/xCT inducers like ceftriaxone (Knackstedt et al. 2010a, Lewerenz et al. 2009), might potentially be effective strategies for treatment in HD. Indeed, ceftriaxone attenuated the clinical phenotype in R6/2 mice (Miller et al. 2008).
Cerebral Ischemia
Interruption of blood flow to the brain, i.e. cerebral ischemia or stroke, is the leading cause of adult disability in the world. Overwhelming evidence indicates that glutamate-mediated excitotoxicity is a major contributor of neuronal death. Yet, inhibitors of post-synaptic glutamate receptors at doses not leading to intolerable side effects, have not proven to be an effective therapeutic option in stroke (Ikonomidou & Turski 2002, Muir & Lees 2003, Ginsberg 2009, Ginsberg 2008). Thus, understanding the source(s) of glutamate contributing to ischemic injury is of paramount importance. Evidence suggests system xc− is one such source.
Positron emission tomography imaging of rat brains after focal experimental cerebral ischemia (transient middle cerebral artery occlusion or MCAO) demonstrated a rapid increase in activity of system xc− that peaked five hours later (Soria et al. 2014). Interestingly, pharmacological inhibition of system xc− reduced oxygen-glucose deprivation-induced neuronal currents (i.e. anoxic depolarizations) as well as cell death in slices and slice cultures, respectively (Soria et al. 2014). In addition, using a mixed cortical cell culture system, it was found that enhanced astrocytic system xc− activity ― while not deleterious in and of itself ― becomes a major contributor of excitotoxic glutamate under conditions of energy deprivation: hypoxia or hypoglycemia (Fogal et al. 2007, Jackman et al. 2012, Jackman et al. 2010).
At present, there are no commercially available selective pharmacological reagents with which to directly demonstrate that inhibiting system xc− activity contributes to cerebral ischemic injury. Nevertheless, carboxyphenylglycine compounds that antagonize both system xc− and mGluR1α (Brabet et al. 1995, Kingston et al. 2002) — which have proven useful to teasing out the contributions of system xc− in vitro — have been utilized in animal experiment ischemia models. Interestingly, mice who were administered one such compound (LY367385) via tail vein three hours after ischemia showed less infarct volume when evaluated one day later (Li et al. 2013). Similar protective effects were found in rats (Moroni et al. 2002) and gerbils (Bruno et al. 1999). Curiously, neuronal injury following MCAO is not reduced in mGluR1α-deficient mice (Ferraguti et al. 1997). Thus, the notion that inhibition of system xc− activity underlies the neuroprotective effects of the carboxyphenylglycine derivatives under the conditions of cerebral ischemia cannot be excluded and should be tested directly. In support of this idea, Hewett and colleagues demonstrate that sut/sut mice were found to be less susceptible to injury that follows transient cerebral ischemia induced by MCAO (SJH, unpublished observations). Hence, in amalgamation, evidence suggests a potential role for system xc− in cerebral ischemic damage.
Multiple Sclerosis
Multiple Sclerosis (MS) is best considered a chronic primary demyelinating disease of autoimmune origin with more than two million people affected worldwide. One of the best studied animal models of autoimmune inflammatory demyelination as found in MS, is experimental autoimmune encephalomyelitis (EAE) elicited by immunization of animals with peptides derived from myelin proteins (reviewed in (Rangachari & Kuchroo 2013)). Alterations in glutamate homeostasis contribute to oligodendrocyte cell death and neurological deficits in animal models of MS (reviewed in (Gonsette 2008)). The source of this glutamate has been a subject of much investigation. Pertinently, cells of the monocyte-macrophage-microglia lineage taken from animals with EAE have higher xCT expression than those taken from control animals (Pampliega et al. 2011). Although these findings were not recapitulated in tissue from mice infected with Theiler’s murine encephalomyelitis virus, another common animal model of MS (Merckx et al. 2015), enhanced xCT expression was found in leukocytes taken from human MS patients as well as in MS post-mortem optic nerve (Pampliega et al. 2011). The potential significance of these findings was demonstrated in vitro. In oligodendrocyte-microglia co-culture, activated microglia increased extracellular glutamate release, which resulted in oligodendrocyte excitotoxicity that was prevented by inhibition of system xc− (Domercq et al. 2007).
Treatment with the unspecific system xc− inhibitor SSZ, reduced the clinical severity of EAE in mice, an effect associated with a reduction in T cell infiltration, reactive gliosis and myelin damage (Evonuk et al. 2015). To further confirm the involvement of system xc− in this effect, sut/sut mice and wildtype littermates were subjected to EAE, to which mice deficient in xCT proved to be largely resistant. Finally, the authors found that myelin-specific CD4+ T helper type 1 cells provoked microglia to release glutamate via the system xc− transporter causing excitotoxic death to mature myelin-producing oligodendrocytes (Evonuk et al. 2015).
Taken together, the findings suggest that microglial system xc− upregulation can produce excitotoxic damage to myelin in the setting of EAE. The additional intriguing observation that pharmacological inhibition of system xc− can also suppress immune cell infiltration suggests that modulation of system xc− either alone or as an add-on therapy might be therapeutically useful in MS.
Epilepsy
Epilepsy is one of the most common neurological disorders, characterized by the occurrence of epileptic seizures as a result of perturbations in the excitation/inhibition balance. The most common and sometimes devastating form, temporal lobe epilepsy, is initiated by an initial insult, often resulting in a status epilepticus. Next, during a latent phase of variable duration, epileptogenesis takes place, a form of synaptic maladaptation finally leading to a reduced threshold for seizures in the epileptic brain. It was recently discussed that glutamate release from astrocytes, although not necessary for the generation of seizures, can modulate the threshold for seizure generation (Steinhäuser et al. 2015). After all, extrasynaptic glutamate cannot only modulate synaptic transmission via activation of pre- and postsynaptic mGluRs, but it can also act on extrasynaptic NMDA receptors and as such increase neuronal excitability. Moreover, several agonists and antagonists of respectively group II and group I mGluRs as well as antagonists of ionotropic glutamate receptors have been shown to exert anticonvulsive properties (for review (Tang 2005, Casillas-Espinosa et al. 2012)).
Increased xCT expression levels were reported in EL mice, a model for generalized seizures (Takaki et al. 2008). Moreover, it was shown that xCT−/− mice were less susceptible for chemically- as well as electrically-induced acute limbic seizures compared to their wildtype littermates (De Bundel et al. 2011). The increased threshold for eliciting limbic seizures in xCT−/− mice was attributed to the reduction in extracellular hippocampal glutamate levels with about 60% in these mice. Recently, Lewerenz et al. reported increased hippocampal xCT expression levels in temporal lobe epilepsy patients and unveiled the pathway that resulted in this increased xCT expression (see above) (Lewerenz et al. 2014). Increased xCT expression in the epileptic brain can as such result in a decreased threshold for seizures, in accordance with the pro-convulsive effect that has been described for N-acetylcysteine (De Bundel et al. 2011). Finally, as described below, increased expression of system xc− in brain tumor cells has been suggested to contribute to peritumoral seizures (Buckingham et al. 2011).
To conclude, whereas the involvement of system xc− in seizure development seems to be straightforward, the potential role of system xc− in epileptogenesis remains elusive.
xCT and glioblastoma
GBM are brain tumors derived from cells of glial lineage. GBMs grow rapidly and relentlessly and invade normal brain tissue. In 1999, Ye et al. reported that the activity of EAATs which take up glutamate and aspartate, is downregulated in human GBM cell lines compared to astrocytes while the activity of system xc− is upregulated (Ye et al. 1999). The combination of impaired glutamate uptake and increased system xc− activity explained the continuous glutamate release observed in these tumor cells in vitro (Ye & Sontheimer 1999). Glutamate released via system xc− in vitro was shown to positively regulate GBM cell migration, most possibly via AMPA receptor-induced calcium signaling (Lyons et al. 2007). Thus, glutamate release via system xc− from GBM cells might contribute to GBM brain tissue invasion.
In a follow up study, the same group could demonstrate that inhibition of system xc− activity prominently decreases cellular GSH levels, slows proliferation and induces cell death in human GBM cell lines in vitro suggesting that in addition to glutamate release cystine import via system xc− might be pathophysiologically important for the growth of GBMs. In addition, the authors observed that high-dose SSZ (16 mg/mouse daily i.p.) also significantly suppressed growth of GBM cells implanted in mouse brains suggesting that this effect is active in vivo (Chung et al. 2005). In contrast to the studies by the Sontheimer lab, Savaskan et al. neither observed that siRNA-mediated knock-down of system xc− nor inhibition using the non-substrate inhibitor (S)-4-carboxyphenylglycine decreased GBM cell proliferation in vitro nor tumor growth in intracerebral implants in vivo. However, the decreased glutamate release induced by knock-down of system xc− was associated with decreased neurodegeneration when tumor cells were co-cultured with brain slices (Savaskan et al. 2008). In addition, both (S)-4- carboxyphenylglycine and xCT knock-down increased survival of mice with intracranially implanted tumors. This effect was associated by a prominently decreased peritumoral edema. Thus, these findings indicate that the detrimental role of system xc− in GBM pathophysiology might rather consist of glutamate-mediated neurotoxicity and edema formation of the peritumoral brain tissue. Surprisingly, in humans high xCT expression was found associated with a rather invasive phenotype consistent with the idea that system xc− fosters GBM cell migration, whereas an edematous phenotype was associated with low xCT expression (Takeuchi et al. 2013), inconsistent with the idea that system xc− activity induces brain edema. However, in this study tumor xCT expression was judged based upon xCT immunoreactivity using paraffin embedded formaldehyde-fixed tumor samples. Tumors were grouped dichotomically in high and low xCT expressing tumors. There was a correlation of a 57kDa band judged to be xCT upon western blotting with immunohistochemistry signals in a subgroup of samples. However, semiquantitative xCT expression, assessed by western blotting, was in average only two-fold higher in samples with high xCT expression when judged by immunohistochemistry when compared to samples with low expression. In addition, values obtained by semiquantiative western blotting for the high and low xCT group were partially overlapping. Astonishingly, the authors observed two-fold longer progression-free survival and a seven months longer overall survival in patients with GBM with low xCT-like tissue immunoreactivity.
Up to 50% of patients with GBM suffer from sometimes debilitating epileptic seizures (Paillas 1991). Buckingham et al. demonstrated that the human GBM cells when implanted into the brain of scid mice induce epileptiform neuronal activity upon electrophysiological recordings in acute slices as well as epileptic seizures detected by EEG/video monitoring (Buckingham et al. 2011). The authors concluded that glutamate released by system xc− in the tumor tissue was involved in the epileptic activity, as SSZ-inhibitable glutamate release was observed in the tumor-containing acute brain slices when 100µM cystine was added. In addition, SSZ decreased epileptiform activity induced in these slice preparation by NMDA receptor disinhibition due to magnesium removal, also in the presence of 100µM cystine. Finally, spontaneous epileptiform EEG activity was prominently decreased within the first hour of intraperitoneal injection of high-dose SSZ (8 mg/mouse) exceeding the maximal orally administered single dose in humans more than 10-fold.
There is no cure for patients suffering from the rapidly growing tumor, which with time becomes resistant against radio- and chemotherapy. Temozolomide is the standard chemotherapeutic compound administered in GBM patients. In vitro, exposure to temozolomide leads to increased GSH levels in GBM cell lines, probably via ATF4- and Nrf2-mediated upregulation of system xc− activity (Chen et al. 2015). Inhibition of system xc− prominently exacerbated the sensitivity of GBM cell lines to temozolomide suggesting that upregulation of xCT is part of the acquired temozolomide resistance in GBMs. Similar observations were made using the prooxidant experimental anticancer drug cannabidiol (Singer et al. 2015). However, in a small clinical trial, adjuvant SSZ given in combination with temozolomide and radiotherapy did not result in increased antitumor activity of radiochemotherapy and was poorly tolerated (Takeuchi et al. 2014). Another phase 1/2 clinical trial using SSZ monotherapy at doses from 1.5 g to 6 g/day had to be terminated prematurely as severe adverse events were encountered frequently including increase in brain edema, increased seizure frequency associated with decreased plasma levels of anticonvulsant drugs without a relevant effect on tumor growth (Robe et al. 2009).
In summary, preclinical models of GBM suggest that system xc− might play a role in GBM pathophysiology by (1) supporting tumor cell growth, (2) fostering GBM cell migration, (3) by inducing peritumoral neurotoxicity, (4) by inducing epileptic seizures and/or (5) by inducing chemotherapy resistance. However, the results obtained using different preclinical models are in part inconsistent. In addition, more rigorous approaches to quantify xCT expression in human tumor samples to unequivocally characterize the association of the clinical course of the disease with xCT expression are highly warranted. Finally, the only approved drug that inhibits system xc−, SSZ, is poorly tolerated in patients receiving GBM standard therapy and progressive GBM at doses much lower than found effective in preclinical models.
Conclusion
Taken together, with exception of HD (see above), there is accumulating evidence from animal models of neurological diseases, which is in part supported by the analysis of human brain tissues, that xCT and thereby system xc− are upregulated during neurodegeneration and contribute to damage to the CNS. Especially, studies in ALS and AD mouse models hint a predominant expression of system xc− in microglia. However, astrocytic system xc− might also play a role. In theory, glutamate release via system xc− will increase extrasynaptic glutamate, especially as glutamate reuptake via EAATs is generally assumed to be perturbed in many neurological diseases (Sheldon & Robinson 2007). This might lead to the activation of extrasynaptic NMDA receptors, which mediate excitotoxicity (figure 4). The modulation of extrasynaptic glutamate and thereby the activity of metabotropic and ionotropic glutamate receptors might also modulate neuronal excitability in epilepsy and behavior.
Inducible and cell type-specific knock-out models of xCT might help to unequivocally identify the cell types responsible for the above described role of system xc− in neurological diseases. In addition, the development of pharmacologically tolerable and highly selective inhibitors of system xc− (Patel et al. 2010, Newell et al. 2014) will represent an important step toward our ability to analyze whether inhibition of system xc− represents a potent therapeutic strategy for the slowing of neural injury when applied during the symptomatic phase of disease in humans.
Acknowledgments
AM is funded by the Medical Foundation Queen Elisabeth, Fund for Scientific Research Flanders (grant G.0384.12N and G.OA65.13N) and the Vrije Universiteit Brussel (SRP40); SB is funded by the Thierry Latran Foundation and the Association pour la Recherche sur la Sclérose Latérale Amyotrophic (ARSLA); LK is funded by NIDA DA033436 and DA037270; SH is funded by NIH/NINDS R01NS051445-07.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- Aβ
amyloid-β
- AA
L-α-aminoadipate
- AARE
amino acid response element
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate
- APP
amyloid precursor protein
- ARE
antioxidant response element
- ATF4
activating transcription factor 4
- EAAT
excitatory amino acid transporter
- EAE
experimental autoimmune encephalomyelitis
- eIF2α
eukaryotic initiation factor 2 α
- GAS
gamma-activated site
- GBM
glioblastoma
- GCN2
general control non-depressible-2
- HCA
L-homocysteate
- HD
Huntington’s disease
- HRI
heme-regulated eIF2α kinase
- Htt
huntingtin
- IGF-1
insulin-like growth factor-1
- IL-1β
Interleukin-1β
- JAK
janus kinase
- LPS
lipopolysaccharide
- MCAO
middle cerebral artery occlusion
- mGluR
metabotropic glutamate receptor
- MS
multiple sclerosis
- NAc
nucleus accumbens
- NF-κB
nuclear factor-κB
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PD
Parkinson’s disease
- PERK
PKR-like endoplasmic reticulum kinase
- PKR
protein kinase R
- Poly I:C
polyinosinic-polycytidylic acid
- ROS
reactive oxygen species
- sAPP
soluble amyloid precursor protein
- SOD-1
Cu/Zn superoxide dismutase
- SSZ
sulfasalazine
- STAT
signal transducer and activator of transcription
- TLR
Toll-like receptor
- TNFα
tumor necrosis factor α
References
- Albano R, Liu X, Lobner D. Regulation of system x(c)- in the SOD1-G93A mouse model of ALS. Exp Neurol. 2013;250:69–73. doi: 10.1016/j.expneurol.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology (Berl) 2014;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002a;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002b;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76:846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentea E, Demuyser T, Van Liefferinge J, Albertini G, Deneyer L, Nys J, Merckx E, Michotte Y, Sato H, Arckens L, Massie A, Smolders I. Absence of system xc- in mice decreases anxiety and depressive-like behavior without affecting sensorimotor function or spatial vision. Prog Neuropsychopharmacol Biol Psychiatry. 2015a;59:49–58. doi: 10.1016/j.pnpbp.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Bentea E, Sconce MD, Churchill MJ, Van Liefferinge J, Sato H, Meshul CK, Massie A. MPTP-induced parkinsonism in mice alters striatal and nigral xCT expression but is unaffected by the genetic loss of xCT. Neurosci Lett. 2015b;593:1–6. doi: 10.1016/j.neulet.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Bianchin MM, Spanis CW, Roesler R, McGaugh JL, Izquierdo I. (+/−)-Alpha-methyl-4-carboxyphenylglycine, a metabotropic glutamate receptor blocker, impairs retention of an inhibitory avoidance task in rats when infused into the basolateral nucleus of the amygdala. Brain Res. 2000;852:436–443. doi: 10.1016/s0006-8993(99)02220-9. [DOI] [PubMed] [Google Scholar]
- Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006a;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006b;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bordi F, Marcon C, Chiamulera C, Reggiani A. Effects of the metabotropic glutamate receptor antagonist MCPG on spatial and context-specific learning. Neuropharmacology. 1996;35:1557–1565. doi: 10.1016/s0028-3908(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Brabet I, Mary S, Bockaert J, Pin JP. Phenylglycine derivatives discriminate between mGluR1- and mGluR5-mediated responses. Neuropharmacology. 1995;34:895–903. doi: 10.1016/0028-3908(95)00079-l. [DOI] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc−) to normal and pathological glutamatergic signaling. Pharmacol Rev. 2012;64:780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington's disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Kingston A, O'Neill MJ, Catania MV, Di Grezia R, Nicoletti F. Neuroprotective activity of the potent and selective mGlu1a metabotropic glutamate receptor antagonist, (+)-2-methyl-4 carboxyphenylglycine (LY367385): comparison with LY357366, a broader spectrum antagonist with equal affinity for mGlu1a and mGlu5 receptors. Neuropharmacology. 1999;38:199–207. doi: 10.1016/s0028-3908(98)00159-2. [DOI] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17:1269–1274. doi: 10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton T, Pratt J, Stutzmann JM, Imperato A, Boireau A. Glutamate uptake is decreased tardively in the spinal cord of FALS mice. Neuroreport. 1998;9:775–778. doi: 10.1097/00001756-199803300-00001. [DOI] [PubMed] [Google Scholar]
- Casillas-Espinosa PM, Powell KL, O'Brien TJ. Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia. 2012;53(Suppl 9):41–58. doi: 10.1111/epi.12034. [DOI] [PubMed] [Google Scholar]
- Chang YF. Lysine metabolism in the human and the monkey: demonstration of pipecolic acid formation in the brain and other organs. Neurochem Res. 1982;7:577–588. doi: 10.1007/BF00965124. [DOI] [PubMed] [Google Scholar]
- Chen L, Li X, Liu L, Yu B, Xue Y, Liu Y. Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-γ-lyase function. Oncol Rep. 2015;33:1465–1474. doi: 10.3892/or.2015.3712. [DOI] [PubMed] [Google Scholar]
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A. 2005;102:10964–10969. doi: 10.1073/pnas.0502856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25:7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci U S A. 1999;96:6377–6381. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, Vermoesen K, Bannai S, Sato H, Michotte Y, Smolders I, Massie A. Loss of system x(c)− does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J Neurosci. 2011;31:5792–5803. doi: 10.1523/JNEUROSCI.5465-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Benz B, Sorg O, Pellerin L, Magistretti PJ. beta-Adrenergic stimulation promotes homocysteic acid release from astrocyte cultures: evidence for a role of astrocytes in the modulation of synaptic transmission. J Neurochem. 1997;68:2386–2394. doi: 10.1046/j.1471-4159.1997.68062386.x. [DOI] [PubMed] [Google Scholar]
- Do KQ, Herrling PL, Streit P, Cuénod M. Release of neuroactive substances: homocysteic acid as an endogenous agonist of the NMDA receptor. J Neural Transm. 1988;72:185–190. doi: 10.1007/BF01243418. [DOI] [PubMed] [Google Scholar]
- Domercq M, Sánchez-Gómez MV, Sherwin C, Etxebarria E, Fern R, Matute C. System xc− and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J Immunol. 2007;178:6549–6556. doi: 10.4049/jimmunol.178.10.6549. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evonuk KS, Baker BJ, Doyle RE, Moseley CE, Sestero CM, Johnston BP, De Sarno P, Tang A, Gembitsky I, Hewett SJ, Weaver CT, Raman C, DeSilva TM. Inhibition of System Xc− Transporter Attenuates Autoimmune Inflammatory Demyelination. J Immunol. 2015 doi: 10.4049/jimmunol.1401108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell MJ, Witkin JM, Falcone JF, Katner JS, Perry KW, Hart J, Rorick-Kehn L, Overshiner CD, Rasmussen K, Chaney SF, Benvenga MJ, Li X, Marlow DL, Thompson LK, Luecke SK, Wafford KA, Seidel WF, Edgar DM, Quets AT, Felder CC, Wang X, Heinz BA, Nikolayev A, Kuo MS, Mayhugh D, Khilevich A, Zhang D, Ebert PJ, Eckstein JA, Ackermann BL, Swanson SP, Catlow JT, Dean RA, Jackson K, Tauscher-Wisniewski S, Marek GJ, Schkeryantz JM, Svensson KA. N-(4-((2-(trifluoromethyl)-3-hydroxy-4-(isobutyryl)phenoxy)methyl)benzyl)-1-methyl-1H-imidazole-4-carboxamide (THIIC), a novel metabotropic glutamate 2 potentiator with potential anxiolytic/antidepressant properties: in vivo profiling suggests a link between behavioral and central nervous system neurochemical changes. J Pharmacol Exp Ther. 2011;336:165–177. doi: 10.1124/jpet.110.172957. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Pietra C, Valerio E, Corti C, Chiamulera C, Conquet F. Evidence against a permissive role of the metabotropic glutamate receptor 1 in acute excitotoxicity. Neuroscience. 1997;79:1–5. doi: 10.1016/s0306-4522(97)00074-2. [DOI] [PubMed] [Google Scholar]
- Figuera-Losada M, Rojas C, Slusher BS. Inhibition of microglia activation as a phenotypic assay in early drug discovery. J Biomol Screen. 2014;19:17–31. doi: 10.1177/1087057113499406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. System x(c)− activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci. 2007;27:10094–10105. doi: 10.1523/JNEUROSCI.2459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick NM, Berth o J, Patel KK, Petr GT, Bakradze E, Smith SB, Rosenberg PA. Dysregulation of system xc(−) expression induced by mutant huntingtin in a striatal neuronal cell line and in R6/2 mice. Neurochem Int. 2014;76:59–69. doi: 10.1016/j.neuint.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–S114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochenauer GE, Robinson MB. Dibutyryl-cAMP (dbcAMP) up-regulates astrocytic chloride-dependent L-[3H]glutamate transport and expression of both system xc(−) subunits. J Neurochem. 2001;78:276–286. doi: 10.1046/j.1471-4159.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Shanmugam AK, Markand S, Zorrilla E, Ganapathy V, Smith SB. Sigma receptor 1 modulates ER stress and Bcl2 in murine retina. Cell Tissue Res. 2014;356:15–27. doi: 10.1007/s00441-013-1774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Jackman NA, Thorn TL, Vought VE, Hewett SJ. Interleukin-1β protects astrocytes against oxidant-induced injury via an NF-κB-Dependent upregulation of glutathione synthesis. Glia. 2015 doi: 10.1002/glia.22828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Jackman NA, Melchior SE, Hewett JA, Hewett SJ. Non-cell autonomous influence of the astrocyte system xc− on hypoglycaemic neuronal cell death. ASN Neuro. 2012;4 doi: 10.1042/AN20110030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. Regulation of system x(c)(−)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia. 2010;58:1806–1815. doi: 10.1002/glia.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Stanton BR, Turner MR, Gray R, Blunt AH, Butt D, Ampong MA, Shaw CE, Leigh PN, Al-Chalabi A. Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol. 2006;253:1642–1643. doi: 10.1007/s00415-006-0195-y. [DOI] [PubMed] [Google Scholar]
- Johri A, Beal MF. Antioxidants in Huntington's disease. Biochim Biophys Acta. 2012;1822:664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce PI, Fratta P, Fisher EM, Acevedo-Arozena A. SOD1 and TDP-43 animal models of amyotrophic lateral sclerosis: recent advances in understanding disease toward the development of clinical treatments. Mamm Genome. 2011;22:420–448. doi: 10.1007/s00335-011-9339-1. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Ankeny DP, Garg SK, Wei P, Guan Z, Lai W, McTigue DM, Banerjee R, Popovich PG. System x(c)(−) regulates microglia and macrophage glutamate excitotoxicity in vivo. Exp Neurol. 2012;233:333–341. doi: 10.1016/j.expneurol.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Griffey K, Johnson MP, Chamberlain MJ, Kelly G, Tomlinson R, Wright RA, Johnson BG, Schoepp DD, Harris JR, Clark BP, Baker RS, Tizzano JT. Inhibition of group I metabotropic glutamate receptor responses in vivo in rats by a new generation of carboxyphenylglycine-like amino acid antagonists. Neurosci Lett. 2002;330:127–130. doi: 10.1016/s0304-3940(02)00751-6. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010a;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010b;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Sato M, Kasakoshi T, Tsutsui T, Sugimoto M, Osaki M, Okada F, Igarashi K, Hiratake J, Homma T, Conrad M, Fujii J, Soga T, Bannai S, Sato H. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J Biol Chem. 2015;290:8778–8788. doi: 10.1074/jbc.M114.625053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme E, Schousboe A, Hertz L, Torgner IA, Svenneby G. Developmental change of endogenous glutamate and gamma-glutamyl transferase in cultured cerebral cortical interneurons and cerebellar granule cells, and in mouse cerebral cortex and cerebellum in vivo. Neurochem Res. 1985;10:993–1008. doi: 10.1007/BF00964635. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Baxter P, Kassubek R, Albrecht P, Van Liefferinge J, Westhoff M-A, Halatsch M-E, Karpel-Massler G, Meakin PJ, Hayes JD, Aronica E, Smolders I, Ludolph AC, Methner A, Conrad M, Massie A, Hardingham GE, Maher P. Phosphoinositide 3-Kinases Upregulate System x(c)(−) via Eukaryotic Initiation Factor 2 alpha and Activating Transcription Factor 4-A Pathway Active in Glioblastomas and Epilepsy. Antioxidants & Redox Signaling. 2014;20:2907–2922. doi: 10.1089/ars.2013.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem. 2009;284:1106–1115. doi: 10.1074/jbc.M807325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Sato H, Albrecht P, Henke N, Noack R, Methner A, Maher P. Mutation of ATF4 mediates resistance of neuronal cell lines against oxidative stress by inducing xCT expression. Cell Death Differ. 2012;19:847–858. doi: 10.1038/cdd.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang N, Sun G, Ding S. Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN Neuro. 2013;5:195–207. doi: 10.1042/AN20130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tan Z, Li Z, Sun Z, Duan S, Li W. Impaired long-term potentiation and long-term memory deficits in xCT-deficient sut mice. Biosci Rep. 2012;32:315–321. doi: 10.1042/BSR20110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linher-Melville K, Haftchenary S, Gunning P, Singh G. Signal transducer and activator of transcription 3 and 5 regulate system Xc− and redox balance in human breast cancer cells. Mol Cell Biochem. 2015;405:205–221. doi: 10.1007/s11010-015-2412-4. [DOI] [PubMed] [Google Scholar]
- Liu R, Althaus JS, Ellerbrock BR, Becker DA, Gurney ME. Enhanced oxygen radical production in a transgenic mouse model of familial amyotrophic lateral sclerosis. Ann Neurol. 1998;44:763–770. doi: 10.1002/ana.410440510. [DOI] [PubMed] [Google Scholar]
- Liu X, Albano R, Lobner D. FGF-2 induces neuronal death through upregulation of system xc−. Brain Res. 2014;1547:25–33. doi: 10.1016/j.brainres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Liu X, Resch J, Rush T, Lobner D. Functional upregulation of system xc− by fibroblast growth factor-2. Neuropharmacology. 2012;62:901–906. doi: 10.1016/j.neuropharm.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Liu X, Rush T, Zapata J, Lobner D. beta-N-methylamino-l-alanine induces oxidative stress and glutamate release through action on system Xc(−) Exp Neurol. 2009;217:429–433. doi: 10.1016/j.expneurol.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37:707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgen V, Resch J, Qualmann K, Raddatz NJ, Panhans C, Olander EM, Kong L, Choi S, Mantsch JR, Baker DA. Behavioral assessment of acute inhibition of system xc (−) in rats. Psychopharmacology (Berl) 2014;231:4637–4647. doi: 10.1007/s00213-014-3612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Gardner LB. Stress-induced inhibition of nonsense-mediated RNA decay regulates intracellular cystine transport and intracellular glutathione through regulation of the cystine/glutamate exchanger SLC7A11. Oncogene. 2014 doi: 10.1038/onc.2014.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, De Bundel D, Vermoesen K, Bannai S, Smolders I, Conrad M, Plesnila N, Sato H, Michotte Y. Dopaminergic neurons of system x(c)(−)-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. Faseb Journal. 2011;25:1359–1369. doi: 10.1096/fj.10-177212. [DOI] [PubMed] [Google Scholar]
- Massie A, Schallier A, Mertens B, Vermoesen K, Bannai S, Sato H, Smolders I, Michotte Y. Time-dependent changes in striatal xCT protein expression in hemi-Parkinson rats. Neuroreport. 2008;19:1589–1592. doi: 10.1097/WNR.0b013e328312181c. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Zusso M, Panaccione I, Turriziani B, Caruso A, Iacovelli L, Noviello L, Togna G, Melchiorri D, Debetto P, Tatarelli R, Battaglia G, Nicoletti F, Giusti P, Girardi P. Synergism between fluoxetine and the mGlu2/3 receptor agonist, LY379268, in an in vitro model for antidepressant drug-induced neurogenesis. Neuropharmacology. 2008;54:428–437. doi: 10.1016/j.neuropharm.2007.10.020. [DOI] [PubMed] [Google Scholar]
- McClatchy DB, Savas JN, Martínez-Bartolomé S, Park SK, Maher P, Powell SB, Yates JR. Global quantitative analysis of phosphorylation underlying phencyclidine signaling and sensorimotor gating in the prefrontal cortex. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh EA, Featherstone DE. Behavioral characterization of system xc− mutant mice. Behav Brain Res. 2014;265:1–11. doi: 10.1016/j.bbr.2014.02.010. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merckx E, Demuyser T, Bentea E, Liefferinge JV, Albertini G, Deneyer L, Michiels T, Massie A. Lack of effect of Theiler's murine encephalomyelitis virus infection on system xc(.) Neurosci Lett. 2015;593:124–128. doi: 10.1016/j.neulet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Mesci P, Zaïdi S, Lobsiger CS, Millecamps S, Escartin C, Seilhean D, Sato H, Mallat M, Boillée S. System xC− is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice. Brain. 2015;138:53–68. doi: 10.1093/brain/awu312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR, Sanacora G. Antidepressant-like effects of ceftriaxone in male C57BL/6J mice. Biol Psychiatry. 2007;61:250–252. doi: 10.1016/j.biopsych.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Attucci S, Cozzi A, Meli E, Picca R, Scheideler MA, Pellicciari R, Noe C, Sarichelou I, Pellegrini-Giampietro DE. The novel and systemically active metabotropic glutamate 1 (mGlu1) receptor antagonist 3-MATIDA reduces post-ischemic neuronal death. Neuropharmacology. 2002;42:741–751. doi: 10.1016/s0028-3908(02)00033-3. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir KW, Lees KR. Excitatory amino acid antagonists for acute stroke. Cochrane Database Syst Rev. 2003:CD001244. doi: 10.1002/14651858.CD001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance MA. Clinical aspects of CAG repeat diseases. Brain Pathol. 1997;7:881–900. doi: 10.1111/j.1750-3639.1997.tb00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell JL, Keyari CM, McDaniel SW, Diaz PJ, Natale NR, Patel SA, Bridges RJ. Novel di-aryl-substituted isoxazoles act as noncompetitive inhibitors of the system Xc(−) cystine/glutamate exchanger. Neurochem Int. 2014;73:132–138. doi: 10.1016/j.neuint.2013.11.012. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillas JE. A review of 2,413 tumours operated over a 30-year period. J Neuroradiol. 1991;18:79–106. [PubMed] [Google Scholar]
- Pampliega O, Domercq M, Soria FN, Villoslada P, Rodríguez-Antigüedad A, Matute C. Increased expression of cystine/glutamate antiporter in multiple sclerosis. J Neuroinflammation. 2011;8:63. doi: 10.1186/1742-2094-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Rajale T, O'Brien E, Burkhart DJ, Nelson JK, Twamley B, Blumenfeld A, Szabon-Watola MI, Gerdes JM, Bridges RJ, Natale NR. Isoxazole analogues bind the system xc− transporter: structure-activity relationship and pharmacophore model. Bioorg Med Chem. 2010;18:202–213. doi: 10.1016/j.bmc.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Sbodio JI, Xu R, Vandiver MS, Cha JY, Snowman AM, Snyder SH. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature. 2014;509:96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piani D, Fontana A. Involvement of the cystine transport system xc− in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol. 1994;152:3578–3585. [PubMed] [Google Scholar]
- Qin S, Colin C, Hinners I, Gervais A, Cheret C, Mallat M. System Xc− and apolipoprotein E expressed by microglia have opposite effects on the neurotoxicity of amyloid-beta peptide 1–40. J Neurosci. 2006;26:3345–3356. doi: 10.1523/JNEUROSCI.5186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari M, Kuchroo VK. Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun. 2013;45:31–39. doi: 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch JM, Albano R, Liu X, Hjelmhaug J, Lobner D, Baker DA, Choi S. Augmented cystine-glutamate exchange by pituitary adenylate cyclase-activating polypeptide signaling via the VPAC1 receptor. Synapse. 2014 doi: 10.1002/syn.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M, Rosenstock TR, Cunha-Oliveira T, Ferreira IL, Oliveira CR, Rego AC. Glutathione redox cycle dysregulation in Huntington's disease knock-in striatal cells. Free Radic Biol Med. 2012;53:1857–1867. doi: 10.1016/j.freeradbiomed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Rickard NS, Ng KT. Blockade of metabotropic glutamate receptors prevents long-term memory consolidation. Brain Res Bull. 1995;36:355–359. doi: 10.1016/0361-9230(94)00222-m. [DOI] [PubMed] [Google Scholar]
- Robe PA, Martin DH, Nguyen-Khac MT, Artesi M, Deprez M, Albert A, Vanbelle S, Califice S, Bredel M, Bours V. Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer. 2009;9:372. doi: 10.1186/1471-2407-9-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem. 2002;277:44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- Sato H, Fujiwara K, Sagara J, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem J. 1995;310(Pt 2):547–551. doi: 10.1042/bj3100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Kuriyama-Matsumura K, Hashimoto T, Sasaki H, Wang H, Ishii T, Mann GE, Bannai S. Effect of oxygen on induction of the cystine transporter by bacterial lipopolysaccharide in mouse peritoneal macrophages. J Biol Chem. 2001;276:10407–10412. doi: 10.1074/jbc.M007216200. [DOI] [PubMed] [Google Scholar]
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun. 2004;325:109–116. doi: 10.1016/j.bbrc.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, Takahashi S, Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–37429. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)−, in the mouse brain. J Neurosci. 2002;22:8028–8033. doi: 10.1523/JNEUROSCI.22-18-08028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, Ganslandt O, Nimsky C, Buchfelder M, Eyüpoglu IY. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med. 2008;14:629–632. doi: 10.1038/nm1772. [DOI] [PubMed] [Google Scholar]
- Schallier A, Smolders I, Van Dam D, Loyens E, De Deyn PP, Michotte A, Michotte Y, Massie A. Region- and Age-Specific Changes in Glutamate Transport in the A beta PP23 Mouse Model for Alzheimer's Disease. Journal of Alzheimers Disease. 2011;24:287–300. doi: 10.3233/JAD-2011-101005. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, Kelly-Scumpia K, Stevens BR. Alpha-lipoic acid effects on brain glial functions accompanying double-stranded RNA antiviral and inflammatory signaling. Neurochem Int. 2014;64:55–63. doi: 10.1016/j.neuint.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E, Judkins J, Salomonis N, Matlaf L, Soteropoulos P, McAllister S, Soroceanu L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. doi: 10.1038/cddis.2014.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaga I, Pomierny B, Krzyżanowska W, Pomierny-Chamioło L, Miszkiel J, Niedzielska E, Ogórka A, Filip M. N-acetylcysteine possesses antidepressant-like activity through reduction of oxidative stress: behavioral and biochemical analyses in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:280–287. doi: 10.1016/j.pnpbp.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Bridges RJ. Sulfasalazine for brain cancer fits. Expert Opin Investig Drugs. 2012;21:575–578. doi: 10.1517/13543784.2012.670634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria FN, Pérez-Samartín A, Martin A, Gona KB, Llop J, Szczupak B, Chara JC, Matute C, Domercq M. Extrasynaptic glutamate release through cystine/glutamate antiporter contributes to ischemic damage. J Clin Invest. 2014;124:3645–3655. doi: 10.1172/JCI71886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhäuser C, Grunnet M, Carmignoto G. Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2014.12.047. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kong Q, Lin Y, Stouffer N, Schulte DA, Lai L, Liu Q, Chang LC, Dominguez S, Xing X, Cuny GD, Hodgetts KJ, Glicksman MA, Lin CL. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer's disease. J Exp Med. 2015;212:319–332. doi: 10.1084/jem.20140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Ueda Y, Doi T, Nagatomo K, Murashima YL, Kannan H. Molecular regulation of antioxidant ability in the hippocampus of EL mice. Brain Res. 2008;1228:1–5. doi: 10.1016/j.brainres.2008.06.041. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Wada K, Nagatani K, Otani N, Osada H, Nawashiro H. Sulfasalazine and temozolomide with radiation therapy for newly diagnosed glioblastoma. Neurol India. 2014;62:42–47. doi: 10.4103/0028-3886.128280. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Wada K, Toyooka T, Shinomiya N, Shimazaki H, Nakanishi K, Nagatani K, Otani N, Osada H, Uozumi Y, Matsuo H, Nawashiro H. Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery. 2013;72:33–41. doi: 10.1227/NEU.0b013e318276b2de. discussion 41. [DOI] [PubMed] [Google Scholar]
- Tang FR. Agonists and antagonists of metabotropic glutamate receptors: anticonvulsants and antiepileptogenic agents? Curr Neuropharmacol. 2005;3:299–307. doi: 10.2174/157015905774322525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- Tsujita T, Peirce V, Baird L, Matsuyama Y, Takaku M, Walsh SV, Griffin JL, Uruno A, Yamamoto M, Hayes JD. Transcription factor Nrf1 negatively regulates the cystine/glutamate transporter and lipid-metabolizing enzymes. Mol Cell Biol. 2014;34:3800–3816. doi: 10.1128/MCB.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shanmugam A, Markand S, Zorrilla E, Ganapathy V, Smith SB. Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via Nrf2 signaling and system xc(−), the Na(+)-independent glutamate-cystine exchanger. Free Radic Biol Med. 2015 doi: 10.1016/j.freeradbiomed.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warita H, Manabe Y, Murakami T, Shiote M, Shiro Y, Hayashi T, Nagano I, Shoji M, Abe K. Tardive decrease of astrocytic glutamate transporter protein in transgenic mice with ALS-linked mutant SOD1. Neurol Res. 2002;24:577–581. doi: 10.1179/016164102101200384. [DOI] [PubMed] [Google Scholar]
- Warren BA, Patel SA, Nunn PB, Bridges RJ. The Lathyrus excitotoxin beta-N-oxalyl-L-alpha,beta-diaminopropionic acid is a substrate of the L-cystine/L-glutamate exchanger system xc−. Toxicol Appl Pharmacol. 2004;200:83–92. doi: 10.1016/j.taap.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Wagner T, Reymann KG, Riedel G. Open field behaviour in rats: role of metabotropic glutamate receptors. Neuroreport. 1995;6:2389–2393. doi: 10.1097/00001756-199511270-00027. [DOI] [PubMed] [Google Scholar]
- Williams LE, Featherstone DE. Regulation of hippocampal synaptic strength by glial xCT. J Neurosci. 2014;34:16093–16102. doi: 10.1523/JNEUROSCI.1267-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yee D. IGF-I regulates redox status in breast cancer cells by activating the amino acid transport molecule xC−. Cancer Res. 2014;74:2295–2305. doi: 10.1158/0008-5472.CAN-13-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- Zheng W, Winter SM, Mayersohn M, Bishop JB, Sipes IG. Toxicokinetics of sulfasalazine (salicylazosulfapyridine) and its metabolites in B6C3F1 mice. Drug Metab Dispos. 1993;21:1091–1097. [PubMed] [Google Scholar]