Abstract
Von Hippel–Lindau (VHL) disease is caused by germline mutation of the VHL tumour suppressor gene. Patients frequently develop multiple nervous system tumours, denominated haemangioblastomas. Analysis of affected autopsy tissues suggests that tumourigenesis propagates from developmentally arrested, embryonic cells and progresses with consistent architectural, cytological, and molecular sequences similar to haemangioblastic formation and differentiation in the embryo. In this study, we analysed 156 nervous system tumours, 139 of which had been surgically resected from 83 VHL patients. We demonstrate that large tumours consistently contain epithelioid components characteristic of haemangioblastic differentiation in comparison to small tumours that solely display a poorly differentiated, mesenchymal structure. We further show exclusive activation of HIF2α in both small mesenchymal tumours and the mesenchymal component of large tumours, whereas activation of HIF1α is associated with epithelioid structure. We also show that the MIB1 proliferative index is variably increased in the epithelioid component of large tumours, with extramedullary haematopoiesis foci within the epithelioid component at 100%. These data provide compelling evidence that nervous system tumourigenesis in VHL disease represents a protracted process of haemangioblastic proliferation and differentiation that parallels haemangioblastic formation and differentiation in the embryo.
Keywords: VHL, haemangioblastoma, HIF1α, HIF2α, VEGF, von Hippel-Lindau, neoplasia, development
Introduction
VHL disease is a tumour suppressor gene syndrome that frequently results in the nervous system tumour haemangioblastoma. VHL disease is caused by a germline mutation of the VHL gene [1]. Subsequent tumourigenesis of nervous system tumours is associated with somatic inactivation of the wild-type VHL allele [2]. Structural analysis of autopsy spinal cord tissues from VHL patients suggests tumourigenesis to progress from developmentally arrested embryonic cells in nerve root tissue [3]. Tumour progression follows specific patterns of architecture, cytology, and molecular expression that resemble haemangioblastic formation and differentiation in the embryo, including haematopoiesis [4]. It could be argued, however, that incidental findings in autopsy tissues may not appropriately represent the process of haemangioblastoma development causing morbidity and mortality in VHL patients. For example, whereas autopsy studies identify pathological changes predominantly along the spinal cord in the VHL nervous system, clinical studies identify the majority of VHL tumours occurring in the cerebellum and brainstem [5]. Furthermore, VHL tumourigenesis in the central nervous system (CNS) proper, including the site of origin, awaits further clarification. In this study, we conducted structural and molecular analyses of tumour specimens surgically resected from VHL spinal cord, cerebellum, and brainstem. This approach allowed us not only to evaluate hypotheses based primarily on previous spinal cord autopsy studies, but also to expand significantly upon them with surgical samples from multiple anatomic sites.
Traditionally, haemangioblastomas are thought to manifest either as one of two distinct histopathological phenotypes or as individual tumours with highly diverse phenotypic variants. One histopathological phenotype has been denominated mesenchymal [4,6,7], capillary [8], reticular [9-13] or juvenile[14,15]. The other phenotype has been denominated epithelioid [8,16], clear cell [14,15] or cellular [8-13]. The remarkably variable and complex histopathological phenotypes of haemangioblastomas in VHL patients are in sharp contrast to the uniform phenotype of their microscopic precursor structures. We therefore tried to understand better the sequence of morphological events leading from the earliest steps of tumourigenesis to pleomorphic haemangioblastoma. With this study, we demonstrate that haemangioblastomatous growth begins with slow and morphologically uniform amplification of immature mesenchymal cells. Eventually, a subset of immature mesenchymal cells undergoes further haemangioblastic differentiation with marked architectural and cytological changes. We also demonstrate that this structural sequence of progression is accompanied by a molecular sequence of differential activation of hypoxia-inducible factors.
Materials and methods
One hundred and thirty-nine tumours from 83 patients with VHL disease were surgically resected between 2002 and 2006 and then analysed in the Surgical Neurology Branch at the National Institutes of Health in accordance with institutional ethical guidelines. Fifty-three tumours were associated with the spinal cord, 21 tumours with the brainstem, and 65 tumours with the cerebellum. Tumour volumes were calculated from measurements of fresh tumour immediately after surgical resection. Seventeen spinal cord tumours from three VHL autopsy patients were also included in this study.
Immunohistochemistry for CD31, CD34, hypoxia-inducible factor (HIF) 1α and HIF2α was performed as previously reported [4]. Immunohistochemical stains for Ki67 (MIB1 1: 200; DAKO, Carpenteria, CA, USA) and neurofilament triplet protein (NFTP; 1: 800; DAKO) were additionally performed according to the manufacturer’s instructions. In all tumour sections, staining for CD31 and CD34 exclusively labelled vascular structures within the tumour tissue. Staining for HIF1α and HIF2α labelled neoplastic VHL-deficient cells. Immunohistochemistry using MIB1 identified the proliferation antigen Ki-67 in proliferative cells. Immunohistochemistry for NFTP identified nerve root axons.
Results
Structural sequence of tumour progression
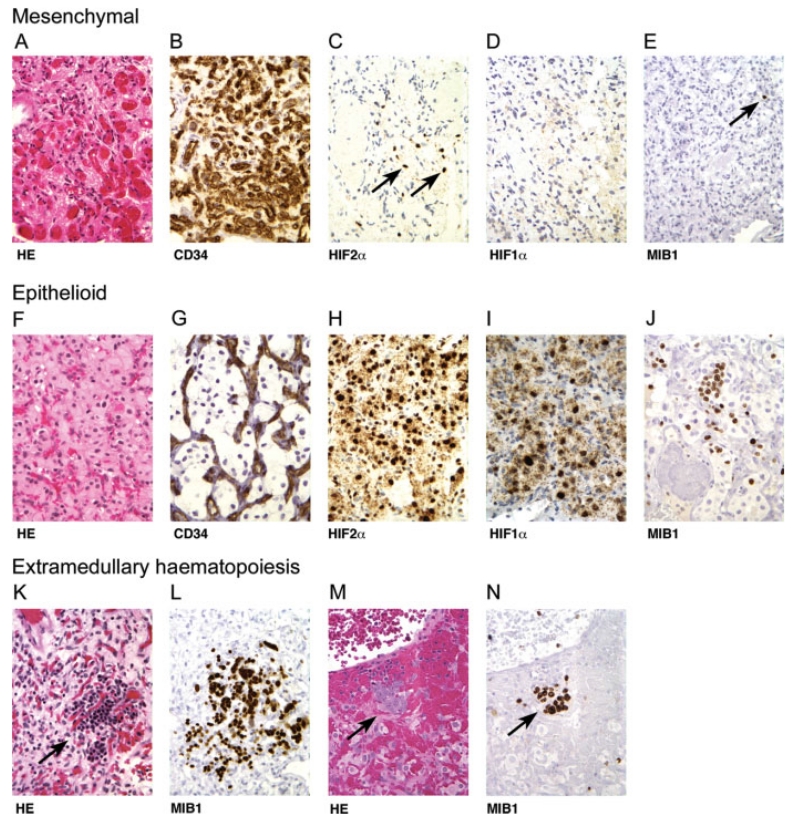
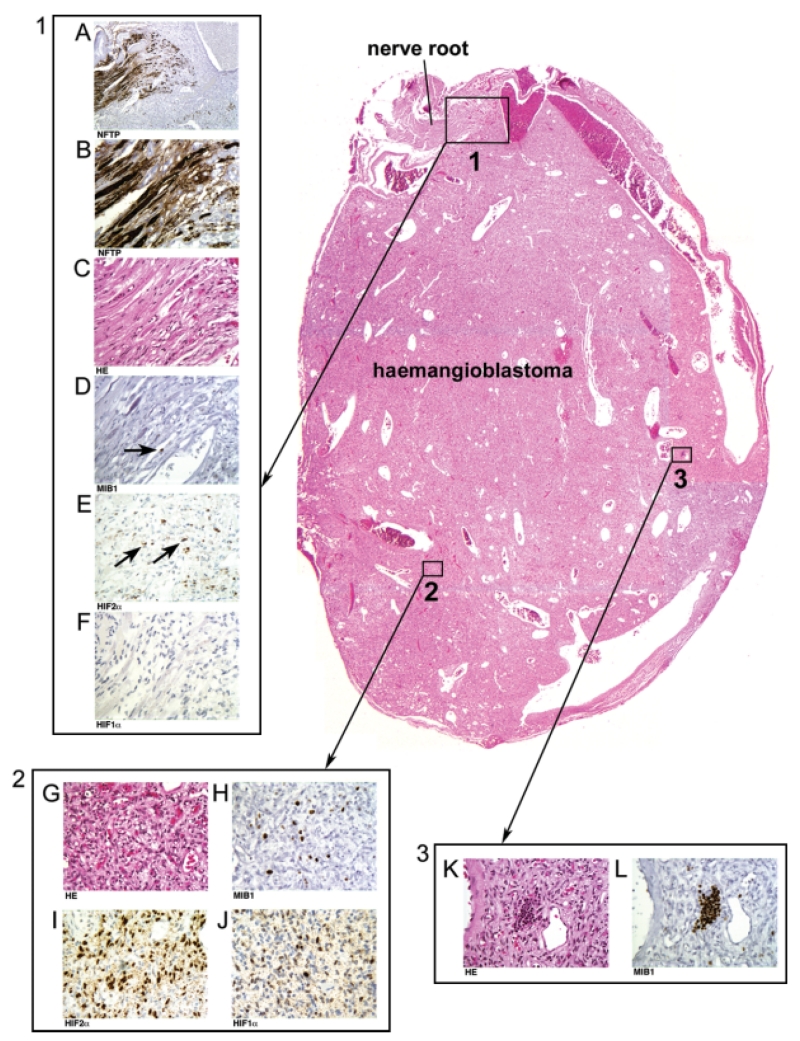
All 156 tumours exhibited areas of mesenchymal structure, either exclusively or in association with areas of epithelioid structure. Mesenchymal areas were composed of small, loosely scattered, individual tumour cells separated by extensive angiogenic vascularization (Figures 1A and 1B). Cytologically, the tumour cells within the mesenchymal areas were poorly differentiated and uniform, variably accompanied by few cells with increased nuclear and cytoplasmic size (Figure 1A). In contrast, epithelioid areas were characterized by large tumour cell clusters encompassed by extensive angiogenesis (Figures 1F and 1G). Cytologically, the tumour cells within the epithelioid areas had prominent cytoplasm and large nuclei (Figure 1F). Furthermore, of the 98 tumours with epithelioid components, one or more foci of extramedullary haematopoiesis were observed in 22 cases, exclusively confined to the epithelioid areas (Figures 1K and 1M). Extramedullary haematopoiesis was never observed in purely mesenchymal tumours or in the mesenchymal component of tumours. Similar to spinal cord tumours at autopsy, surgical tumours at the surface of the spinal cord were attached to nerve root tissues. If sectioned in the appropriate plane, large spinal cord tumours revealed a mesenchymal component in the nerve root that extended into frank tumour containing epithelioid architecture and cytology as well as foci of extramedullary haematopoiesis (Figure 2).
Figure 1.
Two distinct structural presentations of nervous system neoplasia in VHL disease. Tumour tissues may present with mesenchymal (A–E) and epithelioid structures (F–J). All 156 tumours show areas of mesenchymal structure that are composed of small, loosely scattered, individual tumour cells (A) separated by extensive angiogenic vascularisation. (B) Immunohistochemistry for vascular antigen CD34 reveals prominent reactive vascularization, and neoplastic cells are negative for CD34 (vascular antigen CD31 data are not shown). Scattered mesenchymal tumour cells show activation of HIF2α (C), but not HIF1α (D). Immunohistochemistry using MIB1 reveals a low proliferation index with rare, scattered positive cells (E). Ninety-eight of 156 tumours also reveal areas with epithelioid structure (F) that are composed of large tumour cell clusters encompassed by angiogenic vascularisation. (G) Immunohistochemistry for vascular antigen CD34 reveals encompassing reactive vascularization, and neoplastic cells are negative for CD34 (vascular antigen CD31 data are not shown). In epithelioid areas, there is activation of both HIF2α (H) and HIF1α (I). The MIB1 proliferation index is markedly increased, but highly variable in different cell clusters (J). Only in epithelioid tumour areas is there occasional differentiation of haemangioblastic cells into red blood cell precursors forming extramedullary haematopoiesis foci (K, M); all cells within the extramedullary haematopoiesis focus show positive immunoreactivity for the proliferation marker MIB1 on deeper sections (L, N)
Figure 2.
Structural and molecular sequences of nervous system tumour progression in VHL disease, demonstrated in a specimen surgically resected from a cervical nerve root. An intraradicular tumourlet precursor with exclusive mesenchymal structure (box 1) has progressed into a haemangioblastic tumour mass (haemangioblastoma). Swollen and distended axons are shown at lower (A, 10× by objective lens) and higher magnifications (B, 40× by objective lens) by immunohistochemistry for neurofilament triplet protein (NFTP). Adjacent tissue sections show intraradicular mesenchymal neoplastic cells and reactive vascularization (C) with low MIB1 index (D, showing a rare MIB1+ cell). The intraradicular mesenchymal neoplastic cells are positive for HIF2α (E), but negative for HIF1α (F) by immunohistochemistry. The tumour tissue reveals progression into an epithelioid structure characterized by clusters of large tumour cells (box 2, G). The MIB1 proliferation index in individual cell clusters is highly variable (H), but increased compared with the mesenchymal tumour component (box 1, D). Epithelioid neoplastic cells show not only activation of HIF2α (I), but also activation of HIF1α (J). Within epithelioid tumour tissue, red blood cell precursors of the extramedullary haematopoiesis foci (box 3, K) show 100% positive MIB1 immunoreactivity (L)
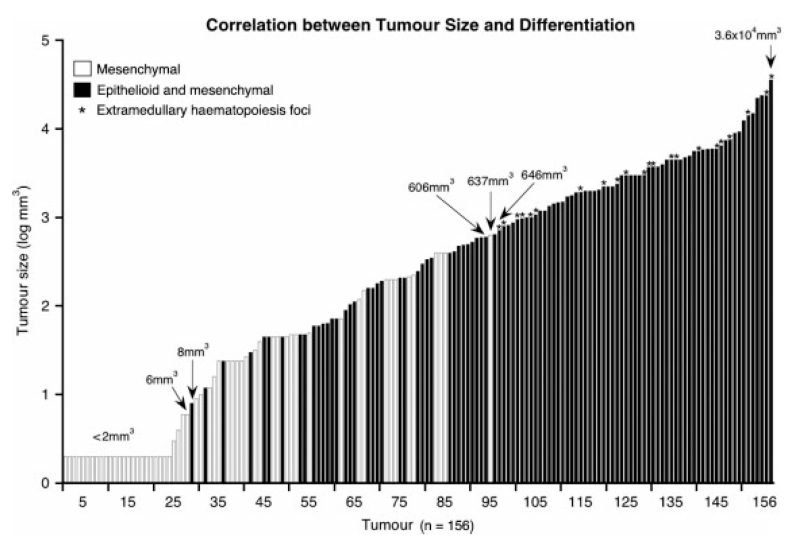
Histological analysis of 156 tumours revealed 58 tumours to display exclusively mesenchymal structure. Ninety-eight tumours contained both mesenchymal and epithelioid structures, with the ratios of mesenchymal to epithelioid components being variable. The correlation between histopathological structure and tumour size is shown in Figure 3. The volume of exclusively mesenchymal tumours ranged from 2 to 637 mm3; the volume of tumours with additional epithelioid differentiation ranged from 8 to 3.6 × 104 mm3. Between 8 and 637 mm3, tumours were either exclusively mesenchymal or both mesenchymal and epithelioid. Above 637 mm3, tumours consistently included epithelioid structure. Furthermore, tumours of 646 mm3 volume or larger size frequently contained foci of extramedullary haematopoiesis.
Figure 3.
Correlation between nervous system tumour size and histopathological structures provides evidence for structural sequence of tumour progression. Tumours smaller than 8 mm3 show only mesenchymal structure. Tumours greater than 637 mm3 always show additional epithelioid structure. Tumours between 8 and 637 mm3 are either exclusively mesenchymal or mesenchymal and epithelioid. Asterisks indicate tumours with foci of extramedullary haematopoiesis, which are consistently confined to areas with epithelioid differentiation
Activation of HIF2α and HIF1α reveals a molecular sequence of tumour progression
Immunohistochemical analysis for the alpha subunits of HIF1 and HIF2 was performed in 19 tumours, 13 being exclusively mesenchymal. Cells within the mesenchymal tumours consistently showed nuclear signal for HIF2α, but not HIF1α (Figures 1C and 1D). In the six tumours with the mesenchymal component continuous with the epithelioid, both HIF1α and HIF2α activation were observed in the epithelioid areas (Figures 1H, 1I, 2I, and 2J), with mesenchymal areas showing only HIF2α activation (Figures 2E and 2F). We conclude from these findings that the earliest stages of VHL tumourigenesis involve mesenchymal structure with exclusive activation of HIF2α, while tumour growth and progression are associated with epithelioid structure and additional activation of HIF1α.
Mesenchymal to epithelioid progression is associated with increased MIB1 proliferation index
Immunohistochemical staining using MIB1 was performed on 25 tumours. The mesenchymal structure was consistently associated with a MIB1 proliferation index of less than 2% (Figures 1E and 2D). The epithelioid structure was associated with variable MIB1 indices between 3% and 30% (Figures 1J and 2H). Further differentiation into red blood cell precursors comprising the extramedullary haematopoiesis foci showed 100% MIB1 positivity (Figures 1L, 1N, and 2L).
Discussion
VHL disease is associated with nervous system tumours in the spinal cord, cerebellum, and brainstem, called haemangioblastomas. There is increasing evidence that the neoplastic cells within haemangioblastomas are VHL-deficient, developmentally arrested, embryonic cells with haemangioblastic differentiation potential. Both haemangioblasts [17-19] and haemangioblastomas [20] are capable of differentiation into red blood cells. Both haemangioblasts [19] and haemangioblastomas [21] are characterized by expression of specific embryonal markers. Both haemangioblasts [18] and haemangioblastomas [22] can be grown and differentiated into red blood cells.
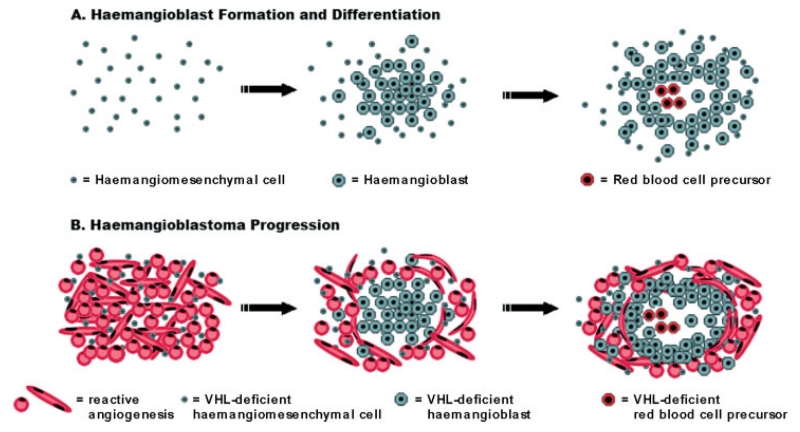
Haemangioblastic differentiation is initiated in mesodermal cells during embryogenesis. While we were unable to retrieve histological data on haemangioblastic differentiation in the contemporary literature, we found several informative studies on the structural sequence of haemangioblastic differentiation in the older literature. Of particular value are observations by Florence Sabin who noted, “When a cell of the mesoderm divides, the daughter cells separate at least enough so that they can be recognized as distinct cells ….” Subsequently, “ … angioblasts give daughter cells that remain together to form dense syncytial masses” [17,23]. In essence, Sabin described and illustrated a ‘mesenchymal to epithelioid’ transition during the formation and differentiation of haemangioblasts. Furthermore, she described the clustering of haemangioblasts to form foci of blood islands comprised of nascent red blood cell precursors producing haemoglobin and capable of becoming free red blood cells [17]. We therefore conclude that the structural sequences of haemangioblastic differentiation according to Sabin and neoplastic haemangioblastomatous differentiation are analogous (Figure 4). Of historic interest, Harvey Cushing and Percival Bailey arrived at the same conclusion after studying Sabin’s work on haemangioblast differentiation, and they therefore designated the neoplastic counterpart as ‘haemangioblastoma’ [8].
Figure 4.
Structural sequence of haemangiomesenchymal differentiation in the embryo (A) and of tumourigenesis in the nervous system of VHL patients (B). Haemangiomesenchymal cells undergo architectural and cytological changes during differentiation to haemangioblasts. Haemangioblasts then cluster to form vessels, plasma, and blood islands comprised of red blood cell precursors (modelled after Sabin [17,23]) (A). Haemangiomesenchymal, VHL-deficient cells undergo similar structural changes, yet differ in the intense reactive angiogenesis beginning in the early stages of tumourigenesis (B)
Morbidity and mortality resulting from haemangioblastoma growth occur via two different mechanisms. Natural history studies indicate that tumour growth may be stagnant for periods of time, followed by rapid solid tumour growth [24]. Other tumours, however, cause morbidity and mortality by expansion of an associated extratumoural cyst [25]. VEGF is up-regulated in the earliest stages of VHL tumourigenesis [4] and appears to influence peritumoural oedema and cyst formation, due to its ability to increase vascular permeability [26]. Millimetre-sized tumours are capable of generating voluminous cysts that disappear after surgical resection of the tumour nodule [25]. Long-term magnetic resonance imaging (MRI) observations show that cyst and solid tumour growth can occur either simultaneously or independently of each other [25]. For this study, we were therefore able to accumulate VHL nervous system tumour tissues of divergent dimensions ranging from less than 2 mm3 to 3.6 × 104 mm3. Frequently, large tumours had been resected after documentation of solid tumour growth on two subsequent MRI scans, whereas small tumour resection was frequently required due to cyst growth. In addition, we included a subset of minute tumours incidentally identified at autopsy from three VHL patients.
Our analysis of 156 tumours revealed that mesenchymal and epithelioid structures do not represent random variation, but rather progressive stages of tumour differentiation. Our conclusion is based on our consistent detection of mesenchymal structure in all tumours and on the exclusive detection of mesenchymal structure in tumours smaller than 8 mm3. The mesenchymal structures were composed of scattered tumour cells with primarily small nuclei and cytoplasm and of intense reactive angiogenic vessels entwined among the cells. The epithelioid structures, with or without haematopoietic differentiation, were consistently observed in tumours of larger size; that is, exceeding 637 mm3. The epithelioid areas were composed of tumour cells with increased nuclear and cytoplasmic size and of intense reactive angiogenic vessels surrounding tumour cell clusters (Figures 1-3). In addition, we detected marked differences of MIB1-associated proliferative activity at different stages of progression. In contrast to small haemangiomesenchymal tumours with rare, scattered, MIB1-positive cells, areas of epithelioid structure and especially foci of extramedullary haematopoiesis showed elevated MIB1 indices (Figures 1 and 2). These findings suggest that downstream haemangioblastomatous differentiation is associated with increased proliferative activity.
Constitutive activation of HIF, resulting from VHL deficiency, significantly affects gene expression patterns and cell signalling pathways, as demonstrated by the up-regulation of VEGF throughout early and late stages of haemangioblastoma progression. Furthermore, the HIF1α and HIF2α subunits can be differentially expressed and although structurally similar, may act in differing capacities in both normal physiology and tumour biology [27]. A previous autopsy tissue study demonstrated that HIF2α, and not HIF1α, activation was associated with early tumourigenesis; HIF1α activation was detected only in further progressed tumours [4]. In this study, we confirmed this result in symptomatic tumours, consistently showing that HIF1α activation does not occur at the early haemangiomesenchymal tumour stage, but rather at the later, further differentiated tumour stage.
In conclusion, we propose that the growth of nervous system tumours in VHL disease follows a sequence of structural and molecular events. These sequences are analogous to those of haemangioblastic differentiation in the embryo, but protracted in time. Further detailed analysis of molecular signatures associated with stages of differentiation may significantly enhance our understanding of tumourigenetic processes.
Acknowledgements
This research was supported, in part, by the Intramural Research Program of the NIH. SB Shively is a doctoral student in the Molecular and Cellular Oncology Program of the Institute for Biomedical Sciences at George Washington University and the Graduate Partnerships Program at the NIH. This work is from a dissertation to be presented to George Washington University in partial fulfilment of the requirements for the PhD degree. We are grateful to Cynthia Harris for skilful immunohistochemistry preparation; to Willie Young, Jim Rainey, and David Kleiner at Autopsy Service, Laboratory of Pathology, NCI; and to Travis Moncrief for work on the illustrations.
Footnotes
No conflicts of interest were declared.
References
- 1.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 2.Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, et al. von Hippel–Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel–Lindau disease. Hum Pathol. 1997;28:540–543. doi: 10.1016/s0046-8177(97)90075-7. [DOI] [PubMed] [Google Scholar]
- 3.Vortmeyer AO, Yuan Q, Lee YS, Zhuang Z, Oldfield EH. Developmental effects of von Hippel–Lindau gene deficiency. Ann Neurol. 2004;55:721–728. doi: 10.1002/ana.20090. [DOI] [PubMed] [Google Scholar]
- 4.Vortmeyer AO, Tran M, Zeng W, Glasker S, Riley C, Tsokos M, et al. Evolution of VHL tumorigenesis in nerve root tissue. J Pathol. 2006;210:374–382. doi: 10.1002/path.2062. [DOI] [PubMed] [Google Scholar]
- 5.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel–Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 6.Stein AA, Schilp AO, Whitfield RD. The histogenesis of hemangioblastoma of the brain. J Neurosurg. 1960;17:751–761. doi: 10.3171/jns.1960.17.4.0751. [DOI] [PubMed] [Google Scholar]
- 7.Ho K. Ultrastructure of cerebellar capillary hemangioblastoma. I. Weibel–Palade bodies and stroma cell histogenesis. J Neuropathol Exp Neurol. 1984;43:592–608. doi: 10.1097/00005072-198411000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cushing H, Bailey P. Tumors Arising from the Blood-Vessels of the Brain. Angiomatous Malformations and Hemangioblastomas. Charles C Thomas; Springfield: 1928. [Google Scholar]
- 9.Omulecka A, Lach B, Alwasiak J, Gregor A. Immunohistochemical and ultrastructural studies of stromal cells in hemangioblastoma. Folia Neuropathol. 1995;33:41–50. [PubMed] [Google Scholar]
- 10.Böhling T, Plate KH, Haltia MJ, Alitalo K, Neumann HPH. Von Hippel–Lindau disease and capillary haemangioblastoma. In: Kleihues P, Cavenee WK, editors. Tumours of the Nervous System. International Agency for Research on Cancer Press; Lyon: 2000. pp. 223–226. [Google Scholar]
- 11.Ishizawa K, Komori T, Hirose T. Stromal cells in hemangioblastoma: neuroectodermal differentiation and morphological similarities to ependymoma. Pathol Int. 2005;55:377–385. doi: 10.1111/j.1440-1827.2005.01841.x. [DOI] [PubMed] [Google Scholar]
- 12.Hasselblatt M, Jeibmann A, Gerss J, Behrens C, Rama B, Wassmann H, et al. Cellular and reticular variants of haemangioblastoma revisited: a clinicopathologic study of 88 cases. Neuropathol Appl Neurobiol. 2005;31:618–622. doi: 10.1111/j.1365-2990.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 13.Rickert CH, Hasselblatt M, Jeibmann A, Paulus W. Cellular and reticular variants of hemangioblastoma differ in their cytogenetic profiles. Hum Pathol. 2006;37:1452–1457. doi: 10.1016/j.humpath.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Silver ML, Hennigar G. Cerebellar hemangioma (hemangioblastoma): a clinicopathological review of 40 cases. J Neurosurg. 1952;9:484–494. doi: 10.3171/jns.1952.9.5.0484. [DOI] [PubMed] [Google Scholar]
- 15.Frank TS, Trojanowski JQ, Roberts SA, Brooks JJ. A detailed immunohistochemical analysis of cerebellar hemangioblastoma: an undifferentiated mesenchymal tumor. Mod Pathol. 1989;2:638–651. [PubMed] [Google Scholar]
- 16.Burger PC, Scheithauer BW, Vogel FS. Surgical Pathology of the Nervous System and its Coverings. 4th edn Churchill Livingstone; Philadelphia: 2002. [Google Scholar]
- 17.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. Anat Rec. 1917;13:199–204. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- 18.Choi K, Kennedy M, Kazarov ACPJ, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 19.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 20.Vortmeyer AO, Frank S, Jeong SY, Yuan K, Ikejiri B, Lee YS, et al. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res. 2003;63:7051–7055. [PubMed] [Google Scholar]
- 21.Gläsker S, Li J, Xia JB, Okamoto H, Zeng W, Lonser RR, et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 2006;66:4167–4172. doi: 10.1158/0008-5472.CAN-05-3505. [DOI] [PubMed] [Google Scholar]
- 22.Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, et al. von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4:333–341. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabin FR. Studies on the origin of the blood vessels and of red blood corpuscles as seen in the living blastoderm of chick during the second day of incubation. Carnegie Contrib Embryol. 1920;9:215–262. [Google Scholar]
- 24.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel–Lindau disease: implications for treatment. J Neurosurg. 2006;105:248–255. doi: 10.3171/jns.2006.105.2.248. [DOI] [PubMed] [Google Scholar]
- 25.Lonser RR, Vortmeyer AO, Butman JA, Gläsker S, Finn MA, Ammerman JM, et al. Edema is a precursor to central nervous system peritumoral cyst formation. Ann Neurol. 2005;58:392–399. doi: 10.1002/ana.20584. [DOI] [PubMed] [Google Scholar]
- 26.Proescholdt MA, Heiss JD, Walbridge S, Muhlhauser J, Capogrossi MC, Oldfield EH, et al. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J Neuropathol Exp Neurol. 1999;58:613–627. doi: 10.1097/00005072-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]