Abstract
The historical view of vascular smooth muscle cells (VSMCs) in atherosclerosis is that ‘aberrant’ proliferation of VSMCs promotes plaque formation, but that VSMCs in advanced plaques are entirely beneficial, for example preventing rupture of the fibrous cap. However, this view has been based on ideas that there is a homogenous population of VSMCs within the plaque, that can be identified separate from other plaque cells (particularly macrophages) using standard VSMC and macrophage immunohistochemical ‘markers’. More recent genetic lineage tracing studies have shown that VSMC phenotypic switching results in less differentiated forms that lack VSMC ‘markers’ including macrophage-like cells, and this switching directly promotes atherosclerosis. In addition, VSMC proliferation may be beneficial throughout atherogenesis, and not just in advanced lesions, whereas VSMC apoptosis, cell senescence, and VSMC-derived macrophage-like cells may promote inflammation. We review the effect of embryological origin on VSMC behavior in atherosclerosis, the role, regulation and consequences of phenotypic switching, the evidence for different origins of VSMCs, and the role of individual processes that VSMCs undergo in atherosclerosis in regard to plaque formation and the structure of advanced lesions. We believe there is now compelling evidence that a full understanding of VSMC behavior in atherosclerosis is critical to identifying therapeutic targets to both prevent and treat atherosclerosis.
Keywords: Atherosclerosis, smooth muscle
Introduction
Atherosclerosis is a chronic progressive inflammatory disease and the leading cause of death worldwide1-3 [http://www.who.int/mediacentre/factsheets/fs310/en/]. The major clinical consequences of atherosclerosis such as myocardial infarction or stroke are not a function of gradual narrowing of the lumen, but rather due to thrombotic events associated with acute rupture or erosion of an unstable plaque. Post-mortem and clinical imaging studies have identified a number of features of plaque instability leading to rupture, including: 1) a thin or fragmented fibrous cap comprising smooth muscle α-actin (ACTA2)-positive cells presumed to be derived from vascular smooth muscle cells (VSMCs); 2) large numbers of cells positive for markers such as CD68 or LGALS3 presumed to be macrophages; and 3) the presence of a large necrotic core containing cells filled with lipid (foam cells), presumed to be macrophages.
These observations underlie the general dogma that atherosclerotic plaques with a preponderance of macrophages and macrophage-derived foam cells relative to VSMCs, particularly within the fibrous cap and shoulder regions, are less stable and more prone to rupture1,2. That is, VSMCs in advanced lesions are generally regarded as having athero-protective plaque-stabilizing properties whereas macrophages are viewed as being athero-promoting and detrimental for plaque stabilization. However, this model may be overly simplistic and probably incorrect due to three major limitations. First, most studies have not unambiguously identified which cells within advanced lesions are derived from VSMCs or macrophages. This is due to reliance on markers that are not specific, are down-regulated, and/or are activated by another cell type in advanced plaques. Second, the role of individual cell types requires identification of the factors and mechanisms that regulate phenotypic transition of VSMCs and monocyte/macrophages, and defining how the functional properties of these phenotypically modulated cells affect disease pathogenesis. Third, VSMCs undergo multiple processes, often simultaneously, at different stages and in different regions of the plaque. The ‘role’ of VSMCs is therefore the additive effect of these processes, and may vary throughout atherogenesis. This review will analyze the effect of embryological origin on VSMC behavior, the evidence for different origins of VSMCs in atherosclerosis, and the roles of specific processes implicated in atherosclerosis, including phenotypic switching, cell proliferation, migration, cell death and cell senescence.
Origin and plasticity of VSMCs within Atherosclerotic Lesions
Embryological origin of VSMCs and susceptibility to atherosclerosis
Lineage tracing studies have established that VSMCs originate from multipotent precursors from a number of developmental origins (reviewed in 4). For example, VSMCs in the ascending aorta, arch and pulmonary trunk as well as head and neck vessels are derived from neural crest, while Islet-1+ progenitors in the secondary heart field contribute to the proximal aortic root. Coronary VSMCs are generated from the epicardium while the descending aorta is predominantly derived from somitic precursors. Although these lineage-specific VSMC populations share considerable phenotypic similarities, there are key differences between them both in their requirement for developmental regulators such as Myocardin-related transcription factor B (MRTF-B)5,6, and the responses of adult cells to key mediators and factors that may be relevant in disease development such as Transforming growth factor-β (TGF-β)7,8.
There is now evidence that these differences between distinct VSMC lineages influence the development of vascular diseases including atherosclerosis. For example, pioneering clinical studies by deBakey and Glaeser9 suggested that the progression of atherosclerotic lesions in response to systemic risk factors differed in four distinct vascular regions, including coronary arteries, the branches of the aorta, the abdominal visceral arteries, and the terminal abdominal aorta. Further studies of early atherogenesis in unselected young populations that died of non-cardiac causes confirmed that disease development in distinct vascular regions responds differently to common risk factors, such as smoking or gender10. Thus, it is possible that there may be basal differences in VSMC susceptibility to systemic risk factors based on embryonic origins, although it seems likely that local vascular hemodynamic and structural factors still have a major role in defining precise patterns of plaque development.
Although one must be cautious in extrapolating results obtained in cultured cells, including VSMCs, to in vivo settings, the confounding effects of flow and local vessel characteristics may be overcome by study of cultured cells from different regions. Indeed, the latter has been aided recently by the generation of lineage-specific VSMCs in vitro from pluripotent stem cells11. For example, the atherosclerosis-resistant thoracic aorta of fat fed ApoE-/- mice has higher expression of a range of Homeobox (Hox) genes than the more atherosclerosis-prone aortic arch, with reciprocal inhibition between HoxA9 and NF-κB12. The resultant high NF-κB activity in the arch and low activity in the thoracic aorta defines a possible regulatory mechanism for this critical inflammatory regulator in atherosclerosis. Differences in Hox gene expression were also seen in an in vitro human embryonic stem cell-derived model, with high HoxA9 expression in paraxial mesoderm-SMCs that corresponded to thoracic aorta and low expression in neuroectoderm-SMCs corresponding to the arch. Thus, the atherosclerosis-susceptibility or resistance seems to be related in part to developmental programming. The challenges now are to further characterize the identity of different VSMC regions by both transcriptional and epigenetic mechanisms, to determine which developmental signatures are preserved in the adult vasculature, and how these mechanisms which define positional identity may regulate the development of atherosclerosis.
Phenotypic Switching of VSMCs in atherosclerosis
VSMCs in the normal arterial media express a range of ‘SMC markers’, conventionally including Smooth muscle cell myosin heavy chain (MYH11), SM22α/tagln, Smooth muscle cell actin (ACTA2), smoothelin and others. VSMCs in culture and in atherosclerosis reduce expression of these markers, and, at least in vitro, acquire increased capacity for cell proliferation, migration and secretion of various extracellular matrix proteins and cytokines (reviewed in13). VSMCs undergoing phenotypic switching can also acquire macrophage markers and properties. This ‘phenotypic switching’ has long been considered of fundamental importance to atherosclerosis, generating a VSMC phenotype that is pro-atherogenic; however, direct interventional studies to prevent phenotypic switching have been lacking.
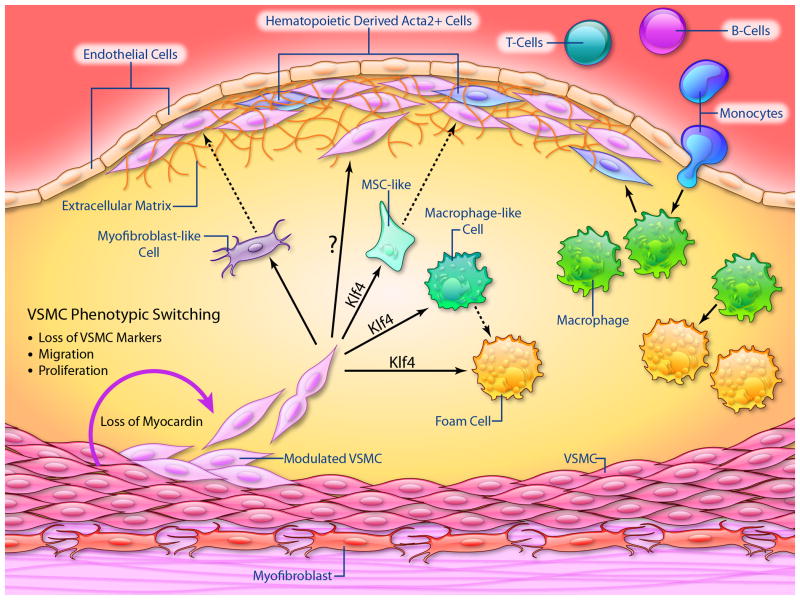
Regulation of VSMC phenotypic switching has been reviewed extensively elsewhere14, but recent studies have defined the role that phenotypic switching actually plays in atherosclerosis and plaque stability, and established that inhibiting VSMC phenotypic switching may be beneficial in advanced atherosclerosis. For example, the myocardin-serum response factor (SRF) regulatory module is a central component of phenotypic regulation that facilitates combinatorial interactions of activating and repressing signals and co-factors that act on most VSMC contractile genes. Myocardin+/- mice on an ApoE-/- background exhibit increased atherosclerosis with increased accumulation of macrophage or macrophage-like cells compared to myocardin+/+ littermates15. Although this was not a VSMC-specific loss of function study, the only cells in the vasculature that express myocardin are VSMCs. Loss of myocardin upregulated a variety of inflammatory pathways to increase macrophage recruitment, or switched VSMCs to a macrophage-like phenotype (see below). Conversely, gain of myocardin function inhibited inflammatory pathways and limited neointimal macrophage accumulation in vivo15.
Similarly, the stem cell and induced pluripotent stem cell factor KLF4 has been shown previously to be required for phenotypic transition of cultured VSMCs in response to Platelet-derived growth factor (PDGF) BB16,17, oxidized phospholipids18,19, or IL1β20, and silences SMC marker genes to inhibit myocardin-dependent gene activation16,21. Loss of KLF4 in VSMCs in vivo is also associated with a transient delay in phenotypic switching following ligation injury22. More recent studies have shown that VSMC-specific conditional knockout of KLF4 does not prevent VSMC phenotypic switching, but markedly reduces plaque size with increased fibrous cap area, an index of increased plaque stability23. Interestingly, KLF4 knockout KO did not alter overall VSMC numbers, but reduced the number of VSMC-derived macrophage-like and mesenchymal stem cell-like cells, indicating that KLF4 regulates the transition towards a ‘macrophage’ phenotype. Indeed, results of KLF4 CHiP-seq analyses on brachiocephalic lesions of SMC-selective KLF4 knockout versus wild-type mice identified a large number of putative SMC KLF4 target genes including many associated with pro-inflammatory processes23.
The switching of VSMCs to macrophage-like cells may be driven by lipid accumulation in the plaque, as cholesterol loading of cultured VSMCs activated multiple pro-inflammatory genes, suppressed expression of VSMC marker genes, activated ‘macrophage markers’, and induced phagocytic activity, all of which were KLF4-dependent23 (Figure 1). However, gene expression of these VSMC-derived macrophage-like cells is distinctly different from classical monocytes, macrophages, and dendritic cells24, and these cells have reduced phagocytic capacity compared with activated peritoneal macrophages. Reduced phagocytosis, for example of apoptotic cells, is evident in advanced atherosclerosis25 and directly promotes formation of the ‘necrotic’ core of the lesion. These studies indicate that SMC-derived macrophage-like cells may promote atherosclerosis by having reduced ability to clear lipids, dying cells, and necrotic debris, and by exacerbating inflammation. Although it has long been postulated that VSMCs within lesions play a beneficial role (reviewed in1,26,27), for example by protecting the fibrous cap from rupture and promoting plaque repair, recent studies show this is an over-simplification, and VSMC function can vary dramatically depending on the nature of the phenotypic transitions.
Figure 1.
Schematic summarizing the current knowledge of the identity and origins of VSMCs, macrophages, and putative derivatives of these cells within advanced atherosclerotic lesions. The solid lines illustrate known pathways that give rise to lesion cells whereas dotted lines with a “?” indicate putative pathways not yet directly validated in animal models or humans. (Illustration Credit: Ben Smith)
Although we have focused on the signals within VSMCs that regulate phenotypic switching, VSMCs synthesize and are embedded in an extracellular matrix (ECM) that separates them from each other, except at defined cell-cell contacts. The conventional view is that ECM suppresses phenotypic switching, keeping VSMCs in a ‘contractile’ state that is less responsive to mitogens. Conversely, breakdown of ECM, collagen, or elastin, for example by matrix metalloproteinases released from macrophages and VSMCs, would promote phenotypic switching and facilitate both cell proliferation and migration28-30. However, the real effects of ECM on VSMCs may be more complex. For example, recent studies have shown that fibronectin deposition at sites of early plaque formation promotes atherosclerosis, but also promotes the formation of the protective fibrous cap31. Similarly, although it is widely believed that phenotypically modulated VSMCs within the fibrous cap produce ECM molecules critical for plaque stabilization, there are no studies that have examined how knockout of a given ECM gene exclusively in VSMCs impacts lesion pathogenesis. Indeed, this is a critical area in need of further studies.
Derivation of VSMCs from within the vessel wall or bone marrow
Although it has long been assumed that differentiated (mature contractile state) VSMCs undergo phenotypic switching during atherogenesis1, direct evidence that VSMCs exhibit phenotypic switching in vivo during atherogenesis has only been proven recently based on rigorous SMC-specific conditional lineage tracing studies23,32,33. These studies showed that >80% of VSMC-derived cells within advanced ApoE-/- mouse plaques lacked detectable expression of commonly used SMC markers such as ACTA2, and that >30% of VSMC-derived cells expressed multiple markers of macrophages including LGALS3/Mac2, CD11b, F4/80 and CD68. Similar studies using a single cell epigenetic assay32 and Y-chromosome lineage tracing in humans who had a cross gender heart transplant showed that nearly 20% of CD68+ cells in advanced coronary plaques are also of SMC not myeloid origin, demonstrating that transition of VSMCs to macrophage-like cells also occurs at a relatively high frequency in human lesions. These studies indicate that the conventional view of the macrophage-rich ‘necrotic core’ may also be incorrect, and that VSMCs and dead VSMCs comprise a substantial component of the core. Interestingly, VSMC-derived cells that lacked detectable expression of ACTA2 expressed markers of mesenchymal stem cells (e.g. Sca1+ CD105+), as well as myofibroblasts (ACTA2+/- PDGFβ receptor+), raising the concept of a progenitor population of SMCs within the vessel wall that selectively proliferate and accumulate in atherosclerosis23 (see below).
These studies clearly indicate that a major fraction of VSMC-derived cells in advanced lesions have previously gone either unidentified or been incorrectly identified as being another cell type. However the converse is also true, that a subset of cells within lesions that express at least some SMC markers are not SMC-derived. For example, a number of studies from 2002 onwards have shown that some SMC marker-positive cells within lesions were of myeloid origin both in mice34 and humans35. Later studies showed that hematopoietic (myeloid)-derived cells can activate early but not late stage markers of SMCs within ApoE-null mouse lesions including ACTA2 and SM22α but not MYH1136, although all of these markers can be expressed by bone marrow-derived cells in culture37. Similarly, studies of cross-gender bone marrow transplant human subjects showed that at least 10-15% of ACTA2+ cells within advanced human coronary artery lesions are of myeloid and not VSMC origin36. In contrast, cross gender BMT lineage tracing and arterial transplantation studies concluded that 100% of ACTA2+ positive cells within lesions of ApoE-/- mice are of local arterial wall origin and not derived from the bone marrow38,39, and lineage tracing using the SM22α promoter indicated that <1% of cells expressing SM22α were of myeloid origin37.
The discrepancy between these studies even in the same species may be due to the assumption that immunohistochemical ‘SMC’ markers are specific for VSMCs, and ‘macrophage’ markers are specific for bone marrow-derived cells. However, recent studies showed that 40% of foam cells within advanced human coronary artery lesions express both the SMC marker ACTA2 and the macrophage marker CD68, although it is unclear if these represent VSMC-derived cells that have activated macrophage markers, are macrophages that have activated SMC markers, or neither40. Again, lineage tracing studies using epigenetic markers showed that 38% of cells within advanced human coronary artery lesions that were dual positive for CD68 and ACTA2 exhibited the SMC-specific MYH11 H3K4diMe epigenetic signature indicating they were of VSMC and not myeloid origin23. Similarly, Y-chromosome lineage tracing studies in cross gender heart transplant coronary artery lesions showed that myeloid cells do not acquire the SMC-specific MYH11 H3K4diMe epigenetic signature and that non-myeloid cells are CD68+. These studies indicate that myeloid cells can acquire some, but not all, SMC markers in advanced plaques, suggesting that they do not behave like vessel-wall derived VSMCs. Indeed, ablation studies indicated that in contrast to vessel-wall derived VSMCs, myeloid-derived SMC-marker positive cells are likely to promote atherosclerosis37.
VSMCs derived from stem and progenitor populations within the vessel wall
A number of studies have suggested several alternative sources of VSMC-like cells within atherosclerotic lesions. For example, Tang et al.41 found that MYH11-expressing medial VSMCs are terminally differentiated and incapable of phenotypic transition during vascular injury and disease, and the existence of a MYH11- medial stem cell population that gives rise to VSMC-like cells within lesions. Both of these findings have been refuted based on technical limitations that have been summarized recently42. These include: 1) the failure to perform high resolution Z-stack confocal analyses to ensure the lineage tracing gene and other marker genes are expressed within the same cell and do not represent signals from overlapping cells, an essential requirement for lineage tracing (see 43 for review); 2) inappropriate reliance on assessing a negative population since it is impossible to ascertain if this represents failed cre-mediated recombination, silencing of the lineage tracing gene, or technical loss of the reporter marker, or whether the cell truly did not express the MYH11 SMC marker gene – i.e. reliable lineage tracing should focus only on assessing a positively labeled cell population; 3) failure to provide rigorous validation that only the cell population of interest (in this case MYH11 expressing mature VSMCs) was labeled at time zero and not other cell types; and 4) methodological concerns including inappropriate fixation and cell permeabilization methods that might result in artificial loss of eGFP, for example validation of their lineage tracing model and image resolution to determine co-localization of markers (reviewed in 42) and functional studies using rigorous lineage tracing methodologies. Indeed, subsequent rigorous lineage tracing studies by several groups have shown that mature MYH11-expressing medial VSMCs are not terminally differentiated, and are capable of phenotypic transition in culture23,32, in atherosclerosis33, and after vascular injury44.
Several groups have postulated that adventitial cells including Sca1+ stem cells45, adventitial pericytes46, and/or adventitial fibroblasts47,48 may contribute to formation of neointimal lesions following vascular injury or within atherosclerotic plaques. Indeed, there is compelling evidence for the existence of a population of Sca1+ adventitial cells that can be induced to activate multiple SMC marker genes in vitro49,50. Moreover it is well established that pericytes and activated myofibroblasts express multiple SMC markers and thus may give rise to VSMC-like cells within lesions. Unfortunately studies to date have relied on use of single markers or panels of markers that do not clearly define the origins of cells nor exclude VSMCs as the source of the cells in question, and as yet there have been no rigorous high-resolution lineage tracing studies of any of these cell populations. Indeed, to our knowledge, no one has identified appropriate cell-specific conditional lineage tracing genes to specifically label these interesting cell populations. Adventitial Sca1+ c-Kit+ cells tagged with LacZ in vitro and then transplanted to the adventitial surface of a vein graft within ApoE-/- mice have been shown to contribute to vein graft neointima formation45. However, this model results in huge decellularization of the media after transplant, and as yet there are no rigorous lineage tracing studies showing that endogenous adventitial cells normally contribute to primary atherosclerotic plaque formation, although this is an area deserving further study.
Clonal nature of the atherosclerotic plaque
Although the most robust lineage tracing studies support a prominent role for phenotypic switching of MYH11+ medial VSMCs in generating the atherosclerotic lesion, a key consideration is whether all medial MYH11+ cells contribute to the intimal VSMCs. In particular, findings of ‘monoclonality’ of human plaques both in historical and recent studies suggested that a subpopulation of medial VSMCs selectively proliferates to cause VSMC accumulation in atherosclerosis, and in particular in the fibrous cap. For example, over 40 years ago Benditt and Benditt found that fibrous caps of atherosclerotic plaques of females heterozygous for the X-linked enzyme glucose-6-phosphate dehydrogenase were monoclonal in nature, while the medial VSMCs were a mixture51. Although subsequent observations showed that normal vessels also comprised a mosaic of monoclonal patches due to the expansion of progenitor cells during normal vessel development52-54, recent lineage tracing studies showed preliminary evidence of clonal expansion of MYH11+ cells during plaque development33. While the latter study did not address the extent to which this occurs nor the cell types generated in the plaque, they do raise critical questions as to the nature of the MYH11+ cells that contribute to atherosclerosis. For example, is there a specific sub-population of MYH11+ progenitors and if so, how could these be identified and what are their molecular characteristics? Alternatively, dynamic fluctuations within MYH11+ medial VSMCs could lead to stochastic development of plaque ‘progenitor’ cells. Clearly further studies are needed to define precisely the extent to which atherosclerotic plaques are derived from a single progenitor and the molecular mechanisms underlying such an event.
VSMC processes in atherosclerosis – evidence and consequences
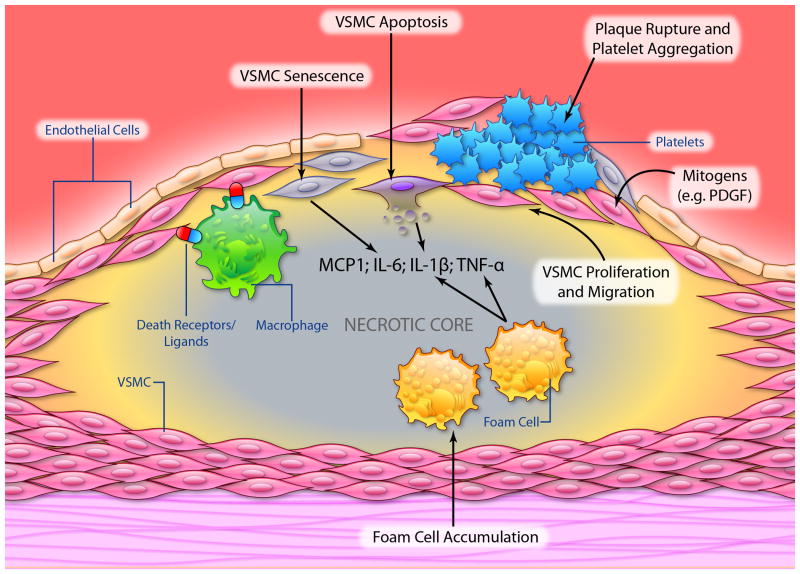
The stability of the atherosclerotic plaque depends upon the thickness of the fibrous cap and the degree of cap inflammation. Plaque rupture is increased by cap thinning promoted by death of VSMCs and breakdown of collagen and extracellular matrix (ECM), which may subsequently lead to myocardial infarction or stroke. However, plaque rupture is frequently sub-clinical, as VSMCs repair the rupture and reorganize the associated thrombus. Indeed, complicated plaques frequently show evidence of multiple ruptures and repair, ultimately resulting in luminal narrowing. Successful plaque repair requires VSMCs to proliferate and synthesize matrix, both properties that are altered by death and cellular senescence. Indeed, the balance of cell proliferation and migration vs. cell death and cell senescence determines the population of VSMCs within the atherosclerotic plaque (Figure 2). The role and regulation of these processes is crucial to both atherogenesis and plaque stability.
Figure 2.
Schematic illustrating a number of processes that VSMCs undergo in advanced atherosclerotic plaques. (Illustration Credit: Ben Smith)
Cell Proliferation
VSMCs in the normal vessel wall have a very low turnover, with barely measureable proliferation indices. Increased cell proliferation is observed during early atherogenesis and upon vascular injury55, and aged VSMCs from rodents also show increased proliferation56-58 compared with cells from younger animals. In contrast, human VSMCs derived from both aged vessels and advanced atherosclerotic plaques undergo reduced proliferation and prolonged population doubling times59,60. This observation corresponds to in vitro findings where plaque VSMCs in culture show decreased percentages in S-phase and increased percentages in G1, consistent with a G1 growth arrest60. Whilst some of the arrest is associated with reduction in responses to mitogens, such as insulin-like growth factor 1 (IGF-1)61, the arrest is mediated by major changes in the expression of various cell cycle regulators, especially those involved in G1-S transition. Thus, increased expression of the cyclin-dependent kinase inhibitors p16INK4a and p2162, decreased cyclin D and cyclin E63, and hypophosphorylation of the retinoblastoma protein (pRB)62-64 are observed in both normal human VSMCs undergoing replicative senescence and human plaque VSMCs. Plaque VSMCs also show reduced expression of the transcription factors E2F1-3 and increased sequestration of E2F1 to pRB62. Importantly, these cell cycle regulators become potential markers of vascular cell senescence. Collectively these observations suggest that in advanced lesions enhancement and not inhibition of VSMC proliferation may be beneficial for plaque stability and progression. Similarly, if VSMC proliferation is predominantly reparative in atherogenesis, enhancement of the ability of VSMCs to proliferate may also be beneficial early in disease.
Cell Migration
The presence of a large number of intimal VSMCs, for example forming a fibrous cap, has been taken as evidence that VSMC migration from the media plays an important role in atherogenesis. However, VSMC migration is a difficult process to quantify in human atherosclerosis, in part because there are no specific markers available, and also because human arteries also contain intimal VSMCs. In rodents, where there are no VSMCs in the intima in the normal vessel, intimal VSMCs must have arisen by migration from the intima or transdifferentiation of invading myeloid cells from the lumen. Indeed, seminal studies from the 1970s demonstrated VSMC migration both directly (via appearance in the intima) and indirectly (via proliferation labeling studies with demonstration of intimal VSMCs that had not proliferated). In contrast, such direct or indirect quantification of migration in human vessels is not possible, and we are left with evidence that human VSMCs can migrate to a variety of stimuli in culture, but the contribution of VSMC migration to the mature atherosclerotic plaque is unclear. Similarly, in humans it is not clear whether migration occurs independently or is dependent upon cell proliferation
Cell Death
The presence of apoptosis in atherosclerotic plaques has been confirmed by a number of studies55,60,65. Apoptotic indices are low in early lesions (Stary grades I-III), but seen with increasing frequency as lesions develop, in both the necrotic core and fibrous cap. Apoptosis is predominantly restricted to macrophages and VSMCs, although all cell types within the vessel wall can undergo apoptosis. However, the same caveats apply to studies on apoptosis and other studies, that interpretation is now limited by their use of markers that are not lineage-specific. Plaque rupture occurs most commonly in the shoulder area of the plaque, a region characterized by reduced VSMCs and increased macrophages. This suggests that VSMC apoptosis, perhaps induced by macrophages through death ligand/death receptor interactions66, may be a central event in plaque rupture and its subsequent sequelae67. Indeed, symptomatic plaques exhibit increased levels of VSMC apoptosis68 compared with stable lesions.
Although apoptosis is seen in vascular disease, these frequencies cannot be transposed into absolutes rates of cell death, as we do not know how long the death process lasts in vivo in diseased vessels, and how much of the death process is associated with positive markers69,70. For example, delay of phagocytosis may result in increased apoptosis being detected25, and positive live cells will be marked if apoptotic bodies retain terminal UTP nick end-labeling (TUNEL)-positivity after engulfment. Although we cannot get accurate rates of apoptosis, VSMC apoptosis in atherosclerosis has profound consequences, promoting multiple features of vulnerable plaques71 such as a thin fibrous cap, enlarged necrotic core, and macrophage infiltration into the cap. Chronic VSMC apoptosis accelerates both atherogenesis and progression of established lesions, promotes calcification, and also induces features of medial degeneration, including medial atrophy, VSMC loss, elastin fragmentation, increased glycosaminoglycans and speckled calcification72. These features are seen in cystic medial degeneration, for example in Erdheim's disease, in Marfan syndrome, and to a lesser extent in normal ageing73,74. Importantly, loss of VSMCs is sufficient alone to trigger all of these secondary features, suggesting that VSMC apoptosis is a primary an early event in these diseases.
VSMC apoptosis in atherosclerosis is also associated with inflammation, whereas in vascular ageing, medial degeneration and remodeling, there is remarkably little inflammation. The recent explanation for this phenomenon may rest on the efficiency of clearance of apoptotic cells and the cytokines released from dead and surrounding live VSMCs. Dying VSMCs release interleukin 1; apoptosis releases IL-1β and necrosis releases IL-1α. Secondary necrosis (after apoptosis) releases both IL-1 α and IL-1β75. Apoptotic VSMCs are normally cleared from the vessel wall in ∼48 hours, as VSMCs themselves are very efficient at clearing apoptotic VSMCs76. However, phagocytosis is delayed in the presence of hyperlipidemia, possibly due to the defective phagocytosis induced when VSMCs undergo phenotypic switching to macrophage-like cells, with the resultant subsequent inflammation that is dependent upon IL-175. Furthermore, a recent study has linked the human 9p21 gene locus, which has been shown to be highly correlated with enhanced cardiovascular disease, with reduced expression of cyclin-dependent kinase inhibitor 2B (CDKN2B) and calreticulin, a ligand required for activation of engulfment receptors on phagocytic cells77. CDKN2B-deficient apoptotic bodies were resistant to efferocytosis and not efficiently cleared by neighboring macrophages77. These data suggest that loss of CDKN2B promotes atherosclerosis by increasing the size and complexity of the lipid-laden necrotic core through impaired efferocytosis.
As described above, recent studies have suggested that bone marrow-derived cells may migrate to the atherosclerotic plaque or neointima after injury and express SMC markers. Indeed, VSMC apoptosis releases Stromal cell-derived factor 1α (SDF-1α) after injury, which may recruit SMC progenitors to sites of arterial injury78. However, bone marrow-derived smooth muscle-like cells are infrequent in primary atherosclerotic plaques, and unlike vessel wall-derived VSMCs, their apoptosis reduces atherosclerosis and reduces plaque inflammation37. In this case, their pro-atherogenic action is also dependent on cytokines released, including Chemokine (C-X-C motif) ligand 16 (CXCL16), IL-6 and Monocyte chemoattractant protein-1 (MCP-1)37, but apoptosis of these cells reduces plaque inflammation. Clearly, whether apoptosis induces inflammation depends upon their origin; vessel wall-derived cells promote inflammation when they undergo apoptosis in atherosclerosis; bone marrow-derived SMC-like cells already have a pro-inflammatory phenotype and their apoptosis reduces inflammation.
Cell Senescence
Cell senescence is defined as the irreversible loss of the ability of cells to divide. There are two general types of cell senescence, replicative senescence and stress-induced premature senescence (SIPS). Replicative senescence occurs with exhaustion of proliferative lifespan over time, a characteristic of aging, and is associated with critically shortened telomeres at chromosomal ends, which then induce a DNA damage response (DDR). In contrast, SIPS is triggered by external stimuli, including oxidizing agents and radiation, which activate the intracellular senescence cascade prematurely. Whilst SIPS shares many morphological and molecular characteristics to replicative senescence, SIPS is not usually characterized by telomere shortening.
As well as altered expression of cell cycle regulators, senescent cells are characterized by ‘specific’ markers, including senescence-associated β galactosidase (SAβG), a lysosomal enzyme seen in senescence of multiple human cell types79. Increased numbers of SAβG-positive cells expressing markers associated with VSMCs, endothelial cells (ECs) and monocyte/macrophages are observed in aged vessels and atherosclerotic lesions when compared to their respective young and normal counterparts62,80, reinforcing the idea that atherosclerosis is associated with premature cellular senescence. However, a word of caution is required when interpreting SAβG staining. In particular, cells with a high lysosomal content, such as macrophage foam cells, show SAβG reactivity that may not reflect senescence.
Shortened telomeres are evident in atherosclerosis, observed in plaque VSMCs62 and ECs81 relative to the normal vessel wall, and in circulating endothelial progenitor cells82. Telomeres are also shorter in leukocytes in patients with atherosclerosis compared to control subjects83,84 and are also inversely correlated to cardiovascular disease risks in patients with subclinical diseases85,86. Short telomeres and low levels of telomerase expression and activity are functionally important in VSMC senescence, as ectopic telomerase expression can dramatically increase lifespan of both plaque and normal VSMCs62. However, some of these effects may be independent of telomeres, as telomeres continue to shorten in these cells and cells replicate with critically short telomeres62. In addition, whilst telomere length mostly reflects previous replication, arterial segments resistant to atherosclerosis, such as internal mammary artery or ascending aorta, have longer telomeres than the aortic regions prone to the disease87,88. This difference is age-independent, suggesting the existence of intrinsic genetic or developmental variations in telomere regulation may underlie location-specific predisposition in atherogenesis.
VSMCs in human plaques or derived from plaques show early senescence and increased susceptibility to apoptosis. These properties would reduce the ability to repair plaques that undergo rupture. Aged rodent aortas also show increased levels of interleukin-6 (IL-6) and aged aortic VSMCs have a higher basal secretion of IL-6 than young VSMCs. Indeed, secretion of a common set of secreted proteins as cells age is a widespread phenomenon, known as the ‘senescence-associated secretory phenotype’ or SASP. Moreover, aged VSMCs exhibit upregulation of chemokines (e.g. CCL2), adhesion molecules (e.g. ICAM-1), and innate immune receptors (e.g. Toll-like receptor 4)89. These properties generate a pro-inflammatory environment, further promoting migration of inflammatory cells. Indeed, experimental induction of VSMC senescence has been shown to promote both plaque progression and features of unstable plaques90.
Although we have discussed these processes separately to review their consequences, many processes occur simultaneously (Figure 2). For example, VSMC phenotypic switching to a macrophage-like cell, VSMC death, and senescence all promote inflammation, monocyte recruitment, and subsequent secretion of VSMC mitogens. VSMC proliferation ultimately generates cell senescence, as do defects in cell death or cell clearance, and lack of clearance of senescent VSMCs may promote cell death. The complex structure of the atherosclerotic plaque therefore reflects the complex cellular and extracellular environment, and both the complementary and competing nature of these processes.
Summary and Conclusions
The role of VSMCs in atherosclerosis has evolved remarkably in the last 30 years. Previously it was thought that aberrant proliferation of VSMCs following phenotypic switching drove atherogenesis, although VSMCs were also protective in advanced lesions, preventing fibrous caps from rupturing and promoting plaque repair. VSMCs expressed VSMC markers or no markers, and macrophages expressed macrophage makers.
More recent studies using lineage tracing and VSMC-specific manipulation of both specific genes and pathways have changed our view. It is highly likely that VSMCs and macrophages within lesions have been mis-identified in many previous studies in the field. This is because VSMC marker-positive cells within lesions can be derived from multiple cell types including macrophages and possibly various adventitial cells, and the majority of VSMC-derived cells within lesions lose expression of SMC markers. Similarly, macrophage marker-positive cells within plaques may not be macrophages or even of myeloid origin, and a large fraction of foam cells dual-positive for SMC and macrophage markers are of VSMC not myeloid origin. A further level of complexity is introduced by findings that cellular proteins and mRNAs can be passed between cells through exosomes or microvesicles, such that a cell can acquire a ‘marker’ by passive transfer (reviewed in 91,92). This problem is not just confined to VSMCs, or even vascular biology. Clearly, if a ‘marker’ can be induced in a different lineage or lost in the same cell type, it is not a marker of that lineage, or implies a property of that cell. Otherwise we end up with a circular argument that a marker is identified if it is expressed in a lineage, and a lineage identified if it expresses a marker, when neither are necessarily true. For example, expression of Sca1 alone does not mean that that cell is a progenitor cell. However, we can conclude that the majority of cells expressing SMC markers in atherosclerosis or after arterial injury derive from the vessel wall and not the bone marrow, although the contribution of endogenous progenitor populations within the media or adventitia is unclear.
Recent studies have also clarified the role of VSMCs in disease. VSMC proliferation in atherosclerosis appears to be predominantly reparative, even in atherogenesis, and not the primary driver of plaque formation. The role of VSMC migration per se in atherosclerosis is still unclear, including adventitial progenitor populations. In contrast, VSMC cell death and cell senescence promote both atherogenesis and multiple features of plaque instability.
A critical challenge for future studies will be to identify the environmental cues within advanced atherosclerotic lesions that regulate phenotypic transitions of VSMCs, as well as each of the major cell types within lesions, and to determine how these might be manipulated therapeutically to reduce plaque burden and increase plaque stability. Our rationale is that development of novel therapeutic approaches for treating atherosclerosis, and reducing major clinical consequences such as MI or stroke, will be dependent on a rigorous understanding of the biology of each of the major cell types that contribute to the pathogenesis of late-stage lesions. We are assuming that certain pathways and targets may have opposing effects on one cell type versus another and that an “ideal” therapeutic target would promote beneficial changes in multiple cell types. This represents a paradigm shift for the atherosclerosis field since therapies to date have largely been focused on drugs such as statins that control blood lipids, which do modestly reduce disease prevalence, and/or anti-inflammatory strategies targeting macrophages and other immune cells, which to date are unproven. An important goal for the future is to identify the factors and mechanisms that can promote beneficial changes in VSMC phenotype and processes that can either augment or replace these more conventional anti-atherosclerotic therapies.
Acknowledgments
Sources of Funding: This work was supported by British Heart Foundation (BHF) grants RG71070, RG79079 to MRB, FS/13/29/30024 to SS, NIH R01 grants HL057353, HL098538, and HL087867 to GKO, the Cambridge NIHR Biomedical Research Centre, and the BHF Centre and Centre for Research Excellence.
Non-standard Abbreviations and Acronyms
- ACTA2
smooth muscle α-actin
- ApoE
apolipoprotein E
- CDKN2B/p15
cyclin-dependent kinase inhibitor 2B
- ECM
extracellular matrix
- KLF4
Kruppel-like factor 4
- IL-1
Interleukin-1
- LacZ
Beta galactosidase gene product
- MYH11
Smooth muscle cell myosin heavy chain
- NF-κB
Nuclear factor kappa B
- PDGF
Platelet-derived growth factor
- SAβG
Senescence-associated β galactosidase
- SASP
Senescence-associated secretory phenotype
- Sca1
Stem cell antigen1
- SIPS
stress-induced premature senescence
- SM22α/tagln
22-kDa smooth muscle cell (SMC) lineage-restricted protein
- SMC
Smooth muscle cell
- TGF-β
Transforming growth factor-β
- VSMC
Vascular smooth muscle cell
Footnotes
Disclosures: None
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death : A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 5.Oh J, Richardson JA, Olson EN. Requirement of myocardin-related transcription factor-B for remodeling of branchial arch arteries and smooth muscle differentiation. Proc Natl Acad Sci USA. 2005;102:15122–15127. doi: 10.1073/pnas.0507346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci USA. 2005;102:8916–8921. doi: 10.1073/pnas.0503741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Developmental Biology. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 8.Xie WB, Li Z, Shi N, Guo X, Tang J, Ju W, Han J, Liu T, Bottinger EP, Chai Y, Jose PA, Chen SY. Smad2 and myocardin-related transcription factor B cooperatively regulate vascular smooth muscle differentiation from neural crest cells. Circ Res. 2013;113:e76–86. doi: 10.1161/CIRCRESAHA.113.301921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBakey ME, Glaeser DH. Patterns of atherosclerosis: effect of risk factors on recurrence and survival-analysis of 11,890 cases with more than 25-year follow-up. Am J Cardiol. 2000;85:1045–1053. doi: 10.1016/s0002-9149(00)00694-9. [DOI] [PubMed] [Google Scholar]
- 10.McGill HC, Jr, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW, Strong JP. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol. 2000;20:836–845. doi: 10.1161/01.atv.20.3.836. [DOI] [PubMed] [Google Scholar]
- 11.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trigueros-Motos L, Gonzalez-Granado JM, Cheung C, Fernandez P, Sanchez-Cabo F, Dopazo A, Sinha S, Andres V. Embryological-origin-dependent differences in homeobox expression in adult aorta: role in regional phenotypic variability and regulation of NF-kappaB activity. Arterioscler Thromb Vasc Biol. 2013;33:1248–1256. doi: 10.1161/ATVBAHA.112.300539. [DOI] [PubMed] [Google Scholar]
- 13.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Ann Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 14.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 15.Ackers-Johnson M, Talasila A, Sage AP, Long X, Bot I, Morrell NW, Bennett MR, Miano JM, Sinha S. Myocardin regulates vascular smooth muscle cell inflammatory activation and disease. Arterioscler Thromb Vasc Biol. 2015;35:817–828. doi: 10.1161/ATVBAHA.114.305218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida T, Gan Q, Shang Y, Owens GK. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am J Physiol Cell Physiol. 2007;292:C886–895. doi: 10.1152/ajpcell.00449.2006. [DOI] [PubMed] [Google Scholar]
- 17.Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1027–1037. doi: 10.1152/ajpheart.01230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida T, Gan Q, Owens GK. Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol. 2008;295:C1175–1182. doi: 10.1152/ajpcell.00288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-kappaB-dependent mechanisms. Physiol Genomics. 2012;44:417–429. doi: 10.1152/physiolgenomics.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmon M, Gomez D, Greene E, Shankman L, Owens GK. Cooperative Binding of KLF4, pELK-1, and HDAC2 to a G/C Repressor Element in the SM22alpha Promoter Mediates Transcriptional Silencing During SMC Phenotypic Switching In Vivo. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.269811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 26.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 28.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 29.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 30.Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110:1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 31.Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Duner P, Nilsson J, Soehnlein O, Fassler R. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Molec Med. 2012;4:564–576. doi: 10.1002/emmm.201200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez D, Shankman LS, Nguyen AT, Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods. 2013;10:171–177. doi: 10.1038/nmeth.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 34.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 35.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048–2057. doi: 10.1161/CIRCULATIONAHA.110.965202. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Stoneman V, Clarke M, Figg N, Xin HB, Kotlikoff M, Littlewood T, Bennett M. Bone Marrow-Derived Smooth Muscle-Like Cells Are Infrequent in Advanced Primary Atherosclerotic Plaques but Promote Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1291–1299. doi: 10.1161/ATVBAHA.110.218578. [DOI] [PubMed] [Google Scholar]
- 38.Bentzon JF, Sondergaard CS, Kassem M, Falk E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation. 2007;116:2053–2061. doi: 10.1161/CIRCULATIONAHA.107.722355. [DOI] [PubMed] [Google Scholar]
- 39.Bentzon JF, Weile C, Sondergaard CS, Hindkjaer J, Kassem M, Falk E. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 40.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 41.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, Giachelli CM, Parmacek MS, Raines EW, Rusch NJ, Speer MY, Sturek M, Thyberg J, Towler DA, Weiser-Evans MC, Yan C, Miano JM, Owens GK. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckingham ME, Meilhac SM. Tracing cells for tracking cell lineage and clonal behavior. Dev Cell. 2011;21:394–409. doi: 10.1016/j.devcel.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6:21. doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tigges U, Komatsu M, Stallcup WB. Adventitial pericyte progenitor/mesenchymal stem cells participate in the restenotic response to arterial injury. J Vasc Res. 2013;50:134–144. doi: 10.1159/000345524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31:2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 48.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 49.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y, Davison F, Ludewig B, Erdel M, Mayr M, Url M, Dietrich H, Xu Q. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106:1834–1839. doi: 10.1161/01.cir.0000031333.86845.dd. [DOI] [PubMed] [Google Scholar]
- 51.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung IM, Schwartz SM, Murry CE. Clonal architecture of normal and atherosclerotic aorta - Implications for atherogenesis and vascular development. Am J Path. 1998;152:913–923. [PMC free article] [PubMed] [Google Scholar]
- 54.Esner M, Meilhac SM, Relaix F, Nicolas JF, Cossu G, Buckingham ME. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133:737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- 55.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 56.Stemerman MB, Weinstein R, Rowe JW, Maciag T, Fuhro R, Gardner R. Vascular smooth muscle cell growth kinetics in vivo in aged rats. Proc Natl Acad Sci USA. 1982;79:3863–3866. doi: 10.1073/pnas.79.12.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hariri RJ, Hajjar DP, Coletti D, Alonso DR, Weksler ME, Rabellino E. Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol. 1988;131:132–136. [PMC free article] [PubMed] [Google Scholar]
- 58.Moon SK, Cha BY, Kim CH. In vitro cellular aging is associated with enhanced proliferative capacity, G1 cell cycle modulation, and matrix metalloproteinase-9 regulation in mouse aortic smooth muscle cells. Arch Biochem Biophys. 2003;418:39–48. doi: 10.1016/s0003-9861(03)00402-8. [DOI] [PubMed] [Google Scholar]
- 59.O'Brien ER, Alpers CE, Stewart DK, Ferguson M, Tran N, Gordon D, Benditt EP, Hinohara T, Simpson JB, Schwartz SM. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993;73:223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- 60.Bennett M, Evan G, Schwartz S. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel VA, Zhang QJ, Siddle K, Soos MA, Goddard M, Weissberg PL, Bennett MR. Defect in insulin-like growth factor-1 survival mechanism in atherosclerotic plaque-derived vascular smooth muscle cells is mediated by reduced surface binding and signaling. Circ Res. 2001;88:895–902. doi: 10.1161/hh0901.090305. [DOI] [PubMed] [Google Scholar]
- 62.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 63.O'Sullivan M, Scott S, McCarthy N, Shapiro LM, Kirkpatrick PJ, MR B. Differential Cyclin E Expression in human in stent stenosis vascular smooth muscle cells identifies targets for selective anti-restenotic therapy. Cardiovascular Res. 2003;60:673–683. doi: 10.1016/j.cardiores.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL. Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ Res. 1998;82:704–712. doi: 10.1161/01.res.82.6.704. [DOI] [PubMed] [Google Scholar]
- 65.Geng Y, Libby P. Evidence for apoptosis in advanced human atheroma: colocalization with interleukin-1b converting enzyme. Am J Path. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 66.Boyle JJ, Weissberg PL, Bennett MR. Human macrophage-induced vascular smooth muscle cell apoptosis requires NO enhancement of Fas/Fas-L interactions. Arterioscler Thromb Vasc Biol. 2002;22:1624–1630. doi: 10.1161/01.atv.0000033517.48444.1a. [DOI] [PubMed] [Google Scholar]
- 67.Bennett MR. Apoptosis of vascular smooth muscle cells in vascular remodelling and atherosclerotic plaque rupture. Cardiovasc Res. 1999;41:361–368. doi: 10.1016/s0008-6363(98)00212-0. [DOI] [PubMed] [Google Scholar]
- 68.Bauriedel G, Hutter R, Welsch U, Bach R, Sievert H, Luderitz B. Role of smooth muscle cell death in advanced coronary primary lesions: implications for plaque instability. Cardiovasc Res. 1999;41:480–488. doi: 10.1016/s0008-6363(98)00318-6. [DOI] [PubMed] [Google Scholar]
- 69.Kockx MM. Apoptosis in the atherosclerotic plaque - Quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- 70.Bennett MR. Life and death in the atherosclerotic plaque. Curr Opin Lipidol. 2010;21:422–426. doi: 10.1097/MOL.0b013e32833d2bfd. [DOI] [PubMed] [Google Scholar]
- 71.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 72.Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 73.Ihling C, Szombathy T, Nampoothiri K, Haendeler J, Beyersdorf F, Uhl M, Zeiher AM, Schaefer HE. Cystic medial degeneration of the aorta is associated with p53 accumulation, Bax upregulation, apoptotic cell death, and cell proliferation. Heart. 1999;82:286–293. doi: 10.1136/hrt.82.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010;10(Suppl 1):S213–220. doi: 10.1111/j.1447-0594.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- 75.Clarke M, Talib S, Figg N, Bennett M. Vascular smooth muscle cell apoptosis induces IL-1-directed inflammation; effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res. 2010;106:363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- 76.Bennett M, Gibson D, Schwartz S, Tait J. Binding and phagocytosis of apoptotic rat vascular smooth muscle cells is mediated in part by exposure to phosphatidylserine. Circ Res. 1995;77:1136–1142. doi: 10.1161/01.res.77.6.1136. [DOI] [PubMed] [Google Scholar]
- 77.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–1097. doi: 10.1172/JCI70391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 79.Dimri G, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medranos E, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 81.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 82.Carracedo J, Merino A, Briceno C, Soriano S, Buendia P, Calleros L, Rodriguez M, Martin-Malo A, Aljama P, Ramirez R. Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. FASEB J. 2011;25:1314–1322. doi: 10.1096/fj.10-173377. [DOI] [PubMed] [Google Scholar]
- 83.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 84.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 85.Panayiotou AG, Nicolaides AN, Griffin M, Tyllis T, Georgiou N, Bond D, Martin RM, Hoppensteadt D, Fareed J, Humphries SE. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis. 2010;211:176–181. doi: 10.1016/j.atherosclerosis.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 86.Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 87.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci USA. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nzietchueng R, Elfarra M, Nloga J, Labat C, Carteaux JP, Maureira P, Lacolley P, Villemot JP, Benetos A. Telomere length in vascular tissues from patients with atherosclerotic disease. J Nutr Health Aging. 15:153–156. doi: 10.1007/s12603-011-0029-1. [DOI] [PubMed] [Google Scholar]
- 89.Song Y, Shen H, Schenten D, Shan P, Lee PJ, Goldstein DR. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2012;32:103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132:1909–19. doi: 10.1161/CIRCULATIONAHA.115.016457. [DOI] [PubMed] [Google Scholar]
- 91.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res. 2014;114:345–353. doi: 10.1161/CIRCRESAHA.113.300858. [DOI] [PubMed] [Google Scholar]