Abstract
Objectives
Our goal was to describe the neurobehavioral phenotype in mucopolysaccharidosis Type IIIB (MPS IIIB). Parents report that behavioral abnormalities are a major problem in MPS III posing serious challenges to parenting and quality-of-life for both patient and parent. Our previous research on MPS IIIA identified autistic symptoms, and a Klüver-Bucy-type syndrome as indicated by reduced startle and loss of fear associated with amygdala atrophy. We hypothesized that MPS IIIB would manifest similar attributes when assessed with the same neurobehavioral protocol.
Methods
Ten patients with MPS IIIB were compared with 9 MPS IIIA patients, all older than 6. 8 younger children with Hurler syndrome (1H) were chosen as a comparison group for the Risk Room procedure; MPS IH does not directly affect social/emotional function and these younger children were closer to the developmental level of the MPS IIIB group. To examine disease severity, cognitive ability was assessed. Four evaluations were used: the Risk Room procedure (to measure social-emotional characteristics, especially fear and startle responses), the Autism Diagnostic Observation Schedule (ADOS), the Sanfilippo Behavior Rating Scale (SBRS), and amygdala brain volumes calculated from manually-traced MRI images.
Results
The two groups are equivalent in severity and show severe cognitive impairment. On the ADOS, the MPS IIIB patients exhibited the same autistic features as IIIA. The IIIB means differed from MPS IH means on most measures. However, the IIIB group did not approach the Risk Room stranger, like the MPS IH group who kept their distance, but unlike the IIIA group who showed no fear of the stranger. On the SBRS, the MPS IIIB patients were described as more inattentive and more fearful, especially of new people than the MPS IIIA. Onsets of some disease characteristics appeared more closely spaced and slightly earlier in MPS IIIB than IIIA.
Conclusions
On most behavioral measures, MPS IIIB patients did not differ substantially from MPS IIIA patients over age six, demonstrating autistic features and a Klüver Bucy-like syndrome including lack of fear and poor attention. Delay in onset of behavioral symptoms was associated with later diagnosis in two patients. Lack of fear, poor attention, and autistic-like symptomatology are as characteristic of MPS IIIB as they are of MPS IIIA. A possible difference is that the some behavioral abnormalities develop more quickly in MPS IIIB. If this is so, these patients may become at risk for harm and present a challenge for parenting even earlier than do those with MPS IIIA. In future clinical trials of new treatments, especially with respect to quality of life and patient management, improvement of these behaviors will be an essential goal. Because very young patients were not studied, prospective natural history documentation of the early development of abnormal behaviors in MPS IIIB is needed.
Keywords: Sanfilippo Syndrome, Type B; Behavior phenotype; Lack of fear and autistic symptoms
Highlights
-
•
MPS IIIB patients are significantly inattentive and hyperactive
-
•
Autistic-like symptoms of impaired social communication are present in MPS IIIB
-
•
MPS IIIB patients have a Klüver-Bucy-like loss of fear
-
•
MPS IIIB and MPSIIIA who are over six years of age show a similar behavioral phenotype
1. Introduction
Mucopolysaccharidosis type IIIB (MPS IIIB) is an autosomal recessive lysosomal disorder associated with severe behavioral abnormalities and progressive dementia. Of the four subtypes of MPS III, it is reported to be the second most common (approximately 1 in 200,000 births) [1], [2] with the exception of south-eastern European countries, where it may be the most common of MPS III types [3], [4], [5].
It is caused by reduced N-acetyl-alpha-d-glucosaminidase (NAGLU; EC 3.2.1.50) catalytic activity, a necessary metabolic step in degradation of the glycosaminoglycan heparan sulfate, leading to accumulation of heparan sulfate and secondary gangliosides in the central nervous system [6], [7].
Behavioral abnormalities in MPS IIIB have been noted but not described in detail [8], [9]. We have previously reported that, in the classic, severe form of MPS IIIA, many characteristics of autism begin to appear at about 3–4 years of age [10]. We also found that the behaviors in MPS IIIA, including increased orality, reduced startle, and reduced fearfulness, present as a variant of Klüver-Bucy syndrome [11], [12]. We also found a significantly greater rate of amygdala atrophy over time associated with the reduced fearfulness [11].
In the present study, we hypothesized that MPS IIIB would display behavioral abnormalities and rate of appearance of behavioral symptoms similar to those of MPS IIIA. If similar patterns do emerge, it could provide guidance for understanding the natural history of the disease and markers for treatment response. If not, expectations for disease and treatment response will need to be altered for MPS IIIB.
2. Methods
2.1. Participants
2.1.1. MPS IIIB patients
During the recruitment phase, due to low incidence of MPS IIIB, accrual was considerably lower than anticipated, so were unable to recruit younger and less impaired children. Thus, our findings are restricted to older patients with more advanced disease.
Parents of 11 children with MPS IIIB, ages 0.5 years to 32 years, were contacted for this cross-sectional neurobehavioral study from a longitudinal natural history (NH) study sponsored by Shire at the University of Minnesota (NCT01509768). The goal of the longitudinal study was to detail the natural course of MPS IIIB and to identify potential endpoints for future treatment trials.
Patients in the NH study met the following criteria: (a) MPS IIIB diagnosis confirmed by enzyme or genetic assay, (b) developmental age ≥ 12 months on the Vineland Adaptive Behavior Scales, Second Edition [13]. Exclusion criteria were: (a) known hyper-sensitivity to anesthesia, (b) history of ameliorative treatment with any investigational drug or with (c) hematopoietic cell transplant, (d) significant non-MPS IIIB-related central nervous system (CNS) impairment, (e) medications that would significantly interfere with performance, (f) any condition or device precluding MRI, and (g) blindness and/or deafness.
Additional inclusion criteria for this neurobehavioral study were being more than two years old and independently ambulatory, which excluded one child from the NH study who was less than one year old for a total sample size of 10. Although the NH study had European sites as well, the neurobehavioral study was only carried out at the baseline visit to the University of Minnesota.
2.1.2. MPS IIIA comparison group
From a previous natural history study of MPS IIIA (NCT01047306), we matched our sample to 9 patients in a similar age range, all over the age of six. This subsample was part of a larger sample of MPS IIIA whose behavioral data have been reported previously [10], [12].
2.1.3. MPS IH comparison group
In addition, the 8 children with MPS I (Hurler syndrome, or MPS IH) who participated in the MPS IIIA neurobehavioral study were selected as a comparison group for this Risk Room procedure [11]. This younger group was selected as they have a similar level of cognitive development.
2.2. Procedures
2.2.1. Neurocognitive assessment
To determine the phenotypic severity and the equivalency of our two samples, cognitive ability scores were utilized. As part of the NH studies mentioned above, two standard instruments, the Bayley Scales of Infant Development, Edition III (BSID-III) [14] or the Kaufman Assessment Battery for Children, Edition II (KABC-II) [15] were selected that cover the anticipated age and ability range. Age equivalent scores (AEqs) were generated from published normative data [14], [15] and a Development Quotient (DQ) was derived by dividing the AEq by chronological age and then multiplying by 100. This approach avoids the “floor” effects in normative tables that makes scores insensitive for severely cognitively impaired children [16], [17], [18]. The specific test administered was chosen according to an algorithm previously described [19].
2.2.2. Risk Room
The rationale for this laboratory procedure is outlined in our description for the MPS IIIA patients [11]. The Risk Room is a part of the Laboratory-Temperament Assessment Battery [20], which evaluates social/emotional behaviors, and specifically fearfulness, in lower functioning children [20]. To test whether the MPS IIIB patients presented with a similar variant of Klüver-Bucy syndrome and to investigate attachment/compliance issues, we assessed participants' 1) locomotor exploration of the Risk Room, 2) response to attractive and mildly frightening objects in the room (scary Halloween masks and a seated male stranger wearing a ball cap and sunglasses), 3) exposure to a 92-dB startle-noise triggered by the patient's first contact with an attractive toy (the “trigger toy”). This test is based on findings that amygdala dysfunction reduces startle in other animals [21], 4) reunion with their mother after her brief absence, and 5) compliance with her directive to pick up and put away a small toy [22].
2.2.3. Autism Diagnostic Observation Schedule
The ADOS is a semistructured observation tool designed to observe and judge the quality of a child's social communication and play and to assess for the presence of any excessively intense interests or repetitive behaviors [23]. The revised algorithms of the ADOS were used [24]. The ADOS yields total scores for social affect and restricted and repetitive behavior, yielding an overall classification indicating behaviors and symptoms consistent with autism, consistent with milder indications of autism spectrum disorder (ASD), or not consistent with ASD (“nonspectrum”).
2.2.4. Sanfilippo Behavior Rating Scale (SBRS)
Parents completed the 68 items in the SBRS [12]. Details of that measure can also be found at z.umn.edu/sbrs. Four cluster scores and two domain scores are generated as well as parental recall of age of onset of behavioral symptoms. The four cluster scores are Movement, Lack of Fear, Social-Emotional, and Executive Function; a higher score denotes more abnormality in each cluster. Each cluster score contains a number of domains and individual items that make up the domain. Two domains are unique and not included in clusters: “Mood, Anger, and Aggression”, and “Orality” (excessive mouthing of objects).
2.2.5. Amygdala and hippocampus volumes
While the patient was under anesthesia during the baseline visit, MRI was acquired on a 3-Tesla Siemens Trio scanner with sequences that included MPRAGE (magnetization-prepared rapid acquisition with gradient echo). Volumetric analyses included manual tracing of amygdala and hippocampus using Brains2, which allows accurate measurement of three-dimensional representations of structures [25]. Our rater's intra-rater reliability was 0.99 and inter-rater reliability was 0.87 [26].
2.2.6. Statistical analyses
Group data were summarized by means and standard deviations for continuous variables and frequency with percentages for categorical variables. Confidence intervals for estimated means within a group were based on a t-distribution; differences in means were evaluated using a t-test with unequal variance and Welch approximation to the degrees of freedom. Confidence intervals for estimated proportions within a group were determined by inverting the score test; differences in proportions between groups were evaluated with chi-squared tests. Survivor analytic techniques (time-to-event analysis) have been used to address the timing of symptom appearance in disease [27]. All analyses were conducted using R v3.1.1 [28].
3. Results
All MPS IIIB patients tested were Caucasian (as were the comparison groups). 6/10 MPS IIIB and 5/9 MPS IIIA patients were male. The average (SD) age for the MPS IIIB patients was 16.3 (9.5) years and for the MPS IIIA patients was 10.0 (4.5) years. Four patients in the IIIB group were over age 20 (ages 22, 23, 28, and 32); the oldest in the IIIA group was 18.4. We analyzed the data with and without the two patients in the IIIB group who were outliers in age, both female siblings, ages 28 and 32, diagnosed within the past two years. The average age at diagnosis was 89 months in the MPS IIIA group and 119 for the III B group. However with the two older patients removed, the average age at diagnosis for the MPS IIIB group was 64 months.
In order to determine whether the two groups differed in phenotypic severity, we report their age equivalent scores on the BSID or the nonverbal scale of the KABC-II. Results are reported in Table 1 with the outliers removed.
Table 1.
Age and cognitive ability (means (M), standard deviations (SD) and range for MPS IIIB and MPS IIIA.
| MPS IIIB (without outliers) |
MPS IIIA |
|||
|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | |
| Age (years) | 12.94 (7.01) | 6.28–23.95 | 9.95 (4.46) | 6.41–18.33 |
| Cognitive age equivalent (months) | 16.25 (8.40) | 8–28 | 16.89 (12.56) | 6–45 |
| Developmental quotienta | 14.75 (13.00) | 3–37 | 14.79 (7.79) | 3–26 |
Calculated using age equivalent score divided by chronological age (Delaney et al.).
The two outliers in age have a chronological age of 27.77 and 31.70 with cognitive age equivalent scores of 21 and 10 months respectively. These low scores do not rule out the possibility they could have attenuated disease as their diagnosis was so late and no early testing was available. The single child who was not included because the age was below the inclusion for the risk room and ADOS, at 1.69 years had an age equivalent score of 17 months yielding a developmental quotient of 84. This is similar to the range of results of the MPS IIIA patients of the same age [29].
3.1. ADOS
The results on the ADOS were very similar to those of the MPS IIIA group (Table 1). Of the 10 MPS IIIB patients, scores for all but one were in the ADOS range consistent with those on the autism spectrum (12 is the cutoff); higher scores indicate more behaviors associated with autism spectrum disorder). The one patient who did not meet ADOS criteria was 6.7 years old and had an ADOS total score of 9; a 6 year old participant had the next highest score exceeding the cutoff with a score of 16. All others had scores between 16 and 23 compared with the MPS IIIA patients whose range of scores was 12–22. The social/affective domain was more affected than the restricted or repetitive behaviors in both groups.
3.2. Risk Room
3.2.1. Comparison with MPS IH
As expected, the MPS IIIB patients differed significantly from the MPS IH comparison group on four of nine measures (Table 2). With the older two patients removed, three of nine measures were statistically significant and one indicated a trend. Although substantial mean differences were found, lack of significance on several measures is due to variability and small N in both groups.
Table 2.
ADOS Scores, mean (SD), for MPS IIIB (with outliers) and IIIA and difference in means.
| MPS IIIB (n = 9)a | MPS IIIA (n = 9) | Difference (95% CI) | P-value | |
|---|---|---|---|---|
| ADOS SOCIAL | 14.3 (4.4) | 15.6 (4.3) | − 1.2 (− 5.6, 3.1) | 0.56 |
| ADOS BEHAVIORAL | 3.8 (1.1) | 3.2 (1.2) | 0.6 (− 0.6, 1.7) | 0.32 |
| ADOS TOTAL | 18.1 (4.3) | 18.8 (3.5) | − 0.7 (− 4.6, 3.3) | 0.72 |
One patient did not have an ADOS evaluation.
3.2.2. Comparison with MPS IIIA
Only a few mean differences are noted overall (p values are in Table 3).
Table 3.
Mean/percent with 95% confidence intervals of risk room scores across groups.
| Variable | MPS IIIB |
MPS IIIA |
MPS IH |
P-value |
P-value |
MPS III Ba |
P-value |
P-value |
|---|---|---|---|---|---|---|---|---|
| (N = 10) | (N = 9) | (N = 8) | A vs B | IH vs. B | (N = 8) | A vs Ba | IH vs. Ba | |
| Move away latency (seconds) | 12.0 (9.0, 15.0) |
25.6 (1.5, 49.6) |
161.7 (0.0, 399.5)2 |
0.232 | 0.167 | 12.5 (8.6, 16.4) |
0.249 | 0.168 |
| Percent time in locomotion | 62.0 (38.7, 85.3) |
59.7 (33.2, 86.2) |
10.0 (0.0, 22.3)2 |
0.882 | < 0.001 | 53.8 (27.7, 79.8) |
0.715 | 0.005 |
| Percent time next to mother | 27.4 (11.3, 43.5) |
28.0 (3.4, 52.6) |
71.5 (41.7101.3)2 |
0.963 | 0.010 | 30.8 (10.7, 50.8) |
0.843 | 0.018 |
| Touch mask (%) | 70.0 (35.4, 91.9) |
77.8 (40.2, 96.1) |
0.0 (0.0, 48.3)2 |
1.000 | 0.027 | 62.5 (25.9, 89.8) |
0.875 | 0.064 |
| Approach stranger (%) | 30.0 (8.1, 64.6) |
66.7 (30.9, 91.0) |
33.3 (6.0, 75.9)2 |
0.255 | 1.000 | 37.5 (10.2, 74.1) |
0.474 | 1.000 |
| Startle (%) | 30.0 (8.1, 64.6) |
33.3 (9.0, 69.1) |
75.0 (35.6, 95.5) |
1.000 | 0.155 | 37.5 (10.2, 74.1) |
1.000 | 0.313 |
| Hold/return to trigger toy (%) | 60.0 (27.4, 86.3) |
77.8 (40.2, 96.1) |
12.5 (0.7, 53.3) |
0.735 | 0.117 | 62.5 (25.9, 89.8) |
0.875 | 0.121 |
| Reunion scoreb | 1.7 (0.9, 2.5) |
2.8 (1.9, 3.6) |
3.7 (3.1, 4.2)2 |
0.052 | < 0.001 | 1.9 (0.8, 2.9) |
0.137 | 0.004 |
| Clean-up compliance (%) | 40.0 (13.7, 72.6) |
22.2 (3.9, 59.8) |
87.5 (46.7, 99.3) |
0.735 | 0.117 | 50.0 (21.5, 78.5) |
0.492 | 0.281 |
Superscript in MPS IH column denotes number missing.
Designates group with outliers (older patients) removed.
0 = ignore mother, 1 = look at mother, 2 = talk to mother without approaching, 3 = approach mother, 4 = make physical contact/hug.
3.2.3. Exploration
The MPS IIIB patients were similar to MPS IIIA and differed notably from the IH patients in exploration of the room and proximity to their mothers. The IIIB patients spent an average of 62% of their time walking around the room (53.8% with the oldest two patients removed), similar to 59.7% for IIIA, compared to 10% for the MPS IH group. The MPS IIIB patients did not differ significantly from the IIIA patients in time spent next to their mother (30.0% and 28.0%, respectively) compared to 71.5% for MPS IH. Similarly 70.0% of the IIIB patients touched a scary mask at least once compared to 77.8% for IIIA and none for the MPS IH group. Removing the older patients did not change the significance values for time next to mother or touching the mask. However, fewer MPS IIIB patients (30%) approached the stranger than did the MPS IIIA group (37.5% with older patients removed) compared to 66.7 although the differences were not statistically significant. The MPS IIIB was similar to the MPS IH group, only 33.3% of whom approached.
3.2.4. Response to startle noise and trigger toy
The proportion of the MPS IIIB patients who startled to the loud noise (30%) was similar to that of the previously tested the IIIA group (33.3%), but significantly less than the 75.0% of the MPS IH patients who startled. Sixty percent of the IIIB group returned to the trigger-toy following the noise compared to 78% of the IIIA group. 12.5% of the MPS IH group returned to the trigger-toy. Removing the two older patients did not substantially change these results.
3.2.5. Reunion score and compliance
During the 1–2 min following the mother's return from her brief exit, the IH group showed the most response to her, the IIIA group showed less response while the IIIB patients acknowledged her the least. When asked to pick up and put away a toy, the IIIB group (40%) was more compliant than the IIIA group (22.2%), but not as compliant as the IH group (87.5%). With the two older patients removed, 50% percent of the IIIB group demonstrated compliance.
3.3. SBRS
3.3.1. Level of behavioral impairment
The MPS IIIB group was similar to the IIIA group in the frequency of items endorsed on three of the four clusters: Movement, Lack of Fear, and Executive Function (Table 4). On the Social-Emotional Cluster, the IIIB patients tended to score higher than IIIA patients, although this trend was nonsignificant. The two MPS III groups did not differ from each other on the two domains, Orality and Mood/Anger/Aggression.
Table 4.
Results from SBRS (range of scores are from 1 to 6 with higher numbers being more abnormal) comparing results from MPS IIIB with MPS IIIA.
| MPS IIIB |
MPS IIIA |
P-value |
MPS IIIBa |
P-value |
|
|---|---|---|---|---|---|
| (N = 10) | (N = 8)b | (N = 8) | |||
| Cluster | |||||
| Movement cluster | 2.2 (0.7) | 2.1 (0.9) | 0.684 | 2.2 (0.8) | 0.766 |
| Lack of fear cluster | 2.3 (0.7) | 2.7 (0.8) | 0.222 | 2.3 (0.7) | 0.261 |
| Social emotional cluster | 2.4 (0.6) | 1.9 (0.6) | 0.151 | 2.3 (0.6) | 0.242 |
| Executive function cluster | 3.6 (1.1) | 3.4 (1.1) | 0.656 | 3.5 (1.2) | 0.861 |
| Unique domains (not in cluster) | |||||
| Mood, Anger, Aggression | 1.5 (1.0) | 2.1 (1.1) | 0.223 | 1.6 (1.1) | 0.320 |
| Orality | 2.5 (1.4) | 3.1 (1.7) | 0.432 | 2.3 (1.5) | 0.346 |
| Domains of interest (within clusters) | |||||
| Safety Consciousness | 3.1 (1.2) | 3.9 (1.4) | 0.223 | 3.3 (1.2) | 0.349 |
| Emotional function | 2.2 (0.9) | 1.5 (0.9) | 0.132 | 2.1 (0.9) | 0.241 |
| Attention | 4.2 (0.7) | 3.5 (0.6) | 0.030 | 4.2 (0.8) | 0.064 |
| Items of interest (in domains/cluster) | |||||
| 26. Goes into unsafe areas | 3.4 (1.7) | 5.1 (1.2)1 | 0.027 | 3.2 (1.9) | 0.039 |
| 28. Less fearful | 2.1 (1.5) | 3.6 (1.6) | 0.058 | 2.1 (1.7) | 0.093 |
| 32. Fearful of new people | 1.2 (1.5)2 | 0.2 (0.7) | 0.117 | 1.0 (1.7) | 0.340 |
| 53. Does not pay attention | 3.9 (1.1) | 2.9 (1.2)1 | 0.095 | 3.9 (1.2) | 0.134 |
Superscripts denote number missing.
Designates group with 2 outliers (older patients) removed.
One patient did not complete the SBRS.
We examined the domains within each cluster, and found that within the Lack of Fear Cluster, the Safety Consciousness domain overall was equally impaired in the IIIB group (with or without the older patients) and the IIIA groups. Within the Social-Emotional Cluster, Emotional Functioning was slightly more impaired in the IIIB group with and without the older patients suggesting more emotional disruption. Within the Executive Function Cluster, the Attention domain was significantly more impaired in the IIIB group. After removing the older two patients, the means remained the same but a statistically significant difference shifted to a trend.
Parent ratings for individual items indicated that IIIB patients were significantly less likely to go into unsafe areas and scored lower for lack of fear than did IIIA patients, but IIIB patients were described as slightly more fearful of new people. Again a trend was present for attention problems to be endorsed more by parents of MPS IIIB patients. When examining the data for the group of IIIB patients without the two older patients, the mean differences did not change. However, because of the lower N, p values changed when these two patients were eliminated.
3.3.2. Ages of symptom onsets
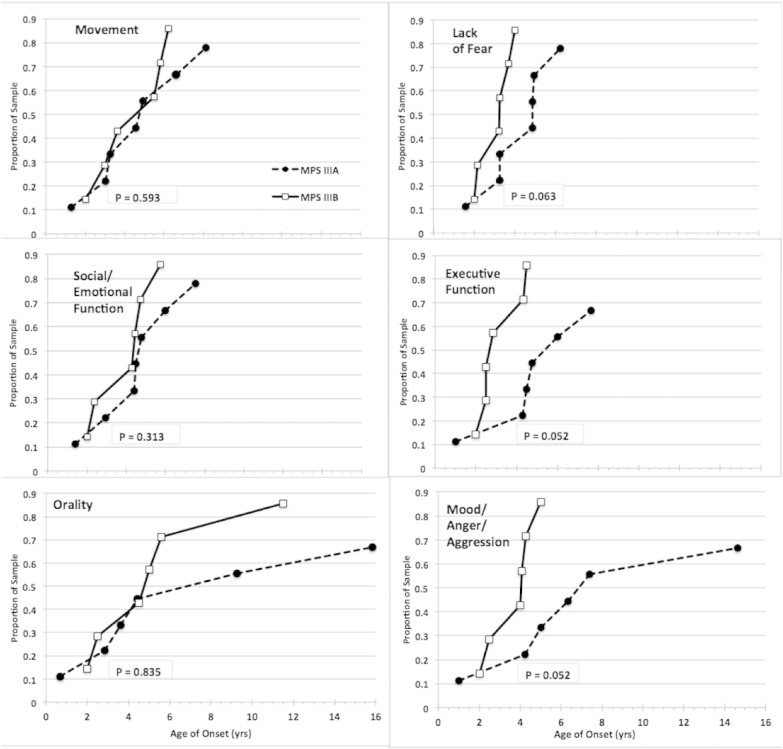
When parents recalled the age of onset of behavioral symptoms, the IIIB patients were described as having a later onset on average than IIIA patients in almost every domain (Table 5). However, when the two older patients were removed, this difference disappeared. Data for 6 MPS IIIB patients (without the oldest two) and 7 MPS IIIA patients were available for a time-to-event analysis of age of onset. Fig 1 plots the successive ages of onset of each behavior characteristic within each group and compares the relative rate of appearance of each characteristic between the two groups. The time-to-event intervals for Executive function and Mood/anger/aggression were shorter within the IIIB group than within the IIIA group. Lack of Fear showed a trend in the same direction.
Table 5.
Age of onset of symptoms in years from SBRS ratings by parents.
| MPS IIIB (N = 10) |
MPS IIIBa (N = 8) |
MPS IIIA (N = 8)b |
||||
|---|---|---|---|---|---|---|
| Mean (SD) age of onset | Mean (SD) age of onset | Mean (SD) age of onset | ||||
| Movement cluster | 8.22 | (6.97) | 4.67 | (2.03) | 4.14 | (3.00) |
| Social-emotional cluster | 8.69 | (8.12) | 4.33 | (2.63) | 4.62 | (3.19) |
| Lack of fear cluster | 5.60 | (4.46) | 3.66 | (1.73) | 3.90 | (2.52) |
| Executive function cluster | 5.26 | (4.05) | 3.89 | (2.26) | 4.56 | (2.97) |
| Domains: | ||||||
| Mood, anger and aggression | 6.40 | (5.17) | 4.38 | (1.36) | 5.78 | (4.08) |
| Orality | 10.64 | (10.11) | 5.08 | (3.65) | 6.43 | (6.38) |
| Mean across all areas | 7.47 | (6.48) | 4.33 | (2.28) | 4.91 | 3.69 |
9.3% of missing values for age of onset in MPS IIIB and 21.2% missing values for MPS IIIA.
Designates group with outliers (older patients) removed.
Sample size is 8 due to one patient missing SBRS.
Fig. 1.
Age of onset data from SBRS as recalled by parents.
Comparison of MPS IIIA and IIIB within-group time-to-event plots for onsets of six behavior clusters and domains. The same age scale is used in all plots. Final sample proportions are < 1.0 due to missing data. Between group differences in the right hand plots show a trend or are significant.
3.4. Amygdala and hippocampus volumes
No meaningful or statistical differences were found in baseline amygdala and hippocampus volumes between IIIB and IIIA patients (Table 6).
Table 6.
Hippocampus and amygdala volumes in ml.
| MPS IIIB (n = 9) | MPS IIIA (n = 9) | |
|---|---|---|
| Left hippocampus | 1.83 (0.29) | 1.84 (0.29) |
| Right hippocampus | 1.89 (0.23) | 1.91 (0.26) |
| Left amygdala | 1.01 (0.11) | 1.04 (0.23) |
| Right amygdala | 1.04 (0.09) | 1.07 (0.24) |
4. Discussion
MPS III is characterized by progressive neurodegeneration and dementia, with death typically occurring in the second decade of life [1]. In MPS IIIA, symptoms become apparent between 2 and 6 years of age, although diagnosis often lags behind the appearance of the earliest symptoms [30]. We have previously reported that MPS IIIA has a characteristic behavioral pattern consisting of autism-like symptoms and what appears to be a variant of Klüver-Bucy Syndrome including orality, reduced startle and fearfulness, and associated with atrophy in amygdala volume [10], [11], [12].
Our primary objectives were to determine how patients with MPS IIIB differ behaviorally from those with MPS IH and, more specifically, from those with IIIA, especially with regard to autistic and Klüver-Bucy symptoms. We were able to characterize the behavior of the MPS IIIB participants in our group; however, our recruited sample was older and showed more disease progression than we anticipated. We were able to select an older group of patients with MPS IIIA in a more comparable age range, which allowed us to compare behavioral symptoms after age 6 between these two disease groups. However, low incidence of MPS IIIB in the U.S. and low accrual in this study, limited our ability to characterize the behavioral course of the disease for patients under six.
In order to ensure that our patients did not have an attenuated phenotype and that they were equivalent in stage of disease, we compared them on measures of cognitive development. Both groups were profoundly cognitively impaired. The two outliers were similarly impaired, but as they were diagnosed later than any other patient in our samples, we did not include them in our comparison. Clearly compared to the patients described by Valstar [31], our sample did not fall into the attenuated group. We surmised that phenotypic cognitive data would be more predictive than mutation or enzyme levels in categorizing our patients as severe or not.
Our samples of MPS IIIA and IIIB did not differ significantly on the ADOS. Both had impaired social communication. As in MPS IIIA, restricted interests, behavioral rigidity, and repetitive behaviors were not as prominent as in individuals with a diagnosis of autism. All but one patient in the MPS IIIB group exceeded the ADOS cutoff, placing their scores in the range consistent with autism (all MPS IIIA patients had exceeded the cutoff score). The two older patients were similar to the other MPS IIIB patients. The phenotypic autistic-like behaviors of both MPS IIIA and B may result in misdiagnosis. Nonetheless, management techniques appropriate for the ASD disorders may be appropriate for MPS III [10].
In the Risk Room procedure, MPS IIIB patients on average initially moved away from their mothers and into the room at the same rate as the IIIA patients, but much more quickly than the MPS IH patients. Also, the total duration of time MPS IIIB patients spent next to their mothers was similar to that of the IIIA patients but significantly less than that of the MPS IH patients suggesting that the older age in both groups (and presumably greater developmental independence) of the MPS III patients could be a significant factor. With the oldest two patients removed, these group means did not change much. Those with MPS IIIB moved around the Risk Room and touched a frightening mask at the same high rates and startled to a loud noise at the same low rates, as the IIIA patients, suggesting that they, too, show Klüver-Bucy symptoms, and possible amygdala involvement [32], [33], [34], [35], [36]. They again differed significantly from the MPS IH patients.
However, the MPS IIIB patients were less like MPS IIIA patients and more similar to the MPS IH patients in their less frequent approach to the stranger even though the difference between MPS IIIB and IIIA is not statistically significant. Note that this is consistent with parental report of significantly more fear of new people in the IIIB group on the SBRS rating scale (see below). Interestingly, despite greater parent-reported attention concerns on the SBRS the MPS IIIB patients were, on average, somewhat more compliant in picking up toys than the IIIA group, although not as compliant as the MPS IH group.
On the SBRS MPS IIIB patients were reported to show slightly less general “lack of fear” than IIIA, consistent with Risk Room findings. Also consistent with this, the MPS IIIA patients were reported to go into unsafe areas more frequently, and the MPS IIIB patients were reported to be more fearful of new people. The consistency between parental reports of stranger fear and patients' behavior observed in the risk room is noteworthy.
SBRS scores also suggest that MPS IIIB patients have significantly more attention difficulties than IIIA. The Mood, Anger and Aggression domain scores were slightly higher in the IIIA patients, and the highest scores overall for both groups were in the Executive Function Cluster indicating significant problems in attention and compliance for both MPS IIIA and IIIB. No significant difference between groups was found in either Movement or Executive Function Clusters. While there were mean differences in the Lack of Fear cluster and differences on specific items, large variability contributed to lack of statistical significance, Similarly, while oral exploration means were higher in IIIA patients, the large variability in these behaviors precludes interpretation.
Examining the entire MPS IIIB group, the SBRS domains showed a later age of onset based on this retrospective data. However, this result was solely the consequence of the age of onset in the two oldest patients; when they are removed this difference disappeared and tended to reverse. These two patients were not only older than the other patients but they were diagnosed much later. It is likely that these two patients represent a different rate of progression than the other patients. By parental report, onset of symptoms other than executive functions (attention) and lack of fear for these two patients was after age 10 with some first presentations as late as their twenties. However, we caution that this is retrospective data in older patients, so a long time has elapsed between symptom onset and caregiver report. When plots of symptom onsets in the remaining MPS IIIB patients were compared to onsets in MPS IIIA (Fig. 1), some disease characteristics appeared to present more rapidly and occur slightly earlier across the IIIB group. Onsets were more rapid for Executive Function and Mood/anger/aggression, with a trend in that direction for Lack of Fear.
There is growing interest in the significance of age at onset of disease, e.g., disorders being more severe, persistent and less amenable to treatment when they appear earlier rather than later in childhood [37], [38], [39]. In some cases, earlier age of onset of the first disease symptom is associated with the earlier appearance of the full range of disease symptoms [38].
Note that for both groups, but especially MPS IIIA patients, some age of onset data were missing. Parents' poor memory for behavior onset may be why they were reluctant to complete this portion of the questionnaire. While missing data may bias these results, which should certainly be viewed as exploratory, they do demonstrate the need for prospective natural history data in the youngest MPS IIIB patients to clarify ages of symptom onset.
Overall, our results suggest that in those over 6 years old, MPS IIIB patients, like MPS IIIA patients, do not fear scary objects but are slightly more fearful of strange people. However, differences in these behaviors presumably related to Klüver-Bucy syndrome are not reflected in their respective amygdala volumes, which are similar in both groups. We also have exploratory evidence of more attention difficulties in the IIIB patients.
Our two oldest patients demonstrate a different course that has a later onset and progresses more slowly, although it is ultimately as severe. Such patients are likely to be identified as having another disorder, such as autism or attention deficit hyperactivity disorder, before their cognitive decline is noticed. For this reason their diagnostic odyssey may be more protracted and locating these patients is more difficult. Misdiagnosis leading to delayed identification of MPS III disorders has been previously documented [40].
We conclude that behavioral patterns in MPS IIIB and IIIA patients over age 6 do differ in a few areas but both demonstrate symptoms associated with autism and Klüver-Bucy syndrome. However, because we were unable to recruit younger patients, unanswered questions remain about the onset and course of the behavioral presentation prior to age 6. In our MPS IIIB sample, and even in the oldest two patients symptom severity does not appear to be attenuated, unlike in the European sample previously described [31].
We caution that because this is an exploratory study, some mean differences in the results we describe lack statistical significance. In rare diseases, lack of power to detect significant effects is a frequent problem. A larger international sample using a multicenter approach is needed to verify these exploratory results. Clearly, longitudinal research is needed, particularly in parts of the world where MPS IIIB is more prevalent.
Funding
-
•
Shire supported this study through an investigator initiated research grant “Characterizing the neurobehavioral phenotype of MPS IIIB,” M. Potegal, P.I. and through a natural history clinical trial “A Longitudinal, Prospective, Natural History Study of Patients with Sanfilippo Syndrome Type A (MPS IIIA)” NCT01047306, C.B. Whitley, P.I.
-
•
The Lysosomal Disease Network (U54NS065768) supported this study through a pilot study called “Characterizing the neurobehavioral phenotype of MPS III,” M. Potegal, P.I. The Lysosomal Disease Network (U54NS065768) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS and the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
-
•
This project was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, UMN-CTSI (UL1TR000114 Dr. Rudser). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Acknowledgements
We thank the participants for their cooperation in this study.
We are also grateful to the Center for Neurobehavioral Development, the Center for Magnetic Resonance Research, and the Minnesota Supercomputer Center for the provision of infrastructure for this research.
References
- 1.Heron B., Mikaeloff Y., Froissart R. Incidence and natural history of mucopolysaccharidosis type III in France and comparison with United Kingdom and Greece. Am. J. Med. Genet. 2011;155A:58–68. doi: 10.1002/ajmg.a.33779. [DOI] [PubMed] [Google Scholar]
- 2.Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Poorthuis B.J., Wevers R.A., Kleijer W.J. The frequency of lysosomal storage diseases in The Netherlands. Hum. Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 4.Pinto R., Caseiro C., Lemos M. Prevalence of lysosomal storage diseases in Portugal. Eur. J. Hum. Genet. 2004;12:87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 5.Beesley C., Moraitou M., Winchester B. Sanfilippo B syndrome: molecular defects in Greek patients. Clin. Genet. 2004;65:143–149. doi: 10.1111/j.0009-9163.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 6.Ellinwood N.M., Wang P., Skeen T. A model of mucopolysaccharidosis IIIB (Sanfilippo syndrome type IIIB): N-acetyl-α-d-glucosaminidase deficiency in Schipperke dogs. J. Inherit. Metab. Dis. 2003;26:489–504. doi: 10.1023/a:1025177411938. [DOI] [PubMed] [Google Scholar]
- 7.Li H.H., Yu W.-H., Rozengurt N. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding α-N-acetylglucosaminidase. Proc. Natl. Acad. Sci. 1999;96:14505–14510. doi: 10.1073/pnas.96.25.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bax M.C., Colville G.A. Behaviour in mucopolysaccharide disorders. Arch. Dis. Child. 1995;73:77–81. doi: 10.1136/adc.73.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valstar M.J., Ruijter G.J.G., Van Diggelen O.P., Poorthuis B.J., Wijburg F.A. Sanfilippo syndrome: a mini-review. J. Inherit. Metab. Dis. 2008;31:240–252. doi: 10.1007/s10545-008-0838-5. [DOI] [PubMed] [Google Scholar]
- 10.Rumsey R., Rudser K., Delaney K. Acquired autistic behaviors in children with MPSIIIA. J. Pediatr. 2014;164:1147–1151. doi: 10.1016/j.jpeds.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potegal M., Yund B., Delaney K. Mucopolysaccharidosis Type IIIA presents as a variant of Klüver–Bucy Syndrome. J. Clin. Exp. Neuropsychol. 2013;35:608–616. doi: 10.1080/13803395.2013.804035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro E., Nestrasil I., Ahmed A. Quantifying behaviors of children with Sanfilippo syndrome: the Sanfilippo Behavior Rating Scale. Mol. Genet. Metab. 2015;114:594–598. doi: 10.1016/j.ymgme.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparrow S.S., Cicchetti D.V. Diagnostic uses of the Vineland Adaptive Behavior Scales. J. Pediatr. Psychol. 1985;10:215–225. doi: 10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- 14.Bayley N (2006) Bayley Scales of Infant and Toddler Development - 3rd ed. San Antonio, TX: Psychological Corporation.
- 15.Kaufman A.S. second ed. American Guidance Service; Circle Pines MN: 2004. Manual for the Kaufman Assessment Battery for Children. (KABC-II) [Google Scholar]
- 16.Volkmar F.R., Sparrow S.S., Goudreau D., Cicchetti D.V., Paul R., Cohen D.J. Social deficits in autism: an operational approach using the Vineland Adaptive Behavior Scales. J. Am. Acad. Child Adolesc. Psychiatry. 1987;26:156–161. doi: 10.1097/00004583-198703000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Staba S.L., Escolar M.L., Poe M. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N. Engl. J. Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 18.Peters C., Balthazor M., Shapiro E.G. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 19.Delaney K., Rudser K., Yund B. Methods in neuropsychological assessment in children with neurodegenerative disease: Sanfilippo syndrome. J. Inherit. Metab. Dis. Rep. 2013 doi: 10.1007/8904_2013_269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buss K.A., Goldsmith H.H. University of Wisconsin, Department of Psychology; Madison: 2000. Manual and normative data for the Laboratory Temperament Assessment Battery—Toddler Version (Tech. Rep.) [Google Scholar]
- 21.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 22.Roberts M.W. Clinic observations of structured parent–child interaction designed to evaluate externalizing disorders. Psychol. Assess. 2001;13:46–58. [PubMed] [Google Scholar]
- 23.Lord C., Rutter M., DiLavore P., Risi S. Western Psychological Services; Los Angeles (CA): 1999. Autism Diagnostic Observation Schedule. [Google Scholar]
- 24.Gotham K., Risi S., Pickles A., Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J. Autism Dev. Disord. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 25.Magnotta V.A., Harris G., Andrease N.C., O'Leary D.S., Yuh W.T., Heckel D. Structural MR image processing using the BRAINS2 tool- box. Comput. Med. Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A., Nestrasil I., Rudser K., Shapiro E.G. Reliability of manual and automated tracing of hippocampal volumes in MPS patients and normal controls: A report of the neuroimaging core of the lysosomal disease network. Mol. Genet. Metab. 2010;99:S9. [Google Scholar]
- 27.Cupples L.A., Terrin N.C., Myers R.H., D'Agostino R.B., Rao D.C. Using survival methods to estimate age-at-onset distributions for genetic diseases with an application to Huntington disease. Genet. Epidemiol. 1989;6:361–371. doi: 10.1002/gepi.1370060206. [DOI] [PubMed] [Google Scholar]
- 28.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: a language and environment for statistical computing. (URL http://www.R-project.org/) [Google Scholar]
- 29.Shapiro E., Nestrasil I., Delaney K. A prospective natural [2] history study of Mucopolysaccharidosis Type IIIA. J. Pediatr. 2016 doi: 10.1016/j.jpeds.2015.11.079. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleary M.A., Wraith J.E. Management of mucopolysaccharidosis type III. Arch. Dis. Child. 1993;69:403–406. doi: 10.1136/adc.69.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valstar M.J., Bruggenwirth H.T., Olmer R. Mucopolysaccharidosis type IIIB may predominantly present with an attenuated clinical phenotype. J. Inherit. Metab. Dis. 2010;33:759–767. doi: 10.1007/s10545-010-9199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tranel D., Gullickson G., Koch M., Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cogn. Neuropsychiatry. 2006;11:219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- 33.Wilensky A.E., Schafe G.E., Kristensen M.P., LeDoux J.E. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meunier M., Bachevalier J., Murray E.A., Málková L., Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur. J. Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 35.LaBar K.S., Gatenby J.C., Gore J.C., LeDoux J.E., Phelps E.A. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 36.Angrilli A., Mauri A., Palomba D. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- 37.Kessler R.C., Amminger G.P., Aguilar-Gaxiola S., Alonso J., Lee S., Ustun T.B. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiatry. 2007;20:359. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Queiro R., Tejón P., Alonso S., Coto P. Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology. 2014;53:1178–1185. doi: 10.1093/rheumatology/ket363. [DOI] [PubMed] [Google Scholar]
- 39.Sproule D.M., Hasnain R., Koenigsberger D., Montgomery M., Darryl C., Kaufmann P. Age at disease onset predicts likelihood and rapidity of growth failure among infants and young children with spinal muscular atrophy types 1 and 2. J. Child Neurol. 2012;27:845–851. doi: 10.1177/0883073811415680. [DOI] [PubMed] [Google Scholar]
- 40.Wijburg F.A., Wegrzyn G., Burton B.K., Tylki-Szymanska A. Mucopolysaccharidosis type III (Sanfilippo syndrome) and misdiagnosis of idiopathic developmental delay, attention deficit/hyperactivity disorder or autism spectrum disorder. Acta Paediatr. 2013;102:462–470. doi: 10.1111/apa.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]