Abstract
The remarkable plasticity and plethora of biological functions performed by macrophages have enticed scientists to study these cells in relation to atherosclerosis for more than 50 years, and major discoveries continue to be made today. It is now understood that macrophages play important roles in all stages of atherosclerosis, from initiation of lesions and lesion expansion, to necrosis leading to rupture and the clinical manifestations of atherosclerosis, to resolution and regression of atherosclerotic lesions. Lesional macrophages are derived primarily from blood monocytes, although recent research has shown that lesional macrophage-like cells can also be derived from smooth muscle cells. Lesional macrophages take on different phenotypes depending on their environment and which intracellular signaling pathways are activated. Rather than a few distinct populations of macrophages, the phenotype of the lesional macrophage is more complex and likely changes during the different phases of atherosclerosis and with the extent of lipid and cholesterol loading, activation by a plethora of receptors, and metabolic state of the cells. These different phenotypes allow the macrophage to engulf lipids, dead cells, and other substances perceived as danger signals; efflux cholesterol to HDL; proliferate and migrate; undergo apoptosis and death; and secrete a large number of inflammatory and pro-resolving molecules. This review article, part of the Compendium on Atherosclerosis, discusses recent advances in our understanding of lesional macrophage phenotype and function in different stages of atherosclerosis. With the increasing understanding of the roles of lesional macrophages, new research areas and treatment strategies are beginning to emerge.
Keywords: Atherosclerosis, Cholesterol, Cytokine, Foam cells, Macrophage
Introduction
Atherosclerosis is initiated by the subendothelial retention of apolipoprotein B-containing lipoproteins, which then triggers a maladaptive, non-resolving inflammatory process that over time drives disease progression1-4. Although the presence of inflammatory cells in lesioned arteries was described as early as in the 1800’s by Rudolph Virchow and others (for a historical perspective see5), it took decades for the role of inflammation in the pathophysiology of atherosclerosis progression to be fully appreciated. The major immune cell in atherosclerotic lesions is the macrophage, the primary origin of which is myeloid progenitor cells in bone marrow. Myeloid progenitor cells develop into circulating monocytes, and in certain setting, the spleen acts as a reservoir for monocytes infiltrating atherosclerotic lesions, at least in the mouse6. Interestingly, the production of monocytes in the bone marrow is stimulated by several cardiovascular risk factors, including hypercholesterolemia, leading to monocytosis (an increased number of circulating monocytes)7,8, which itself is an independent risk factor for atherosclerotic disease9. Circulating monocytes then enter sites of arterial hemodynamic stress by adhering to the endothelial cells lining the lumen of susceptible arteries10. Once the monocytes have entered the subendothelial space, they differentiate to lesional macrophages11.
In addition, it is now recognized that a population of myeloid cells that have features and markers of both dendritic cells and macrophages are present in the artery wall in pre-lesional, susceptible arterial sites in humans, rabbits and mice11. It is not known if these cells help promote atherogenesis, although a pro-atherogenic role seems likely given that the number of these early cells correlates with susceptibility of atherosclerosis and that they can accumulate lipid and proliferate12.
In this part of the Compendium, we will focus on lesional macrophage phenotype and function and the contribution of macrophages to the different stages of atherosclerosis. Examples of different stages of mouse and human atherosclerotic lesions and the presence of macrophages are shown in Figure 1.
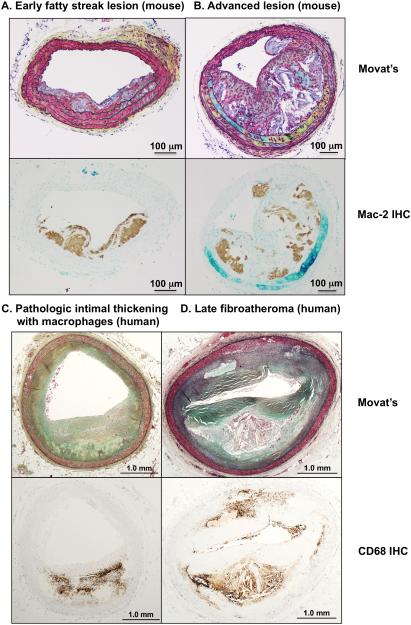
Figure 1. Cross-sections of different stages of lesions.
A-B. Cross-sections of lesions in the brachiocephalic artery of Ldlr−/− mice fed a high-fat diet. A. Early fatty streak lesion with some smooth muscle cell involvement. B. Advanced lesion containing large necrotic cores in macrophage-rich areas. Macrophages are visualized by anti-Mac-2 immunohistochemistry (brown reaction product) on adjacent sections. C-D. Cross-sections of lesions of human coronary arteries. C. Pathologic intimal thickening with macrophages. D. Human advanced lesion (late fibroatheroma with macrophages and necrotic core). Macrophages are visualized by anti-CD68 immunohistochemistry (brown reaction product) on adjacent sections. Panels C-D are from Atherosclerosis, 241, Otsuka et al.140 Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study, pages 772-82, Copyright (2015), with permission from Elsevier. Sections were stained by Movat’s pentachrome. Scale bar, 100 μm (A-B); 1 mm (C-D).
Lesional macrophages take on different phenotypes depending on microenvironmental cues and activated intracellular signaling pathways
Serving to defend the organism from infection, macrophages have developed remarkable plasticity, notably, the ability to promote inflammation when needed and to turn the inflammatory response off when it is no longer needed. Thus, macrophages have the ability to assume inflammatory properties or inflammation-suppressing and reparative properties that aid in the resolution phase of inflammation. Without this plasticity, an organism would either not be able to effectively fight infection or would be unable to heal after infection. In experimental systems, the classical inflammatory macrophage phenotype has been termed M1 (analogous to the T cell nomenclature Th1) and is often induced by incubating macrophages in vitro with a combination of interferon-γ (IFN-γ) and the toll-like receptor 4 (TLR4) ligand lipopolysaccharide (LPS). LPS is a component of the cell wall of Gram-negative bacteria, whereas IFN-γ is produced primarily by natural killer cells and Th1 T cells following infection. When a resting macrophage encounters these stimuli, it initiates a strong inflammatory program, which includes production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-12 and tumor necrosis factor-α (TNF-α), chemokines, such as monocyte chemoattractant protein-1 (MCP-1 or CCL2) to attract more monocytes, and inducible nitric oxide synthase (iNOS). The latter produces large quantities of nitric oxide, which helps kill pathogens. In addition, these macrophages produce reactive oxygen species through NADPH oxidase activation, which aid in pathogen destruction. Thus, the purpose of this inflammatory response is to markedly promote inflammation and kill pathogens.
Several subsets of alternatively activated macrophage populations, termed M2 (analogous to Th2 T cells) macrophages, have also been identified in vitro. The M2 phenotype can be induced by incubating macrophages with IL-4 and IL-13, both of which inhibit the M1 phenotype and prompt the macrophage to produce pro-resolving molecules, such as IL-10 and transforming growth factor-β (TGF-β). IL-4 and IL-13 are produced by Th2 T cells and are involved in tissue remodeling and repair. Moreover, IL-4 is a strong stimulus for macrophage proliferation13.
The M1 and M2 classification of macrophage phenotypes is based on in vitro model systems with unknown relevance to in vivo states, and pure M1 and M2 macrophages almost certainly do not occur in atherosclerotic lesions, where macrophages are exposed to a plethora of stimuli that will result in different macrophage functions and cell surface markers. Important attempts have been made to define a framework and consensus markers to describe macrophage activation14, but more work is needed to characterize macrophage phenotypes, especially in vivo. The atherosclerotic lesional environment has been characterized as Th1-dominant based on studies with advanced human endarterectomy lesions, where IFN-γ is more abundant than IL-415. However, the M1/M2 paradigm is now being reassessed16.
Several other macrophage populations have been suggested to be present in atherosclerotic lesions. These include the M(Hb) and Mhem populations17, which are resistant to lipid loading and are induced by exposure to haemoglobin–haptoglobin complexes and haem in vitro, respectively; the Mox macrophage population, which is induced by exposure to oxidized phospholipids in vitro and is characterized by high expression of heme oxygenase-118; and the M4 population, which is induced by the chemokine CXCL419. Recently, IL-17A-stimulated macrophages were suggested to constitute a new macrophage population distinct from M1, M2, and M4 macrophages20.
We propose that the phenotype of lesional macrophages cannot be classified into predetermined subsets but rather is a consequence of the lesional microenvironment and the activation of specific intracellular signaling pathways. For example, activation of TLR4 can result in NF-κB activation, activation of ERK, p38 MAPK, JNK, and interferon response genes, each of which has different downstream effects. Activation of the IL-4 receptor causes activation of STAT6 (signal transducer and activator of transcription 6), which can suppress TLR4 signaling21. Cross-talk among different signaling pathways is therefore likely to further impact macrophage phenotype. Thus, lesional macrophage phenotype can likely change rapidly as the microenvironment and intracellular signaling pathways change, for example by increased exposure to lipids or inflammatory stimuli. Indeed, laser capture microdissection of lesional CD68-positive macrophages in apolipoprotein E-deficient (Apoe−/−) mice has demonstrated that injection of LPS results in large increases in Ccl2, Vcam1, and Icam1 mRNA levels as early as 4 h after injection22. As such, lesional macrophages can best be viewed as representing a wide continuum of phenotypes and functions.
Homeostatic imbalance in intracellular lipids, metabolites and pro-inflammatory versus pro-resolving mediators impacts macrophage phenotype and function
The microenvironment in lesions is likely to be different in different areas of the lesion and in different stages of lesion development. The lesional microenvironment is also affected by systemic factors, such as dyslipidemia, low-grade inflammation associated with diabetes and autoimmune disease, and infection. We will briefly discuss three likely and highly interdependent contributors to macrophage phenotype: cholesterol and lipid-loading, metabolic state, and the balance between pro-inflammatory and pro-resolving mediators.
Cholesterol and lipid-loading affect macrophage phenotype
Hyperlipidemia is a well-known cause of cardiovascular disease and a strong driver of atherosclerosis in humans and animal models. One of the most commonly used mouse models of atherosclerosis—the LDL receptor-deficient (Ldlr−/−) mouse - takes advantage of this fact. These mice lack the ability to effectively clear LDL from the circulation and therefore develop large atherosclerotic lesions containing macrophage foam cells23. Statins are currently the most effective drugs used in the prevention and treatment of cardiovascular events. The beneficial effects of statins are due primarily to their ability to lower plasma LDL cholesterol levels, although statins also exert anti-inflammatory effects that may be important in suppressing atherosclerosis in certain settings. Cholesterol-rich lipoproteins accumulate in arteries4 and are taken up by macrophages and dendritic-like cells. When macrophages take up more lipoprotein-derived cholesterol than they excrete, the intracellular free cholesterol is converted into cholesteryl ester (CE), which accumulates in lipid droplets and results in the foam cell morphology observed by early pathologists.
Modified LDL, aggregated lipoproteins, and other substances are taken up by a number of different scavenger receptors expressed by macrophages and by macro-pinocytosis and phagocytosis24 (Figure 2A). These include scavenger receptor type 1 (SR-A), CD36, SR-BI, LOX1 25, and LDL receptor-related protein 1 (LRP1) 26. Apoe−/− mice deficient in SR-A27 or CD3628 in all tissues are largely protected from atherosclerosis. However, this effect appears to be influenced by the background of the mouse or possibly other factors, as SR-A- or CD36-deficient Apoe−/− or Ldlr−/− mice backcrossed into the C57BL/6 background showed no reduction of atherosclerosis and abundant lesional macrophage foam cells29. Recent studies have implicated SR-A in mediating proliferation of lesional macrophages30, CD36 in inhibiting migration and promoting macrophage spreading and attachment31 and coordinating inflammasome activation32, and SR-A and CD36 in promoting apoptosis, lesion necrotic core expansion and inflammatory gene expression33. The latter effects were observed in the absence of effects on lesion size and foam cell formation33. Therefore, in addition to promoting lipid uptake, SR-A and CD36 appear to have important signaling functions that affect atherosclerosis progression34.
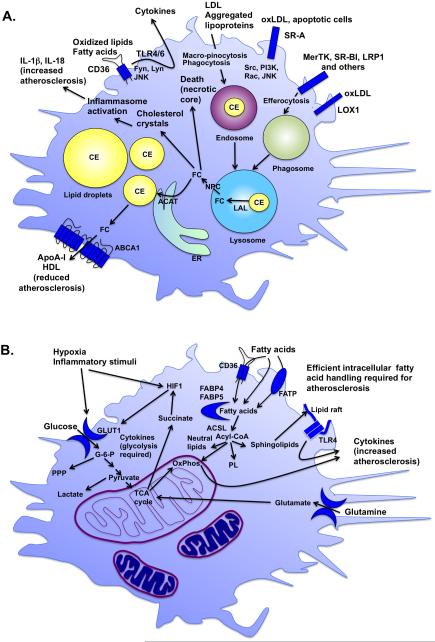
Figure 2. Lipids and metabolism determine lesion macrophage phenotype and function.
A. Lipoproteins impact macrophage phenotype. Lipoproteins are taken up by macrophages through macro-pinocytosis, phagocytosis and scavenger receptors, including SR-A, CD36, SR-BI, and LOX1. The scavenger receptors also have signaling capacities. As the lipoproteins are degraded in endosomes-lysosomes, CE is converted to free cholesterol (FC) by lysosomal acid lipase (LAL) and then redistributed to other cellular compartments through NPC (Niemann-Pick disease, type C) proteins. FC is converted back to CE by acyl-CoA cholesterol acyltransferase (ACAT) in the ER and then again to FC before it can be effluxed from the cell through the cholesterol exporters ABCA1 and ABCG1 to apolipoprotein A-I (ApoA-I) or HDL. FC can exert detrimental effects if it is not re-esterified to CE in lipid droplets or effluxed through ABCA1 and ABCG1. For example, cholesterol crystal formation leads to inflammasome activation, and FC accumulation causes ER stress and cell death. These processes promote progression of lesions of atherosclerosis and necrotic core expansion. B. The metabolic state of the macrophage influences its phenotype. Increased glycolysis initiated by glucose entry into the cell through glucose transporters, such as GLUT1, is required for the inflammatory phenotype. Hypoxia and inflammatory stimuli increase glycolysis in part to allow the cell to generate sufficient energy under anaerobic conditions, but also to fight pathogens and infection. TCA cycle intermediates also play important roles in inflammatory activation of macrophages. For example, the amino acid glutamine replenishes the TCA cycle to produce succinate, which in turn stabilizes the hypoxia-inducible factor 1 (HIF1) complex. Whereas a relative increase in fatty acid oxidation is associated with a more resolving macrophage phenotype, fatty acids and their metabolites exert a number of important effects in macrophages. Fatty acids are taken up by the cell through CD36 and fatty acid transport proteins (FATP) or by transport across the plasma membrane, and are readily converted to acyl-CoAs by a group of enzymes with acyl-CoA synthetase activity, including long-chain acyl-CoA synthetases (ACSLs). Acyl-CoAs are channeled to different fates in the cell, including oxidative phosphorylation (OxPhos), neutral lipids, phospholipids (PL), sphingolipids, or are used for protein modification or signaling. Sphingolipids are found in lipid rafts and are believed to promote inflammatory activation by TLRs present in these rafts. Reduced levels of intracellular fatty acids or acyl-CoAs or reduced OxPhos lead to reduced inflammatory activation and atherosclerosis.
Whether foam cell formation per se is a pro-atherosclerotic event likely depends on the overall environment in which the lipid loading occurs. First, to the extent that cholesterol is stored as CE in cytoplasmic neutral lipid droplets, the membranes of the cells are protected from the cytotoxic effects of free cholesterol. Second, in a non-inflammatory environment, lipid loading of macrophages may prevent inflammatory activation by activating liver X receptors (LXRs)35. The main LXR ligand in lipid-loaded macrophages was identified as desmosterol, a precursor of cholesterol in the Bloch pathway of cholesterol biosynthesis. The somewhat counterintuitive accumulation of a cholesterol precursor in lipid-loaded macrophages is due to a compensatory downregulation of cholesterol biosynthetic enzymes, and especially 24-dehydrocholesterol reductase, which is responsible for cholesterol synthesis from desmosterol35. The physiologic evolution of these protective processes may be related to a fundamental role of macrophages in clearing apoptotic cells (a process termed "efferocytosis"), which results in the delivery of large amounts of cholesterol to macrophages. Thus, it is plausible that cholesterol from dead cells engulfed by efferocytosis contributes to inhibition of endogenous cholesterol biosynthesis through a similar pathway, resulting in downregulation of cholesterol biosynthesis enzymes, desmosterol accumulation and subsequent LXR activation in efferocytes.
An example where this LXR-mediated physiologic process goes awry can be found with macrophages devoid of NPC1, the protein that is defective in patients with Niemann-Pick disease, type C1. NPC1 disease is a rare autosomal neurodegenerative disease resulting from the accumulation of cholesterol and glycerolipids in late endosomes and lysosomes (Figure 2A). Ldlr−/− mice deficient in NPC1 in bone marrow-derived cells are characterized by increased atherosclerosis, and macrophages from these mice exhibit decreased synthesis of putative LXR ligands and decreased expression of cholesterol transporters (ABCA1 and ABCG1), which are induced by LXRs, and impaired cholesterol efflux36. Thus, a combination of spill-over of free cholesterol or other lipid species into non-lipid droplet compartments, together with reduced production of endogenous ligands for LXR and other anti-atherosclerotic transcription factors, likely explains the adverse effects of cholesterol loading under certain conditions.
Many studies have demonstrated that the balance between CE storage and efflux of free cholesterol determines the inflammatory phenotype of macrophages and that too much free cholesterol acts, at least in part, by disrupting the normal function of intracellular membranes and the plasma membrane. Thus, accumulation of unesterified free cholesterol causes inflammatory activation of macrophages by promoting endoplasmatic reticulum (ER) stress37 and subsequent calcium leakage into the cytosol38; TLR4 activation by cholesterol enrichment of lipid rafts; inflammasome activation by cholesterol crystals39,40; and lysosome dysfunction41. The lysosome is crucial in degrading LDL as lipoprotein-derived CEs in lysosomes are converted to free cholesterol by lysosomal acid lipase (LAL) (Figure 2A). Lysosomal free cholesterol is then re-esterified by acyl-CoA cholesterol acyltransferase (ACAT) in the ER and stored in lipid droplets in the cytoplasm. Mice lacking ACAT1 in bone marrow-derived cells exhibit massive xanthomatosis, and atherosclerotic lesions in these mice show a paucity of macrophage foam cells42. This is likely due to macrophage death, as excess free cholesterol due to ACAT deficiency is cytotoxic.43
The cholesterol efflux transporters ABCA1 and ABCG1 promote cholesterol efflux from cells by transporting free cholesterol and phospholipids to free apolipoprotein A-I (ApoA-I) or HDL (Figure 2A). Thus, ABCA1- and ABCG1-deficiency causes inflammatory activation of macrophages and promotes atherosclerosis44-47. Importantly, the cholesterol efflux capacity of HDL is inversely associated with the incidence cardiovascular events in human subjects48. A major role of ABCA1 and ABCG1 is to prevent free cholesterol accumulation in hematopoietic stem cells in the bone marrow, thereby preventing their proliferation and subsequent leukocytosis in response to fat-feeding in mice49, but, in addition, these transporters almost certainly have important local effects in lesional macrophages. Thus, an imbalance in cholesterol metabolism, rather than just increased lipid droplet formation per se, is likely to promote atherosclerosis by impacting various inflammatory processes in macrophages. Conversely, inflammation inhibits cholesterol efflux from macrophages and reverse cholesterol transport in vivo50, demonstrating that inflammation and reduced cholesterol efflux may participate in a detrimental amplification cycle.
Together, these findings show that the balance among uptake, intracellular processing, and efflux of cholesterol is carefully tuned and that disturbances in this complex machinery can result in macrophage dysfunction, altered activation of nuclear receptors, inflammatory activation, and atherosclerosis. Human relevance still needs to be established for much of this work. Furthermore, few of the atherosclerosis studies in this area used mouse models in which expression of the protein being studied was restricted to macrophages, leaving the possibility that some of the effects could have been mediated by other immune cells, bone marrow progenitor cells, or other cell types.
Macrophage metabolic state and phenotype – a bidirectional relationship
Inflammation and metabolism are inextricably linked, and the metabolic phenotype of a macrophage can determine its inflammatory phenotype and vice versa. We define macrophage “metabolic phenotype” as a phenotype induced by substrates and intermediates used in energy metabolism. M1 and M2 macrophages in vitro demonstrate important differences in metabolism in that M2 macrophages rely more on fatty acid oxidation whereas M1 macrophages rely on an increase in glycolysis51. Furthermore, fatty acid oxidation appears to be required for the M2 phenotype51, and glycolysis is required for inflammatory activation and survival of activated macrophages52-54. However, increased glycolysis through overexpression of the glucose transporter GLUT1 in myeloid cells is not sufficient to promote inflammatory activation in vivo or atherosclerosis53. Inhibiting glycolysis in activated macrophages can result in increased apoptosis and thereby could increase necrotic core formation.
What factors might regulate macrophage metabolism in lesions in vivo?
Modulators of glucose metabolism
Inflammatory mediators are well known to induce several enzymes required for glucose uptake and glycolysis in macrophages (Figure 2B). It is therefore tempting to speculate that conditions associated with low-grade inflammation, such as diabetes and metabolic syndrome and several autoimmune diseases, are associated with altered metabolism in lesional macrophages. Furthermore, hypoxic conditions are believed to occur in advanced human atherosclerotic lesions. The presence of hypoxia in mouse lesions, which are much smaller than human lesions, is less certain. What is clear, however, is that hypoxia-inducible factor 1 (HIF1α and HIF1β) is present in both human and mouse atherosclerotic lesions55,56. HIF1 is induced by hypoxia, but also by inflammatory stimuli, such as LPS. Interestingly, succinate generated primarily from glutamine has been shown to stabilize the HIF1 complex and induce IL-1β57. The HIF1α and HIF1β complex induces transcription of genes involved in stimulating angiogenesis, inflammation and metabolism56-58. One of the genes induced by HIF1 in macrophages is GLUT1. The effect of hypoxia on atherosclerosis appears to be detrimental, with a recent study showing necrotic core expansion and defective macrophage efferocytosis in advanced mouse lesions59. Thus, inflammatory mediators and perhaps hypoxia promote an inflammatory macrophage phenotype associated with increased glucose uptake and metabolism.
Role of fatty acid handling
Inflammatory mediators can also increase proteins and enzymes involved in intracellular fatty acid handling. Fatty acids and their downstream lipid mediators have important effects on macrophage phenotype, and, like with cholesterol, the balance between uptake and metabolism as well as cellular localization are key. Free fatty acids enter the cell, in part through CD36 and fatty acid transport proteins (FATPs), or by flip-flopping across the plasma membrane, and are quickly bound to intracellular fatty acid binding proteins (FABPs) or are esterified into their acyl-CoA derivatives (Figure 2B). Macrophages express the fatty acid binding proteins FABP4 (aP2) and FABP5 (Mal1). Macrophages deficient in FABP4 or FABP5 exhibit reduced inflammatory activation, and inhibition of FABP4 or FABP5 results in reduced atherosclerosis60-62. This phenotype is associated with reduced inflammatory activation (NF-κB activity) and increased peroxisome proliferator-activated receptor-γ (PPARγ) activity, perhaps because free unsaturated fatty acids can act as PPARγ ligands. Conversion of free fatty acids into their acyl-CoA derivatives traps them in the cell because of the large hydrophilic CoA moiety and thereby promotes their entry into a variety of different pathways, including fatty acid oxidation, phospholipid synthesis and reacylation, diacylglycerol and triacylglycerol synthesis, esterification of free cholesterol into CEs, and sphingolipid metabolism63. Long-chain acyl-CoA synthetases (ACSLs) catalyze this reaction of acyl-CoA synthesis from free fatty acids and CoA. One of the ACSL isoforms expressed in macrophages, ACSL1, is markedly upregulated by inflammatory mediators and is required for atherosclerosis in diabetic mice64. Moreover, macrophages deficient in serine palmitoyltransferase subunit 2 (SPT) exhibit reduced levels of sphingomyelin in lipid rafts, which results in reduced TLR4 activity and reduced atherosclerosis65. Fatty acids might promote inflammatory processes by several different mechanisms, including altered activation of nuclear receptors, altered generation of bioactive lipid mediators, and facilitation of TLR4 activation through organization of TLR4 receptor complexes within lipid raft domains. A similar mechanism has been proposed for how free cholesterol promotes TLR4 activation46. Finally, omega-3 fatty acids and arachidonic acid can be converted into classes of lipids called specialized pro-resolving mediators (SPMs), that quell the inflammatory response and promote tissue repair and healing.66 Examples include lipoxins, resolvins, protections, and maresins. Inflammation resolution is defective in advanced atherosclerosis, perhaps in part due to defective SPM synthesis or action, and treatment of athero-prone mice with resolving mediators suppresses plaque progression.67-69
Role of oxidative phosphorylation
Finally, excessive oxidative phosphorylation can induce mitochondrial oxidative stress (Figure 2B), which in turn can promote an inflammatory macrophage phenotype and atherosclerosis. The role of mitochondrial oxidative stress in atherosclerosis was recently addressed by taking advantage of a mouse model in which mitochondrial oxidative stress is quenched by a mitochondria-targeted catalase construct. This study demonstrated that fat-fed Ldlr−/− mice have increased mitochondrial oxidative stress in lesional macrophages and that quelling mitochondrial oxidative stress in hematopoietic cells suppresses atherosclerosis and cytokine production by decreasing the activation of NF-κB70.
Together, these studies demonstrate that macrophage metabolism, phenotype, inflammation and atherosclerosis are inextricably linked and that by directing flow of metabolites to specific fates in macrophages, it is possible to alter their overall phenotype.
The balance between pro-inflammatory and pro-resolving mediators governs lesional macrophage phenotype
The net pro-atherogenic effect of lesional macrophages can be understood as a delicate balance between inflammatory and pro-resolving processes in these cells71, as illustrated in Figure 3.
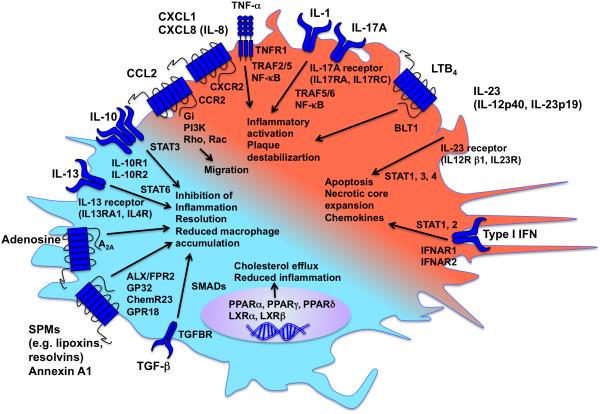
Figure 3. Pro-inflammatory and pro-resolving mediators balance macrophage phenotype.
A large number of pro-inflammatory and pro-resolving mediators act on macrophages through binding to specific receptors and activation of intracellular signaling pathways. The balance between pro-inflammatory mediators (indicated by red) and pro-resolving mediators (indicated by turquoise) determines the macrophage phenotype. Effects on atherosclerosis are indicated by arrows.
Pro-inflammatory, pro-atherosclerotic, cytokines
Macrophages can release pro-inflammatory cytokines and chemokines, including CCL2, IL-1, IL-6, IL-12, IL-15, IL-18, and TNF-α72. CCL2 and its receptor CCR2 was the first chemokine-receptor unit shown to be required for initiation of atherosclerosis in mice through promoting monocyte recruitment into lesions73-75. Another chemokine receptor, CXCR2, was also shown early to be pro-atherogenic: fat-fed Ldlr−/− mice transplanted with bone marrow from CXCR2-deficient mice exhibited reduced atherosclerosis and lesion macrophage accumulation76. This receptor binds a number of chemokines of the CXC family, including CXCL1 and CXCL8 (IL-8). Both CCR2 and CXCR2 are seven-transmembrane G-protein-coupled receptors that act to activate PI3-kinase, and the small G-proteins Rac and Rho, which mediate cytoskeletal rearrangements and monocyte migration into lesions. Other monocyte receptors playing a role in the recruitment of monocytes into lesions of atherosclerosis under different conditions include CCR1, CCR5, and CX3CR177,78.
The list of cytokines and chemokines promoting atherosclerosis in mice has grown considerably. A recent addition is IL-17A, which promotes monocyte adhesion and sensitation of antigen-presenting cells to LPS20. IL-17A binds to its receptor, which is composed of two subunits (IL-17RA and IL-17RC), and activates a signaling pathway similar to that of IL-1 and TNF-α, culminating in rapid activation of NF-κB and increased production of inflammatory mediators. These observations are in agreement with studies showing that bone marrow transplantation of TNFR1 (TNF receptor 1) knockout mice into fat-fed Ldlr−/− mice leads to smaller lesions, smaller macrophage lesion area, and reduced CCL2 levels.79 Likewise, Apoe−/− mice deficient in IL-1β exhibit smaller lesions and reduced aortic Ccl2 mRNA80. NF-κB is activated by IκB kinase (IKK)-mediated degradation of the IκB subunit, which sequesters NF-κB in the cytosol. Following IkB degradation, liberated NF-κB subunits translocate to the nucleus to mediate their effects on transcription. It was therefore surprising that deleting IKK in myeloid cells resulted in increased atherosclerosis in Ldlr−/− mice81. Reduced expression of IL-10, which exhibits anti-inflammatory/pro-resolving and anti-atherosclerotic properties,82 might explain this result. Moreover, TRAF5, one of the signaling molecules downstream of TNFR1 and the IL-17A receptor, has been shown to be athero-protective by preventing monocyte recruitment83. These findings further highlight the complexity and cross-talk of intracellular signaling pathways.
IL-23 is another interleukin recently demonstrated to have important effects on lesions: IL-23 promotes necrotic core expansion by mediating macrophage apoptosis84. The IL-23 receptor is composed of IL-12p40 and IL-23p19 subunits and activates a signaling pathway more similar to type I interferons (IFN), leading to apoptosis and necrotic core expansion through STAT transcription factors, including STAT1 (Figure 3). Consistent with this notion, IFNβ in myeloid cells promotes atherosclerosis and formation of necrotic cores in mice,85 and hematopoietic STAT1-deficiency results in reduced atherosclerosis in concomitant with reduced macrophage foam cell formation86.
A causative role for inflammation in promoting cardiovascular events in humans has not yet been established. However, evidence supporting a role for inflammation comes from studies demonstrating an increased risk of cardiovascular disease across a range of chronic inflammatory disorders and association between cardiovascular risk and severity of inflammation87. Furthermore, some of the cardioprotective effects of statins may be due to their anti-inflammatory effects, particularly when administered soon after the onset of acute coronary syndromes88.
Pro-resolving, anti-atherosclerotic, mediators
Macrophages also release pro-resolving mediators, such as IL-10 and TGF-β72 (Figure 3). Other examples of chemokines with pro-resolving functions in mouse models of atherosclerosis include IL-13 and IL-27, which limit accumulation of macrophages in lesions89,90, and CXCL5, which promotes cholesterol efflux by inducing ABCA1 in macrophages and also limits macrophage accumulation in lesions91. Annexin A1, acting through the receptor FPR2, has emerged as another pro-resolving protein with the ability to prevent myeloid cell recruitment into lesions of atherosclerosis in mice92, and some studies indicate that adenosine binding to its receptor (A2A) can be added to this growing group of pro-resolving molecules93. Furthermore, SPMs generated from arachidonic acid or omega-3 fatty acids (fish oils), including lipoxins and resolvins, mediate resolution of inflammation. The balance between the synthesis of the pro-inflammatory and pro-atherogenic leukotriene B4 (LTB4) and the pro-resolving lipoxin A4 in macrophages is regulated by the subcellular localization of 5-lipoxygenase, the enzyme responsible for generation of both lipid mediators from arachidonic acid94. The subcellular localization of 5-lipoxygenase is in turn regulated by resolvin D1, which is derived from omega-3 fatty acids.
Nuclear receptors
Finally, the inflammatory phenotype of macrophages can be markedly suppressed by transcription factors, transcriptional transactivators and repressors, and epigenomic processes. Peroxisome proliferator-activated receptors (PPARα, PPARγ and PPARδ) in macrophages are now well-known to reduce inflammatory activation and atherosclerosis in mice95-97. The PPARγ activators thiazolidinediones, such as rosiglitazone and pioglitazone, have been used as insulin sensitizers. However, rosiglitazone was found to have adverse effects on cardiovascular endpoints, and other glitazones have other negative effects. Systemic PPARγ activation is therefore not currently a promising strategy to reduce lesion inflammation. Fibrates, such as clofibrate and fenofibrate, act as PPARα activators and are used for the treatment of elevated blood lipids. Like the glitazones, fibrates have shown no significant beneficial effect on cardiovascular endpoints. Other nuclear receptor transcription factors also suppress the inflammatory phenotype of macrophages and atherosclerosis, including LXRα and LXRβ98,99. As discussed above, LXRs act as cholesterol sensors and induce genes involved in reverse cholesterol transport (ABCA1 and ABCG1) to promote cholesterol efflux and inhibit inflammatory activation of macrophages100 (Figure 3).
The balance between pro-inflammatory and pro-resolving mediators in lesional macrophages is likely to affect their function as well as atherosclerosis.
Transdifferentiation of smooth muscle cells to macrophage-like lesion cells
In the past few years, there have been significant discoveries related to the role of smooth muscle cells and their contribution to macrophage-like foam cells in murine and human atherosclerosis. Although the origin of lesional foam cells was believed to be the smooth muscle cell by some investigators as early as in the 1950’s101, this concept has recently been investigated by using lineage-tracing. These studies have demonstrated that smooth muscle cells make up a much larger part of lesional foam cells than previously thought and that these cells can also express macrophage markers, such as CD68102-104. The role of these smooth muscle-derived foam cells in atherosclerosis and plaque rupture will now have to be reevaluated.
The role of macrophages in lesion initiation and progression
Macrophage accumulation within the subendothelium or neointima constitutes one of the first steps in atherogenesis (Figure 4). Fatty streak lesions with macrophages can be observed in human fetal aortas, especially if the mother is hypercholesterolemic105. These early macrophages accumulate in susceptible regions of arteries due to endothelial adhesion molecule expression and the presence of apoB lipoproteins in the subendothelium4. Expression of chemokines by endothelial cells and macrophages recruit additional monocytes by interacting with monocyte receptors, including CCR2, CCR5, CX3CR1 and CXCR2,8 if the arterial insult, e.g. hyperlipidemia, diabetes, or smoking, persists. The macrophages ingest modified lipids and other substances that accumulate in the subendothelium. The mechanisms governing atherogenesis and formation of advanced lesions are in part distinct. For example, macrophage apoptosis is protective in early lesions, resulting in reduced lesion size due to efficient efferocytosis by neighboring macrophages, but promotes lesion size and development of necrotic cores in advanced lesions because of defective efferocytosis (below)106. Early lesions can resolve, perhaps by macrophages leaving the lesion or, as alluded to above, by efferocytosis.
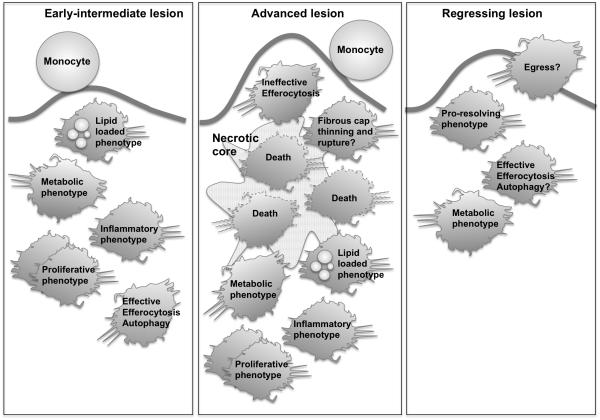
Figure 4. Roles of macrophages in different stages of lesions.
In early fatty streak lesions, monocytes and macrophages gather lipoproteins and become lipid-loaded foam cells. Inflammatory mediators and changes in macrophage metabolism govern lesional macrophage phenotype. We define “metabolic phenotype” as a phenotype induced by substrates and intermediates used in energy metabolism, i.e. generation of ATP from nutrients. Some macrophages become apoptotic, but are effectively cleared by efferocytosis. Hyperlipidemia also promotes proliferation of macrophages, which may contribute to lesion growth. In advanced lesions, the ability of lesional macrophages to effectively efferocytose dying macrophages is defective, and inflammatory processes and macrophage death are rampant, contributing to necrotic core expansion and plaque rupture. Macrophages also secrete proteases, which might promote plaque rupture. In regressing lesions, lesional macrophages are reduced, perhaps due to egress or reduced monocyte infiltration, and perhaps due to improved efferocytosis and a pro-resolving macrophage phenotype. The remaining macrophages adopt a phenotype characterized by altered gene expression, and lipid loaded macrophages are reduced due to reduced lipid uptake and/or increased efflux.
The fatty streak or pathologic intimal thickening with macrophages grows primarily by accumulation of more macrophages and by expansion of macrophages foam cells (Figures 1A, 1C). Local proliferation of macrophages can also contribute to the accumulation of cells, at least in fat-fed mice and rabbits30,107,108, although monocyte recruitment is likely to dominate in early lesions whereas macrophage proliferation takes on a more important role in more advanced lesions30. Arrest of macrophage proliferation results in reduced lesion size30,109.
The role of macrophages in advanced necrotic lesions
In advanced lesions (Figures 1B, 1D, 4), macrophage apoptosis is increased, due in part to increased ER stress110, which can be induced for example by free cholesterol111 or fatty acids112. Macrophages undergoing ER stress are more susceptible to apoptosis induced by oxidized phospholipids or lipoproteins through pathways that involve CD36, TLR2113, SR-A and STAT138. Most importantly, advanced lesional macrophages have a defect in clearing these apoptotic cells, which contributes to plaque necrosis and increased inflammation due to release of inflammatory mediators from uncleared, post-apoptotic necrotic cells114, 135.
Efferocytosis is mediated by the interaction of apoptotic cell recognition motifs, macrophage receptors, such as MerTK (MER proto-oncogene, tyrosine kinase), and molecules that bridge these two components115,116. It is possible that the impaired ability of macrophages to efferocytose apoptotic cells in advanced lesions is due to impaired function of one or more of these proteins. For example, MerTK is shed from macrophages activated by TLR4 through a pathway that involves NADPH oxidase and disintegrin and metalloproteinase ADAM17117, and there is evidence that this process occurs in advanced human atheroma118. In addition, primary necrosis of macrophages in lesions can occur through a pathway that is mediated by RIP3 (receptor interacting protein 3, a mediator of necrosis)119. Together, macrophage apoptosis, necrosis, and reduced efferocytosis contribute to expanding necrotic cores, which make the lesion unstable and more likely to rupture or fissure.
Macrophage autophagy (“self-eating” - the intracellular processes by which a cell transports cytoplasmic components, such as damaged and dysfunctional organelles and aggregates, into the lysosomal lumen for degradation and recycling120) triggered by increased ER stress has been shown to protect against lesion necrosis. For example, inhibition of autophagy by silencing the E3 ubiquitin ligase ATG5 (autophagy protein 5) results in increased macrophage apoptosis and NADPH oxidase-mediated oxidative stress and, in addition, renders the apoptotic cells less well recognized by efferocytes121. Furthermore, defective autophagy in macrophages is associated with pro-atherogenic inflammasome activation in response to cholesterol crystals and larger atherosclerotic lesions in Apoe−/− mice122 and also reduced cholesterol efflux through ABCA1123. Autophagy has therefore emerged as an important process that may protect lesional macrophages from apoptosis, inflammasome activation, and cholesterol accumulation.
Macrophages have also been suggested to contribute to fibrous cap thinning and plaque rupture by the secretion of matrix metalloproteinases124, although this has been difficult to prove in mouse models because mice do not exhibit the type of plaque rupture that occurs in humans. Intraplaque hemorrhage is sometimes present in association with macrophage accumulation in advanced human lesions125 and is also seen in mice, particularly in advanced lesions of diabetic mice126. It is possible that macrophages contribute to intraplaque hemorrhage in advanced lesions through the secretion of proteases.
Together, these studies demonstrate that macrophages play an important role in the development of advanced lesions, in particular necrotic core formation, which destabilizes the lesion and thereby promotes acute clinical cardiovascular events.
The role of macrophages in lesion regression and resolution
Regression of lesions can be induced in hyperlipidemic mouse models by aggressive lipid lowering and in diabetic mice by blood glucose lowering127-130. The regression in these models is characterized by reduced lesion macrophage content (Figure 4) and altered gene expression in the remaining CD68-positive cells. CD68-positive cells from regressing lesions exhibit elevated levels of Arg1 and Cd163 (genes often used as markers of the M2 phenotype) and reduced levels of Ccl2 and Tnfa mRNA127. However, there is also upregulation of the inflammatory cytokines/chemokines Cxcl2 and Il1b127, suggesting that the macrophage phenotype is not entirely anti-inflammatory or possibly that CD68-positive smooth muscle cells contribute to some of the differences. It remains somewhat controversial as to whether macrophages leave the lesion during regression or whether the reduced macrophage content is due primarily to a reduced recruitment of new monocytes11,131-133. The latter concept was recently supported by regression studies in diabetic mice, in which the impaired regression was due to increased recruitment rather than reduced egress129. It is possible that macrophage egress and reduced monocyte recruitment both occur in regressing lesions, and that their relative contribution to lesion regression differs in different states. Improved efficiency of efferocytosis and autophagy may also contribute to the reduced macrophage content in regressing lesions. Furthermore, it is quite possible that macrophage proliferation is reduced in regressing lesions due to a reduced engagement of SR-A when lipid levels fall. The extent to which these mechanisms are operative in human subjects is as yet unknown.
Novel macrophage-based treatment possibilities and future directions
Lipid lowering by statins is effective in preventing cardiovascular events and would be even more effective if medication compliance and safety issues enabled earlier and more robust LDL lowering. This is particularly the case when additional cardiovascular risk factors are present, including smoking and diabetes, where very low levels of LDL are usually necessary to prevent atherosclerotic disease. Perhaps the new availability of PCSK9 inhibitors will help narrow this therapeutic gap. In the meantime, however, we can ask whether therapeutic measures targeting pro-atherogenic processes in macrophages, particularly inflammation or defective resolution, can be additive or synergistic with lipid-lowering therapy in terms of cardiovascular risk reduction. It is possible that agents that suppress systemic inflammation will be effective134,135, but suppression of inflammation is likely to be detrimental in the long-term due to increased susceptibility of infection. Furthermore, systemic inhibition of inflammatory processes might have effects that are very different from those of local inhibition of the lesional macrophage inflammatory phenotype.136 Increasing atherosclerosis resolution might provide novel therapeutic opportunities because pro-resolving mediators, unlike direct inhibitors of inflammatory cytokines or chemokines, are less likely to compromise host defense136. Recent studies in mice have demonstrated the potential promise of proresolving therapy for atherosclerosis67,92.
A more macrophage-specific strategy is to target these cells by using nanoparticles. Nanoparticles can be designed to release their cargo in lesions for activation of macrophage cell-surface receptors or for internalization by macrophages. The former was the strategy used for delivering a pro-resolving mediator to lesions67. Other studies in mice have demonstrated beneficial effects on atherosclerosis using nanoparticles to interfere with scavenger receptor-mediated oxidized lipid uptake137, statin-loaded nanoparticles that have anti-inflammatory effects in lesions138, and nanoparticles encapsulating CCR2-silencing short interfering RNA that prevents monocyte recruitment to lesions139. Development of nanoparticles or other treatment strategies to prevent necrosis or improve efferocytosis in advanced lesions might lead to prevention of plaque rupture. Future work will be needed to determine the feasibility of treating humans with nanoparticles for long periods of time.
Although our understanding of the phenotypes and functions of macrophages in different stages of atherosclerosis has increased markedly since Virchow made his seminal observations, many important questions remain. For example, more work is needed to understand how lesional macrophages and atherosclerosis are governed by events in the bone marrow and spleen versus local factors in lesions, the role of smooth muscle transdifferentiation into macrophage-like cells in lesions, and whether targeting macrophages using new forms of therapy has promise in suppressing plaque progression and acute clinical events.
Translation of findings from mouse studies to human disease
As we have highlighted in this review, many of the concepts that have been studied in depth in vitro and in animal models have their basis in human observations. Examples include the importance of retained apoB lipoproteins in initiating the atherogenic process and the protection against cardiovascular disease by treatments that lower those lipoproteins; the overall role of inflammatory macrophages and a maladaptive inflammatory response in lesion progression; the association of HDL function, i.e., ability to efflux cholesterol from macrophages, with protection from cardiovascular disease; and the association of hypoxia, ER stress, oxidative stress, cell death, and defective efferocytosis with advanced lesions. Indeed, the appreciation of the role of macrophages and inflammation in atherosclerosis has led to two current human trials131, 132, one using anti-IL-1β and the other low-dose methotrexate, which target the inflammatory response. Moreover, ongoing clinical trials using resolving mediator therapy in other inflammatory diseases in humans may set the stage for their use to prevent the progression of atherosclerosis. A clear challenge that lays ahead in the translational work in this area is how to assess efficacy in humans before committing to very expensive and long-term endpoint trials. As such, parallel developments in macrophage-based imaging and biomarker studies are essential as the mechanistic and preclinical studies progress.
Supplementary Material
Acknowledgments
We apologize to our many colleagues whose important work we were unable to cite due to space limitations. We thank Drs. Renu Virmani and Kazuyuki Yahagi, CVPath Institute, Inc., Gaithersburg, MD, for providing high-resolution images of human atherosclerotic lesions.
Sources of Funding: The authors are supported by the National Institutes of Health, under awards R01HL062887, P01HL092969, R01HL126028 and DP3DK108209 (KEB); R01HL107497, R01 HL075662, and Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C (IT). Research in KEB’s laboratory is also supported in part by a Grant-in-Aid from the American Heart Association (14GRNT20410033), by Novo Nordisk A/S, and by the Diabetes Research Center at the University of Washington (P30DK017047).
Nonstandard Abbreviations and Acronyms
- ACAT
acyl-CoA cholesterol acyltransferase
- ACSL
acyl-CoA synthetase
- ApoE
apolipoprotein E
- CCL2
monocyte chemoattractant protein-1
- CE
cholesteryl ester
- FABP
fatty acid binding protein
- IFN
interferon
- IL
interleukin
- LAL
lysosomal acid lipase
- LDLR
low-density lipoprotein receptor
- LPS
lipopolysaccharide
- SPM
specialized pro-resolving mediators
- SR-A
scavenger receptor type 1
- TLR
toll-like receptor
Footnotes
Disclosures: None
Subject Terms:
Atherosclerosis
Lipids and Cholesterol
Cell Signaling/Signal Transduction
Inflammation
Metabolism
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12:10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 20.Erbel C, Akhavanpoor M, Okuyucu D, et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol. 2014;193:4344–4355. doi: 10.4049/jimmunol.1400181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 22.Trogan E, Choudhury RP, Dansky HM, Rong JX, Breslow JL, Fisher EA. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2002;99:2234–2239. doi: 10.1073/pnas.042683999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crucet M, Wust SJ, Spielmann P, Luscher TF, Wenger RH, Matter CM. Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36. Atherosclerosis. 2013;229:110–117. doi: 10.1016/j.atherosclerosis.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Lillis AP, Muratoglu SC, Au DT, Migliorini M, Lee MJ, Fried SK, Mikhailenko I, Strickland DK. LDL Receptor-Related Protein-1 (LRP1) Regulates Cholesterol Accumulation in Macrophages. PLoS One. 2015;10:e0128903. doi: 10.1371/journal.pone.0128903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 28.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricci R, Sumara G, Sumara I, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 35.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JR, Coleman T, Langmade SJ, Scherrer DE, Lane L, Lanier MH, Feng C, Sands MS, Schaffer JE, Semenkovich CF, Ory DS. Niemann-Pick C1 protects against atherosclerosis in mice via regulation of macrophage intracellular cholesterol trafficking. J Clin Invest. 2008;118:2281–2290. doi: 10.1172/JCI32561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 38.Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emanuel R, Sergin I, Bhattacharya S, Turner JN, Epelman S, Settembre C, Diwan A, Ballabio A, Razani B. Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler Thromb Vasc Biol. 2014;34:1942–1952. doi: 10.1161/ATVBAHA.114.303342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Accad M, Smith SJ, Newland DL, Sanan DA, King LE, Jr., Linton MF, Fazio S, Farese RV., Jr. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J Clin Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tannahill GM, O'Neill LA. The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett. 2011;585:1568–1572. doi: 10.1016/j.febslet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Nishizawa T, Kanter JE, Kramer F, et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014;7:356–365. doi: 10.1016/j.celrep.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tawakol A, Singh P, Mojena M, et al. HIF-1alpha and PFKFB3 Mediate a Tight Relationship Between Proinflammatory Activation and Anerobic Metabolism in Atherosclerotic Macrophages. Arterioscler Thromb Vasc Biol. 2015;35:1463–1471. doi: 10.1161/ATVBAHA.115.305551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vink A, Schoneveld AH, Lamers D, Houben AJ, van der Groep P, van Diest PJ, Pasterkamp G. HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Folco EJ, Sukhova GK, Quillard T, Libby P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res. 2014;115:875–883. doi: 10.1161/CIRCRESAHA.115.304437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsch E, Theelen TL, Demandt JA, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–2553. doi: 10.1161/ATVBAHA.114.304023. [DOI] [PubMed] [Google Scholar]
- 60.Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, Sulsky R, Robl JA, Parker RA, Hotamisligil GS. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babaev VR, Runner RP, Fan D, Ding L, Zhang Y, Tao H, Erbay E, Gorgun CZ, Fazio S, Hotamisligil GS, Linton MF. Macrophage Mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-gamma-regulated genes. Arterioscler Thromb Vasc Biol. 2011;31:1283–1290. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annu Rev Nutr. 2014;34:1–30. doi: 10.1146/annurev-nutr-071813-105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A. 2012;109:E715–724. doi: 10.1073/pnas.1111600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty M, Lou C, Huan C, Kuo MS, Park TS, Cao G, Jiang XC. Myeloid cell-specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis. J Clin Invest. 2013;123:1784–1797. doi: 10.1172/JCI60415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra220. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–1133. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 75.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 76.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 78.Soehnlein O, Drechsler M, Doring Y, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xanthoulea S, Gijbels MJ, van der Made I, Mujcic H, Thelen M, Vergouwe MN, Ambagts MH, Hofker MH, de Winther MP. P55 tumour necrosis factor receptor in bone marrow-derived cells promotes atherosclerosis development in low-density lipoprotein receptor knock-out mice. Cardiovasc Res. 2008;80:309–318. doi: 10.1093/cvr/cvn193. [DOI] [PubMed] [Google Scholar]
- 80.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 81.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A, Mallat Z. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 83.Missiou A, Kostlin N, Varo N, et al. Tumor necrosis factor receptor-associated factor 1 (TRAF1) deficiency attenuates atherosclerosis in mice by impairing monocyte recruitment to the vessel wall. Circulation. 2010;121:2033–2044. doi: 10.1161/CIRCULATIONAHA.109.895037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Subramanian M, Thorp E, Tabas I. Identification of a non-growth factor role for GM-CSF in advanced atherosclerosis: promotion of macrophage apoptosis and plaque necrosis through IL-23 signaling. Circ Res. 2015;116:e13–24. doi: 10.1161/CIRCRESAHA.116.304794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ, Kalinke U, Weber C, Lutgens E, de Winther MP. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Agrawal S, Febbraio M, Podrez E, Cathcart MK, Stark GR, Chisolm GM. Signal transducer and activator of transcription 1 is required for optimal foam cell formation and atherosclerotic lesion development. Circulation. 2007;115:2939–2947. doi: 10.1161/CIRCULATIONAHA.107.696922. [DOI] [PubMed] [Google Scholar]
- 87.Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–844. doi: 10.1161/CIRCULATIONAHA.114.009990. [DOI] [PubMed] [Google Scholar]
- 88.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 89.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koltsova EK, Kim G, Lloyd KM, Saris CJ, von Vietinghoff S, Kronenberg M, Ley K. Interleukin-27 receptor limits atherosclerosis in Ldlr−/− mice. Circ Res. 2012;111:1274–1285. doi: 10.1161/CIRCRESAHA.112.277525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rousselle A, Qadri F, Leukel L, Yilmaz R, Fontaine JF, Sihn G, Bader M, Ahluwalia A, Duchene J. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123:1343–1347. doi: 10.1172/JCI66580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, Doring Y, Zarbock A, Soehnlein O. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ Res. 2015;116:827–835. doi: 10.1161/CIRCRESAHA.116.305825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol. 2013;4:85. doi: 10.3389/fimmu.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fredman G, Ozcan L, Spolitu S, Hellmann J, Spite M, Backs J, Tabas I. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc Natl Acad Sci U S A. 2014;111:14530–14535. doi: 10.1073/pnas.1410851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 96.Babaev VR, Ishiguro H, Ding L, Yancey PG, Dove DE, Kovacs WJ, Semenkovich CF, Fazio S, Linton MF. Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;116:1404–1412. doi: 10.1161/CIRCULATIONAHA.106.684704. [DOI] [PubMed] [Google Scholar]
- 97.Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, Zou Y, Hwang H, Kang H, Curtiss L, Evans RM, Lee CH. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calkin AC, Tontonoz P. Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1513–1518. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cookson FB. The origin of foam cells in atherosclerosis. Br J Exp Pathol. 1971;52:62–69. [PMC free article] [PubMed] [Google Scholar]
- 102.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 104.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551–1559. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 105.Napoli C, D'Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 107.Rosenfeld ME. Macrophage proliferation in atherosclerosis: an historical perspective. Arterioscler Thromb Vasc Biol. 2014;34:e21–22. doi: 10.1161/ATVBAHA.114.303379. [DOI] [PubMed] [Google Scholar]
- 108.Lamharzi N, Renard CB, Kramer F, Pennathur S, Heinecke JW, Chait A, Bornfeldt KE. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: potential role of glucose-oxidized LDL. Diabetes. 2004;53:3217–3225. doi: 10.2337/diabetes.53.12.3217. [DOI] [PubMed] [Google Scholar]
- 109.Sayin VI, Khan OM, Pehlivanoglu LE, Staffas A, Ibrahim MX, Asplund A, Agren P, Nilton A, Bergstrom G, Bergo MO, Boren J, Lindahl P. Loss of one copy of Zfp148 reduces lesional macrophage proliferation and atherosclerosis in mice by activating p53. Circ Res. 2014;115:781–789. doi: 10.1161/CIRCRESAHA.115.304992. [DOI] [PubMed] [Google Scholar]
- 110.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 112.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tao H, Yancey PG, Babaev VR, Blakemore JL, Zhang Y, Ding L, Fazio S, Linton MF. Macrophage SR-BI Mediates Efferocytosis via Src/PI3K/Rac1 Signaling and Reduces Atherosclerotic Lesion Necrosis. J Lipid Res. 2015 doi: 10.1194/jlr.M056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cdelta, and p38 mitogen-activated protein kinase (MAPK) J Biol Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garbin U, Baggio E, Stranieri C, Pasini A, Manfro S, Mozzini C, Vallerio P, Lipari G, Merigo F, Guidi G, Cominacini L, Fratta Pasini A. Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovasc Res. 2013;97:125–133. doi: 10.1093/cvr/cvs301. [DOI] [PubMed] [Google Scholar]
- 119.Lin J, Li H, Yang M, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Sergin I, Razani B. Self-eating in the plaque: what macrophage autophagy reveals about atherosclerosis. Trends Endocrinol Metab. 2014;25:225–234. doi: 10.1016/j.tem.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Libby P. Collagenases and cracks in the plaque. J Clin Invest. 2013;123:3201–3203. doi: 10.1172/JCI67526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 126.Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.MacDougall ED, Kramer F, Polinsky P, Barnhart S, Askari B, Johansson F, Varon R, Rosenfeld ME, Oka K, Chan L, Schwartz SM, Bornfeldt KE. Aggressive very low-density lipoprotein (VLDL) and LDL lowering by gene transfer of the VLDL receptor combined with a low-fat diet regimen induces regression and reduces macrophage content in advanced atherosclerotic lesions in LDL receptor-deficient mice. Am J Pathol. 2006;168:2064–2073. doi: 10.2353/ajpath.2006.051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nagareddy PR, Murphy AJ, Stirzaker RA, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Willecke F, Yuan C, Oka K, Chan L, Hu Y, Barnhart S, Bornfeldt KE, Goldberg IJ, Fisher EA. Effects of High Fat Feeding and Diabetes on Regression of Atherosclerosis Induced by Low-Density Lipoprotein Receptor Gene Therapy in LDL Receptor-Deficient Mice. PLoS One. 2015;10:e0128996. doi: 10.1371/journal.pone.0128996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207 e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 136.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewis DR, Petersen LK, York AW, Zablocki KR, Joseph LB, Kholodovych V, Prud'homme RK, Uhrich KE, Moghe PV. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc Natl Acad Sci U S A. 2015;112:2693–2698. doi: 10.1073/pnas.1424594112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duivenvoorden R, Tang J, Cormode DP, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Otsuka F, Kramer MC, Woudstra P, Yahagi K, Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR, Joner M, Virmani R. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis. 2015;241:772–782. doi: 10.1016/j.atherosclerosis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.