Abstract
Mitochondria are highly dynamic cellular organelles, with the ability to change size, shape and position over the course of a few seconds. Many of these changes are related to the ability of mitochondria to undergo the highly co-ordinated processes of fission (division of a single organelle into two or more independent structures) or fusion (the opposing reaction). These actions occur simultaneously and continuously in many cell types, and the balance between them regulates the overall morphology of mitochondria within any given cell. Fission and fusion are active processes which require many specialized proteins, including mechanical enzymes that physically alter mitochondrial membranes, and adaptor proteins that regulate the interaction of these mechanical proteins with organelles. Although not fully understood, alterations in mitochondrial morphology appear to be involved in several activities that are crucial to the health of cells. In the present chapter we discuss the mechanisms behind mitochondrial fission and fusion, and discuss the implications of changes in organelle morphology during the life of a cell.
Introduction
In addition to their classic role as generators of ATP, mitochondria are central to several vital cellular functions. Following their identification as the site of oxidative phosphorylation 60 years ago [1], mitochondria have also been implicated in the regulation of programmed cell death, the biosynthesis of haem complexes, calcium signalling, the oxidation of fatty acids and, more recently, as a platform for signal transduction in the innate immune response. In keeping with the magnitude of these responsibilities, mitochondria are themselves highly regulated and dynamic organelles, having the ability to change both form and function rapidly to meet the physiological needs of the cell. Alterations in mitochondrial morphology have been recorded for over 100 years, with early light microscopists describing changes in mitochondrial size, number and position under many different conditions [2]. However, with the advent of electron microscopy and its ability to unearth the secrets of cellular ultrastucture, the dynamic aspects of mitochondrial form were overshadowed for many years by pictures of organelles in fixed tissue samples. Consequently, the textbook image of a mitochondrion became that of a static and unvarying organelle marooned in the cytoplasm. It has only been in the last 15 years, with great advances in live-cell-imaging techniques, that we have begun to re-appreciate the amazingly vibrant nature of mitochondria. The search for the mechanisms that control alterations in mitochondrial morphology has led to the realization that the changes we observe are not only indicative of organelle function, but in many cases actually regulate the ability of mitochondria to carry out their numerous roles.
Changes in the number and size of mitochondria in a cell are largely controlled by two opposing forces: fission and fusion [3]. The former involves the separation of a single mitochondrion into two or more daughter organelles, while the latter results in a single mitochondrion being formed from previously discrete and independent structures. While superficially straightforward, these processes require a high degree of co-ordination. For example, fission involves the separation of both the OMM (outer mitochondrial membrane) and IMM (inner mitochondrial membrane) and their rejoining in the correct orientation, without significant loss of soluble proteins from the mitochondrial matrix or intermembrane space. The process also requires the partitioning of mitochondrial proteins and mtDNA (mitochondrial DNA) so that each daughter organelle can function normally. The appearance of the mitochondrial population within any given cell is a result of the balance between the forces of fission and fusion, with a relative increase in one or the other leading to changes in overall morphology (Figure 1) [4]. At a base level, mitochondrial fission is necessary prior to cytokinesis, as mitochondria cannot be formed de novo. Prior to cell division, the mitochondrial population must also divide and localize to both sides of the division plane, so that each new cell can receive a portion of the organelles. However, mitochondrial fission and fusion is observed continuously in many non-dividing cells, indicating that it must be necessary for other functions.
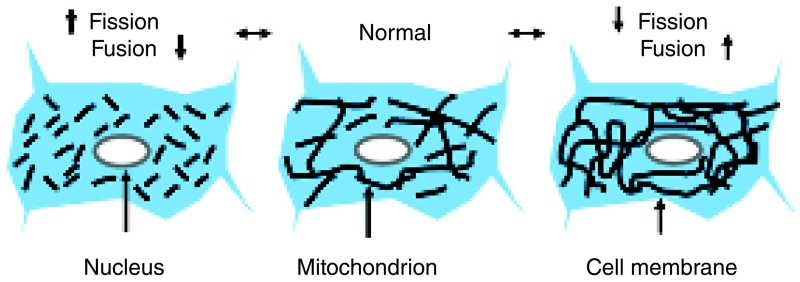
Figure 1. Balance of fission and fusion dynamically regulates mitochondrial morphology.
The overall morphology of mitochondria within any given cell is dynamically controlled by a balance between organelle fission and fusion. In many cell types, mitochondria form a network of interconnected tubules, along with multiple independent organelles (centre). Continual fission and fusion events lead to rapid changes in morphology in discrete areas; however, when these two processes are balanced, the overall mitochondrial morphology observed appears relatively constant. When the balance between fission and fusion is disrupted, caused by a relative increase or decrease in either process, the overall mitochondrial morphology can change markedly. In fusion-deficient cells (left-hand side), continued fission leads to the break-up of the mitochondrial network, leading to an increase in smaller independent organelles. Conversely, in fission-deficient cells (right-hand side), the relative increase in fusion events leads to a highly interconnected network of mitochondrial tubules.
Mitochondrial morphology: clues from evolutionary history
The mechanisms that carry out mitochondrial fission and fusion reflect, to a large degree, the evolutionary history of the organelle. Mitochondria are descended from α-proteobacteria, and became part of the present-day eukaryotic cell through an endosymbiotic event approx. 2 billion years ago [5]. Studies of extant proteobacteria, such as Escherichia coli, show that cell division is carried out by proteins such as FtsZ (filamentous temperature-sensitive mutant Z), a bacterial tubulin homologue [6]. FtsZ localizes to the inner surface of the cell membrane at division sites, where it forms a ring structure (Z ring) that enables constriction and scission of the parent into two daughter cells. FtsZ is a GTPase, which can hydrolyse GTP to GDP to provide a source of energy; however, it is unknown whether the Z ring produces the contractile forces to divide the cell, or whether it acts as a scaffold for other proteins to effect the final scission [7]. As mitochondria are direct descendents of proteobacteria, it would be logical to assume that they may have retained this system in their own division. Indeed lower eukaryotes, such as the algae Mallomonas splendens and Cyanidioschyzon merolae, and the slime mould Dictyostelium discoideum, utilize FtsZ for this purpose, with another homologue of the protein performing the same task for the algal photosynthetic plastids [8–10]. However, FtsZ has been lost from the mitochondrial division apparatus of animals, fungi and higher plants, and has been replaced by a system involving eukaryotic-derived proteins. This shift is likely to have occurred prior to the evolutionary divergence of the eukaryotic kingdoms, as many of the proteins involved are shared (orthologous) between animals, fungi and plants.
Mitochondrial fission in higher eukaryotes
The change from the prokaryotic fission system, to that seen in extant higher eukaryotes, may have been a passive or an active process during the evolution of endosymbiosis. Over time, much of the mitochondrial genome was passed horizontally from the organelle to the host nucleus [5], allowing the eukaryotic cell to gain transcriptional control over most aspects of mitochondrial function. While the transfer may have led to the passive loss of the FtsZ system by a transmission error, the need for the new cell to regulate the energetic potential of mitochondria is likely to have necessitated the evolution of a host-controlled mechanism. As such, the eukaryotic cell appears to have evolved a mitochondrial fission system that shares many features with the scission of membrane vesicles during endocytosis.
In endocytosis, components entering the cell are surrounded by the plasma membrane, which then buds off to form a vesicle that can move into the cytosol. The scission of the membrane is carried out by dynamin, a mechanical enzyme that forms tight spirals around the neck of vesicles to constrict and then cleave them off. Dynamin, like FtsZ, is a large GTPase that uses the energy released from GTP hydrolysis to provide the mechanical forces required in division systems [11]. Many of the proteins involved in mitochondrial fission and fusion are members of the dynamin GTPase protein family. The dynamin homologues involved in mitochondrial fission, called Dnm1 in yeast and Drp1 (dynamin-related protein 1) in mammals, are predominantly found in the cytosol. During fission events, they cycle to the OMM at scission sites, where they perform the late steps of fission [12]. At these scission sites, dynamin forms large homomultimeric complexes that encircle the mitochondrion in spirals, which then constrict in an energy-dependent manner until membrane fission occurs. Studies of Dnm1 in vitro show that the spirals match the minimal diameter of constricted mitochondria in yeast during fission, indicating that Dnm1 is tailored to the process of mitochondrial fission [13]. Mutations in both Dnm1 and Drp1 block mitochondrial fission, upsetting the balance between fission and fusion that controls overall mitochondrial morphology [14,15]. This leads to the formation of networks of highly interconnected mitochondrial tubules, as mitochondrial fusion continues as normal (Figure 1).
Dynamin is recruited to the OMM by a series of adaptor proteins, which were initially identified by genetic screens in yeast. Fis1 (fission 1) is an OMM protein, which is anchored to the organelle via a transmembrane domain in its C-terminus [16]. The remainder of the protein faces the cytosol, where it interacts, perhaps indirectly, with Drp1 to initiate fission (Figure 2). In yeast, this interaction is mediated by a second set of adaptors, Mdv1 and Caf4, which can bind both Fis1 and Dnm1 to allow their association [17–19]. Loss of either Fis1 or Mdv1, and to a lesser extent Caf4, prevents Dnm1 from associating with the mitochondria, and hence blocks mitochondrial division. In mammals there is no homologue of Mdv1 or Caf4, therefore the association between Fis1 and Drp1 at the outer membrane may be more direct, as evidenced by their ability to bind in vitro [20]. RNAi (RNA interference) knockdown of Fis1 protein levels in mammalian cells leads to an increase in the interconnectivity of mitochondrial tubules, similar to those seen in Drp1 mutants, whereas overexpression of Fis1 has been shown to increase mitochondrial fragmentation [20]. However, as both RNAi and overexpression can result in experimental artefacts, a concrete link between Fis1 and mitochondrial division in mammalian cells will require the creation and characterization of a true genetic knockout, as seen in Fis1-null yeast models.
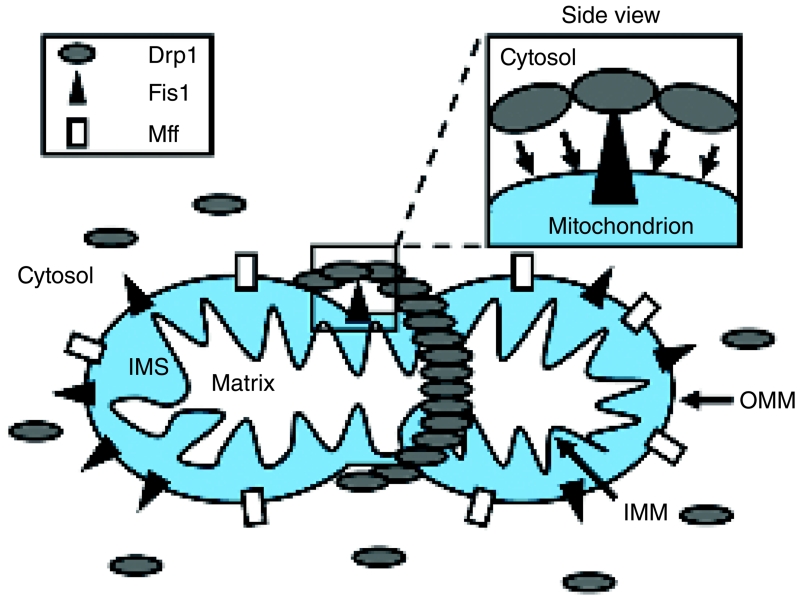
Figure 2. Mitochondrial fission in mammalian cells.
The two major proteins involved in mammalian mitochondrial fission are Fis1 (black triangle) and Drp1 (grey oval), and loss of either protein limits fission. Fis1 is an adaptor protein located in the OMM, where it is anchored by a C-terminal transmembrane domain. The remainder of Fis1 consists of protein–protein interaction sites which protrude into the cytosol. The majority of Drp1 is localized in the cytosol, from where it shuttles back and forth to the OMM during fission events. Drp1 interacts with Fis1 at fission sites, where it is proposed to form a collar that encircles the mitochondrion. The Drp1 collar tightens around the mitochondrion, and this constriction leads to a severing of the OMM and fission into two independent organelles. What regulates the site of fission, and how fission of the IMM is carried out, is currently unknown. Mff is a second OMM protein, which is anchored at its C-terminus like Fis1. Loss of Mff blocks mitochondrial fission; however, its mode of action, and whether it interacts with Drp1 to aid fission, is currently unknown. IMS, intermembrane space.
Two recently discovered proteins have also been implicated in the control of mitochondrial fission. In plants (which, like mammals, have Fis1 and Drp1 proteins but no Mdv1 or Caf4 homologues), ELM1 is localized to the OMM. Mutations in ELM1 lead to a decrease in mitochondrial fission, and the appearance of elongated mitochondrial tubules with many constrictions, indicating that division is blocked after the early events have taken place, but before final scission [21]. This was confirmed by the finding that in wild-type plants, the dynamin homologue was found at mitochondrial fission sites, while it remained in the cytosol in ELM1 mutants, indicating that ELM1 is required for the mitochondrial placement of dynamin [21]. It remains to be determined whether ELM1 acts in a manner analogous to Mdv1 (i.e. bridging the gap between Fis1 and dynamin), or whether ELM1 recruits dynamin to fission sites directly. In humans, another OMM protein, Mff, has been found to be involved in the regulation of fission (Figure 2). Mff was discovered in an RNAi screen for mitochondrial morphology mutants in Drosophila, and the human protein retains the same function [22]. Like Fis1, Mff is anchored at the membrane by a C-terminal transmembrane domain, and knockdown of protein levels by RNAi leads to an elongated mitochondrial network [22]. However, in vitro studies show that Mff is found in a different complex to both Fis1 and Drp1, indicating that it may affect mitochondrial fission through some unknown pathway.
Mitochondrial fusion
As noted above, mitochondrial fusion is also dependent on dynamin-related GTPases. The first genetic evidence of the mitochondrial fusion machinery came from studies of Drosophila spermatogenesis where, following meiosis, the entire mitochondrial population of the cell fuses into two organelles, forming a spherical structure called the nebenkern. The nebenkern, which in cross-section electron micrographs resembles an onion slice due to the concentric circles of the mitochondria, is disrupted in flies with a mutation in a large GTPase located on the OMM [23]. This protein, dubbed fuzzy onions due to the appearance of the altered nebenkern, prevents the fusion of the mitochondrial population. Fuzzy onions has homologues in yeast (Fzo1) and mammals (Mfn1 and Mfn2; Mfn is mitofusin), where it performs a similar function in controlling outer membrane fusion [24,25]. The proteins are tethered to the outer membrane by two transmembrane domains, with the rest of the protein facing the cytosol (Figure 3). The cytosolic portion contains the GTPase domain, along with two coiled-coil protein-interaction domains that mediate homotypic binding of Fzo/Mfn proteins on neighbouring mitochondria [26]. The docking of two adjacent mitochondria via these proteins is required for fusion of the outer membrane, and mutation of Fzo/Mfn genes leads to a loss of fusion and an increase in fragmented mitochondria. Interestingly, plants do not have any fuzzy onion mitochondrial homologues, yet still carry out mitochondrial fusion [27], indicating that a separate system must have evolved in this kingdom.
Figure 3. Mitochondrial fusion in mammalian cells.
The major fusion proteins of mammalian mitochondria are Mfn1 and Mfn2 (black symbol) and OPA1 (grey symbol). Mfn proteins are localized to the OMM and aid the tethering of two discrete mitochondria at the early stages of fusion, through homotypic coiled-coil protein-interaction domains. These proteins are also hypothesized to provide the mechanical forces involved in outer membrane fusion. mitoPLD (white symbol) is also localized to the OMM, with its catalytic domain facing the cytosol. Topological and enzymatic studies suggest that it may insert into the membranes of neighbouring mitochondria, changing the properties of the membrane lipids to aid fusion. OPA1 is localized in the IMM and intermembrane space, and co-ordinates the fusion of the IMM. OPA1 is processed into several different cleavage isoforms by mitochondrial proteases, such as PARL (blue symbol), with each variant proposed to have different roles in fusion events.
IMM fusion is controlled by a final dynamin-like GTPase, called Mgm1 in yeast and OPA1 (optic atrophy 1) in mammals, which is found in the intermembrane space or associated with the inner membrane [28,29]. Mutations in OPA1 cause dominant optic atrophy, an hereditary autosomal disease which results in progressive vision loss. There are eight variants of OPA1 in human cells, formed as the result of differential splicing of transcripts from a single gene [30]. These peptides undergo further proteolytic processing in the mitochondria, resulting in a variety of long and short OPA1 isoforms. In the yeast homologue Mgm1, a combination of long and short isoforms is required to allow inner membrane fusion, indicating that proteolysis is essential for protein function [31]. A number of proteins have been implicated in OPA1 processing, including PARL (presenilin-associated rhomboid-like; a member of the rhomboid family of proteases), whose yeast homologue Rbd1/Pcp1 performs the same function on Mgm1 [31–34]; Yme1, an AAA-metalloprotease found in the intermembrane space [35,36]; and paraplegin, another AAA protease located in the IMM [37]. In addition to facilitating inner membrane fusion, OPA1 regulates the morphology of mitochondrial cristae [34], which is implicated in processes such as apoptosis (see below).
In vivo and in vitro studies of mitochondrial fusion have revealed the general energetic requirements for the system to function. Analysis of the yeast components in vitro have shown that the fusion of both the inner and outer membranes are mechanically distinct [38]; however, there is an interaction between the outer membrane GTPase Fzo1 and the inner membrane Mgm1, which is mediated through an adaptor protein, Ugo1 [39]. While there may be a direct interaction in human cells with the homologous proteins, particularly Mfn1 and OPA1, there is no human homologue of Ugo1. The fusion of both membranes requires GTP hydrolysis to provide energy; however, the inner membrane also requires the maintenance of the electrochemical membrane potential [38]. This may be related to the fact that the human inner membrane GTPase, OPA1, undergoes increased proteolytic processing when membrane potential is lost, turning it into an inactive form [37]. This change in OPA1 results in a reduction of mitochondrial fusion, thereby linking energetics with organelle morphology. Changes to the biophysical properties of the lipids in mitochondrial membranes are now being seen as another aspect in the regulation of organelle morphology. For example, RNAi knockdown of a mitochondrial phospholipase, mitoPLD, leads to the fragmentation of the mitochondrial network [40]. MitoPLD is localized to the OMM by a C-terminal anchor, with a catalytic domain facing the cytosol. MitoPLD can hydrolyse cardiolipin, a major lipid component of the IMM [40], and therefore mitoPLD may function by altering the lipid structure of membranes in fusing mitochondria. The importance of lipids in mitochondrial fusion is strengthened by the finding that correct assembly of the long and short isoforms of yeast Mgm1 in vitro is enhanced by the presence of cardiolipin [41]. Overall, it is clear that there is a high level of regulation in mitochondrial fusion processes, and that joining the two membranes from separate mitochondria requires co-ordination on several levels.
Mitochondrial morphology and the regulation of cellular processes
As stated above, mitochondrial fission and fusion occurs continuously in many cell types, including in cells that are not merely increasing organelle number prior to cell division. Mutations in OPA1 and Mfn2 cause diseases, therefore their normal operation in mitochondrial dynamics must provide an important benefit to the cell. One major role of mitochondrial fission and fusion is likely to be as a means of sharing organelle contents, in order to replace damaged or lost components [42]. As a result of their bioenergetic processes, mitochondria produce many chemicals (e.g. reactive oxygen species) that can damage themselves and the rest of the cell. By fusing to a healthy organelle, a damaged mitochondrion may be able to receive components to compensate for damaged contents and/or repair its function. For example, the mitochondrial genome (mtDNA) is packaged into condensed structures called nucleoids, from which essential mitochondrial proteins (such as electron transport chain complexes) are encoded. While the majority of individual organelles normally contain at least one nucleoid, mitochondria in fusion-deficient cells progressively lose their mtDNA, most likely as a result of the inability to share materials during fusion [43].
Both fission and fusion proteins are required for the normal development of offspring, with Caenorhabditis elegans worms lacking Drp1 dying prior to adulthood [44], and mice lacking either Drp1, Mfn1, Mfn2 or OPA1 unable to survive past early gestation [45–48]. However, a direct link between these deaths and changes in mitochondrial morphology remains to be established, as these proteins may have roles in other processes. However, mitochondrial morphology is directly connected to the death of cells, through its role in the regulation of apoptosis. Apoptosis is a type of programmed cell death, which involves the regulated destruction and removal of damaged or superfluous cells [49]. Mitochondria participate in the early stages of apoptosis, mainly by releasing factors such as cytochrome c from their intermembrane space into the cytosol. Cytochrome c is recognized by cytosolic receptors, which act to switch on a cascade of cysteine proteases called caspases, which in turn degrade cellular components in an orderly fashion to kill the cell [50].
Mitochondrial morphology is tightly linked to apoptosis in two ways. First, at an early stage of the apoptotic programme (concurrent with cytochrome c release), Drp1 is recruited to mitochondria and the organelles within the dying cell rapidly fragment [51]. RNAi knockdown of the fission proteins Fis1 and Drp1 prevents both the fragmentation of mitochondria and the release of cytochrome c, thereby protecting the cell from apoptosis [3]. Conversely, overexpression of OPA1 prevents cytochrome c release, by maintaining tight mitochondrial cristae junctions that inhibit the movement of cytochrome c from the intermembrane space [52]. The second link between mitochondrial morphology and cell death is through the Bcl-2 family of apoptotic proteins. The Bcl-2 family contains pro-survival proteins, including Bcl-2 and Bcl-xL, and pro-death proteins, such as Bax and Bak, which oppose the actions of each other [50]. While Bcl-2, Bcl-xL and Bak are normally found on the OMM, Bax resides in the cytosol in healthy cells. However, once a pro-death signal is received by the cell, Bax, like Drp1, translocates to mitochondria and, importantly, co-localizes with Drp1 and Mfn2 at mitochondrial fission sites [53]. Translocation of Bax, mitochondrial fragmentation and cytochrome c release occur almost concurrently; however, RNAi knockdown of Drp1 prevents the latter two steps, even if Bax translocation goes ahead [53], showing the importance of mitochondrial morphology. To square the circle, it has recently been shown that Bcl-2 family proteins regulate mitochondrial morphology in healthy, non-apoptotic cells. In mice cells lacking both Bax and Bak, mitochondria are fragmented under normal conditions, relative to wild-type controls, and it appears that Bax has the ability to control Mfn2 distribution on the OMM to influence mitochondrial fusion [54]. In addition, mammalian Bcl-xL increases the rate of both fission and fusion in neurons, while the C. elegans Bcl-2 homologue CED-9 promotes mitochondrial fusion [55–57].
Mitochondrial morphology also changes as a function of the cell cycle. As discussed above, mitochondria cannot be formed de novo, so there must be a partitioning of the mitochondrial population prior to cell division, so that each daughter cell can receive a usable complement of organelles. As the normal morphology of mitochondria in many cell types is that of an elongated network of organelles, there must be a fission step to free up mitochondria to pass to the next generation. However, prior to this step there is actually a massive fusion of mitochondria to form a single reticulated organelle. For example, in undifferentiated tobacco protoplast cells, all the discrete mitochondria fuse together a few hours prior to cell division, forming a single, giant organelle reticulum [58]. As the cells progress from G1- to S-phase, the network breaks up and the mitochondrial population returns to its normal state. Visualization of mtDNA before and after this process shows that fusion allows the once randomly arranged nucleoids to be evenly apportioned across the mitochondrial population [58], further indicating the importance of mitochondrial fusion. Recent research in mammalian cells takes these findings even further, and demonstrates that changes in mitochondrial morphology may actually help regulate the cell cycle. In rat kidney cells, mitochondria also fuse into a single massive organelle at the G1–S-phase boundary. However, by altering mitochondrial properties, it is possible to force the cell to alter its cell cycle [59]. For example, depolarization of the inner membrane (which blocks mitochondrial fusion and leads to fragmented organelles) stops the movement from G1- to S-phase, indicating that cell-cycle progression is reliant on mitochondrial fusion. This is confirmed by the fact that the opposite effect (blocking fission by RNAi inhibition of Drp1) forces cells to move into the S-phase prematurely [59]. Phosporylation of Drp1 by the cell-cycle-specific kinase Cdk1/cyclin B, during mitosis, promotes the fragmentation process, prior to the redistribution of mitochondria into daughter cells [60]. Further work is now required to establish whether the changes in cell cycle observed are a direct result of mitochondrial dynamics, or whether they are a downstream effect of perturbing overall mitochondrial function at such an energy-dependent time.
Conclusions
Over the last 15 years, we have rapidly gained (or indeed regained) a wide appreciation of the dynamic nature of mitochondria, both from a biochemical and genetic viewpoint. As our knowledge of mitochondrial morphology has grown, so has our understanding that the changes we observe are not just the passive redistributions of organelles, but in many cases are actually the driving force behind numerous cell biology processes. As the pace of these discoveries continues to grow, it would appear that we have not yet exhausted the mystery behind these organelles.
Summary.
Mitochondria are dynamic organelles that can change size, shape and position within cells in a very short period of time.
Changes in size and shape are often related to mitochondrial fission and fusion events. These events are carried out by a specialized set of mechanical proteins in a highly regulated manner, and the balance between fission and fusion controls the overall morphology of mitochondria within any given cell.
Mitochondrial fission and fusion are critical events in a number of cellular processes, and removal of either can compromise the long-term viability of the cell.
Acknowledgments
Work in the authors’ laboratories is funded by the intramural research programmes of the National Heart, Lung and Blood Institute (I.S.) and National Institute of Neurological Disorders and Stroke (R.J.Y.), National Institutes of Health.
References
- 1.Kennedy EP, Lehninger AL. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. J. Biol. Chem. 1949;179:957–972. [PubMed] [Google Scholar]
- 2.Lewis MR, Lewis WH. Mitochondria in tissue cultures. Science. 1914;39:330–333. doi: 10.1126/science.39.1000.330. [DOI] [PubMed] [Google Scholar]
- 3.Suen D-F, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 1999;149:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmer C. On the origin of eukaryotes. Science. 2009;325:666–668. doi: 10.1126/science.325_666. [DOI] [PubMed] [Google Scholar]
- 6.Bi E, Lutkenhaus J. FtsZ ring structure associated with cell division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 7.Dajkovic J, Mukherjee A, Lutkenhaus J. Investigation into FtsZ assembly by SulA and development of a model for FtsZ polymerization. J. Bacteriol. 2008;190:2513–2526. doi: 10.1128/JB.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beech PL, Nheu T, Schultz T, Herbert S, Lithgow T, Gilson PR, McFadden GI. Mitochondrial FtsZ in a chromophyte alga. Science. 2000;287:1276–1279. doi: 10.1126/science.287.5456.1276. [DOI] [PubMed] [Google Scholar]
- 9.Takahara M, Takahashi H, Matsunaga S, Miyagishima S, Takano H, Sakai A, Kawano S, Kuroiwa T. A putative mitochondrial FtsZ gene is present in the unicellular primitive red alga Cyanidioscyizon merolae. Mol. Gen. Genet. 2001;264:452–460. doi: 10.1007/s004380000307. [DOI] [PubMed] [Google Scholar]
- 10.Gilson PR, Beech PL. Cell division protein FtsZ: running rings round bacteria, chloroplasts and mitochondria. Res. Microbiol. 2001;152:3–10. doi: 10.1016/s0923-2508(00)01162-1. [DOI] [PubMed] [Google Scholar]
- 11.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 12.Lackner LL, Nunnari JM. The molecular mechanism and cellular functions of mitochondrial division. Biochim. Biophys. Acta. 2009;1792:1138–1144. doi: 10.1016/j.bbadis.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, Nunnari JM. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J. Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsuga D, Keegan BR, Brisch E, Thatcher JW, Hermann GJ, Bleazard W, Shaw JM. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tieu Q, Nunnari J. Mdv1p is a WD repeat protein that interacts with the dynamin-related GTPase, Dnm1p, to trigger mitochondrial division. J. Cell Biol. 2000;151:353–366. doi: 10.1083/jcb.151.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerveny KL, McCaffery JM, Jensen RE. Division of mitochondria requires a novel DNM1-interacting protein, Net2p. Mol. Biol. Cell. 2001;12:309–321. doi: 10.1091/mbc.12.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin EE, Graumann J, Chan DC. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J. Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arimura S, Fujimoto M, Doniwa Y, Kadoya N, Nakazono M, Sakamoto W, Tsutsumi N. Arabidopsis ELONGATED MITOCHONDRIA1 is required for localization of DYNAMIN-RELATED PROTEIN3A to mitochondrial fission sites. Plant Cell. 2008;20:1555–1566. doi: 10.1105/tpc.108.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 24.Hermann GJ, Thatcher JW, Mills JP, Hales KG, Fuller MT, Nunnari J, Shaw JM. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 26.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 27.Arimura S, Yamamoto J, Aida GP, Nakazono M, Nobuhiro T. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7805–7808. doi: 10.1073/pnas.0401077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ED, Wagner JA, Gorsich SW, McCaffery JM, Shaw JM, Nunnari JM. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 2000;151:341–352. doi: 10.1083/jcb.151.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- 30.Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum. Genet. 2001;109:584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- 31.Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS. Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J. Biol. Chem. 2003;278:27781–27788. doi: 10.1074/jbc.M211311200. [DOI] [PubMed] [Google Scholar]
- 32.McQuibban GA, Saurya S, Freeman M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature. 2003;423:537–541. doi: 10.1038/nature01633. [DOI] [PubMed] [Google Scholar]
- 33.Sesaki H, Southard SM, Hobbs AE, Jensen RE. Cells lacking Pcp1p/Ugo2p, a rhomboid-like protease required for Mgm1p processing, lose mtDNA and mitochondrial structure in a Dnm1p-dependent manner, but remain competent for mitochondrial fusion. Biochem. Biophys. Res. Commun. 2003;308:276–283. doi: 10.1016/s0006-291x(03)01348-2. [DOI] [PubMed] [Google Scholar]
- 34.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D’Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J. Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meeusen S, McCaffery JM, Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- 39.Sesaki H, Jensen RE. UGO1 encodes an outer mitochondrial membrane protein required for mitochondrial fusion. J. Cell Biol. 2001;152:1123–1134. doi: 10.1083/jcb.152.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat. Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 41.Devay RM, Dominguez-Ramirez L, Lackner LL, Hoppins S, Stahlberg H, Nunnari J. Coassembly of Mgm1 isoforms requires cardiolipin and mediates mitochondrial inner membrane fusion. J. Cell Biol. 2009;186:793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP1 controls severing of the mitochondrial outer membrane. Mol. Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, Nicols PP, Boulton ME, Votruba M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 2007;16:1307–1318. doi: 10.1093/hmg/ddm079. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 48.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009;186:808–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J. Clin. Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell. Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 51.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 52.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 53.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 55.Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB, 3rd, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, Hardwick JM. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J. Cell Biol. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 57.Rolland SG, Lu Y, David CN, Conradt B. The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. J. Cell Biol. 2009;186:525–540. doi: 10.1083/jcb.200905070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheahan MB, McCurdy DW, Rose RJ. Mitochondria as a connected population: ensuring continuity of the mitochondrial genome during plant cell dedifferentiation through massive mitochondrial fusion. Plant J. 2005;44:744–755. doi: 10.1111/j.1365-313X.2005.02561.x. [DOI] [PubMed] [Google Scholar]
- 59.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]