Abstract
Introduction
Prolonged glucocorticoid use may increase the risk of adverse safety outcomes, including cardiovascular events. The European League Against Rheumatism and the Canadian Rheumatology Association advise tapering glucocorticoid dose as rapidly as clinically feasible. There is a paucity of published data on RA that adequately describe concomitant treatment patterns.
Methods
ACTION (AbataCepT In rOutiNe clinical practice) is a non-interventional cohort study of patients from Europe and Canada that investigated the long-term retention of intravenous abatacept in clinical practice. We assessed concomitant glucocorticoids in patients with established RA who had participated in ACTION and received ≥1 biological agent prior to abatacept initiation.
Results
The analysis included 1009 patients. Glucocorticoids were prescribed at abatacept initiation in 734 (72.7%) patients at a median 7.5 mg/day dose (n=692). Of the patients who remained on abatacept at 24 months, 40.7% were able to decrease their dose of glucocorticoids, including 26.9% who decreased their dose from >5 mg/day to ≤5 mg/day.
Conclusion
Reduction and/or cessation of glucocorticoid therapy is possible with intravenous abatacept in clinical practice.
Keywords: Rheumatoid Arthritis, Corticosteroids, DMARDs (biologic)
Key messages.
What is already known about this subject?
Low-dose glucocorticoids are an important treatment option in rheumatoid arthritis (RA), with proven clinical, functional and structural benefits.
Both the European League Against Rheumatism and the Canadian Rheumatology Association advise tapering glucocorticoid dose as rapidly as clinically feasible to minimise long-term safety concerns.
What does this study add?
This paper reports concomitant glucocorticoid use in patients with established RA who participated in the ACTION (AbataCepT In rOutiNe clinical practice) study and received at least one biologic agent prior to abatacept initiation.
For patients who remained on intravenous (IV) abatacept at 24 months, 40.7% were able to decrease their dose of glucocorticoids, including 26.9% who decreased their dose from >5 mg/day to ≤5 mg/day.
How might this impact on clinical practice?
This is the first study to describe concomitant glucocorticoid treatment patterns with abatacept in a real-world clinical setting and demonstrates that reduction and/or cessation of glucocorticoid therapy is possible with IV abatacept in clinical practice.
In patients with rheumatoid arthritis (RA) and poor prognosis, current treatment recommendations from the European League Against Rheumatism (EULAR) suggest initiating a biological agent in combination with methotrexate, with or without glucocorticoids, after failure of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).1 Low-dose glucocorticoids (≤5 mg/day prednisone or equivalent)1 remain an important treatment option given their capacity to increase clinical, functional and structural efficacy when combined with csDMARDs.1 However, EULAR1 and the Canadian Rheumatology Association2 advise tapering the glucocorticoid dose as rapidly as clinically feasible to minimise safety concerns associated with prolonged use.1–3
ACTION (AbataCepT In rOutiNe clinical practice) is a non-interventional, international, multicentre cohort study to assess the long-term retention and effectiveness of intravenous abatacept in patients with RA in clinical practice in Europe and Canada (used in accordance with local licensing).4 5 The study design, ethics approvals, baseline demographics, disease and clinical characteristics, and primary outcomes have been reported elsewhere.6
Patients with established, moderate-to-severe RA, who were naïve to biological therapy or had received ≥1 previous biological agent, were enrolled prospectively (Cohort A; May 2008–December 2010) and followed for up to 24 months or up to 6 months after abatacept discontinuation.6 We report patterns of concomitant glucocorticoid use over 24 months in biological-experienced patients (primarily from Europe); the majority of patients in cohort A experienced failure of at least one prior biological agent (89.2%) and it is the data from this subset that are reported here. Patients were included if they remained on abatacept at 24 months and had a clinical visit within the predefined 24-month time point, with glucocorticoid dose data available at initiation and 24 months. Glucocorticoid dose was assessed using the median of the area under the curve (mg/day) and glucocorticoid use was stratified by dose.
From May 2008 to December 2010, 1137 patients were enrolled and 1131 were evaluable. For biological-experienced patients (n=1009; analysis population), 82.7% were female, the mean (SD) age was 56.2 (12.4) years, disease duration was 11.8 (9.3) years and the 28-joint Disease Activity Score (erythrocyte sedimentation rate; calculated) was 5.7 (1.2). In total, 407/1009 (40.3%) remained on abatacept at 24 months. Baseline characteristics for patients who completed 2 years of follow-up were similar to the analysis population (see online supplementary table).
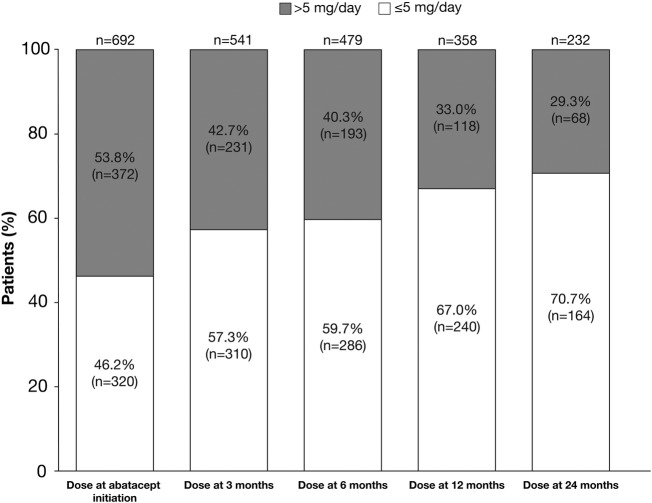
Concomitant glucocorticoids were used in 734/1009 (72.7%) patients. The proportion of patients with glucocorticoid dose >5 mg/day decreased over time from 53.8% at abatacept initiation to 29.3% at 24 months (figure 1). For patients on abatacept at 24 months with available data (n=216), 8.8% increased and 40.7% decreased the glucocorticoid dose. Specifically, 26.9% decreased their glucocorticoid dose from >5.0 to ≤5.0 mg/day and 4.6% increased their dose from ≤5.0 to >5.0 mg/day. Glucocorticoid dose was also assessed using different dose cut-offs, including 7.5 mg/day, with similar findings (see online supplementary figure). The 5 mg/day dose data are reported here as it was the most stringent dose cut-off used.
Figure 1.
The proportion of patients taking glucocorticoid dose ≤5 mg/day or >5 mg/day at the initiation of abatacept and 3, 6 and 12 months after the initiation of abatacept treatment, among patients who had received at least one prior biological agent.
Median glucocorticoid dose (range) at abatacept initiation was 7.5 (1.0–250.0) mg/day in patients with available data on glucocorticoid dose at initiation (n=692), and 6.0 (2.0–30.0) mg/day in patients with available data at both abatacept initiation and 24 months (n=217) versus 5.0 (0.5–25.0) mg/day (n=232) at 24 months.
Studies that adequately describe concomitant use of glucocorticoids and biological agents are relatively few,7 although some have shown that successful treatment strategies that include biological agents can lead to glucocorticoid dose reduction over time.8–10 Real-world analyses and registry data show wide variation in glucocorticoid prescribing patterns.11–13
Over 24 months after abatacept initiation, 40.7% of patients were able to reduce their glucocorticoid dose; a reduction in median glucocorticoid dose mostly occurred within the first 3 months following abatacept initiation. As the ACTION study focused on abatacept retention, reasons for glucocorticoid dose changes were not collected. Other limitations include the observational design, potential for referral and channelling bias and lack of an active comparator. Nevertheless, this is the first prospective study to analyse concomitant glucocorticoid treatment patterns and doses with abatacept in a real-world setting. The reduction and/or cessation of glucocorticoids was possible in patients with established RA and prior exposure to biological agents who were treated with intravenous abatacept. The clinical importance of these findings is reflected in current EULAR recommendations wherein glucocorticoids can be considered part of the initial 6-month combination treatment with the recommendation to taper as rapidly as clinically feasible.1
Acknowledgments
The authors would like to thank all the physicians who participated in the ACTION study. The ACTION study was funded by Bristol-Myers Squibb. Clinical research organisations involved in the ACTION study were Archemin BVBA, Inventiv Health Clinical, TFS Trial Form Support Srl and Winicker Norimed. Statistical analysis support was provided by Stat Process and Guillaume Desachy (Excelya). Gilbert L'Italien (Worldwide Health Economics and Outcomes Research, Bristol-Myers Squibb, at the time of the study) provided input into the design and interpretation of this study. Nathalie Schmidely (Centre of Observational Research and Data Sciences, Bristol-Myers Squibb) provided input into the design of this study. Professor Xavier Mariette (Hôpital Bicêtre, Paris, France) provided input into statistical analysis and interpretation of this analysis. The first draft of the manuscript was prepared by academic and industry authors, with professional medical writing and editorial assistance provided by Lauren Bradley and Catriona McKay at Caudex, and funded by Bristol-Myers Squibb. The academic authors vouch for the completeness and accuracy of the data and data analyses, and for the fidelity of the study to the protocol.
Footnotes
Contributors: RA and CP made a substantial contribution to the conception and design of the study, and to the analysis and interpretation of data. HN, H-ML MC, CR and MLB made a substantial contribution to the conception and design of the study, to the acquisition of data, and to the analysis and interpretation of data. MG made a substantial contribution to the acquisition of data, and to the analysis and interpretation of data. MTN made a substantial contribution to the acquisition of data. WGB made a substantial contribution to the acquisition of data. GRB made a substantial contribution to the acquisition of data, and to the analysis and interpretation of data. H-HP made a substantial contribution to the acquisition of data, and to the analysis and interpretation of data. KP made a substantial contribution to the acquisition of data. YE made a substantial contribution to the analysis and interpretation of data. All the authors have read and approved the final manuscript.
Competing interests: RA has received research grants from Bristol-Myers Squibb and has been a member of the speakers’ bureau for Bristol-Myers Squibb. HN has been a consultant for Abbott, Bristol-Myers Squibb, Chugai, Essex, MSD, Novartis, Pfizer, Roche, UCB and Wyeth; and has been a member of the speakers’ bureau for Abbott, Bristol-Myers Squibb, Chugai, Essex, MSD, Novartis, Pfizer, Roche, UCB and Wyeth. MG has no disclosures to declare. H-ML has been a consultant for and a member of the speakers’ bureau for Bristol-Myers Squibb. MTN declares that the Jan van Breemen Research Institute has received research grants from Abbott, Bristol-Myers Squibb, Pfizer, Roche and UCB; that he has been a consultant for Bristol-Myers Squibb, MSD, Pfizer, Roche, Schering-Plough, UCB and Wyeth; and has been a member of the speakers’ bureau for Abbott, Bristol-Myers Squibb, Pfizer and Roche. WGB has received research grants from Abbott, Amgen, Bristol-Myers Squibb, Janssen, Lilly, Merck, Novartis, Pfizer, Proctor and Gamble, Roche, Sanofi-Aventis, Schering, Takeda, UCB, Warner Chilcott and Wyeth. GRB has been a consultant for Abbott, Bristol-Myers Squibb, Roche, Merck and Pfizer; has received research grants from Abbott, Bristol-Myers Squibb and Roche; and has been on the speakers’ bureau for Abbott. H-HP has no disclosures to declare. KP has been on the speakers’ bureau for Pfizer, Amgen, MSD, Bristol-Myers Squibb and Abbott. MC is a consultant for Bristol-Myers Squibb. CP is a consultant for Bristol-Myers Squibb. CR is an employee of Bristol-Myers Squibb. YE is a consultant for Bristol-Myers Squibb. MLB is an employee of Bristol-Myers Squibb and holds stock options in the company.
Ethics approval: Please find a full list in online supplementary file.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bykerk VP, Akhavan P, Hazlewood GS et al. . Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol 2012;39:1559–82. 10.3899/jrheum.110207 [DOI] [PubMed] [Google Scholar]
- 3.Roubille C, Richer V, Starnino T et al. . The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9. 10.1136/annrheumdis-2014-206624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orencia (250 mg powder for concentrate for solution for infusion) Summary of Product Characteristics. http://www.medicines.org.uk/emc/medicine/19714/SPC/. (accessed 30 Jul 2015).
- 5.Product monograph: Orencia (abatacept). http://www.bmscanada.ca/static/products/en/pm_pdf/ORENCIA_EN_PM.pdf. (accessed 30 Jul 2015).
- 6.Nüßlein H, Alten R, Galeazzi M et al. . Real-world effectiveness of abatacept for rheumatoid arthritis treatment in European and Canadian populations: a 6-month interim analysis of the 2-year, observational, prospective ACTION study. BMC Musculoskelet Disord 2014;15:14 10.1186/1471-2474-15-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.André V, le Goff B, Leux C et al. . Information on glucocorticoid therapy in the main studies of biological agents. Joint Bone Spine 2011;78:478–83. 10.1016/j.jbspin.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Nebro A, Irigoyen MV, Ureña I et al. . Effectiveness, predictive response factors, and safety of anti-tumor necrosis factor (TNF) therapies in anti-TNF-naive rheumatoid arthritis. J Rheumatol 2007;34:2334–42. [PubMed] [Google Scholar]
- 9.Naumann L, Huscher D, Detert J et al. . Anti-tumour necrosis factor {alpha} therapy in patients with rheumatoid arthritis results in a significant and long-lasting decrease of concomitant glucocorticoid treatment. Ann Rheum Dis 2009;68:1934–6. 10.1136/ard.2009.111807 [DOI] [PubMed] [Google Scholar]
- 10.Hetland ML, Lindegaard HM, Hansen A et al. . Do changes in prescription practice in patients with rheumatoid arthritis treated with biological agents affect treatment response and adherence to therapy? Results from the nationwide Danish DANBIO Registry. Ann Rheum Dis 2008;67:1023–6. 10.1136/ard.2007.087262 [DOI] [PubMed] [Google Scholar]
- 11.Sokka T, Kautiainen H, Toloza S et al. . QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis 2007;66:1491–6. 10.1136/ard.2006.069252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galloway JB, Hyrich KL, Mercer LK et al. . Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50:124–31. 10.1093/rheumatology/keq242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchesoni A, Zaccara E, Gorla R et al. . TNF-alpha antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci 2009;1173:837–46. 10.1111/j.1749-6632.2009.04621.x [DOI] [PubMed] [Google Scholar]