Abstract
The importance of hemoglobin A1c (HbA1c) as an indicator of mean glycemia and risks for complications in patients with diabetes mellitus was established by the results of long-term clinical trials, most notably the Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS), published in 1993 and 1998 respectively. However, clinical application of recommended HbA1c targets that were based on these studies was difficult due to lack of comparability of HbA1c results among assay methods and laboratories. Thus, the National Glycohemoglobin Standardization Program (NGSP) was initiated in 1996 with the goal of standardizing HbA1c results to those of the DCCT/UKPDS. HbA1c standardization efforts have been highly successful; however, a number of issues have emerged on the “long and winding road” to better HbA1c, including the development of a higher-order HbA1c reference method by the International Federation of Clinical Chemistry (IFCC), recommendations to use HbA1c to diagnose as well as monitor diabetes, and point-of-care (POC) HbA1c testing. Here, we review the past, present and future of HbA1c standardization and describe the current status of HbA1c testing, including limitations that healthcare providers need to be aware of when interpreting HbA1c results.
Keywords: HbA1c, diabetes, standardization, glycated hemoglobin
1. Introduction
Diabetes mellitus is characterized by chronic hyperglycemia and is a major cause of retinopathy, nephropathy and neuropathy. The burden of diabetes is increasing globally, particularly in developing countries, with over 346 million people diagnosed worldwide [1]. Hemoglobin A1c (HbA1c) is an important measure of glycemic status that has become fundamental to managing patients with diabetes. HbA1c is being used not only to guide diabetes treatment but to assess quality of care, and to predict risk for development and progression of diabetes complications. More recently, it has also been recommended for use in diagnosis.
There have been many advances in the treatment of diabetes, two of the most important being the development of portable blood glucose meters that can be used for self-monitoring of blood glucose by patients, and the establishment of HbA1c as an important long-term indicator of glycemia. These developments have allowed patients and their healthcare providers to better assess (and therefore better manage) glycemic status. Another important development has been the establishment of a definitive link between glycemia and the risks for diabetes complications. Until the early 1990s there was much debate over whether blood glucose levels in persons with diabetes could be maintained close to normal long-term, and whether doing so would actually prevent or delay the development of complications. However, the results of long-term prospective trials published in the 1990s provided irrefutable proof that diabetes complications are directly related to mean glycemia as measured by HbA1c [2, 3]. This in turn has led to recommendations that patients achieve specific HbA1c target levels in order to minimize risks for complications. However, the initial lack of HbA1c standardization made it difficult to utilize these targets in clinical practice due to substantial variability in results among methods and laboratories [4–6]. Fortunately, efforts to standardize HbA1c testing have been highly successful, resulting in much improvement in the comparability of results.
The purpose of this report is to review the “long and winding road” to better HbA1c measurements for optimal diabetes care. Along the way there have been “detours” that, while distracting from the end goal of improving the quality of HbA1c measurements, have nonetheless helped to better define the optimal use of HbA1c and set goals for improvement.
2. Background
In 1955 Kunkel and Wallenuis reported the separation of minor hemoglobin components on starch block electrophoresis [7]. Several investigators subsequently utilized ion-exchange chromatography to separate several minor hemoglobin components which were eventually labeled HbA1a, HbA1b, HbA1c, HbA1d and HbA1e in order of their elution from the column [8–10]. In 1968 Rahbar and colleagues reported that HbA1c levels were elevated in subjects with diabetes [11]. During the 1970s and early 1980s the structure and formation of HbA1c were further characterized [12–14]. Of the fast-moving hemoglobins originally identified by cation-exchange chromatography in the 1950s, HbA1c is present in the greatest quantity in both diabetic and non-diabetic individuals. HbA1c is a specific glycated hemoglobin (GHB) formed by the interaction of glucose and the amino-terminal valine of one or both beta chains of HbA in a multistep condensation reaction of glucose with an amine moiety of hemoglobin [15]. Glycation of hemoglobin also takes place at sites other than the ends of the beta chains, such as the epsilon amino groups of lysine residues and the amino-terminal valines of the alpha chains. These GHBs, unlike HbA1c, cannot be separated from non-glycated hemoglobins by methods that separate molecules based on charge differences. It was not until the early 1980s that these other glycated components, in addition to HbA1c, could be measured using boronate affinity chromatography [16, 17].
GHBs, including HbA1c, are formed continuously from HbA at a rate proportional to the ambient glucose concentration. Therefore, the amount of GHB is a function of the erythrocyte lifespan and the glucose levels to which the erythrocytes have been exposed. HbA1c is thus a “weighted” average of blood glucose levels during the preceding 120 days, meaning that glucose levels in the preceding 30 days contribute substantially more to the level of HbA1c than do glucose levels 90–120 days earlier [18]. This explains why the level of HbA1c can increase or decrease relatively quickly with large changes in glucose; it does not take 120 days to detect a clinically meaningful change in HbA1c following a clinically significant change in average glucose. HbA1c is free of the large fluctuations that can occur with glucose measurements.
As the popularity of HbA1c testing increased, so did the number of assays available for the clinical laboratory. In the early 1970s Trivelli, et al [19] measured HbA1c by cation-exchange chromatography but this method was too laborious for routine clinical laboratories. The method was subsequently modified for routine use, and in the late 1970s ion-exchange mini-columns became available; ion-exchange HPLC methods soon followed. Methods using agar gel electrophoresis, boronate affinity chromatography and immunoassay were subsequently developed and by the early 90s there were several methods available which used different method principles and measured different glycated components (HbA1, HbA1c, total GHB). The principle of all methods is to either separate the glycated and nonglycated forms of hemoglobin or separate HbA1c specifically from the other hemoglobins. This is accomplished based on either differences in charge (e.g. by ion-exchange HPLC with measurement of HbA1c) or structure (e.g. by immunoassay with measurement of HbA1c or by boronate affinity chromatography with measurement of total GHB). Unfortunately, there was no consensus on either a reference method or reference material and absolute numbers generated in one laboratory could not be compared easily to numbers generated in another laboratory, even if both used the same basic assay method and/or the same glycated component [5].
3. HbA1c: A Link to Clinical Outcomes in Diabetes
Before 1993, HbA1c was used in a general way for estimating the level of glycemic control, e.g. lower HbA1c meant lower average glucose. Studies published in the late 1980s and early 1990s indicated a link between glycemic control and diabetes complications [20–24], and the European NIDDM Policy Group published a consensus document in 1988 that included targets for blood glucose control[25], but there were no explicit HbA1c target levels (due largely to an acknowledged lack of HbA1c standardization) and not everyone was convinced that tighter control of glucose levels led to better outcomes. It was not until the publication of the results of the Diabetes Control and Complications Trial (DCCT) in 1993 that the importance of HbA1c as an indicator of both mean glycemia and corresponding outcome risks was firmly established [2].
The DCCT was a large (1441 patients followed over a mean of 6.5 years) prospective long-term randomized trial that provided definitive proof that intensive glycemic control significantly reduces the risk of long-term diabetes complications and allowed establishment of specific HbA1c treatment goals. Soon after the publication of the results of the DCCT, the American Diabetes Association (ADA) recommended HbA1c of 7% and an action limit of 8% as a general treatment goal for all patients with diabetes. However, lack of standardization made it difficult for healthcare providers to utilize these HbA1c targets in clinical practice since there was no way of knowing how their patient’s test results compared to those from the DCCT. Proficiency testing results from 1993 showed that there was considerable variability within and between methods and differences in the analytes reported (HbA1c, HbA1 or total GHB); for example, a result of 10% by one method could be a 15% by another [26].
Because of the positive impact standardization of HbA1c determinations would have on the care of diabetic patients, the American Association for Clinical Chemistry (AACC) Standards Committee established a HbA1c Standardization Subcommittee in April 1993. The goal of the subcommittee was to develop a plan for HbA1c standardization that would ultimately allow individual clinical laboratories to relate their HbA1c assay results to those of the DCCT, where relationships of HbA1c values to mean blood glucose and to risks for developing chronic diabetic complications had been established.
Although the DCCT was completed in 1993, the HbA1c assay systems from the study were to remain in place as part of another National Institutes of Health-sponsored long-term diabetes study called the Epidemiology of Diabetes Interventions and Complications (EDIC); the EDIC would follow the DCCT patients for many years to come. In order to initiate a standardization program in a timely fashion, the subcommittee recommended that the DCCT reference method be used as an interim reference method while studies were being performed to evaluate candidate reference methods and to develop purified HbA1c standards. Standardizing HbA1c results to DCCT values would allow individual clinical laboratories to provide diabetic patients and their health-care providers with test results that could be related directly to risks for development and/or progression of chronic diabetes complications. Although the DCCT included only patients with Type 1 diabetes, results from a similar study in Type 2 diabetes, the United Kingdom Prospective Diabetes Study (UKPDS) [3] also showed a direct relationship between glycemic control (measured by HbA1c) and risk for complications. Fortunately, HbA1c results from the DCCT and UKPDS were linked to a common reference.
4. The NGSP Reference Method and Standardization
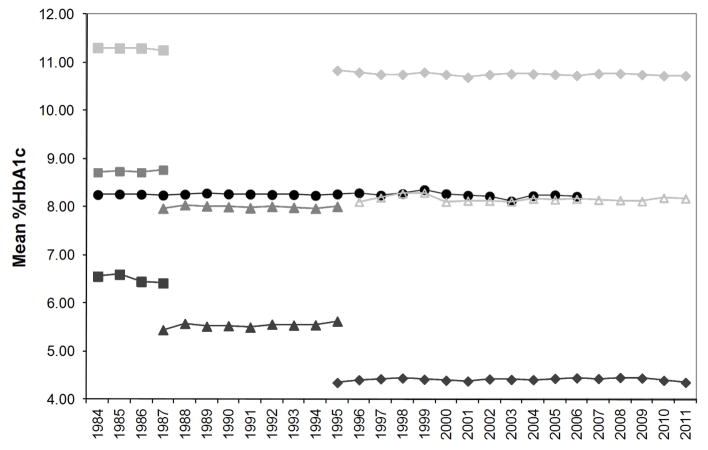
It was recognized that the HbA1c results reported by the DCCT Reference Method were not “true” values since there was known to be some nonspecificity in the measurement. Nevertheless, expediency, consistency over time, and a direct relationship with clinical outcomes were considered to be most important. The long-term stability of this method is shown in Figure 1.
Figure 1.
Mean HbA1c measured by the CPRL for one very long term QC specimen and six additional QC specimens measured for shorter periods. Each point represents the mean of 34–653 measurements during each year of use. For each point the CV was <3%.
Early efforts to standardize HbA1c/GHB results among clinical laboratories by using a “universal calibrator” proved feasible with some assay methods [27]. Later studies showed, however, that such an approach, although simple, did not work for a number of existing methods [28]. It was found that materials prepared for use as calibrators, quality control materials, and proficiency-testing samples are often subjected to preparative processes that may cause them to yield results that differ appreciably from those of patient specimens (matrix effects). Since an important goal was to allow standardization of most existing and future assay methods, it was proposed that, for most assay methods, standardization to the DCCT reference could be performed best at the manufacturing level, where the most appropriate materials and standardization format for each method could be determined. It was also proposed that verification of method standardization should be based on fresh sample comparisons with the DCCT Reference Method in order to avoid any potential matrix effects due to the use of processed materials.
5. The NGSP Network and Process
The NGSP HbA1c standardization program began in 1996 to implement the recommendations of the AACC subcommittee. The NGSP approach to HbA1c assay standardization was modeled after the US Cholesterol Reference Method Laboratory Network program [29]. The Cholesterol program was based on performing split-sample comparisons with the cholesterol reference method and thus provided a means for manufacturers to establish traceability to the National Reference System for Cholesterol. For HbA1c standardization, a network of reference laboratories is calibrated to the DCCT Reference values.
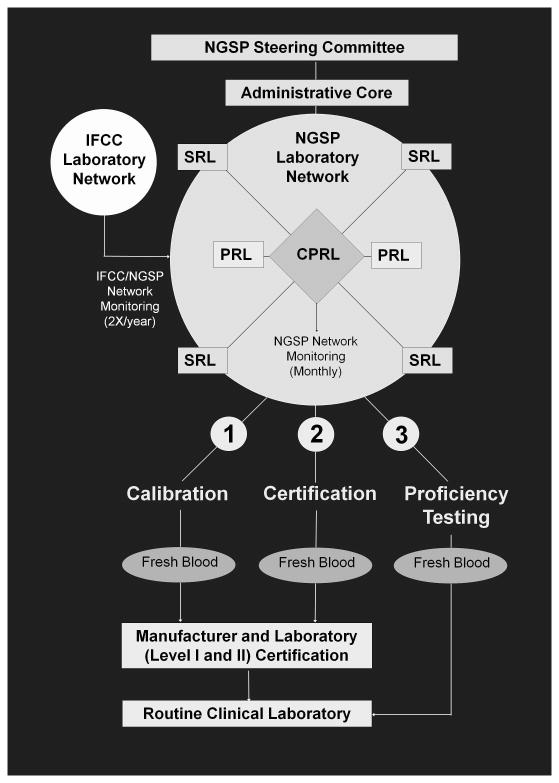
The NGSP network and process are shown in figure 2. The NGSP consists of a Steering Committee and a network of reference laboratories including the Central Primary Reference Laboratory (CPRL), backup Primary Reference Laboratories (PRLs) and Secondary Reference Laboratories (SRLs). The Steering Committee works with the Laboratory Network to implement the HbA1c Standardization Program according to the protocol. The committee is responsible for reviewing policy/protocol changes and quarterly reports submitted by the Laboratory Network. The NGSP Network consists of an Administrative Core (NETCORE), a CPRL, two PRLs, one in the US and one in Europe, and eight SRLs, three in the US, four in Europe, and one in Asia. The NETCORE coordinates the certification process and communicates directly with the Steering Committee. The Core analyzes all certification and network monitoring data, sends reports to the Steering Committee, and issues certificates to laboratories and manufacturers.
Figure 2.
NGSP Network and Process: CPRL, Central Primary Reference Laboratory; PRL, Primary Reference Laboratory; SRL, Secondary Reference Laboratory
The CPRL and PRLs analyze HbA1c using the same Bio-Rex 70 cation-exchange assay method; the CPRL is located in the same laboratory that was used in the DCCT. The CPRL set the initial calibration for the standardization program based on the “set-point” used in the DCCT. In this way, clinical results could match those reported in the DCCT/EDIC and UKPDS which would facilitate use of the treatment goals recommended by the ADA [30]. The PRLs serve as back-up laboratories for the CPRL to ensure that the CPRL function continues without interruption in the event that the CPRL can no longer meet the needs of the program. PRLs and SRLs calibrate their assays so that results from fresh blood specimens match with those of the CPRL. The CPRL administers a monthly monitoring program for all NGSP network laboratories using frozen whole blood pools. The SRLs work directly with manufacturers to assist them in calibrating their methods, and provide data for certification of traceability to the DCCT. The SRLs use highly precise commercial methods that utilize different method principles (including ion-exchange HPLC, boronate affinity HPLC, immunoassay, and capillary electrophoresis) but use a different calibration scheme than that which is provided by the manufacturer. Specific network certification and monitoring criteria are described on the NGSP website [31].
The three major processes of the NGSP are also shown in figure 2. NGSP network laboratories can assist manufacturers with the calibration of their methods. Once calibrated, methods can be certified by the manufacturer. The certification process consists of an exchange of 40 fresh or frozen whole blood samples representing a specified range of HbA1c values between a manufacturer and an NGSP SRL of their choice. A manufacturer is awarded a Certificate of Traceability if 37/40 of the individual results are within 7% of the SRL mean. Each certificate is effective for one year from the date of certification. In order to maintain continuous certification, the certification process must be repeated annually. A summary of NGSP certification and monitoring activities is shown in Table 1. Criteria for both certification and monitoring have tightened over the years since the NGSP began. Individual laboratories can also be certified if they choose; these laboratories are usually participating in clinical trials or performing high volume testing. The certification process for laboratories is the same as that for manufacturers, but there are two levels of laboratory certification. Level II certification criteria are the same as those for manufacturers. Level I certification is tighter; 38/40 of the individual results must be within 7% of the SRL mean. Level I laboratories are also monitored quarterly using the same process and criteria used for monthly monitoring of NGSP network laboratories.
Table 1.
NGSP Certification and Monitoring
| Certification Type | # samples compared | Certification criteria | Monitoring (yes/no) | Monitoring Protocol |
|---|---|---|---|---|
| Network Lab | 100 | CV<3%; 95%CI bias within ±3% at 6 and 9% HbA1c | Yes | 10 Samples Monthly |
| Manufacturer | 40 | 37 of 40 results within ± 7% | No | - |
| Level I Lab | 40 | 38 of 40 results within ± 7% | Yes | 10 Samples Quarterly |
| Level II Lab | 40 | 37 of 40 results within ± 7% | No | - |
The third component of the NGSP process is surveillance of the CAP HbA1c proficiency data. This process is critical for monitoring the success of the NGSP. The CAP GH-2 survey is performed twice a year. Beginning in 1998, fresh whole blood samples were used and target values assigned by the mean of NGSP SRLs. Grading became accuracy-based in 2007 and the acceptance criteria have since been gradually tightened to the current limit of +/−7% of the NGSP target (+/−6% limit is planned to begin in 2013). This has added momentum for laboratories and manufacturers to improve the quality of HbA1c testing as we move further down the road to better HbA1c.
6. Global Standardization and The IFCC network
In 1995 the IFCC Working Group on HbA1c Standardization, which included members of the NGSP Steering Committee, was initiated to develop a higher order reference method and reference materials for HbA1c measurement that would fulfill the requirements of the European Directive [32] for HbA1c methods to show traceability to higher order reference methods. Although the NGSP CPRL method is listed as a Reference Method in the database of the Joint Committee on Traceability in Laboratory Medicine [33], the method is not the highest order reference method available. The IFCC method would be an estimate of “true value” with documented unbroken traceability to pure reference materials. The IFCC established a laboratory network that includes 14 laboratories/methods at the time of this report. Each laboratory uses 1 or both of the 2 approved IFCC methods, namely HPLC – mass spectrometry or HPLC – capillary electrophoresis [34, 35], with results being essentially identical between them since they use the same primary reference materials for calibration. The IFCC also offers manufacturers matrix-appropriate materials (frozen whole blood) with values assigned by the IFCC network to establish and check traceability [36].
The IFCC Reference Method for HbA1c was approved by IFCC national members in 2001. However, there was a major obstacle in the implementation of the IFCC program because there was a bias between the NGSP and IFCC results; IFCC results were between 1.5 and 2% HbA1c lower than NGSP results across the measurement range. Despite the fact that the IFCC network and process provided traceability to a “true value”, and the IFCC method would become the anchor for international standardization, there were concerns expressed by the NGSP and major clinical organizations that changing the numbers reported in routine practice could cause confusion. One set of results could be confused with the other causing misinterpretation that could adversely affect patient care. This concern, plus the fact that there was no evidence that a change in reported results from NGSP to IFCC would improve the quality of patient care, led to years of debate about which numbers should be reported in clinical practice.
In 2007 the IFCC, ADA, European Association for the Study of Diabetes (EASD), and the International Diabetes Federation issued a consensus statement on the worldwide standardization of HbA1c [37]. It recognized that the IFCC reference system should be the anchor for worldwide standardization, but also recommended that HbA1c be reported in both IFCC and NGSP units. To avoid any confusion, IFCC results were to be reported in mmol/mol. IFCC numbers reported in mmol/mol would now be approximately ten times higher than NGSP results, which would continue to be reported as a percent. It was also agreed that values could be reported as estimated average glucose (eAG), as previously recommended by several clinical organizations, if the outcome of a forthcoming study examining the relationship between average blood glucose and HbA1c showed this to be feasible [38]. Although subsequent publication of results from this study established a linear relationship between average glucose and HbA1c and declared that HbA1c results could be reported as eAG, many experts believed there was too much variability in the HbA1c/glucose relationship and the eAG should not be reported [39]. An updated consensus statement issued in 2010 no longer included the recommendation to report eAG [40].
Despite the recommendations in the consensus statements, the decision of how HbA1c will be reported is being done country by country. At this point some countries have decided to report HbA1c in NGSP %, some have decided to report in IFCC mmol/mol, some report both units, and some have not yet decided. Only the US has recommended reporting eAG along with HbA1c, and the US plans to continue to report HbA1c as NGSP%. Despite the lack of global consensus for reporting HbA1c there is now a clear way to relate the different reported results. With the use of a master equation based on many years of NGSP/IFCC Network to network comparisons (NGSP = [0.09148 * IFCC] + 2.152), NGSP and IFCC units can easily be converted from one to the other. This equation is continuously monitored to insure that the relationship between the “true values” (IFCC) and clinical studies/treatment goals (NGSP/DCCT) remains stable. The NGSP and IFCC Reference Systems serve different but complementary purposes, with the former applying defined acceptable limits for method performance that are based on clinical requirements and the latter providing traceability to an accuracy base. There are still unanswered questions about how all these changes will affect global standardization of HbA1c. There is some evidence that a change in the scale of HbA1c results can affect patient glycemic control. This psychological effect was reported in Sweden when there was a change from the Swedish Mono-S calibration to numbers comparable to those from the DCCT; after adjusting for the higher DCCT assay calibration, patient HbA1c results actually decreased significantly indicating improvement of glycemic control [41]. Conversely, when the laboratory changed back to their Mono-S calibration patient glycemic control worsened. Although the IFCC change from % to mmol/mol will increase patients’ HbA1c results, it is difficult to predict what effect such a large increase (almost 10 fold) will have on patient care. Much will depend on how the changes are introduced country by country to healthcare providers and to their patients.
7. Use of HbA1c for Diagnosis
Historically, every scheme for the diagnosis of diabetes has relied on measurement blood glucose in timed samples (i.e. fasting or after a glucose load). The use of HbA1c for diabetes diagnosis had been debated for many years [42]. In the past HbA1c was considered but not accepted for use in diagnosis due in part to lack of standardization. However, in 2009, an International Expert Committee recommended HbA1c for diagnosis [43], stating that compared with the measurement of glucose, the HbA1c assay was at least as good at defining the level of hyperglycemia at which retinopathy prevalence increases. The HbA1c test also had some practical and technical advantages compared to glucose. Following the Expert Committee report, the ADA recommended that HbA1c levels ≥6.5% could be used to diagnose diabetes and that the diagnosis should be confirmed with a repeat HbA1c test. In addition, the ADA recommendations stated that a HbA1c level ≥5.7% but <6.5% is indicative of prediabetes, or risk for progression to diabetes [44]. Other clinical organizations, including the World Health Organization and EASD have subsequently recommended HbA1c for diagnosis although their recommendations are not all identical to those of the ADA [45, 46]. Although the recommendations to use HbA1c for diagnosis represents a clinically useful step along the road to better HbA1c, these recommendations have prompted fierce debate about how diabetes should be defined and whether HbA1c should be used for diagnosis [47]. Oral glucose tolerance testing (OGTT) is clearly more sensitive than HbA1c but HbA1c shows more specificity; although there is overlap, the different diagnostic tests do not identify all of the same individuals. The HbA1c test would miss some people diagnosed by OGTT but many that can be diagnosed with HbA1c (e.g. from a non-fasting sample) may not be tested at all if they need to be fasting for a fasting glucose test or an OGTT. Clearly there are advantages and disadvantages to the different diagnostic tests.
Along with debate about whether or not HbA1c should be used for diagnosis, there was another roadblock. In the US, although physicians have actually been using HbA1c as a diagnostic tool for many years, the claims made by manufacturers of HbA1c methods do not cover their use for diagnosis. In order for a diagnosis claim to be included in product inserts, a new claim will have to be approved by the US Food and Drug Administration (FDA) and this has proven to be a difficult task. More than two years after the first clinical recommendation to use HbA1c for diagnosis, discussions continue regarding the criteria that should be used for approval of diagnosis claims.
8. The Current Status of HbA1c Measurement
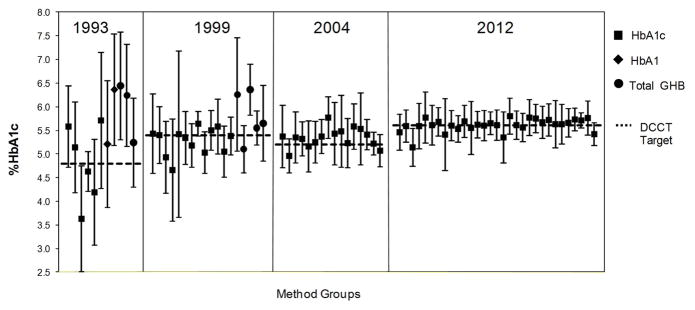
The NGSP uses data from the CAP HbA1c proficiency testing program to assess the success of standardization and the improvement in HbA1c measurement. Figure 3 shows CAP data from HbA1c surveys in 1993, 1999, 2004 and 2012. It is clear that despite all of the obstacles on the way to better HbA1c measurement, there has been considerable progress made since 1993 when the DCCT ended. There has been a steady increase in the number of methods and laboratories that have been certified since the NGSP was initiated in 1996. As of mid 2012 approximately 110 methods and 110 laboratories were certified. This continuous rise in certification documents the ability of manufacturers to continuously improve their methods with progressive tightening of certification criteria. The increase in both manufacturer and laboratory certifications also reflects continued demand for methods and laboratories that can meet the needs of diabetes care teams, clinical research and clinical trials. A list of NGSP certified methods and laboratories (updated monthly) is available on the NGSP website [48].
Figure 3.
Mean of each method compared to the NGSP/DCCT target (dotted lines) in 1993, 1999, 2004, and 2012 based on CAP GH2 survey data. Symbols represent the mean; error bars are ±2SD.
In determining goals for HbA1c measurement, it is important to realize that there was only a 2% HbA1c difference between mean HbA1c in the DCCT intensive and standard treatment groups; this small difference translated into a very significant decrease in the risk for many diabetic complications; 34–76%, 56%, and 60% for retinopathy, albuminuria, and neuropathy, respectively. Clinically, HbA1c is used not only to determine if glycemic control is improving or worsening (HbA1c relative to a previous result) but also to determine how close the patient is to their HbA1c goal (HbA1c relative to a fixed limit). Thus, in determining the analytical performance required for optimal clinical use, both bias from a reference and imprecision must be considered. In general, based on ADA and National Institute for Health and Clinical Excellence treatment guidelines, 0.5% HbA1c is considered a clinically significant change [49, 50] and specific treatment goals for HbA1c are recommended. Lind, et al [51], in a study of the temporal relationship between HbA1c and diabetic complications in the DCCT, found that for a reduction in HbA1c from 8.3% to 8.0%, only 13 patients would need to be treated during the 9 to 12 years after diagnosis in order to prevent one event of retinopathy. In addition, HbA1c is now recommended for prediabetes and diabetes diagnosis with cutoffs of 5.7 and 6.5% respectively. Therefore, goals for HbA1c assay precision and bias must be stringent in order to meet diagnostic and clinical expectations.
In 2012 most method means are close to the NGSP target but some still have significant bias (figure 3). Within-method variability is still also quite high for some methods but very small for others. For some methods (approximately 30%), pass rates at the current CAP limit of ±7% were between 95 and 100% while others, with more bias and/or variability, had lower pass rates. However, 90% of laboratories were using methods with between-laboratory CVs ≤3.5% (based on the 2012 CAP data for a sample in the 7% HbA1c range) and the overall pass rate was ~95%. Therefore 95% of laboratories can provide a result within 0.5% HbA1c of the ADA 7% HbA1c target (7% of 7% HbA1c is ~0.5% HbA1c). It should be noted that NGSP certification is performed by the manufacturer, typically using a single lot of reagents and calibrators, while the CAP survey involves individual laboratories which may be utilizing many different reagent and/or calibrator lots. This could explain why performance of some methods in the field may not be adequate even though all the current methods listed on the CAP report have been certified.
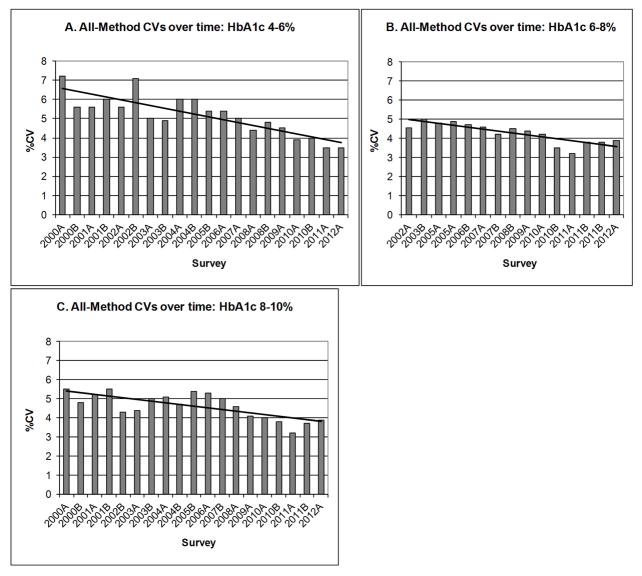
Recent guidelines for the laboratory analysis in the diagnosis and management of diabetes [52] recommend that within-laboratory CV should ideally be less than 2% and between-laboratory CV should be less than 3.5%. It is encouraging that most (but certainly not all) CAP survey participants are using methods that can provide within-laboratory CVs of <2%, and most laboratories are using methods with between-laboratory, within-method CVs of <3.5%. Figure 4 shows overall CVs (all methods’ results combined) for each individual CAP sample from 2000 through 2012 separated into three categories based on HbA1c level (4–6%, 6–8%, 8–10%). This data shows that the all-method CVs have been decreasing since 2000 and that some CVs in past few surveys are down to 3.5%. Although the CAP survey participants are mostly from the US (~90%), similar methods are used in Europe and many other developed countries. It is difficult to know the status of HbA1c measurement in other areas of the world that may have methods that are more localized and possibly not NGSP certified. The goal of 3.5% for all-method results is close to being achieved.
Figure 4.
CVs for all HbA1c results on the GH2 CAP surveys between 2000 and 2012 for samples with assigned HbA1c values of (A) 4–6%, (B) 6–8%, and (C) 8–10%. The bold solid line represents the trend line.
9. Point-of-Care (POC) Testing
An important issue in efforts to improve the quality of HbA1c testing is the status of point-of-care (POC) HbA1c methods. CAP survey data indicate that POC methods for HbA1c, as a group, do not necessarily perform worse than many laboratory-based HbA1c methods. However, POC methods are CLIA-waived, meaning that laboratories or physicians’ offices that use these methods are not required to participate in CAP or other proficiency surveys and the vast majority choose not to do so. We therefore have little or no CAP proficiency data for most POC methods and therefore have little knowledge of how these methods actually perform in the hands of end-users. This is a major concern given the widespread use of POC tests and the fact that recent reports indicate that some POC methods have significant issues in terms of assay performance and lot-to-lot variation [53, 54].
10. Limitations of HbA1c Testing
For the vast majority of patients with diabetes, HbA1c provides an excellent measure of glycemic control. Similarly, for most people HbA1c can be used for diagnosing diabetes. However, in certain situations HbA1c may be unreliable. These include any condition that alters erythrocyte life span (e.g. renal anemia with erythropoietin use, hemolytic anemia), severe iron-deficiency anemia, and recent blood transfusions; such conditions will affect HbA1c results regardless of assay methodology. In addition, hemoglobin variants or adducts can cause method-specific interferences. Investigators have also reported a certain degree of between-individual variability in HbA1c resulting from a number of potential factors such as age or race [54–56], although the issue of whether these differences are caused by direct HbA1c interference or actual differences in mean glycemia is a subject of debate.
10.1 Factors that interfere with HbA1c Testing
The most common analytical interferences are Hb variants, elevated fetal hemoglobin (HbF) and Hb adducts. The most common Hb variants are hemoglobins S, C, D and E. These variants do not cause any hemolytic disease in the heterozygous form so glycation should not be affected. For each of these variants some, but not all, HbA1c methods are affected, thus this interference is both method and variant-specific and it is difficult to generalize the effect of variants based upon method type and substitution [57]. For this reason the NGSP has attempted to evaluate all methods for interference from these four hemoglobin variants. Results of all published studies have been reviewed and a summary (including references) is shown on the NGSP website [58]. In some cases reported results do not agree; this may be due to differences among reagent lots and method changes over time for a particular method. A summary of these results for most of the current assay methods is shown in Table 2. Based on evaluations of some immunoassays and the fact that the HbD and HbE mutations are distant from the N-terminus of the hemoglobin beta chain where the antibody binds to HbA1c, in the absence of specific data it has been assumed that there is no clinically significant interference from HbE and HbD for HbA1c immunoassays. Similarly, based on data from several immunoassay and boronate affinity methods, in the absence of specific method data it can generally be assumed that both immunoassay and boronate affinity methods show interference from HbF levels above ~10–15% [59]. This interference is likely due to a lower glycation rate for HbF compared with HbA; HbF has gamma chains, for which the terminus is a glycine residue, in place of beta chains where the valine terminal residues can be glycated to form HbA1c. Thus, for boronate affinity methods, since HbF is glycated to a lesser extent, the glycated fraction will be lower than for people without elevated HbF. For immunoassay, lack of glycation at the terminus of the gamma chain of HbF results in less antibody binding, while the total Hb measurement includes HbF as well as HbA, leading to a lower HbA1c result. Elevated HbF affects some but not all ion-exchange methods. Many of these methods can report the percent of HbA1c relative to the amount of total HbA while excluding variant or HbF peaks. The degree of interference depends on how well HbF or other variant peaks are separated from the HbA1c and HbA0 peaks, and these separations may be dependent upon column or reagent lots. Several less common Hb variants have also been evaluated with some methods [60–63].
Table 2.
Interference from Hb Variants and Elevated HbF
| Method | Interference from HbC | Interference from HbS | Interference from HbE | Interference from HbD | Interference from elevated HbF |
|---|---|---|---|---|---|
| Abbott Architect/Aeroset | Yes | Yes | a | a | b |
| Arkray ADAMS A1c HA-8180V (Also sold by Menarini) | No | No | HbA1c not quantified | HbA1c not quantified | No |
| Axis-Shield Afinion | No | No | No | No | b |
| Bayer A1cNOW | Yes | Yes | No | No | b |
| Beckman AU system | Yes | Yes | No | No | b |
| Beckman Synchron System | No | No | No | No | b |
| Bio-Rad D-10 | No | No | No | No | Yes >10% HbF |
| Bio-Rad Variant II NU | - | - | No | No | Yes >10% HbF |
| Bio-Rad Variant II Turbo | No | No | Yes | Yes | Yes >5% HbF |
| Bio-Rad Variant II Turbo 2.0 | No | No | No | No | Yes >25% HbF |
| Bio-Rad in2it | Yes | No | Yes | No | b |
| Ortho-Clinical Vitros | No | No | No | No | b |
| Roche Cobas Integra Gen.2 | No | No | No | No | b |
| Roche/Hitachi (Tina Quant II) | No | No | No | No | b |
| Sebia Capillarys 2 Flex Piercing | No | No | No | No | - |
| Siemens Advia A1c | No | No | a | a | b |
| Siemens DCA 2000 | No | No | No | No | Yes >10% |
| Siemens Dimension | No | No | No | No | b |
| Tosoh G7 | Yes | No | Yes | No | No |
| Tosoh G8 | No | No | Yes | No | No |
| Trinity (Primus) HPLC (affinity) | No | No | No | No | Yes >15% HbF |
In the absence of specific method data, it can generally be assumed that immunoassay methods do not have clinically significant interference from HbE and HbD because the E and D substitution are distant from the N-terminus of the hemoglobin β chain
In the absence of specific method data, it can generally be assumed that both immunoassay and boronate affinity methods show interference from HbF levels above ~10–15%
10.2 Factors that affect the interpretation of HbA1c Results
There are several factors that affect the interpretation of the HbA1c result relative to glycemic control. For example, in most individuals a HbA1c result of 7% is roughly equivalent to an average plasma glucose of 154 mg/dL [38]. But for someone with an altered red cell lifespan (e.g. as in renal anemia) or altered glycation rate (as in iron deficiency anemia) a 7% HbA1c could actually relate to a different average glucose. One of the most common conditions that will affect HbA1c result interpretation is in cases of renal failure [64] where results can be 1–2% HbA1c low compared to patients with normal kidney function [64–66]. Since it is critical to maintain adequate glycemic control in these patients, misinterpretation of results could result in higher than expected risk of complications.
Another condition that is fairly widespread in developing countries and appears to lead to artificially high HbA1c results is iron deficiency anemia; iron replacement therapy lowers HbA1c back to expected levels [67, 68]. The possible mechanism for this interference may be that an increase in malondialdehye in patients with iron deficiency anemia enhances the glycation of hemoglobin [69]. Therefore, alternative measures must be used for glycemic assessment in the presence of significant iron deficiency anemia until the condition is successfully treated.
11. Conclusions
The availability of more accurate and precise HbA1c methods facilitates better diabetes care. While the test does not substitute for day to day testing of blood glucose, it is a useful index of long-term glycemia in most patients with diabetes and offers an alternative to timed glucose testing for diagnosis of diabetes. HbA1c is also a powerful risk predictor. In 1968 when it was discovered that HbA1c was increased in people with diabetes, few could have envisioned that more than 40 years later, measurement of HbA1c would become an indispensible part of both routine management of diabetes and diagnosis. Considerable progress has been made in standardizing HbA1c measurements since landmark clinical trials (e.g. DCCT, UKPDS) clarified the relationship between glycemic control (measured by HbA1c) and complications. But there is still more to do. With the tightening of NGSP and proficiency testing criteria, industry has continued to improve HbA1c methods. Insuring that adequate methodology that is traceable to the IFCC and NGSP is available worldwide is still a concern, especially in the developing world. Interferences with HbA1c measurement and interpretation will continue to be investigated. Long term epidemiological studies such as the EDIC continue to highlight the importance of tight glycemic control while other studies define the limits of tight control in some patient populations [70]. We now have more and better tools for managing patients with diabetes but there is still much to be learned about how to apply these tools and specifically how to use the HbA1c test to achieve better patient outcomes.
Abbreviations
- HbA1c
hemoglobin A1c
- DCCT
Diabetes Control and Complications Trial
- UKPDS
United Kingdom Prospective Diabetes Study
- NGSP
National Glycohemoglobin Standardization Program
- IFCC
International Federation of Clinical Chemistry
- GHB
Glycated hemoglobin
- ADA
American Diabetes Association
- AACC
American Association for Clinical Chemistry
- CPRL
Central Primary Reference Laboratory
- PRL
Primary Reference Laboratory
- SRL
Secondary Primary Reference Laboratory
- NETCORE
Network Administrative Core
- EASD
European Association for the Study of Diabetes
- eAG
estimated average glucose
- OGTT
Oral glucose tolerance test
- FDA
Food and Drug Administration
- POC
Point of Care
Contributor Information
Randie R. Little, Email: littler@health.missouri.edu.
Curt Rohlfing, Email: rohlfingc@health.missouri.edu.
References
- 1.World Health Organization. WHO Diabetes Media centre. 2012. [Google Scholar]
- 2.DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. New Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–851. [PubMed] [Google Scholar]
- 4.Baynes JW, Bunn HF, Goldstein D, et al. National Diabetes Data Group: report of the expert committee on glucosylated hemoglobin. Diabetes Care. 1984;7:602–606. doi: 10.2337/diacare.7.6.602. [DOI] [PubMed] [Google Scholar]
- 5.Little RR, Wiedmeyer HM, England JD, Naito HK, Goldstein DE. Interlaboratory comparison of glycohemoglobin results: College of American Pathologists Survey data. Clin Chem. 1991;37:1725–1729. [PubMed] [Google Scholar]
- 6.Weykamp CW, Penders TJ, Miedema K, Muskiet FA, van der Slik W. Standardization of glycohemoglobin results and reference values in whole blood studied in 103 laboratories using 20 methods. Clinical Chemistry. 1995;41:82–86. [PubMed] [Google Scholar]
- 7.Kunkel HG, Wallenius G. New hemoglobin in normal adult blood. Science. 1955;122:288. doi: 10.1126/science.122.3163.288. [DOI] [PubMed] [Google Scholar]
- 8.Huisman TH. Studies on the heterogeneity of hemoglobin. XI. Chromatographic studies of intermediate forms of oxy- and ferrihemoglobin. Arch Biochem Biophys. 1966;113:427–434. doi: 10.1016/0003-9861(66)90209-8. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder WAHW. In: Structural Chemistry and Molecular Biology. Rich ADN, editor. San Francisco: 1968. [Google Scholar]
- 10.Clegg MDSW. A chromatographic study of the minor components of normal adult human hemoglobin. J Am Chem Soc. 1959;81:6065–6069. [Google Scholar]
- 11.Rahbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta. 1968;22:296–298. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- 12.Bookchin RM, Gallop PM. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968;32:86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- 13.Bunn HF. Nonenzymatic glycosylation of protein: relevance to diabetes. Am J Med. 1981;70:325–330. doi: 10.1016/0002-9343(81)90769-5. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro R, McManus MJ, Zalut C, Bunn HF. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980;255:3120–3127. [PubMed] [Google Scholar]
- 15.Bunn HF, Haney DN, Gabbay KH, Gallop PM. Further identification of the nature and linkage of the carbohydrate in hemoglobin A1c. Biochemical and Biophysical Research Communications. 1975;67:103–109. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- 16.Mallia AK, Hermanson GT, Krohn RI, Fujimoto EK, Smith PK. Preparation and Use of a Boronic Acid Affinity Support for Separation and Quantitation of Glycosylated Hemoglobins. Analytical Letters. 1981;14:649–661. [Google Scholar]
- 17.Klenk DC, Hermanson GT, Krohn RI, et al. Determination of glycosylated hemoglobin by affinity chromatography: comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clinical Chemistry. 1982;28:2088–2094. [PubMed] [Google Scholar]
- 18.Tahara Y, Shima K. The response of GHb to stepwise plasma glucose change over time in diabetic patients. Diabetes Care. 1993;16:1313–1314. doi: 10.2337/diacare.16.9.1313. [DOI] [PubMed] [Google Scholar]
- 19.Trivelli LA, Ranney HM, Lai HT. Hemoglobin components in patients with diabetes mellitus. New England Journal of Medicine. 1971;284:353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- 20.Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Two-year experience with continuous subcutaneous insulin infusion in relation to retinopathy and neuropathy. Diabetes. 1985;34(Suppl 3):74–79. doi: 10.2337/diab.34.3.s74. [DOI] [PubMed] [Google Scholar]
- 21.Feldt-Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet. 1986;2:1300–1304. doi: 10.1016/s0140-6736(86)91433-9. [DOI] [PubMed] [Google Scholar]
- 22.Feldt-Rasmussen B, Mathiesen ER, Jensen T, Lauritzen T, Deckert T. Effect of improved metabolic control on loss of kidney function in type 1 (insulin-dependent) diabetic patients: an update of the Steno studies. Diabetologia. 1991;34:164–170. doi: 10.1007/BF00418270. [DOI] [PubMed] [Google Scholar]
- 23.Reichard P, Berglund B, Britz A, Cars I, Nilsson BY, Rosenqvist U. Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med. 1991;230:101–108. doi: 10.1111/j.1365-2796.1991.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 24.Brinchmann-Hansen O, Dahl-Jorgensen K, Sandvik L, Hanssen KF. Blood glucose concentrations and progression of diabetic retinopathy: the seven year results of the Oslo study. BMJ. 1992;304:19–22. doi: 10.1136/bmj.304.6818.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti KGMM, Gries FA. Management of non-insulin-dependent diabetes mellitus in Europe: a concensus view. Diabet Med. 1988;5:275–281. doi: 10.1111/j.1464-5491.1988.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 26.CAP. Electrophoresis Chromatography Survey set EC-A CAP. 1993. [Google Scholar]
- 27.Little RR, England JD, Wiedmeyer HM, et al. Interlaboratory standardization of glycated hemoglobin determinations. Clinical Chemistry. 1986;32:358–360. [PubMed] [Google Scholar]
- 28.Little RR, Wiedmeyer HM, England JD, et al. Interlaboratory standardization of measurements of glycohemoglobins. Clin Chem. 1992;38:2472–2478. [PubMed] [Google Scholar]
- 29.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clinical chemistry. 2000;46:1762–1772. [PubMed] [Google Scholar]
- 30.American Diabetes Association. Implications of the Diabetes Control and Complications Trial. Diabetes Care. 1993;16:1517–1520. doi: 10.2337/diacare.16.11.1517. [DOI] [PubMed] [Google Scholar]
- 31.NGSP. NGSP Protocol Overview. 2012. [Google Scholar]
- 32.Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Off J L. 1998;331:1–37. [Google Scholar]
- 33.Joint Commission on Traceability in Laboratory Medicine. 2012 [Google Scholar]
- 34.Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 35.Weykamp C, John WG, Mosca A, et al. The IFCC reference measurement system for HbA1c: a 6-year progress report. Clin Chem. 2008;54:240–248. doi: 10.1373/clinchem.2007.097402. [DOI] [PubMed] [Google Scholar]
- 36.IFCC Network on HbA1c. 2012 [Google Scholar]
- 37.Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30:2399–2400. doi: 10.2337/dc07-9925. [DOI] [PubMed] [Google Scholar]
- 38.Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barth JH, Marshall SM, Watson ID. Consensus meeting on reporting glycated haemoglobin and estimated average glucose in the UK: report to the National Director for Diabetes, Department of Health. Ann Clin Biochem. 2008;45:343–344. doi: 10.1258/acb.2008.200815. [DOI] [PubMed] [Google Scholar]
- 40.Hanas R, John G. 2010 consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Clinical chemistry. 2010;56:1362–1364. doi: 10.1373/clinchem.2010.150540. [DOI] [PubMed] [Google Scholar]
- 41.Hanas R. Psychological impact of changing the scale of reported HbA(1c) results affects metabolic control. Diabetes Care. 2002;25:2110–2111. doi: 10.2337/diacare.25.11.2110. [DOI] [PubMed] [Google Scholar]
- 42.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 43.International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Diabetes Association. Standards of Medical Care in Diabetes 2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus:Abbreviated report of a WHO consultation. 2011. [PubMed] [Google Scholar]
- 46.AACE/ACE. American Association of Clinical Endocrinologists/American College of Endocrinology Statement on the Use of Hemoglobin A1c for the Diagnosis of Diabetes. Endocrine Practice. 2010;16:155–156. doi: 10.4158/EP.16.2.155. [DOI] [PubMed] [Google Scholar]
- 47.Bonora E, Tuomilehto J, Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184–190. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.NGSP. Certified Methods and Laboratories. 2012. [Google Scholar]
- 49.American Diabetes Association. Standards of Medical Care in Diabetes - 2009. Diabetes Care. 2009;32(suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institute for Health and Clinical Excellence (NICE) Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. [PubMed] [Google Scholar]
- 51.Lind M, Oden A, Fahlen M, Eliasson B. The shape of the metabolic memory of HbA1c: re-analysing the DCCT with respect to time-dependent effects. Diabetologia. 2010;53:1093–1098. doi: 10.1007/s00125-010-1706-z. [DOI] [PubMed] [Google Scholar]
- 52.Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34:e61–99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56:44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]
- 54.Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30:2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pani LN, Korenda L, Meigs JB, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31:1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiener K, Roberts NB. Age does not influence levels of HbA1c in normal subject. QJ Med. 1999;92:169–173. doi: 10.1093/qjmed/92.3.169. [DOI] [PubMed] [Google Scholar]
- 57.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin [Review] Clin Chem. 2001;47:153–163. [PubMed] [Google Scholar]
- 58.NGSP. HbA1c Assay Interferences. 2012. [Google Scholar]
- 59.Little RR, Rohlfing CL, Hanson SE, et al. The effect of increased fetal hemoglobin on 7 common HbA1c assay methods. Clin Chem. 2012;58:945–947. doi: 10.1373/clinchem.2012.181933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scuderi RT, Griffin TL, Mehta SP, Herold DA, Fitzgerald RL. Interference with hemoglobin A(1C) determination by the hemoglobin variant Shelby. Am J Clin Pathol. 2007;128:440–444. doi: 10.1309/WPY5UHR424VUHG8A. [DOI] [PubMed] [Google Scholar]
- 61.Nyenwe EA, Fisher JN. A mistaken diagnosis of type 2 diabetes due to hemoglobin N-Baltimore. Am J Med Sci. 2008;336:524–526. doi: 10.1097/MAJ.0b013e318164bcd3. [DOI] [PubMed] [Google Scholar]
- 62.Jain N, Kesimer M, Hoyer JD, et al. Hemoglobin Raleigh results in factitiously low hemoglobin A1c when evaluated via immunoassay analyzer. Journal of Diabetes & its Complications. 2011;25:14–18. doi: 10.1016/j.jdiacomp.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 63.van den Ouweland JM, van Daal H, Klaassen CH, van Aarssen Y, Harteveld CL, Giordano PC. The silent hemoglobin alpha chain variant Hb Riccarton [alpha51(CE9)Gly-->Ser] may affect HbA1c determination on the HLC-723 G7 analyzer. Clin Chem Lab Med. 2008;46:827–830. doi: 10.1515/CCLM.2008.169. [DOI] [PubMed] [Google Scholar]
- 64.Peacock TP, Shihabi ZK, Bleyer AJ, et al. Comparison of glycated albumin and hemoglobin A(1c) levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 65.Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 66.Freedman BI, Shenoy RN, Planer JA, et al. Comparison of glycated albumin and hemoglobin A1c concentrations in diabetic subjects on peritoneal and hemodialysis. Peritoneal Dialysis International. 2010;30:72–79. doi: 10.3747/pdi.2008.00243. [DOI] [PubMed] [Google Scholar]
- 67.Tarim O, Kucukerdogan A, Gunay U, Eralp O, Ercan I. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357–362. doi: 10.1046/j.1442-200x.1999.01083.x. [DOI] [PubMed] [Google Scholar]
- 68.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112:126–128. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 69.Sundaram RC, Selvaraj N, Vijayan G, Bobby Z, Hamide A, Rattina Dasse N. Increased plasma malondialdehyde and fructosamine in iron deficiency anemia: effect of treatment. Biomed Pharmacother. 2007;61:682–685. doi: 10.1016/j.biopha.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Bloomgarden ZT. Glycemic control in diabetes: a tale of three studies. Diabetes Care. 2008;31:1913–1919. doi: 10.2337/dc08-zb09. [DOI] [PMC free article] [PubMed] [Google Scholar]