Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive motor dysfunction and loss of large motor neurons in the spinal cord and brain stem. While much research has focused on mechanisms of motor neuron cell death in the spinal cord, degenerative processes in skeletal muscle and neuromuscular junctions (NMJs) are also observed early in disease development. Although recent studies support the potential therapeutic benefits of targeting the skeletal muscle in ALS, relatively little is known about inflammation and glial responses in skeletal muscle and near NMJs, or how these responses contribute to motor neuron survival, neuromuscular innervation, or motor dysfunction in ALS. We recently showed that human mesenchymal stem cells modified to release glial cell line-derived neurotrophic factor (hMSC-GDNF) extend survival and protect NMJs and motor neurons in SOD1G93A rats when delivered to limb muscles. In this study, we evaluate inflammatory and glial responses near NMJs in the limb muscle collected from a rat model of familial ALS (SOD1G93A transgenic rats) during disease progression and following hMSC-GDNF transplantation. Muscle samples were collected from pre-symptomatic, symptomatic, and end-stage animals. A significant increase in the expression of microglial inflammatory markers (CD11b and CD68) occurred in the skeletal muscle of symptomatic and end-stage SOD1G93A rats. Inflammation was confirmed by ELISA for inflammatory cytokines interleukin-1 β (IL-1β) and tumor necrosis factor-α (TNF-α) in muscle homogenates of SOD1G93A rats. Next, we observed active glial responses in the muscle of SOD1G93A rats, specifically near intramuscular axons and NMJs. Interestingly, strong expression of activated glial markers, glial fibrillary acidic protein (GFAP) and nestin, was observed in the areas adjacent to NMJs. Finally, we determined whether ex vivo trophic factor delivery influences inflammation and terminal Schwann cell (TSC) response during ALS. We found that intramuscular transplantation of hMSC-GDNF tended to exhibit less inflammation and significantly maintained TSC association with NMJs. Understanding cellular responses near NMJs is important to identify suitable cellular and molecular targets for novel treatment of ALS and other neuromuscular diseases.
Keywords: Amyotrophic lateral sclerosis (ALS), SOD1G93A rats, inflammation, glial activation, skeletal muscle
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal, rapidly progressing neurodegenerative disease caused by the selective loss of motor neurons in the spinal cord and brain stem (Ajroud-Driss and Siddique, 2015; Peters et al., 2015). The cause and process by which motor neurons die as ALS progresses is complex and incompletely understood. Nearly 90% of all ALS cases arise sporadically while the remaining 10% follow familial lines. Of the familial cases, approximately 20% can be attributed to one of over 160 mutations within the gene encoding the ubiquitously expressed human superoxide dismutase 1 (SOD1), the first gene linked to ALS neurotoxicity (Rosen et al., 1993). Other important genes in which ALS-causing mutations can arise have since been described including C9ORF72 and TDP-43 (Ajroud-Driss and Siddique, 2015). Overexpression of mutated human SOD1 gene in rodents produces a neuropathic phenotype similar to the ALS disease state in humans (Gurney et al., 1994; Howland et al., 2002; Nagai et al., 2001; Philips and Rothstein, 2015). While the exact mechanism of pathology remains elusive, multiple pathologies have been implicated as contributing factors to motor neuron death during ALS. These include protein misfolding and aggregation (Blokhuis et al., 2013), defects in axonal transport, glutamate excitotoxicity (Bogaert et al., 2010), oxidative stress (Barber and Shaw, 2010), mitochondrial dysfunction (Duffy et al., 2011), and abnormal astrocyte activation (Hall et al., 1998; Radford et al., 2015).
Despite the enigmatic nature of the ALS mechanism of motor neuron pathology, previous studies have demonstrated that direct muscle delivery of neuroprotective trophic/growth factors is effective to support neuromuscular connections, axon integrity, and motor neuron survival (Azzouz et al., 2004; Kaspar et al., 2003; Mohajeri et al., 1999). Specifically, our group has demonstrated the therapeutic benefits of ex vivo gene therapy (stem cell-based growth/trophic factor delivery) targeting the skeletal muscles in a rat model of familial amyotrophic lateral sclerosis (SOD1G93A transgenic rats) (Krakora et al., 2013; Suzuki et al., 2008). Human mesenchymal stem cells (hMSCs) constitutively secreting glial cell line-derived neurotrophic factor (GDNF) prevented degeneration of motor neurons and associated neuromuscular junctions (NMJs), and slowed ALS progression when delivered to skeletal muscle of SOD1G93A transgenic rats (Suzuki et al., 2008). Most recently, we delivered a combination of GDNF and vascular endothelial growth factor (VEGF) to muscle using hMSCs which further slowed disease progression in SOD1G93A rats (Krakora et al., 2013). While these studies demonstrated a significant ability of GDNF and VEGF to slow motor neuron degeneration and preserve skeletal muscle function, the question of how these growth factors and/or grafted hMSCs protect the motor endplate neuromuscular connection and motor neuron remains. To answer this question, it is important to understand how growth factors and hMSCs influence skeletal muscle degeneration during disease progression and it is logical to expect that the NMJs are the central affected structures.
The NMJ is a structure made up of the motor axon terminals, the muscle, and other supporting cells including terminal Schwann cells (TSCs). TSCs, also known as peri-synaptic Schwann cells, are glial cells found at the NMJ with known functions in synaptic transmission, synaptogenesis, and nerve regeneration (Moloney et al., 2014). NMJ dissociation (the separation of the TSC and motor axon from the motor endplate of the muscle) is a hallmark process of ALS and precedes symptom onset in ALS rodent models and human patients (Dupuis and Loeffler, 2009; Fischer et al., 2004; Krakora et al., 2012). While it is unclear whether NMJ dissociation occurs prior to or after motor neuron death, mounting evidence suggests that it plays a larger role in the progression of ALS than previously thought. Furthermore, little is known about the role of TSCs at the NMJs during ALS progression and pathology. Normally, TSCs play an important role supporting the synapse by taking up excess neurotransmitter, modulating neurotransmitter release, and lending trophic support. This role is analogous to the glial cells of the central nervous system (Feng and Ko, 2008). However, in the limb muscles of end-stage ALS patients, TSCs exhibit abnormal expressions of glial markers such as glial fibrillary acidic protein (GFAP), p75 neurotrophin receptor, and S100β (as known as S100 calcium binding protein B) (Liu et al., 2013). It is possible that progressive distal degeneration of the NMJs occurs early and is followed by axonal degeneration and motor neuron degeneration which would support a “dying back” hypothesis (Krakora et al., 2012).
Inflammation could play a role in NMJ dissociation during ALS progression but the exact role and mechanism is relatively unknown. Inflammation is recognized to play a role in motor neuron death and has been shown to accompany motor neuron degeneration in the central nervous system (Evans et al., 2013; Philips and Robberecht, 2011). As ALS progresses, pro- and anti-inflammatory cytokines increase in the cerebrospinal fluid of patients (Evans et al., 2013; Mitchell et al., 2009). The source of inflammatory factors is thought to be glial. High levels of microglial activation have been observed in the spinal cord of ALS rodent models (Beers et al., 2011; Boillee et al., 2006), as well as the spinal cord and brain stem of ALS patients (Evans et al., 2013). Inflating and activated macrophages are increased in the ventral root and sciatic nerve of ALS mice as the disease progresses (Chiu et al., 2009; Dibaj et al., 2011). Interestingly, macrophage activation is observed in the peripheral sciatic nerve before symptom onset and then steadily increased through end stage (Graber et al., 2010). Furthermore, the presence of inflammatory responses in degenerating peripheral nerve axons is also reported as an early event that occurs prior to the onset of clinical signs of motor weakness.
Inflammation and abnormal glial activation in the spinal cord and peripheral nerve fibers are both early events that occur prior to clinical signs of motor weakness in ALS; however, it is unknown whether inflammation and glial responses occur within the skeletal muscle and near NMJs, and how these responses contribute to motor neuron survival and neuromuscular innervation in ALS. A previous study using endpoint ALS mice revealed an increase in macrophage presence in innervating motor axon fascicles (Chiu et al., 2009); however, it is unclear whether these macrophages are causal or resolving (cleaning up debris from the degenerating axon). In this study, we first evaluate the time course of inflammatory and glial responses in the skeletal muscle near neuromuscular connections in the limb muscle of a rat model of familial ALS (SOD1G93A transgenic). We also ask how intramuscular GDNF delivery using hMSCs influences inflammation and glial responses in the skeletal muscle of SOD1G93A rats.
Materials and Methods
SOD1G93A transgenic rats
Female SOD1G93A transgenic rats exhibiting slow disease progression were used in this study (Suzuki et al., 2007b). The SOD1G93A transgenic male founders, originally obtained from Taconic (Hudson, NY) (Howland et al., 2002), were crossed with wild type female Sprague-Dawley rats to maintain colonies. While colony drift was previously observed in this transgenic rat line (Suzuki et al., 2007b), we have developed a genomic PCR screen and breeding schedule and now maintain stable lines that show similar disease onset and survival. Only animals showing disease onset before 150 days were used as breeders in order to minimize genetic drift in the colony that could increase variability in onset age of symptoms and end-stage age. Care was taken initially to select rats from sires that exhibited only hindlimb symptom onset. Rats exhibiting forelimb symptom onset were excluded from this study. Rats were maintained in a room with controlled illumination, temperature (23±1 °C), and humidity. All animals were given free access to food and water. Heterozygous SOD1G93A progeny were identified by genomic PCR screen using tail snip DNA and primers specific for human SOD1. All animal work in the present study was carried out in accordance with the guidelines for University of Wisconsin-Madison and National Institutes of Health standards of animal care.
Muscle section preparation
ALS rats were sacrificed at pre-symptomatic (<80 days old), symptomatic (100–120 days old), and endpoint stages (n=4–5 for each time point). Hind limb muscles (tibalis anterior; TA) were dissected and flash-frozen in super-cooled isopentane. The muscles were sectioned at 20 μm using a cryostat and placed on glass slides for staining. We confirmed that all symptomatic animals exhibited the Basso-Beattie-Bresnahan (BBB) locomotor rating score of 17 or lower (Krakora et al., 2013). Rats were considered at end-stage (endpoint) when they no longer exhibited reflexes allowing them to right themselves within 30 seconds. Our recent data from this colony indicates the median to reach disease onset and end-stage is 100 and 161 days, respectively (Hayes-Punzo et al., 2012).
Immunohistochemistry
The muscle sections were fixed with ice-cold methanol for 10 min or 4% paraformaldehyde-phosphate-buffered saline (PFA-PBS) for 20 min, and then incubated with primary antibodies at 4 °C for overnight. The following primary antibodies were used to probe for macrophages: anti-CD11b (OX-42, 1:500, mouse monoclonal, MorphoSys, Planegg, Germany), or anti-CD68 (ED1, 1:500, mouse monoclonal, AbD Serotec, Raleigh, NC). The following antibodies were used to visualize and probe markers of TSCs and activated glial cells: anti-glial fibrillary acidic protein (GFAP, 1:200, rabbit polyclonal, Dako, Glostrup, Denmark), anti-nestin (1:50, rabbit polyclonal, Sigma-Aldrich), anti-S100β (1:100, mouse monoclonal, Sigma-Aldrich), anti-4E2 (3G2) (neuromuscular junction and reactive Schwann cell associated antigen; 1:400, mouse monoclonal, Developmental Studies Hybridoma Bank, Iowa City, IA), and anti-GDNF family receptor alpha 1 (GFRα1, 1:50, rabbit polyclonal, Sigma-Aldrich) antibodies. To identify endplate distribution, the muscle sections were incubated with α-bungarotoxin (α-BTX) conjugated with fluorescence marker Alexa Fluor 594 (1:1000, Life Technologies, Carlsbad, CA) or CF405S (1:500, Biotium, Hayward, CA) with primary antibodies. After incubation with the primary antibodies, the sections were rinsed in PBS and incubated at room temperature for 1 hour with secondary antibodies conjugated to Alexa Fluor 488 or Cy3 (1:1000, Jackson ImmunoResearch Laboratories, West Grove, PA). All images were optimized by using a Nikon Eclipse 80i fluorescence microscope with a digital camera (DS-QiIMC, Nikon, Tokyo, Japan). The relative intensity of CD11b-positive signals was densitometrically evaluated using NIH ImageJ software. The number of S100β-positive endplates was presented as a percentage of the total number of counted NMJs. Approximately 50 endplates were exhaustively analyzed.
IL-1β and TNF-α ELISA
TA muscle was dissected and flash-frozen in super-cooled isopentane as described above. The tissues (approximately 50–80 mg) were then homogenized using a tissue homogenizer in 0.5 ml of ice-cold homogenization buffer containing protease inhibitors (40 mM Tris-HCl pH 7.5, 1 mM EDTA, 5 mM EGTA, 0.5% Triton X-100, 1 mM PMSF, and 10 μg/ml leupeptin). Protein concentrations were determined using a DC Protein Assay kit (Bio-Rad, Hercules, CA). The concentrations of rat IL-1β and TNF-α protein were measured by ELISA (DuoSet, R&D Systems, Minneapolis, MN).
Western blotting
Muscle homogenates (n=3–4 for each group) were prepared as describe above and loaded into wells of a 10% polyacrylamide gel and subjected to SDS-PAGE. Proteins were electrotransferred to a PVDF membrane and incubated with anti-GFAP (1:500, mouse monoclonal, Covance, Princeton, NJ) and anti-GAPDH antibodies (1:5,000, rabbit monoclonal, Cell Signaling Technology Inc,, Danvers, MA). Following secondary incubation with horse anti-mouse peroxidase-tagged antibody (1:5000, Vector Laboratories, Burlingame, CA), and incubation with enhanced chemiluminescence incubation (Thermo Scientific, Rockford, IL), the membrane was exposed to film. The protein band signals on the film were scanned and densitometrically analyzed using ImageJ 1.42q software.
Ex vivo GDNF delivery in SOD1G93A rats
Preparation of hMSCs expressing GDNF (hMSC-GDNF) and intramuscular transplantation of hMSCs in SOD1G93A rats were performed as described previously (Krakora et al., 2013; Suzuki et al., 2008). Briefly, hMSC-GDNF were prepared by lentiviral infection for constitutive expression of human GDNF (Krakora et al., 2013; Suzuki et al., 2008). Pre-symptomatic female SOD1G93A rats (90 days old; n = 4–5 animals/group) were administered intramuscular injections of a local anesthetic, bupivacaine hydrochloride (BVC; Sensorcaine-MPF, AstraZeneca, London, UK), to induce focal muscle injury and enhance hMSC integration and survival in the limb muscle prior to transplantation (Krakora et al., 2013; Suzuki et al., 2008). BVC was injected unilaterally into the TA muscle. At time-points of 24 hours, 1 week, and 2 weeks after the BVC injection, wild-type hMSC (hMSC-WT) or hMSC-GDNF (150,000 cells in 50 μl) were injected into the same muscle. All animals were immunosuppressed with cyclosporine which was necessary to prevent rejection of the transplanted hMSCs (10 mg/kg/day, Sandimmun; Novartis, Basel, Switzerland) (Borel et al., 1994). The animals were sacrificed at symptomatic stage (≅153 days old or 9 weeks after the first hMSC injection). The muscle sections were prepared as described above.
Statistical analysis
Prizm software (Graphpad software Inc., La Jolla, CA) was used for all statistical analyses. All ELISA data were analyzed by one-way functional ANOVA using Newman-Keuls post hoc test. Differences were considered significant when P<0.05.
Results
Inflammation is increased in the skeletal muscle of symptomatic and end-stage SOD1G93A rats
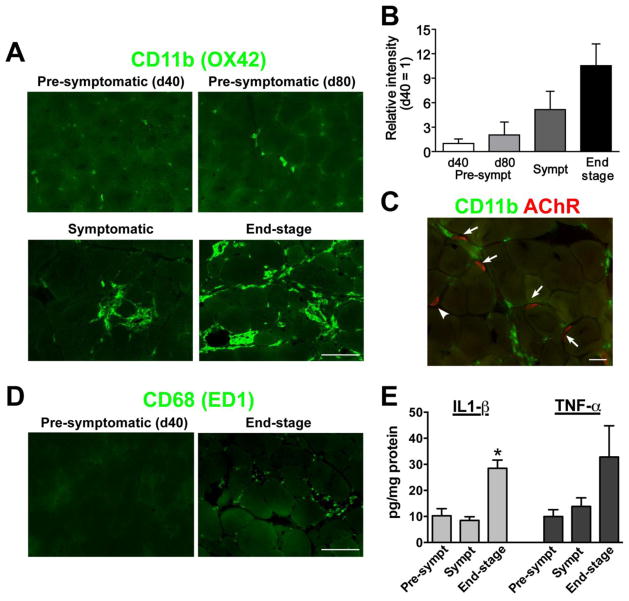
In this study, we first evaluated the time course of the inflammatory response found in the skeletal muscle of SOD1G93A rats (Fig. 1). Muscle samples were collected from early (40 days of age) or late pre-symptomatic (80 days), symptomatic (approximately 120 days old), and end-stage animals. The samples were sectioned and stained for CD11b, a glycoprotein known also known as integrin alpha-M (Robinson et al., 1986). CD11b is implicated in various adhesive interactions of monocytes, macrophages and granulocytes. In this study, we used the OX-42 antibody, a mouse monoclonal clone against rat CD11b (Robinson et al., 1986). At early pre-symptomatic stage (40 days of age), there were few CD11b-positive macrophages located in the skeletal muscle (Fig. 1A). The presence of CD11b-positive cells gradually increased as the disease progressed through late pre-symptomatic (80 days), symptomatic (120 days), and end-stage (Fig. 1A). Specifically, most of CD11b-positive cells resided in the laminar layer of the myofibers. Densitometirc analysis revealed that the level of inflammation in symptomatic and endpoint SOD1G93A rats were 6 and 10 times higher compared to 40-day-old animals, respectively (Fig. 1B). To determine how CD11b-expressing macrophages were distributed near endplates, alpha-bungarotoxin (α-BTX) conjugated with a fluorescence marker was used to identify acetylcholine receptor of the muscle endplates. We found that many CD11b-postive cells were located near endplates in the muscle of symptomatic SOD1G93A rats (Fig. 1C). Additionally, we performed immunostaining against CD68 (ED1 clone), another marker for cells of the macrophage lineage (Holness and Simmons, 1993), and confirmed activated immune cells in the muscle of end-stage SOD1G93A rats (Fig. 1D).
Figure 1. Active inflammatory responses occur near degenerating neuromuscular junctions (NMJs) and axons in symptomatic and end-stage SOD1G93A rats.
(A) CD11b-positive macrophages were identified in the hindlimb muscle of symptomatic and end-stage SOD1G93A transgenic rats. OX-42, a mouse monoclonal antibody clone against rat CD11b, was used for immunostaining. (B) Densitometric analyses indicated that the level of inflammation in symptomatic and endpoint SOD1G93A rats was higher compared to 40-day-old animals. (C) In symptomatic SOD1G93A rats, CD11b-postive signal was identified near AChR-positive endplates. (D) Immunostaining against another macrophage marker CD68 (ED1 clone) showed inflammation in the muscle of end-stage SOD1G93A rats. (E) The concentration of inflammatory cytokines IL1-β and TNF-α was increased in the muscle of end-stage animals. Scale bars: 50 μm in A and D; 20 μm in C. *: P<0.05 vs. pre-symptomatic and symptomatic.
We next measured the levels of inflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). Muscle homogenates were prepared from the TA of SOD1G93A rats (n=4 for each group) at pre-symptomatic (Day 60), symptomatic (Day 120) and end-stage and analyzed by ELISA for IL-1β and TNF-α expression. Although the concentration of IL-1β was unchanged between pre-symptomatic and symptomatic rats, the homogenates from end-stage animals contained approximately three times more IL-1β (28.5 ± 3.2 pg/mg) compared to pre-symptomatic (10.3 ± 2.7 pg/mg) and symptomatic animals (8.5 ± 1.4 pg/mg) (Fig. 1E; P<0.05). There was a similar trend in TNF-α levels (10.0 ± 2.6 pg/mg at Day 60; 13.9 ± 3.3 pg/mg at Day 120; 32.8 ± 12.0 pg/mg at end-stage). However, due to high variance among the end-stage samples we could not conclusively claim significance (Fig. 1E). Together, these results demonstrate a significant increase in inflammation occurred in the skeletal muscle of symptomatic and end-stage SOD1G93A rats.
Increased expression of GFAP and nestin near endplates of SOD1G93A rats follows ALS disease progression
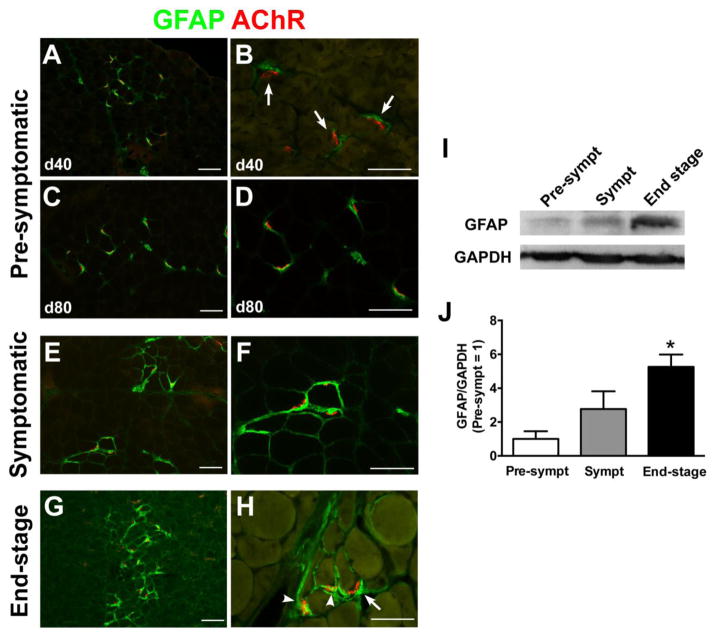
Adjacent serial sections to those used in Fig. 1 were stained for GFAP, a glial intermediate filament component considered to be a marker of active glial cells (including TSCs, astrocytes, and Schwann cells) (Yang and Wang, 2015). At the early pre-symptomatic period (40 days of age), GFAP protein was tightly expressed in the pre-synaptic compartments adjacent to AChR-positive endplates (Fig. 2A and B). The expression of GFAP protein gradually increased following disease progression from late pre-symptomatic (80 days old) (Fig. 2C and D), symptomatic (Fig. 2E and F) to end stage (Fig. 2G and H). Notably, strong GFAP expression was observed in a wide area around endplates and extended along with axons in end-stage animals. Furthermore, western blotting for GFAP protein confirmed a steady and significant increase in relative GFAP expression (Fig. 2I and J).
Figure 2. GFAP expression is increased in the muscle of SOD1G93A rats following disease progression.
(A–H) GFAP immunostaining in the hindlimb muscle of SOD1G93A rats. GFAP expression was limited near AChR-positive endplates at 40 days (A, B) and 80 days (C, D) of age. However, GFAP expression gradually increased in symptomatic (E, F) and end stage (G, H). (I) Representative band images of western blotting for GFAP and GAPDH expression. (J) Densitometric analyses of western blotting data supported that GFAP was increased in the muscle homogenates of end-stage rats. Scale bar: 100 μm in A, C, E, G; 50 μm in B, D, F, H. *: P<0.05 vs. pre-symptomatic and symptomatic.
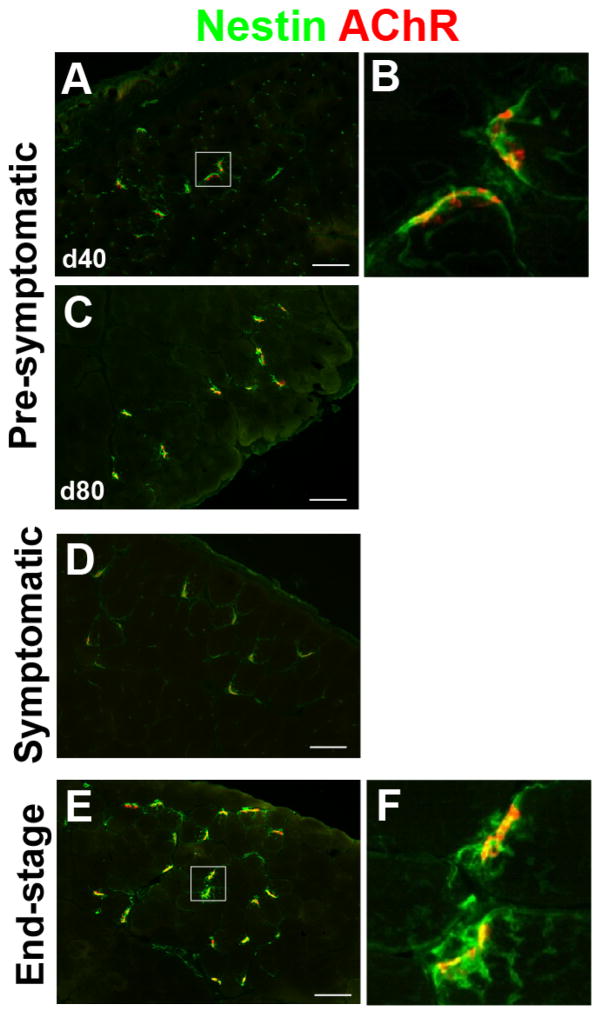
Nestin is an intermediate filament that localizes to the muscle fiber underneath the NMJs of innervated muscle (Kang et al., 2007). Upon denervation, muscle fiber nestin expression decreases. Simultaneously, TSCs associated with the denervated NMJs begin to express nestin. We examined nestin expression patterns in the NMJs of SOD1G93A rats during ALS progression (Fig. 3). Nestin was found to overlap endplates in pre-symptomatic muscles (Fig. 3A–C) and was not altered at the symptomatic stage (Fig. 3D) compared to the pre-symptomatic animals. In end-stage animals, nestin was upregulated specifically in the pre-synaptic area of NMJs and took on morphology similar to that of activated Schwann cells (Fig. 3E and F). Together, the shifts in expression patterns of GFAP and nestin proteins follow the expected pattern of denervation associated with disease progression in SOD1G93A rats.
Figure 3. An intermediate filament nestin is detected near NMJs of SOD1G93A rat muscle at end-stage.
Nestin protein expression overlapped with endplates in pre-symptomatic (40 day in A, B; 80 days in C) and symptomatic stage muscles (D). Nestin was upregulated specifically in the pre-synaptic area of NMJs at end-stage (E, F). Scale bar: 100 μm.
TSCs dissociate from NMJ as ALS progresses
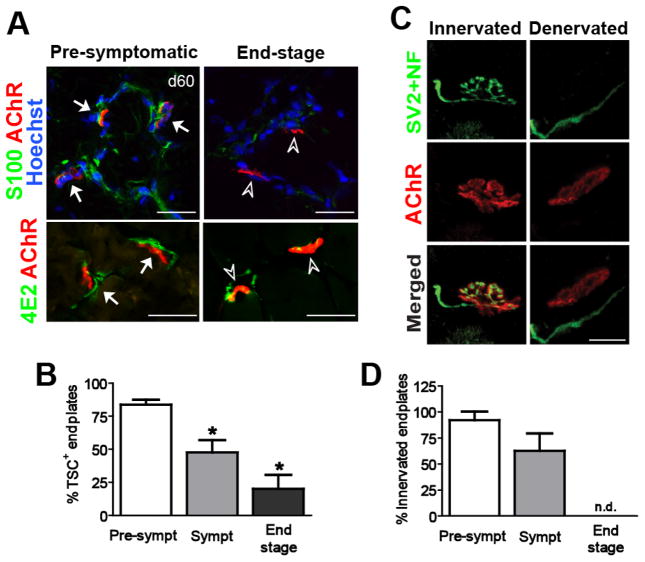
We next analyzed TSC localization relative to the NMJ by co-labeling for a TSC marker (S100β) and motor endplates. In pre-symptomatic SOD1G93A rats, S100β signals overlapped the pre-synaptic endplates (Fig. 4A). Approximately 80% of endplates were S100β positive at this time point (Day 60) (Fig. 4B). The number of S100β-positive endplates was then gradually decreased in symptomatic and end-stage ALS rats (Fig. 4B). This dissociation correlated with a loss in endplate innervation in serially adjacent muscle sections (Fig. 4C and D). Immunostaining with 4E2 (another marker of TSCs) (Astrow et al., 1994) also showed a reduction of positive signals associated with endplates in the end-stage animals (Fig. 4A).
Figure 4. TSC survival is associated with the level of NMJ innervation.
(A) Immuno-labeling of TSCs using anti-S100β and 4E2 (Schwann cell associated antigen) antibodies. S100β and 4E2-positive TSCs overlapped with pre-synaptic area of endplates (arrows) in pre-symptomatic rats. However, these positive signals were weak or undetectable in end-point animals (arrowheads). (B) The number of S100β-positive endplates was significantly decreased at symptomatic (Sympt) and end-stage compared to pre-symptomatic stage (Pre-sympt). (C) The reduction of S100β-positive endplates was associated with the level of innervated endplates. Scale bar: 20 μm. *: P<0.05 vs. pre-symptomatic. n.d.: not detected.
Intramuscular delivery of GDNF using hMSCs enhanced TSC association with NMJs in SOD1G93A rats
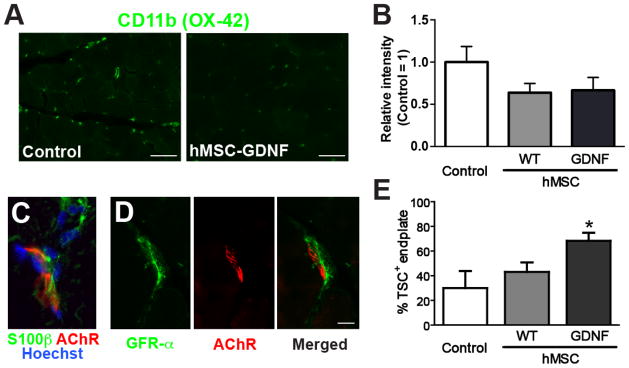
We tested whether GDNF delivery using hMSCs influenced inflammation and TSC response during ALS. hMSCs genetically modified to secrete GDNF (hMSC-GDNF) were unilaterally injected in pre-injured TA of pre-symptomatic SOD1G93A rats (90 days old). Control groups received wild-type hMSCs (naïve cells with no GDNF over-expression; hMSC-WT) or vehicle. The animals were sacrificed at the symptomatic stage (≅153 days old). CD11b staining intensity was reduced in TA muscle sections by approximately 36% and 34% when transplanted with hMSC-WT and hMSC-GDNF respectively (Fig. 5A and B). Further, we hypothesized that direct delivery of GDNF via hMSCs could increase the percent of NMJ-associated TSCs. In adjacent sections, a subunit of GDNF receptor GFRα1 was detected in the S100β+ pre-synaptic area of NMJs in pre-symptomatic SOD1G93A rats (Fig. 5C and D). We also counted S100β+ endplates in the TA muscle of hMSC-transplanted SOD1G93A rats and found that hMSC-GDNF significantly increased the number of TSC-positive endplates (P<0.05 vs. Control and hMSC-WT; Fig. 5E).
Figure 5. Direct delivery of GDNF using hMSCs influences inflammation and TSC survival in SOD1G93A rats.
hMSCs genetically modified to secrete GDNF, were transplanted in the hindlimb muscle of pre-symptomatic SOD1G93A rats. (A) Representative images of CD11b in symptomatic muscle injected with vehicle control or GDNF-secreting hMSCs. (B) Relative intensity of CD11b-positive signals in the muscle sections. TSCs labeled with S100β (C), and GFRα1 (D) at the pre-synaptic area of neuromuscular connections. (E) Direct delivery of GDNF using hMSCs significantly increased TSCs. Scale bars: 100 μm in A; 10 μm in D. *: P<0.05 vs. Control.
Discussion
In this study, we demonstrate that activated inflammation and abnormal glial responses occur in the limb muscle of familial ALS model rats, specifically near denervated NMJs. We previously reported that while over 80% of NMJs were innervated in pre-symptomatic SOD1G93A rats up to 80 days old, this number gradually decreased to the point where all NMJs were denervated by end-stage (Suzuki et al., 2007a). Although most ALS research has focused on mechanisms of motor neuron cell death, degeneration is also observed in skeletal muscle, particularly at the neuromuscular connection. While recent studies potentially support the therapeutic benefits targeting the skeletal muscle in ALS (Krakora et al., 2013; Suzuki et al., 2008), it has not been fully determined how inflammation and glial responses proceed in skeletal muscle and near NMJs in ALS model animals at different time points. Although further study is necessary to determine the possible roles of inflammation and glial responses in ALS patients, this animal study provides useful insight into the biological events occurring within the skeletal muscle during ALS.
Inflammation is recognized to play a role in ALS-related motor neuron pathology and has been shown to accompany motor neuron degeneration in the central and peripheral nervous systems of rodent models of ALS and human patients. In the spinal cord, this upregulation of inflammation is thought to be due to activation of microglia primed for activity whose subsequent activation plays a role in ALS pathology (Dibaj et al., 2011). In this study, we examine a time course of inflammatory responses in the skeletal muscle near NMJs in the limbs of transgenic rats carrying the SOD1G93A mutation. Markers of inflammation are significantly increased in the limb muscle as ALS progressed until end stage. The progressive increase in CD11b expression is similar to what has been previously observed in the ventral nerve root and sciatic nerve of SOD1G93A rats (Graber et al., 2010). Similarly, we found that CD68 expression is elevated in the TA near the NMJs at end stage. CD68 has also been observed in the ventral nerve roots of SOD1G93A rats before the onset of symptoms (Graber et al., 2010), in the peripheral nerves of SOD1G93A mice (Chiu et al., 2009; Dibaj et al., 2011) and around the innervating axon terminals of SOD1G93A mouse diaphragm (Chiu et al., 2009). Inflammation markers IL1-β and TNF-α are also elevated in end stage muscle in this study. IL1-β is secreted by activated microglia and speeds motor neuron pathology in ALS. Also, the systemic blockade of the IL-1 receptor has been shown to slow disease progression in SOD1G93A mice (Meissner et al., 2010). TNF-α is secreted by activated microglia and promotes an imbalance of AMPA and NMDA excitatory receptors compared to inhibitory GABA receptors in the motor neuron. This imbalance is thought to increase susceptibility of the motor neurons to excitotoxicity which is one possible mechanism involved in motor neuron pathology (Olmos and Llado, 2014). While inflammatory cytokines are observed at the NMJ in this study, it is important to note that inflammatory responses can vary by region and exhibit heterogeneous expression as exhibited in the spinal and cortical tissue of SOD1G93A rats (Nikodemova et al., 2014). It is possible that inflammatory responses at the NMJ may also be heterogeneous and regional.
We also characterize the response of resident glial cells adjacent to axons and TSCs adjacent to NMJs as ALS progresses and motor control is lost. Specifically, we monitored the expression of the intermediate filament proteins GFAP and nestin. GFAP is commonly used as a marker of astrocyte activation in the central nervous system (Yang and Wang, 2015). GFAP expression has been shown to be increased in sciatic nerves damaged by crush or cut and in rodent models of ALS preceding and during symptom onset through end stage (Keller et al., 2009). Similarly, GFAP has been found to be elevated in the serum of patients with motor and sensory neuropathies (Notturno et al., 2009). These reports support the idea that GFAP expression correlates with the degeneration of motor axons and denervation of NMJs. Further, we observed a marked increase in NMJ-associated nestin expression as ALS progressed. Nestin is known to be expressed near the post-synaptic nuclei of innervated muscle fibers but not in the presynaptic axon or TSCs. Following denervation, post-synaptic nestin expression drops off while nestin expression in TSCs increases dramatically (Kang et al., 2007).
In adults, TSCs play an important role in maintaining the NMJ (Feng and Ko, 2008). We examined TSCs in SOD1G93A rat muscle as ALS progressed. As expected, colocalization of AChRs and TSCs was lost as exhibited by S100β and 4E2 staining. We also observed small increases in nestin and GFAP in pre-symptomatic muscle that increased dramatically as symptoms progressed toward end stage. An increase in glial GFAP expression has been observed in spinal cord and peripheral nerves of SOD1G93A mice with spikes of increased expression at distinct time points beginning before symptom onset (Keller et al., 2009). The data we present here also demonstrate a general increase in GFAP expression at the NMJ beginning before gross loss of motor function develops. TSCs do not normally express appreciable levels of GFAP. In cases of denervation or axonal damage, TSCs enter a dedifferentiated state during which they upregulate GFAP as part of the repair/reinnervation process (Cheng and Zochodne, 2002; Kang et al., 2014). Indeed nerve crush and cut can induce elevated GFAP expression at the NMJ (Keller et al., 2009). Despite multiple studies describing GFAP expression increase in the central nervous system of ALS animal models and patients, GFAP expression increase has not been observed in either the limb muscles or extra-ocular muscles of human ALS patients who have reached end stage (Liu et al., 2013). The combination of the steady increase in nestin and GFAP expressions in TSC as ALS progresses and simultaneous loss of TSC colocalization with AChRs matches previous findings in SOD1G93A rodent models and human ALS patients (Hegedus et al., 2008; Pun et al., 2006) and correlates with loss of motor function.
We next used hMSCs to deliver GDNF to muscle of SOD1G93A rats and analyzed the effect on inflammation and glial response. We previously described a significant delay in symptom onset and an increase in overall survival of SOD1G93A rats following intramuscular transplantation of hMSCs overexpressing GDNF (Krakora et al., 2013; Suzuki et al., 2008). Interestingly, we found that both hMSC-GDNF and hMSC-WT tended to exhibit less inflammation, suggesting that GDNF may not be directly linked to inflammation regulation. Several studies have demonstrated that hMSCs show significant immunomodulatory effects following transplantation (De Miguel et al., 2012; Vercelli et al., 2008). Because inflammation severity correlates with disease progression, it is possible that the trend toward less inflammation following hMSC transplantation is due to an overall slowing of disease progression as we have previously observed. The increase in inflammation markers at the NMJs may indicate inflammation-based activation of peripheral macrophages similar to microglial activation in the central nervous system as ALS progresses. Macrophage activity may play a role in NMJ and axonal degeneration during ALS. Our study suggests a correlation between an increase in inflammatory markers and markers of TSCs dissociation from NMJs; however, it has also been suggested that the elevated macrophage presence and activity found in the peripheral nerves as ALS progresses, may be due to increased cellular debris as motor axons degenerate and that macrophages are simply present to clean up (Chiu et al., 2009; Dibaj et al., 2011). Indeed, the possibility exists that the preservation of NMJ integrity may result in reduced inflammation. In this study, an immunosuppressive reagent cyclosporine was administered to all animals to prevent the rejections of grafted hMSCs. Based on our preliminary observations, cyclosporine itself did not cause obvious differences in disease progression and animal survival in our ALS rat models (Suzuki et al., 2007a). However, it is still necessary to elucidate how cyclosporine influences inflammation in the muscle and the effects of hMSC-based GDNF delivery following disease progression.
Finally, we found that hMSC-GDNF, but not hMSC-WT, prevented TSC dissociation from the NMJs in SOD1G93A animals. Motor neurons express GDNF family receptor alpha (GFRα1) and GDNF is a known critical factor for motor neuron survival and development (Henderson et al., 1994; Tovar et al., 2014). Interestingly, we found that GFRα1 is also expressed in TSCs and observed a significant preservation of TSC overlap with endplates in the hMSC-GDNF transplanted muscles compared to those transplanted with the hMSC-WT. These data further support that GDNF preserves tripartite NMJ integrity and suggest that GDNF plays a direct role in preserving TSC association with the NMJ. Subsequently, the preservation of NMJ integrity would potentially lead to further motor neuron protection and could be the mechanism by which delivered GDNF delays ALS symptom onset. Further studies are necessary to fully explore this possibility and to determine underlying mechanisms of NMJ and motor neuron protection by hMSC-GDNF.
Highlights.
Macrophage-mediated inflammation is increased at the NMJs in ALS rats following disease progression
Strong expressions of reactive glial markers are observed near NMJs
TSCs dissociate when NMJ integrity is lost
Ex vivo GDNF delivery alters inflammation and maintains TSC-NMJ association
Acknowledgments
This work was supported by grants from NIH (R01NS091540 to M.S.), US Department of Defense, the ALS Association, and the University of Wisconsin Foundation.
Abbreviations
- α-BTX
alpha-bungarotoxin
- AChR
acetylcholine receptor
- ALS
amyotrophic lateral sclerosis
- ANOVA
analysis of variance
- BBB
Basso-Beattie-Bresnahan hindlimb locomotor rating
- BVC
bupivacaine hydrochloride
- CD11b
cluster of differentiation 11b
- CD68
cluster of differentiation 68
- C9ORF72
chromosome 9 open reading frame 72
- EDTA
Ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GDNF
glial cell line-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- GFRα1
GDNF family receptor α 1
- hMSC
human mesenchymal stem cell
- IL-1β
interleukin-1β
- NMJ
neuromuscular junction
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PFA
paraformaldehyde
- PMSF
phenylmethylsulfonyl fluoride
- SDS-PAGE
sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- SOD1
superoxide dismutase 1
- S100
calcium binding protein
- TA
tibialis anterior
- TDP-43
TAR DNA binding protein 43
- TNF-α
tumor necrosis factor-α
- TSC
terminal Schwann cell
- VEGF
vascular endothelial growth factor
Footnotes
The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajroud-Driss S, Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim Biophys Acta. 2015;1852:679–684. doi: 10.1016/j.bbadis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Astrow SH, Son YJ, Thompson WJ. Differential neural regulation of a neuromuscular junction-associated antigen in muscle fibers and Schwann cells. J Neurobiol. 1994;25:937–952. doi: 10.1002/neu.480250804. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Beers DR, Zhao W, Liao B, Kano O, Wang J, Huang A, Appel SH, Henkel JS. Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain, behavior, and immunity. 2011;25:1025–1035. doi: 10.1016/j.bbi.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ. Protein aggregation in amyotrophic lateral sclerosis. Acta neuropathologica. 2013;125:777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert E, d’Ydewalle C, Van Den Bosch L. Amyotrophic lateral sclerosis and excitotoxicity: from pathological mechanism to therapeutic target. CNS Neurol Disord Drug Targets. 2010;9:297–304. doi: 10.2174/187152710791292576. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. 1976. Agents Actions. 1994;43:179–186. doi: 10.1007/BF01986686. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–329. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Phatnani H, Kuligowski M, Tapia JC, Carrasco MA, Zhang M, Maniatis T, Carroll MC. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20960–20965. doi: 10.1073/pnas.0911405106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel MP, Fuentes-Julian S, Blazquez-Martinez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- Dibaj P, Steffens H, Zschuntzsch J, Nadrigny F, Schomburg ED, Kirchhoff F, Neusch C. In Vivo imaging reveals distinct inflammatory activity of CNS microglia versus PNS macrophages in a mouse model for ALS. PloS one. 2011;6:e17910. doi: 10.1371/journal.pone.0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy LM, Chapman AL, Shaw PJ, Grierson AJ. Review: The role of mitochondria in the pathogenesis of amyotrophic lateral sclerosis. Neuropathology and applied neurobiology. 2011;37:336–352. doi: 10.1111/j.1365-2990.2011.01166.x. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Loeffler JP. Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models. Curr Opin Pharmacol. 2009;9:341–346. doi: 10.1016/j.coph.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Evans MC, Couch Y, Sibson N, Turner MR. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Feng Z, Ko CP. The role of glial cells in the formation and maintenance of the neuromuscular junction. Ann NY Acad Sci. 2008;1132:19–28. doi: 10.1196/annals.1405.016. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Graber DJ, Hickey WF, Harris BT. Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:8. doi: 10.1186/1742-2094-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hayes-Punzo A, Mulcrone P, Meyer M, McHugh J, Svendsen CN, Suzuki M. Gonadectomy and dehydroepiandrosterone (DHEA) do not modulate disease progression in the G93A mutant SOD1 rat model of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2012;13:311–314. doi: 10.3109/17482968.2012.654393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. The Journal of physiology. 2008;586:3337–3351. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson LC. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci US A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Tian L, Mikesh M, Lichtman JW, Thompson WJ. Terminal Schwann cells participate in neuromuscular synapse remodeling during reinnervation following nerve injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:6323–6333. doi: 10.1523/JNEUROSCI.4673-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, Ponomareva O, Mignone J, Enikolopov G, Rimer M, Thompson W. Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:5948–5957. doi: 10.1523/JNEUROSCI.0621-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- Keller AF, Gravel M, Kriz J. Live imaging of amyotrophic lateral sclerosis pathogenesis: disease onset is characterized by marked induction of GFAP in Schwann cells. Glia. 2009;57:1130–1142. doi: 10.1002/glia.20836. [DOI] [PubMed] [Google Scholar]
- Krakora D, Macrander C, Suzuki M. Neuromuscular junction protection for the potential treatment of amyotrophic lateral sclerosis. Neurol Res Int. 2012;2012:379657. doi: 10.1155/2012/379657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, Zimprich C, Aebischer P, Svendsen CN, Suzuki M. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:1602–1610. doi: 10.1038/mt.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JX, Brannstrom T, Andersen PM, Pedrosa-Domellof F. Distinct changes in synaptic protein composition at neuromuscular junctions of extraocular muscles versus limb muscles of ALS donors. PloS one. 2013;8:e57473. doi: 10.1371/journal.pone.0057473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13046–13050. doi: 10.1073/pnas.1002396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RM, Freeman WM, Randazzo WT, Stephens HE, Beard JL, Simmons Z, Connor JR. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology. 2009;72:14–19. doi: 10.1212/01.wnl.0000333251.36681.a5. [DOI] [PubMed] [Google Scholar]
- Mohajeri MH, Figlewicz DA, Bohn MC. Intramuscular grafts of myoblasts genetically modified to secrete glial cell line-derived neurotrophic factor prevent motoneuron loss and disease progression in a mouse model of familial amyotrophic lateral sclerosis. Hum Gene Ther. 1999;10:1853–1866. doi: 10.1089/10430349950017536. [DOI] [PubMed] [Google Scholar]
- Moloney EB, de Winter F, Verhaagen J. ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front Neurosci. 2014;8:252. doi: 10.3389/fnins.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Small AL, Smith SM, Mitchell GS, Watters JJ. Spinal but not cortical microglia acquire an atypical phenotype with high VEGF, galectin-3 and osteopontin, and blunted inflammatory responses in ALS rats. Neurobiology of disease. 2014;69:43–53. doi: 10.1016/j.nbd.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notturno F, Capasso M, DeLauretis A, Carpo M, Uncini A. Glial fibrillary acidic protein as a marker of axonal damage in chronic neuropathies. Muscle Nerve. 2009;40:50–54. doi: 10.1002/mus.21323. [DOI] [PubMed] [Google Scholar]
- Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014;2014:861231. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters OM, Ghasemi M, Brown RH., Jr Emerging mechanisms of molecular pathology in ALS. J Clin Invest. 2015;125:1767–1779. doi: 10.1172/JCI71601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- Philips T, Rothstein JD. Rodent Models of Amyotrophic Lateral Sclerosis. Curr Protoc Pharmacol. 2015;69:5 67 61–65 67 21. doi: 10.1002/0471141755.ph0567s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Radford RA, Morsch M, Rayner SL, Cole NJ, Pountney DL, Chung RS. The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. Front Cell Neurosci. 2015;9:414. doi: 10.3389/fncel.2015.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Molecular therapy: the journal of the American Society of Gene Therapy. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. 2007a;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Tork C, Shelley B, McHugh J, Wallace K, Klein SM, Lindstrom MJ, Svendsen CN. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotrophic lateral sclerosis: official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2007b;8:20–25. doi: 10.1080/17482960600982447. [DOI] [PubMed] [Google Scholar]
- Tovar YRLB, Ramirez-Jarquin UN, Lazo-Gomez R, Tapia R. Trophic factors as modulators of motor neuron physiology and survival: implications for ALS therapy. Front Cell Neurosci. 2014;8:61. doi: 10.3389/fncel.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli A, Mereuta OM, Garbossa D, Muraca G, Mareschi K, Rustichelli D, Ferrero I, Mazzini L, Madon E, Fagioli F. Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2008;31:395–405. doi: 10.1016/j.nbd.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends in neurosciences. 2015 doi: 10.1016/j.tins.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]