For non-ambulatory children with severe physical and cognitive disabilities (SPCD), being lifted and transported become more challenging with increasing size. Reduction in length and overall size for select children with SPCD can be accomplished by growth attenuation therapy (GAT), which reduces linear growth by administering hormones (eg, oestrogen) that accelerate epiphyseal closure. There are no reliable data describing attitudes and practices of paediatric endocrinologists regarding GAT for children with SPCD.
In collaboration with the Pediatric Endocrine Society (PES), an anonymous questionnaire about GAT was emailed to PES physician members (n=1100, 92% from the USA). Responses were stripped of identifiable information. Consent was implied by responding. The study received institutional review board exemption status at the University of Wisconsin–Madison.
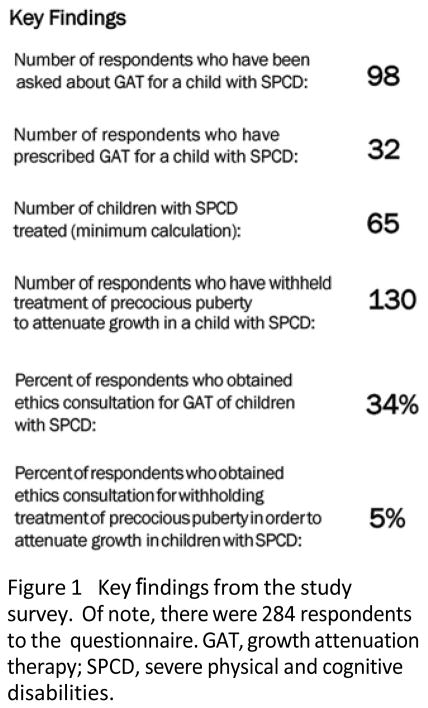
Two hundred and eighty-four PES members responded to the survey (response rate=26%). Ninety-nine respondents had either been asked to prescribe or had prescribed GAT to a child with SPCD. Of these, 92 (94%) said requests came from the patient’s family and 34 reported seeking ethics consultation.
Thirty-two respondents had prescribed GAT to a child with SPCD, most recalling doing so one to five times (n=28, 88%), yielding a conservative estimate of at least 65 children with SPCD treated. Oral oestrogen was the most common sex hormone therapy used for GAT (n=21, 66%).
Withholding of interventions that suppress precocious puberty in a child with SPCD in order to reduce ultimate linear growth was reported by 130 respondents (46%). Of these, only seven (5%) sought ethics consultation. Key findings are highlighted in figure 1.
Figure 1.
Key findings from the study survey. Of note, there were 284 respondents to the questionnaire. GAT, growth attenuation therapy; SPCD, severe physical and cognitive disabilities.
Previous surveys of GAT practices1,2 focused on prescriptions of GAT to healthy tall-statured girls, not on treatment of children with SPCD. Results from this present survey show, for the first time, that instances of prescribing GAT for children with SPCD are not limited to the single existing case report. There is a need for collaborative investigation of therapeutic growth attenuation strategies, reporting of outcomes, and discussion among stakeholders to develop evidence-based guidance for patients and families. The demonstration here that approximately one in three responding paediatric endo-crinologists have been asked about GAT for a child with SPCD provides an opportunity for further investigation regarding efficacy of GAT in length reduction, quality of life improvement and the possible risks of therapy.3
The main limitations of this study were (1) brevity of the questionnaire, which was designed to optimise ease of participation and therefore maximise the response rate and (2) the possibility that respondents may not be representative of paediatric endocrinologists overall.
In conclusion, paediatric endocrinolo-gists are receiving inquiries regarding GAT from families of children with SPCD more commonly than previously realised and at least 65 children with SPCD have received GAT. Most of the responding paediatric endocrinologists view GAT as an appropriate therapeutic modality in certain circumstances. Systematic investigation of risks and benefits of GAT in children with SPCD would enrich paediatric endocrinologists’ guidance for families who are already inquiring about GAT and would assist in developing consensus on a responsible and thoughtful approach to GAT within the paediatric endocrinology community.
Acknowledgments
The authors would like to thank Jens Eickhoff, PhD, for his assistance with statistical analysis and the Pediatric Endocrine Society for facilitating distribution of the questionnaire.
Footnotes
Contributors: AJP: Conceptualised and designed the study and questionnaire instrument, carried out the initial analyses, drafted the initial manuscript and approved the final manuscript as submitted. NF and DBA: Contributed to the design and analyses, critically reviewed and revised the manuscript and approved the final manuscript as submitted.
Competing interests: None declared.
Ethics approval: University of Wisconsin Institutional Review Board.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Conte FA, Grumbach MM. Estrogen use in children and adolescents: a survey. Pediatrics. 1978;62(6 Pt 2):1091–7. [PubMed] [Google Scholar]
- 2.Barnard ND, Scialli AR, Bobela S. The current use of estrogens for growth-suppressant therapy in adolescent girls. J Pediatr Adolesc Gynecol. 2002;15:23–6. doi: 10.1016/s1083-3188(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 3.Wilfond BS, Miller PS, Korfiatis C, et al. Navigating growth attenuation in children with profound disabilities. Children’s interests, family decision-making, and community concerns. Hastings Cent Rep. 2010;40:27–40. doi: 10.1002/j.1552-146x.2010.tb00075.x. [DOI] [PubMed] [Google Scholar]