Abstract
Lung cancer is the most common cause of cancer-related deaths worldwide and non-small cell lung cancer (NSCLC) accounts for ~85% of all lung cancer. While recent research has shown that cancer stem cells (CSC) exhibit radioresistant and chemoresistant properties, current cancer therapy targets the bulk of the tumor burden without accounting for the CSC and the contribution of the tumor microenvironment. CSC interaction with the stroma enhances NSCLC survival, thus limiting the efficacy of treatment. The aim of this study was to elucidate the role of CSC and the microenvironment in conferring radio- or chemoresistance in an in vitro tumor model for NSCLC. The novel in vitro three-dimensional (3D) NSCLC model of color-coded tumor tissue analogs (TTA) that we have developed is comprised of human lung adenocarcinoma cells, fibroblasts, endothelial cells and NSCLC cancer stem cells maintained in low oxygen conditions (5% O2) to recapitulate the physiologic conditions in tumors. Using this model, we demonstrate that a single 5 Gy radiation dose does not inhibit growth of TTA containing CSC and results in elevated expression of cytokines (TGF-α, RANTES, ENA-78) and factors (vimentin, MMP and TIMP), indicative of an invasive and aggressive phenotype. However, combined treatment of single dose or fractionated doses with cisplatin was found to either attenuate or decrease the proliferative effect that radiation exposure alone had on TTA containing CSC maintained in hypoxic conditions. In summary, we utilized a 3D NSCLC model, which had characteristics of the tumor microenvironment and tumor cell heterogeneity, to elucidate the multifactorial nature of radioresistance in tumors.
INTRODUCTION
Despite the advances in cancer therapy, lung cancer continues to be the leading cause of cancer-related mortality, causing more deaths than the three most common cancers: colon, breast and prostate, combined. Approximately 220,000 new cases occur every year in the United States and more than 1.1 million people worldwide die from lung cancer annually (1). Non-small cell lung cancer (NSCLC) accounts for 85% of the lung malignancies (2) with ~40% of the diagnosed NSCLC cases in stage IV and ~25% in stage III. When the disease is diagnosed at an advanced stage, a more aggressive treatment plan is required (2). Although fractionated radiotherapy and platinum-based chemotherapy have been the mainstay of treatment for stage III NSCLC over the past decade, there are still a number of concerns regarding multimodal therapy. These include selection of the appropriate chemotherapy agent(s) and optimizing schedules for fractionated radiotherapy (2–5). Preclinical models that could predict therapeutic response in patients are key in developing new forms of therapy or improving existing therapies in a disease where the probability of long-term survival remains low.
Most cancer therapies focus on the tumor cancer cells while failing to consider the role of the tumor microenvironment in regulating tumor growth and metastasis (6, 7). Components of the tumor microenvironment, including endothelial cells, immune cells and cancer-associated fibroblasts that assist in formation of the extracellular matrix (ECM), play a crucial role in regulating tumor cell function and disease progression (8). These non-neoplastic components of the tumor microenvironment facilitate tumor growth through the production of extracellular matrices, cytokines, growth factors, mechanical cues and vascular networks for nutrient and waste exchange (6). Therefore, it is important to understand the role of the tumor microenvironment in natural and acquired tumor resistance to anticancer therapies.
Among the normal cells in the human body, the microenvironment plays an integral part in maintaining the normal stem cells in a quiescent state while preserving their potential for proliferation and differentiation (9). Studies in cancer suggest that stromal cells in the tumor microenvironment express factors that regulate self-renewal and differentiation of tumor cells possessing stem-like features, commonly known as cancer stem cells (CSC) (9). While characterization of the CSC niche for various solid tumors has proven to be technically difficult, identification of the biological processes utilized by CSC to interact with the microenvironment will yield crucial information on the role of CSC in treatment failure and tumor recurrence (9).
Despite the widespread use of fractionated radiotherapy and platinum-based chemotherapy for the treatment of NSCLC, tumor cell resistance to treatment remains a major factor limiting successful outcomes (10). Conventional radiotherapy targets the bulk of the tumor burden but fails to prevent proliferation of the CSC within the tumor (10). These CSC, which exhibit innate resistance to radiotherapy, can survive typical treatment regimens and repopulate the tumor (11).
In our previous work, we developed an in vitro 3D breast co-culture model to study the effects of different treatment options on tumor and stromal cells (12, 13). The 3D co-cultures generated robust spheroids with an extracellular matrix providing a physiologically relevant tumor model, which recapitulated the graded hypoxia, pH and interstitial pressure of the tumor microenvironment that are not represented in two-dimensional (2D) monolayer cell cultures (13, 14).
Most of the existing knowledge related to cellular response to therapy is based on experiments conducted at an atmospheric O2 concentration of ~21%. However, tissue O2 concentrations are considerably lower than the atmospheric O2 levels and, depending on the tissue or cell population, the O2 concentration varies between 1 to 14% (15). Low concentrations of O2 increase the stability of the HIF-1α transcript (16, 17) and support the stem cell phenotype (18, 19). Therefore, in vitro studies from cells require a platform that more reliably represents the in situ normoxia for insights into the actual tumor physiology and response to therapy. In the current study, the 3D NSCLC model consists of tumor tissue analogs (TTA) that are formed by the co-culture of red fluorescent protein (RFP)-labeled A549 NSCLC tumor cells, green fluorescent protein (GFP)-labeled human neonatal dermal fibroblasts, human pulmonary artery endothelial cells and NSCLC with cancer stem cells in “hanging drops” of media at 5% O2. These TTA were used to study the effect of radiotherapy and cisplatin treatment on tumor cell and microenvironment growth with the objective of investigating the contribution of CSC to therapeutic resistance.
MATERIALS AND METHODS
Chemicals and Reagents
Cisplatin [cis-diammineplatinum(II) dichloride] was purchased from Sigma-Aldrich, LLC (St. Louis, MO). Quick coating solution used to coat the flask for human neonatal dermal fibroblast (HNDF) cells was purchased from Angio-Proteomie (Shrewsbury, MA). Monoclonal mouse anti-vimentin was purchased from Dako, Inc. (Carpinteria, CA) and rabbit anti-vimentin polyclonal antibody was purchased from Bioss, Inc. (Woburn, MA).
Cell Lines and Culture
Red fluorescence protein-expressing A549 human NSCLC epithelial cell line (A549-RFP) was obtained from AntiCancer, Inc. (San Diego, CA); the human pulmonary artery endothelial cells (HPAEC) from Lonza (Walkersville, MD); and human neonatal dermal fibroblast cells expressing green fluorescent protein (HNDF-GFP) were purchased from Angio-Proteomie. The HNDF cells were isolated from normal neonatal forehead skin tissue samples and transfected with GFP-lentiviral particles at passage one, selected using puromycin (1 mg/ml) and maintained in DMEM containing 5% fetal bovine serum (FBS). Human NSCLC with cancer stem cells prescreened for CD133, CD 44, SSEA-3/4 and their ability to form tumors <1,000 cells in mice were obtained from Celprogen, Inc. (Torrance, CA).
The A549 cells were cultured in DMEM high glucose media (Invitrogen™, Carlsbad, CA), supplemented with 10% FBS, 1% penicillin-streptomycin and 1% glutaMAX™ (Invitrogen). The HPAEC were cultured in the recommended media, EGM™-2 BulletKit™ (Lonza Inc., Walkersville, MD). The human neonatal dermal fibroblasts (HNDF) were grown in flasks precoated with quick coating solution and maintained in DMEM high glucose containing 5% FBS, 1X GlutaMAX and 1% Pen Strep (Invitrogen, Carlsbad, CA). The human NSCLC stem cells were incubated in the M36107-34S media and cultured on pre-coated ECM E36107-34-T25 (Celprogen, Torrance, CA) flasks precoated with human lung cancer stem cell extracellular matrix at 5% oxygen supply. All cell lines were maintained at 37°C and 5% CO2 equilibrated with atmospheric O2 in a humidified incubator containing 20% O2 unless otherwise stated. The multicellular 3D co-cultures were maintained either under normoxic conditions (20% oxygen supply; hereafter referred to as normoxia) or in an inflatable hypoxia chamber with 5% oxygen supply (hereafter referred to as hypoxia). All 3D cultures were maintained at 37°C in a humidified atmosphere with 5% CO2.
Clonogenic Assay
Cells (200–500) were plated in 6-well culture plates and exposed to increasing radiation doses (0, 2.5, 5 and 10 Gy) in triplicate. After irradiation, plates were incubated at 37°C and 20% oxygen supply (normoxia) or in the inflatable hypoxia chamber with 5% oxygen supply (hypoxia) for 10–12 days until large colonies (>1 mm or >50 cells) formed. The colonies were fixed and stained with 0.5% crystal violet (Sigma-Aldrich) in 50% methanol/water. Colonies were counted using ImageJ Software (National Institutes of Health, Bethesda, MD) and confirmed by manual counting.
Plating efficiency (PE) and survival fraction (SF) were calculated using the following equations (20):
Surviving fraction was determined for the lung cancer stem cells after irradiation. Survival fractions were calculated from n = 3 samples for each experiment.
Tumor Tissue Analogs
Three-dimensional tumor tissue analogs were generated by co-culturing of four different cell types: A549-RFP tumor cells; HPAEC endothelial cells; and HNDF-GFP cells with or without CSC in hanging drops of media, as previously described (13). Briefly, the single cell suspension mix of A549-RFP cells, HNNDF-GFP cells and HPAEC cells at a ratio of 1.2:1:1 (3,200 cells/20 μl) was dispensed on the inside of the lid of each well of a 48-well cell culture plate (Greiner Cellstar, BioExpress, Kaysville, UT). For tumor tissue analogs with cancer stem cells (TTA + CSC) the cell suspension mix of A549-RFP cells, HNNDF-GFP cells, HPAEC cells and CSC was at a ratio of 1:1:1:0.2 (3,200 cells/20 μl). Once initiated in the hanging drops they were incubated for ~10 days to enable the formation of a tissue-like morphology as a result of the intercellular interaction. This was monitored by imaging the TTA using confocal microscopy. Subsequently, depending on the experimental design 5–12 TTA/group received the respective treatment. The TTA were transferred 24–48 h later to optically clear cell-repellent plates (Greiner) in fresh media to assess response to 5 Gy irradiation and/or chemotherapy (cisplatin) 5 days after treatment.
Patient-derived CSC were included in developing the 3D NSCLC tumor model for several reasons. The A549 cell line has been in culture since 1972 and the traditional marker CD133 identified for the selection and characterization of lung cancer stem cells (21) is no longer effective in sorting the stem cell-like subpopulation from the A549 cells (22). Therefore, in the human 3D NSCLC model used to develop the tumor tissue analogs in this study, which are comprised of A549 tumor cells and microenvironment components (endothelial cells and fibroblasts) grown in hypoxic O2 (5%) concentrations, we opted for patient-derived human NSCLC with cancer stem cells that were well characterized for several stem cell markers including serial dilution assays for growth and metastasis (Celprogen). Another pertinent reason for inclusion of patient-derived CSC in the experimental design utilizing 3D co-cultures was the exceedingly low occurrence (0.5–1%) of stem cells in a traditional 2D monolayer cell culture of A549 cells (9). While this percentage of A549 stem cells when compared to the tumor cell population may increase in the 3D hypoxic experimental conditions for the TTA, obtaining a significant number of A549 stem cells that would allow us to assess the experimental end points would have been difficult. The enhanced ability of NSCLC with cancer stem cells to undergo rapid self renewal, particularly in hypoxic conditions (23, 24), enabled us to readily distinguish them from the remaining cell population in the TTA.
Tumor/Cell X-Ray Irradiation
The Varian 21EX Platinum TrueBeam™ X-Ray System (Palo Alto, CA) was used for irradiations (Department of Radiation Medicine, UK Albert B. Chandler Hospital, Lexington, KY). The instrument was set at a radiation dose rate of 1.018 ± 0.10 Gy/min at 150 kV and 6.6 mA.
Microscopy and Immunostaining of Intact TTA with Vimentin
The 3D TTA comprised of A549-RFP cells, HNDF-GFP and unlabeled HPAEC endothelial cell line with or without the unlabeled human NSCLC stem cells were imaged at 10× and 20× magnification using a FV1000 laser scanning confocal fluorescence microscope. The presence of tdTomato and GFP in the TTA were imaged by excitation with argon laser at 532 and 453 nm, respectively. The bright field images taken at 10× were used to compare the size of the TTA in different environmental conditions and cell composition. Images were processed using FV10-ASW 1.7 viewer software (Olympus Imaging America Inc., Center Valley, PA). Captured images were analyzed for sizing using Olympus image analyses software and ImageJ, v. 1.47.
For immunoprobing with vimentin the intact TTA were fixed in 4% paraformaldehyde and washed with phosphate buffered saline (PBS). Blocking was performed in 5% BSA for 30 min, followed by incubation in 1:450 dilution of rabbit anti-vimentin polyclonal antibody (Bioss, Inc.) for 1 h and then later in Alexa Fluor® 633-labeled donkey-anti rabbit secondary antibody at a dilution of 1:500 for 30 min. The TTA were transferred to glass bottom plates for acquisition of confocal images at 10× and 20× magnification using a FV1000 laser scanning confocal microscope (Olympus Imaging America).
Histogel Embedding
Tumor tissue analogs were collected and fixed in 10% formalin for 2 h, washed with PBS and dehydrated twice with 50 and 70% ethanol for 15 min each. The dehydrated TTA were transferred to the cryomold (Thermo Fisher Scientific Inc., Waltham, MA) and embedded in HistoGel™ (Thermo Scientific™, Kalamazoo, MI) liquefied by heating at 60° ± 5°C. The TTA containing Cryomolds® were subsequently solidified ice for 10 min. The HistoGel blocks with TTA were wrapped within a piece of Bio-Wrap™ (Leica Biosystems, Buffalo Grove, IL) and placed into tissue biopsy cassette (Thermo Fisher Scientific) for processing. Tissue biopsy cassettes containing HistoGel blocks were dehydrated by grades of alcohol and then cleaned with xylene and impregnated with paraffin. Processed HistoGel blocks were then removed from the Bio-Wrap and placed in wax (Paraplast X-TRA®, Sigma-Aldrich) to prepare paraffin blocks. Sections (4–5 μm) were cut with a microtome (Leica Biosystems).
Immunohistochemistry for Vimentin
Tumor tissue analogs grown for 10 days under hypoxia received 5 Gy irradiation and then were stained for vimentin on day 5 after treatment. The 4–5 μm TTA sections were placed on the positively charged plus slides (Fisher Scientific, Pittsburgh, PA) and probed with monoclonal mouse anti-vimentin V9 antibody using the automated IHC (Dako, Inc. FLEX System). Briefly, sections were deparaffinized in xylene and hydrated stepwise through ethanol to water. Antigen retrieval was performed using a high pH antigen retrieval buffer (Dako, Inc.) in a decloaking chamber (Biocare Medical, Concord, CA) according to the manufacturer's instructions. Slides were incubated in pre-diluted monoclonal mouse anti-vimentin antibody clone 9 (1:400) for 10 min at room temperature (no. IR630; Dako, Inc.) followed by cell labeling with the EnVision+ kit (Dako, Inc.) according to the manufacturer's instructions. Signal was visualized with 3,3′-diaminobenzidine (DAB) followed by a light hematoxylin counterstain. Immunoprobed sections were then coverslipped with Permount™ and air dried before images were captured with a digital camera (AxioCam, Carl Zeiss Microscopy GmbH, Gottingen, Germany) mounted on an Axioskop 2 Plus microscope (Carl Zeiss Microscopy) at 40× magnification.
Cisplatin and Radiation Treatment
The TTA in hanging drops on day 10 after co-culture were treated as follows: 1. control (vehicle treatment); 2. cisplatin (2.5 μM); 3. cisplatin (2.5 μM) + radiation (5 Gy); 4. radiation (5 Gy); 5. cisplatin (2.5 μM) + radiation (2.5 Gy×2). For combination treatments, a 2 h incubation of TTA with cisplatin (2.5 μM) was followed by either a 5 Gy dose or the first fractionated 2.5 Gy dose. The second fractionated dose was delivered after 24 h. Cell culture media in all treatment groups was replaced at 24 h after each treatment. Each treatment group had an average of 12 TTA that were monitored every alternative day and final images were captured at the experiment end point (day 8).
MMP-TIMP Assay
Approximately 12 TTA from each treatment group were harvested on day 8 of treatment. The cell pellets of 12 TTA were washed with PBS and incubated in 100 μl of lysis buffer at 4°C for 1 h. The cell debris was cleared by centrifugation for 15 min at 12,000g and the supernatant was collected. The protein concentration of the lysates was determined using Micro BCA™ Protein Assay Kit (Thermo Scientific Pierce, Rockford, IL) and absorbance was measured at 562 nm by Spectramax® Microplate Reader (Molecular Devices, Sunnyvale, CA). In this study, we used the Discovery Assay® for quantification of human matrix metalloproteinase (MMPs) and human tissue inhibitors of metalloproteinases (TIMPs) (Eve Technologies Corp., Calgary, Canada). The multiplexing analysis was performed using the Human MMP 9-plex and Human TIMP 4-plex Multiplex Kit (R&D Systems™, Minneapolis, MN) and read with a Luminex® 100™ system (Austin, TX). The assay was performed by Eve Technologies Corp. according to the manufacturer's protocol. The 9-plex and 4-plex consisted of: MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-12, MMP-13, TIMP-1, TIMP-2, TIMP-3 and TIMP-4. The assay sensitivities of these markers range from 0.28–63.5 pg/ml for the two panels. Individual analyte values are available in the R&D protocols.
Multiplex Analysis of Cytokines
The culture media of all TTA groups were analyzed for cytokine/chemokine using a Human Cytokine/Chemokine Array (Eve Technologies). The multiplex assay was performed at Eve Technologies with the Bio-Plex™ 200 system (Bio-Rad Laboratories Inc., Hercules, CA), and a Milliplex® Human Cytokine Kit (EMD Millipore, St. Charles, MO) according to the supplier's protocol. Briefly, this high-throughput assay utilizes 64 fluorescently-labeled distinct bead populations. Target analyte detection begins on the surface of a bead coupled to a capture antibody that binds a specific target analyte. During the incubation of biological samples with the multiplexed beads in a single microwell, any target analyte in the biological sample is bound to a bead via the analyte-specific capture antibody. A detection antibody cocktail, containing biotinylated antibodies that bind to any target analyte captured onto the bead, is introduced. The reaction mix is then incubated with streptavidinphycoerythrin conjugate, the fluorescent reporter molecule, to complete the reaction on the surface of each microsphere. The beads were analyzed for the presence of the reporter molecule in a Bio-Plex 200. The 64-plex consisted of EGF, eotaxin, FGF-2, Flt-3 ligand, fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IL-1ra, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, MCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, Platelet-derived growth factor-AA (PDGF-AA), PDGF-AB/BB, RANTES, Transforming growth factor alpha (TGF-α), TNF-α, TNF-β, vascular endothelial growth factor (VEGF), sCD40L, eotaxin-2, MCP-2, BCA-1, MCP-4, I-309, IL-16, TARC, 6CKine, eotaxin-3, LIF, TPO, SCF, TSLP, IL-33, IL-20, IL-21, IL-23, TRAIL, CTACK, SDF-1a+B, ENA-78, MIP-1d, IL-28A. The assay sensitivities of these markers range from 0.1 to 55.8 pg/ml.
Statistical Analysis
Statistical significance of difference in means was determined using a parametric two-sample t test with unequal variance (α = 0.05; GraphPad Prism software; GraphPad Software Inc., LaJolla, CA). Data are expressed as mean ±1 SD of at least three different experiments. Two-tailed unpaired t tests with Welch's correction was used to determine statistical significance. The level of significance was accepted at *P < 0.05 or as mentioned.
RESULTS
Cancer Stem Cells in the Hypoxic Niche Are More Radioresistant
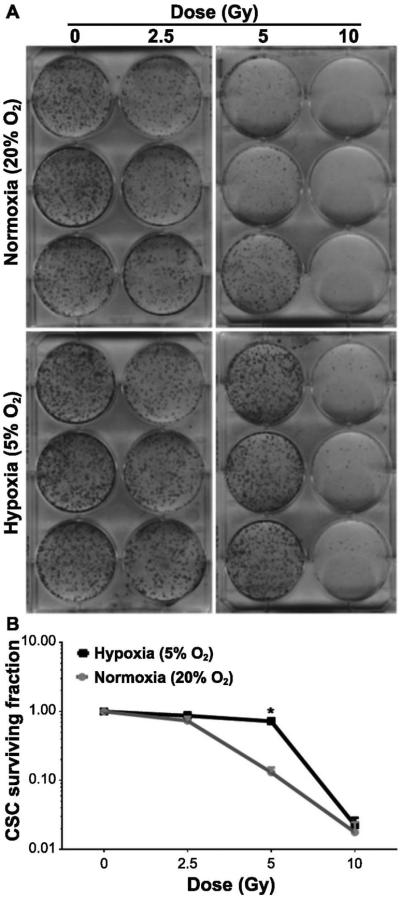
Clonogenic survival studies were performed to understand the effect of physiological oxygen levels on the response of CSC to an increasing dose of radiation. These experiments revealed that the CSC maintained in hypoxic conditions were significantly more resistant to a radiation dose of 5 Gy or less compared to CSC growing in normoxia (Fig. 1A and B). Given that the CSC preferentially survive in the hypoxic niche the subsequent studies to investigate the radioresistant properties of CSC in the context of the tumor microenvironment were designed using a single radiation dose of 5 Gy.
FIG. 1.
The hypoxic microenvironment increases CSC radioresistance. Panels A and B: CSC maintained in normoxia (20% O2) and hypoxia (5% O2) were exposed to increasing doses of radiation, as indicated. Cells were further maintained for 14 days after the media was replaced. Colonies (>50 cells) were stained and counted (see Materials and Methods). Data represent means ± SD (n = 3). Significant change in the surviving fraction of the CSC population maintained in hypoxic and normoxic conditions after 5 Gy irradiation (recorded as *P < 0.01).
Impact of Radiation Exposure on Cell Composition of TTA in Normoxia and Hypoxia
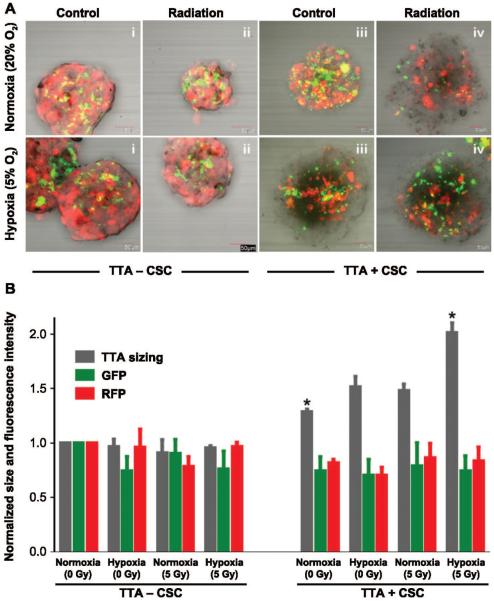
In this study, two distinct types of TTA were developed to understand the impact of cancer stem cells in the lung cancer microenvironment. The 3D co-cultures comprised of RFP-expressing human A549 NSCLC cells, HNDF-GFP, HPAEC with and without NSCLC with cancer stem cells were established in hanging drops of media. The TTA containing A549, fibroblast and endothelial cells without CSC were termed TTA − CSC. TTA containing A549, fibroblast and endothelial cells with CSC were termed TTA + CSC. The 3D co-cultures were maintained in either a hypoxic or normoxic environment. By day 10 of the initial co-culture, the different cell types had arranged into robust TTA and received a 5 Gy dose. Differences in cell composition were visually identified through confocal fluorescence microscopy on day 5 after treatment. While the A549 cells and fibroblasts were detected via the overexpression of RFP and GFP, respectively, both endothelial cells and CSC were colorless. Quantitation of the TTA size, and RFP and GFP fluorescence intensity in the different treatment groups revealed the impact of hypoxia and radiation to preferentially select the colorless cell population and cause the enhanced growth of TTA + CSC (Fig. 2B). Proliferation of the colorless cells was presumed to be primarily CSC due to their enhanced ability to self renew (24) and proliferate in 2D cell culture (data not shown).
FIG. 2.
The radiation-induced active proliferation of CSC in tumor tissue analogs (TTA) maintained in hypoxia accounts for the robust growth of TTA. Panel A: Confocal images of TTA maintained in normoxia 20% (upper images) and hypoxia 5% (lower images) with and without radiation treatment. Treatment regimens shown in the upper and lower images, from left to right, are as follows: i. TTA − CSC control; ii. TTA − CSC 5 Gy radiation; iii. TTA + CSC control; and iv. TTA + CSC 5 Gy radiation. Panel B. Graphical representation of size, green and red fluorescence of the TTA normalized to the untreated control (TTA − CSC) maintained in normoxic conditions. While the mere presence of CSC in the TTA under normoxic conditions resulted in a significant increase in size, radiation treatment and hypoxic microenvironment caused a further increase in the size of the TTA + CSC, with *P < 0.005 (n = 3). Abbreviations are as follows: TTA − CSC (A549, endothelial and fibroblast), TTA + CSC (A549, endothelial and fibroblast with CSC). The images are an overlay of red (tumor cells) and green (fibroblasts) fluorescence. The endothelial cells and CSC do not express any fluorescent protein.
While the confocal images of the TTA − CSC grown in normoxic (20% O2) and hypoxic (5% O2) conditions and the quantitation for red and green fluorescence intensity revealed no notable change in growth with similar compositions of cell types, the TTA + CSC appeared to have altered cell compositions, particularly with an increased CSC population, when grown in hypoxia (5% O2) (Fig. 2A and B). Radiation exposure induced a decrease in the size of the TTA − CSC in normoxia, which was not as notable under hypoxic conditions. When TTA + CSC were grown in normoxia, we observed that CSC became the dominant cell type only after 5 Gy irradiation. In contrast, when TTA + CSC were grown in hypoxia, CSC represented the dominant and proliferating cell type in both control and radiation treatment groups. These results indicate that both hypoxia and radiation promote the selection and proliferation of lung cancer stem cells grown in a 3D in vitro model of NSCLC.
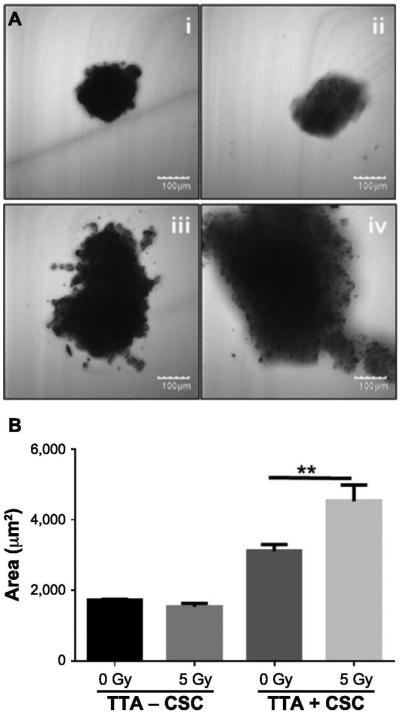
Growth of TTA + CSC Maintained in Hypoxia (5% O2) is Further Increased after Radiation Exposure
Radiation therapy is expected to shrink tumors as a result of the decrease in cell proliferation and cell death. To observe the impact of radiation on the size of TTA with and without CSC when maintained in hypoxia, the TTA received a single 5 Gy dose. Phase contrast images were taken 5 days after treatment. The images were used to measure TTA size by area analysis using ImageJ software. While irradiated and nonirradiated TTA − CSC had comparable mean areas (1,784 um2 and 1,642 um2, respectively), TTA + CSC exhibited a mean area of 3,163 μm2 without irradiation and 4,219 μm2 with irradiation (P < 0.008, Fig. 3). These results further confirm that the growth of TTA comprised of CSC in a hypoxic tumor microenvironment remains unaffected after 5 Gy irradiation.
FIG. 3.
The presence of CSC in TTA maintained in a hypoxic environment (5% O2) results in increased TTA growth, which is further enhanced after irradiation. Panel A: Phase-contrast images of TTA using confocal microscopy under the following treatment regimens (left to right): i. TTA−CSC control; ii. TTA−CSC 5 Gy radiation; iii. TTA + CSC control; and iv. TTA + CSC 5 Gy radiation. Panel B. Sizing of tumor tissue analogs grown in hypoxia by area analysis conducted in ImageJ. Mean area values are shown. Abbreviations are as follows: TTA−CSC (A549 lung adenocarcinoma cells, pulmonary endothelial cells and fibroblast), TTA + CSC (A549 lung adenocarcinoma cells, pulmonary endothelial and fibroblast with CSC). (n = 4 TTA were utilized per treatment to assess spheroid size). **P < 0.008 indicates a statistically significant difference between the treatments.
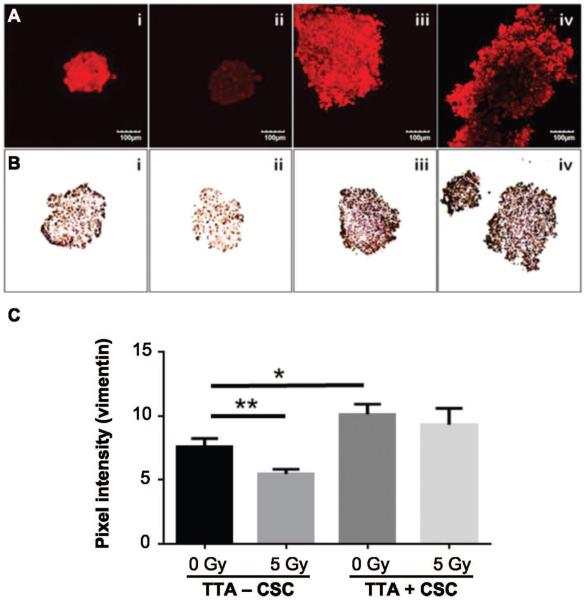
Elevated Expression of Vimentin in TTA with CSC is not Altered after Radiation Exposure
Vimentin is a protein released by cells into the extracellular matrix that contributes to the structural integrity of cells and tissues (25). Increased vimentin expression is associated primarily with metastatic phenotype and poor prognosis (26, 27). Tumor cells that survive the ionizing radiation express cancer stem cell markers and have an invasive phenotype with high levels of vimentin (11). The average pixel intensities of TTA groups incubated in the vimentin antibody were obtained using ImageJ software (Fig. 4A and C). The mean pixel intensities showed a 25% decrease in vimentin expression in TTA − CSC groups after irradiation (P < 0.009). In contrast, the TTA + CSC control group demonstrated a 30% increase in vimentin expression from baseline (relative to the TTA − CSC control group, P < 0.01). We hypothesize that this increase in vimentin expression is a consequence of the presence of CSC in the TTA. However, after irradiation the TTA + CSC groups demonstrated a nonsignificant 8% decrease in expression levels of vimentin (Fig. 4C). Here again we believe that the presence of CSC to be the primary cause for the radioresistance of TTA + CSC. Imaging results were validated with immunohistochemistry (DAB) for vimentin of a 5 μm TTA section fixed in HistoGel (Fig. 4B). Elevated expression of vimentin in TTA + CSC with no significant decrease after irradiation indicates the radioresistant characteristic contributed by the lung CSC in the hypoxic microenvironment.
FIG. 4.
Radiation treatment did not notably alter the increased expression of vimentin in TTA with CSC grown in hypoxia. TTA − CSC and TTA + CSC maintained in hypoxia at 5 days after 5 Gy irradiation were incubated with rabbit anti-vimentin polyclonal antibody followed by anti-rabbit secondary Alexa Fluor 633 (red, panel A) and monoclonal mouse anti-vimentin (Dako, Inc.) followed by subsequent DAB staining (panel B). Treatment regimens are as follows (left to right for panels A and B): i. TTA − CSC control; ii. TTA − CSC 5 Gy radiation; iii. TTA + CSC control; iv. TTA + CSC 5 Gy radiation. Panel C: Intensity analysis of antibody staining for vimentin in the respective groups shown in panel A. The average pixel intensity for vimentin in the different treatment groups was analyzed using ImageJ software. Mean ratio values are shown. Abbreviations are as follows: −CSC (A549, endothelial, fibroblast), +CSC (A549, endothelial, fibroblast, CSC). (n = 4 TTA were used per treatment assess spheroid size.) **P < 0.009 and *P < 0.01 indicate a statistically significant difference between the treatments.
Inhibition of TTA + CSC Growth in Hypoxia by Low-Dose Fractionated Irradiation and Cisplatin Treatment
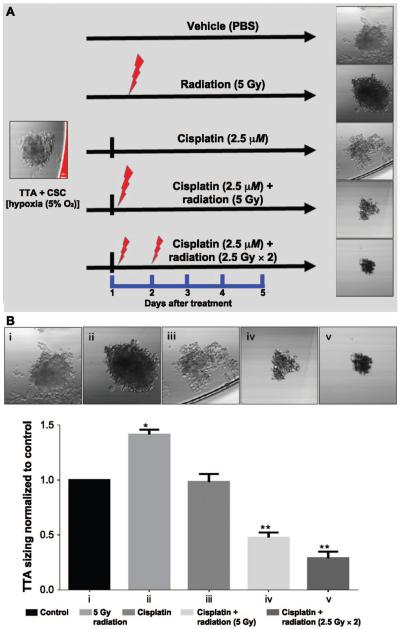
In this experiment, we investigated the effect of fractionated irradiation combined with cisplatin on TTA + CSC, as described in Materials and Methods. Figure 5A shows a comparison of the different treatment groups and the response of TTA + CSC in hypoxic states at day 5 after treatment. The confocal images of the TTA + CSC taken 5 days after treatment and their ImageJ analysis for TTA sizing (Fig. 5B) revealed a notable decrease in size of the TTA + CSC when treated with fractionated radiation (2.5 Gy × 2) and cisplatin. These treatment groups were further analyzed for changes in the molecular signatures in lysates and culture media of TTA + CSC that were representative of the invasive and aggressive phenotype of NSCLC.
FIG. 5.
Combined cisplatin and fractionated radiation treatment inhibits the growth of TTA + CSC in a hypoxic environment. Panel A: A schematic representation of TTA + CSC maintained in the hypoxic microenvironment and treated with different combinations of radiation and/or cisplatin. Panel B: Confocal phase-contrast images (upper images) of TTA + CSC grown in hypoxia (5%) after various treatment regimens (left to right): i. control; ii. single 5 Gy dose; iii. 2.5 μM cisplatin; iv. 2.5 μM cisplatin + single 5 Gy dose; v. 2.5 μM cisplatin + fractionated doses (2.5 Gy × 2). The images were taken on treatment day 5. ImageJ software analysis of sizing in TTA + CSC maintained in hypoxia revealed a significant decrease (P < 0.01) with combined cisplatin and single dose or fractionated doses compared to radiation treatment alone.
Expression Profile of Proteins in TTA + CSC and Cytokines Released Associated with the Invasive Phenotype of Lung Cancer after Treatment with Radiation Alone and in Combination with Cisplatin
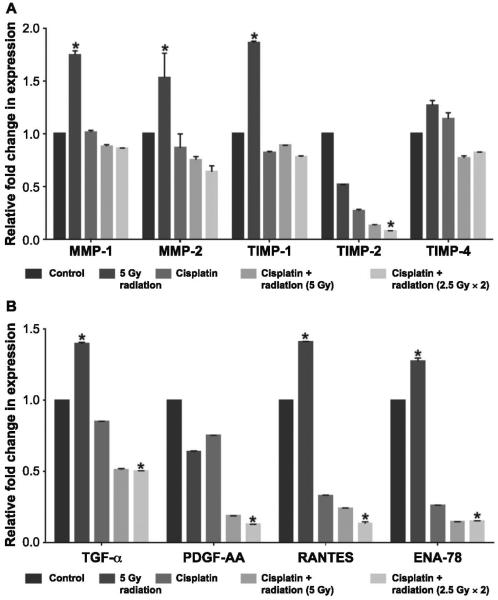
The TTA + CSC were treated with one of the following regimens: 1. untreated (control); 2. single radiation dose (5 Gy); 3. cisplatin (2.5 μM); 4. cisplatin (2.5 μM) + single radiation dose (5 Gy); and 5. cisplatin (2.5 μM) + fractionated radiation (2.5 Gy×2). The combined radiation and treatments were administered as described earlier. The lysates of 15 TTA + CSC from each treatment group were collected after 5 days of treatment and underwent molecular profiling for human matrix metalloproteinase and human tissue inhibitors of metalloproteinases. The MMP and TIMP data were analyzed and expression ratios were normalized to control groups with a value of 1 (Fig. 6A). Radiation exposure alone caused an increase in the expression of MMP-1, MMP-2 and TIMP-1 in the TTA + CSC lysates. This overexpression was normalized after treatment with cisplatin alone or in combination with radiation. While the expression of TIMP-4 did not show much variation in all treatment groups, the TIMP-2 expression was notably reduced (0.08) after combination treatment of cisplatin and radiation compared to untreated controls (1.0; Fig. 6A).
FIG. 6.
Single doses or fractionated doses combined with cisplatin in the TTA + CSC reverts the expression profile of proteins and cytokines associated with the invasive phenotype in lung cancer. Panel A: Quantification of pixel intensity as a measure of protein expression from TTA + CSC lysates 5 days after treatment incubated with the human MMP and TIMP multiplex antibody arrays. Panel B: Quantification of pixel intensity as a measure of protein expression from the culture media maintaining TTA + CSC 5 days after treatment and incubated with the human cytokine/chemokine antibody arrays. The results are presented as mean ± SE of three different determinations. *Significant difference (P < 0.05) in the treatment group compared to the untreated control group.
The culture media collected from the different TTA treatment groups were also analyzed for 64 human cytokines and chemokines using the human cytokine discovery array. TGF-α, PDGF-AA, RANTES and ENA-78 were four cytokines in the discovery array associated with the invasive and aggressive phenotype of lung cancer that demonstrated measurable changes in their levels in culture media. The expression profiles of these cytokines in response to the treatment regimens in TTA + CSC as described earlier are shown in Fig. 6B. The expression levels of cytokines trended downward in all treatment groups. Among TGF-α, RANTES and ENA-78, it was observed that a single 5 Gy dose increased expression levels, while other treatment groups appeared to suppress this increase and show a decrease in expression compared to the control group. PDGF-AA showed a progressive decrease in expression correlating with the aggressiveness of the treatment regimens, while sCD40L exhibited a notable increase in expression with cisplatin and a single 5 Gy dose. Decreased expression of RANTES was most prominent in the combined cisplatin and fractionated dose compared to the control or treatment of TTA + CSC with radiation alone.
DISCUSSION
The overall five-year progression-free survival for lung cancer has been slow to improve over the past three decades (28), due in part to the inability of existing tumor models to accurately simulate the biological processes that occur in lung cancer patients. Cancer stem cells form a subpopulation of the heterogeneous tumor mass with the ability to self renew and survive the current treatment modalities of chemotherapy and radiotherapy (29). An understanding of the tumor and its microenvironment-specific mechanisms that protect or sensitize the CSC from chemo-radiation therapies would serve as a valuable tool in predicting the therapeutic response in lung cancer patients. While the adenocarcinomic A549 cell line isolated by Giard et al. (30) has been extensively used as a valid NSCLC model to discover and develop various anti-cancer agents, it fails to incorporate the tumor:stroma crosstalk, which is known to play a critical role in the formation of the cancer stem cell niche (31). The rationale for use of patient-derived CSC in the human 3D NSCLC model to develop tumor tissue analogs presented in this study has been described in Materials and Methods. The inclusion of CSC in the 3D tumor model is that radiation modifies the cell composition of the TTA in favor of a CSC-dominant phenotype. These findings closely correlate with the existing understanding that CSC are a key player in tumor radioresistance (32–34).
The resistance phenomena seen in CSC require a hypoxic environment to adequately confer therapy resistance and metastatic potential to the population of tumor cells (23–33) (Fig. 1). Mimicking the physiological condition for the CSC was paramount in designing the 3D NSCLC model, which includes endothelial cells and fibroblasts, recreating conditions suitable for the intercellular crosstalk and formation of an extracellular matrix as evidenced in our previous studies (6, 12, 13). A preliminary study was conducted to assess the differences in cell composition seen in TTA − CSC and TTA + CSC in hypoxic 5% O2 and normoxic 20% O2 conditions (Fig. 2). As anticipated, TTA + CSC demonstrated increased proliferative potential and hypoxic conditions further promoted growth. It is important to note that while the 3D TTA develop their own hypoxic gradient, the lower O2 concentration (5%) reflects an in situ normoxia that is seen in normal human physiology (15). Culturing the TTA in hypoxic conditions served to recapitulate the in situ environment that the tumor and tumor microenvironment would typically thrive in within the human body. In line with the intrinsic resistance mechanisms seen in CD133+ cells (32), our studies on TTA + CSC show resistance to radiation and a moderate response to cisplatin treatment (Figs. 2 and 5). The TTA + CSC, maintained in hypoxia at 5 days after a single 5 Gy dose, displayed a CSC-dominant and radioresistant phenotype resulting in TTA growth that was significantly more pronounced than the nonirradiated control group maintained in normoxia (Figs. 2 and 3). While treatment with a single agent showed limited efficacy in reducing the size of TTA, co-administration of cisplatin with either single dose or fractionated doses resulted in a marked decrease in the size of TTA that included the CSC (TTA + CSC) (Fig. 5). The results from the TTA + CSC studies indicate an additive or synergistic treatment response that aligns with new evidence of the synergistic pro-apoptotic and autophagic killing effect of radiation when administered in conjunction with cisplatin (35). Our efforts are now directed toward developing cytotoxic assays to complement the decrease in TTA size for more reliable prediction of the effect of chemo-radiation therapy alone and in combination in our 3D NSCLC tumor model.
As a marker of the epithelial-to-mesenchymal transition (EMT), vimentin overexpression has been correlated with increased tumor growth and invasion (27–36). It has been reported that the expression of vimentin was observed to be a common occurrence in NSCLC and has been associated with dedifferentiation and metastases (27). Due to the increased expression of vimentin observed in the TTA + CSC (Fig. 4), we hypothesize that the inclusion of CD133+ CSC and stromal elements in the 3D model to provide a more comprehensive in vitro depiction of NSCLC by accounting for both the ECM and EMT elements. The lack of decrease in vimentin expression after irradiation of TTA + CSC is indicative of the intrinsic radioresistance contributed by the CSC.
While the prognostic value of vimentin expression is still controversial, matrix metalloproteinases have been associated with poor prognosis through their role in tumor progression, invasion, metastasis, tumorigenesis, angiogenesis and tumor growth (27–37). Conventional understanding of MMPs views their function in the context of a balance among the MMP proteins and their physiological inhibitors present in the ECM, TIMPs (37). Inhibition of MMPs occurs through noncovalent binding of MMPs with the TIMPs in a 1:1 stoichiometric complex (37). While it has been traditionally believed that TIMPs played a key role in inhibiting tumorigenic and metastatic phenotype of cancer cells through extracellular suppression of tumor invasion, new studies have show an increase in tumor progression with elevation of TIMP expression (37–39). The role of TIMPs appears to be multifunctional, and paradoxical effects on tumor progression have been seen in these new studies (37). In particular, TIMP-1 has been studied as a potential prognostic indicator in NSCLC and has been seen to possess growth-factor-like properties and anti-apoptotic activity (37). In accordance with the newfound understanding of the paradoxical effects of TIMPs, studies on TTA + CSC demonstrated increased expression of MMPs-1, —2 and TIMPs-1, —4 after a single 5 Gy dose, indicating an increase in expression of markers for tumor invasiveness due to survival of radioresistant CSC (Fig. 6A). This result was found to correlate with the findings by Tsutsumi et al. (40) where mRNA expression levels of MMPs-1, —2, —9 were seen to increase in tumor cells surviving a 10 Gy dose of radiation. Treatment with cisplatin alone or in combination with radiation resulted in lowering of this increased expression of MMP-1, —2 and TIMP-1, —2. This effect was, however, not observed in the expression of TIMP-4, most likely due to the chemoresistant properties of the CSC. The increase in MMP and TIMP expression due to radiation treatment alone was either attenuated or decreased in the combinatorial regimens of cisplatin with single dose or fractionated doses in TTA + CSC. Efforts are now underway to correlate the expression of the MMPs and TIMPs in response to the two treatment modalities, cisplatin and radiation, with the subsequent cellular toxicity in the NSCLC model with CSC.
Cytokines and chemokines produced by tumor and stromal cells have also been implicated in the progression and neovascularization of the tumor (41). Examination of cytokine and chemokine expression levels in culture media maintaining TTA + CSC after irradiation supported a proliferative and invasive phenotype. This effect was reversed after the combined cisplatin and radiation treatment of TTA + CSC (Fig. 6B). TGF-α is a ligand for the epidermal growth factor receptor (EGFR) that promotes the proliferation of epithelial cells (42). During cellular oncogenesis TGF-α is secreted by tumor cells for maintenance of the transformed phenotype and angiogenesis. TGF-α levels in the current study were found to increase in the culture media of the TTA + CSC exposed to a single 5 Gy dose (Fig. 6B). These results correlate with earlier studies where radiotherapy induces release of TGF-α in human lung cancer cells and their murine xenografts (43, 44). However, the co-administration of both single doses and fractionated doses with cisplatin attenuated the increase in TGF-α levels in the culture media of TTA + CSC and served to limit the level of radiation-induced proliferation of the CSC.
Platelet-derived growth factor-AA, a prognostic indicator for NSCLC, is postulated to directly contribute to angiogenic responses in addition to modulating the expression of VEGF, a key factor regulating tumor angiogenesis (45). The TTA + CSC studies demonstrate a downward trend of PDGF-AA expression correlating with the aggressiveness of therapy. PDGF-AA levels were highest in the untreated group, followed by the irradiated group, and were lowest in the group treated with combined cisplatin and fractionated radiation (2.5 Gy×2), suggesting its potential role in the inhibition of tumor angiogenesis with the combinatorial therapy.
The recently published literature reported that inflammatory chemokines like RANTES (CCL5) are not merely the gate keepers of inflammation and immunity but are important constituents of the tumor microenvironment with tumor promoting roles. Cancer cells secrete CCL5 or induce fibroblasts to secrete RANTES (CCL5) that binds to its receptor, CCR5, on tumor and stromal cells (including the endothelial cells) to sustain their proliferation and recruit immunosuppressive T-reg cells and monocytes to induce neoangiogenesis and metastasis (46, 47). The increase in the size of TTA + CSC and elevated expression of RANTES released in the culture media after a single 5 Gy dose is indicative of the tumor-promoting potential of radiation monotherapy. Lowering of RANTES level in the culture media after aggressive combinatorial regimens supported the enhanced cell killing in the TTA + CSC.
Epithelial-neutrophil activating peptide (ENA-78) is a CXC chemokine that plays an important role in the regulation of angiogenesis in NSCLC (48). Tumor growth was found to correlate with elevated expression of ENA-78 in a tumor xenograft of A549 NSCLC cells in SCID mice. Immunization of the NSCLC tumor-bearing mice with anti-ENA-78 antibody resulted in a reduced tumor growth, tumor vascularity and metastases (48). Our data demonstrated higher levels of ENA-78 in culture media of TTA + CSC exposed to a single 5 Gy radiation dose. This effect was attenuated with the administration of cisplatin, and co-administration of cisplatin with both single and fractionated radiotherapy lowered the levels of ENA-78 secreted into the culture media of TTA + CSC.
We surmise from our data that the 3D human NSCLC tumor tissue analogs serve as a realistic platform for the investigation of novel radiotherapy and chemotherapy treatment regimens. Crucial to this model was the inclusion of CSC that exhibit a similar invasive profile paralleling what is observed clinically in human patients (21). Using the in vitro model we have demonstrated that tumor cells, particularly CSC, survive radiation monotherapy and exhibit enhanced invasiveness due to the overexpression of MMPs, TIMPs and associated cytokines/chemokines, thereby indicating that radiation monotherapy is not sufficient for the treatment of NSCLC. Contrary to the conventional understanding of TIMPs' anti-invasive properties, executed by their ability to regulate the inhibition of MMPs (49–51), there are several published studies where increased TIMP expression is associated with tumor growth and progression (39, 52–54). Our results of TIMP-1 overexpression in response to radiation treatment in the TTA + CSC (Fig. 6A) indicate the tumor-promoting ability of TIMP-1 may also play a role in regulating the proliferation of CSC. Efforts are underway to understand the functional role of MMPs and TIMPs in the growth and invasive phenotype of TTA + CSC. These studies with TTA on the treatment regimens prevalent in NSCLC substantiate their use as physiologically relevant models for the investigation of treatment response in CSC that co-exist in the tumor microenvironment.
ACKNOWLEDGMENTS
This project was supported by the National Cancer Institute [grant nos. R21CA173609 (MU) and R25CA153954]. We acknowledge the 2014 SIT travel award from the Radiation Research Society to RC. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
REFERENCES
- 1.Cancer Facts and Figures. American Cancer Society; 2015. bit.ly/1tMudoF. [Google Scholar]
- 2.Price A. Emerging developments of chemoradiotherapy in stage III NSCLC. Nat Rev Clin Oncol. 2012;9:591–8. doi: 10.1038/nrclinonc.2012.135. [DOI] [PubMed] [Google Scholar]
- 3.Jett JR, Schild SE, Keith RL, Kesler KA, American College of Chest Physicians Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:266S–76S. doi: 10.1378/chest.07-1380. [DOI] [PubMed] [Google Scholar]
- 4.Robinson LA, Ruckdeschel JC, Wagner H, Jr., Stevens CW, American College of Chest Physicians Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:243S–65S. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 5.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 6.Upreti M, Jyoti A, Sethi P. Tumor microenvironment and nanotherapeutics. Transl Cancer Res. 2013;2:309–19. doi: 10.3978/j.issn.2218-676X.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faurobert E, Bouin AP, Albiges-Rizo C. Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol. 2015;27:64–70. doi: 10.1097/CCO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 9.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen GH, Murph MM, Chang JY. Cancer stem cell radioresistance and enrichment: where frontline radiation therapy may fail in lung and esophageal cancers. Cancers (Basel) 2011;3:1232–52. doi: 10.3390/cancers3011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, et al. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelialmesenchymal transition phenotypes. Mol Cancer. 2013;12:94. doi: 10.1186/1476-4598-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Upreti M, Jamshidi-Parsian A, Apana S, Berridge M, Fologea DA, Koonce NA, et al. Radiation-induced galectin-1 by endothelial cells: a promising molecular target for preferential drug delivery to the tumor vasculature. J Mol Med (Berl) 2013;91:497–506. doi: 10.1007/s00109-012-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upreti M, Jamshidi-Parsian A, Koonce NA, Webber JS, Sharma SK, Asea AA, et al. Tumor-endothelial cell three-dimensional spheroids: New aspects to enhance radiation and drug therapeutics. Transl Oncol. 2011;4:365–76. doi: 10.1593/tlo.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain RK, Forbes NS. Can engineered bacteria help control cancer? Proc Natl Acad Sci U S A. 2001;98:14748–50. doi: 10.1073/pnas.261606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–5. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 16.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–19. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 17.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271(4 Pt 1):C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 18.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–35. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanovic Z, Hermitte F, Brunet de la Grange P, Dazey B, Belloc F, Lacombe F, et al. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%) Stem Cells. 2004;22:716–24. doi: 10.1634/stemcells.22-5-716. [DOI] [PubMed] [Google Scholar]
- 20.Franken NA, Rodermond HM, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–19. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 21.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–6. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X, Li M, Wang X, Wang Y, Ma D. Both CD133+ and CD133− subpopulations of A549 and H446 cells contain cancer-initiating cells. Cancer Sci. 2009;100:1040–6. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M, Zhang Y, Zhang H, Wang S, Zhang M, Chen X, et al. Hypoxia-induced cell stemness leads to drug resistance and poor prognosis in lung adenocarcinoma. Lung Cancer. 2015;87:98–106. doi: 10.1016/j.lungcan.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–20. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 25.Franke WW, Grund C, Kuhn C, Jackson BW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation. 1982;23:43–59. doi: 10.1111/j.1432-0436.1982.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 26.Havel LS, Kline ER, Salgueiro AM, Marcus AI. Vimentin regulates lung cancer cell adhesion through a VAV2-Rac1 pathway to control focal adhesion kinase activity. Oncogene. 2014;34:1979–90. doi: 10.1038/onc.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dauphin M, Barbe C, Lemaire S, Nawrocki-Raby B, Lagonotte E, Delepine G, et al. Vimentin expression predicts the occurrence of metastases in non small cell lung carcinomas. Lung Cancer. 2013;81:117–22. doi: 10.1016/j.lungcan.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Joo KM, Jin J, Nam DH. Cancer stem cells and their mechanism of chemo-radiation resistance. Int J Stem Cells. 2009;2:109–14. doi: 10.15283/ijsc.2009.2.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–23. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 31.Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumour Biol. 2014;35:3945–51. doi: 10.1007/s13277-013-1561-x. [DOI] [PubMed] [Google Scholar]
- 32.Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755–7. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- 33.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8:545–54. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 34.Desai A, Webb B, Gerson SL. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother Oncol. 2014;110:538–45. doi: 10.1016/j.radonc.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Ma S, Liu M, Hou Y, Liang B, Su X, et al. Synergistic killing of lung cancer cells by cisplatin and radiation via autophagy and apoptosis. Oncol Lett. 2014;7:1903–10. doi: 10.3892/ol.2014.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aljada IS, Ramnath N, Donohue K, Harvey S, Brooks JJ, Wiseman SM, et al. Upregulation of the tissue inhibitor of metalloproteinase-1 protein is associated with progression of human non-small-cell lung cancer. J Clin Oncol. 2004;22:3218–29. doi: 10.1200/JCO.2004.02.110. [DOI] [PubMed] [Google Scholar]
- 38.Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993;59:329–41. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–52. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 40.Tsutsumi K, Tsuda M, Yazawa N, Nakamura H, Ishihara S, Haga H, et al. Increased motility and invasiveness in tumor cells that survive 10 Gy irradiation. Cell Struct Funct. 2009;34:89–96. doi: 10.1247/csf.09006. [DOI] [PubMed] [Google Scholar]
- 41.Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta. 2014;1845:182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Siegfried JM. Detection of human lung epithelial cell growth factors produced by a lung carcinoma cell line: use in culture of primary solid lung tumors. Cancer Res. 1987;47:2903–10. [PubMed] [Google Scholar]
- 43.Chung EJ, Urick ME, Kurshan N, Shield W, 3rd, Asano H, Smith PD, et al. MEK1/2 inhibition enhances the radiosensitivity of cancer cells by downregulating survival and growth signals mediated by EGFR ligands. Int J Oncol. 2013;42:2028–36. doi: 10.3892/ijo.2013.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–7. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 45.Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65:7241–8. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 46.Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strieter RM, Belperio JA, Burdick MD, Sharma S, Dubinett SM, Keane MP. CXC chemokines: angiogenesis, immunoangiostasis, and metastases in lung cancer. Ann N Y Acad Sci. 2004;1028:351–60. doi: 10.1196/annals.1322.041. [DOI] [PubMed] [Google Scholar]
- 48.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest. 1998;102:465–72. doi: 10.1172/JCI3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brand K. Cancer gene therapy with tissue inhibitors of metalloproteinases (TIMPs) Curr Gene Ther. 2002;2:255–71. doi: 10.2174/1566523024605564. [DOI] [PubMed] [Google Scholar]
- 50.Brand K, Baker AH, Perez-Canto A, Possling A, Sacharjat M, Geheeb M, et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res. 2000;60:5723–30. [PubMed] [Google Scholar]
- 51.Elezkurtaj S, Kopitz C, Baker AH, Perez-Canto A, Arlt MJ, Khokha R, et al. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinases-1 in the liver: efficient protection against T-cell lymphoma and colon carcinoma metastasis. J Gene Med. 2004;6:1228–37. doi: 10.1002/jgm.637. [DOI] [PubMed] [Google Scholar]
- 52.Kahlert C, Bandapalli OR, Schirmacher P, Weitz J, Brand K. Invasion front-specific overexpression of tissue inhibitor of metalloproteinase-1 in liver metastases from colorectal cancer. Anticancer Res. 2008;28:1459–65. [PubMed] [Google Scholar]
- 53.Gong Y, Scott E, Lu R, Xu Y, Oh WK, Yu Q. TIMP-1 promotes accumulation of cancer associated fibroblasts and cancer progression. PLoS One. 2013;8:e77366. doi: 10.1371/journal.pone.0077366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rojiani MV, Ghoshal-Gupta S, Kutiyanawalla A, Mathur S, Rojiani AM. TIMP-1 overexpression in lung carcinoma enhances tumor kinetics and angiogenesis in brain metastasis. J Neuropathol Exp Neurol. 2015;74:293–304. doi: 10.1097/NEN.0000000000000175. [DOI] [PubMed] [Google Scholar]