Abstract
Objective
Children and adolescents with critical cyanotic congenital heart disease (CHD) are at risk for deficits in aspects of executive function (EF). The primary aim of this investigation was to compare EF outcomes in three groups of children/adolescents with severe CHD and controls (ages 10–19 years).
Method
Participants included 463 children/adolescents with CHD [dextro-transposition of the great arteries (TGA), n = 139; tetralogy of Fallot (TOF), n = 68; and, single-ventricle anatomy requiring Fontan procedure (SVF), n = 145] and 111 controls, who underwent laboratory and informant-based evaluation of EF skills.
Results
Rates of EF impairment on D-KEFS measures were nearly twice as high for CHD groups (75–81%) than controls (43%). Distinct EF profiles were documented between CHD groups on D-KEFS tasks. Deficits in flexibility/problem-solving and verbally-mediated EF skills were documented in all three CHD groups; visuo-spatially-mediated EF abilities were impaired in TOF and SVF groups, but preserved in TGA. Parent, teacher, and self-report ratings on the BRIEF highlighted unique patterns of metacognitive and self-regulatory concerns across informants.
Conclusions
CHD poses a serious threat to EF development. Greater severity of CHD is associated with worse EF outcomes. With increased understanding of the cognitive and self-regulatory vulnerabilities experienced by children and adolescents with CHD, it may be possible to identify risks early and provide individualized supports to promote optimal neurodevelopment.
Keywords: congenital heart defects, executive function, transposition of great vessels, tetralogy of Fallot, single-ventricle, Fontan procedure
Congenital heart disease (CHD) includes a diverse array of conditions affecting the structural and/or functional integrity of the heart. Dextro-transposition of the great arteries (TGA), tetralogy of Fallot (TOF), and single-ventricle conditions such as hypoplastic left heart syndrome (HLHS) are among the most serious forms of critical cyanotic CHD (Marino et al., 2012), each requiring early and intensive medical and surgical intervention(s) to sustain life. In TGA, the major blood vessels connecting the systemic and pulmonary blood supplies are transposed. In TOF, four cardiac abnormalities (ventricular septal defect, pulmonary stenosis, right ventricular hypertrophy, and an overriding aorta) undermine heart function and blood flow. In single-ventricle conditions such as HLHS, one ventricle of the heart fails to develop and thus is unable to circulate oxygenated blood to the body; staged palliative surgeries, typically culminating in the Fontan procedure, are often indicated. The incidence of critical cyanotic CHD is approximately 3/1000 live births (Hoffman & Kaplan, 2002).
Severe CHD poses a serious threat to brain development. The pathophysiological mechanisms underlying neurologic injury in CHD are diverse and not yet fully understood, including not only potential hypoxic/ischemic cascades triggered by hypoperfusion during cardiac surgery but also a wide range of genetic, prenatal, and other pre- and post-operative factors. In at least some forms of severe CHD, atypical brain development is evident prenatally, as early as 25- to 30-weeks gestation (Clouchoux et al., 2012; Limperopoulos et al., 2010). Infants with CHD exhibit high rates of microcephaly, hypotonia, and atypical state regulation on clinical examination, and neuroimaging abnormalities such as ischemic infarcts and white matter injury (periventricular leukomalacia) are present in up to 59% prior to surgery (Owen et al., 2011). Relative to controls, the brains of full-term infants with TGA or HLHS are smaller and less mature structurally than those of typically-developing infants (Licht et al., 2009), with reduced grey matter volumes particularly in the frontal lobe (Watanabe et al., 2009). Adolescents with corrected TGA (Bellinger et al., 2011) and those with TOF (Bellinger et al., 2014a) exhibit much higher rates of structural MRI abnormalities than controls. Fractional anisotropy on diffusion tensor imaging is significantly reduced in adolescents with TGA, particularly within deep cerebral, cerebellar, and midbrain white matter (Rivkin et al., 2013).
Behavioral studies further evidence the adverse impact of CHD on the developing brain. Within the context of Low Average to Average overall cognitive abilities (Karsdorp et al., 2007), children/adolescents with CHD, as a group, face increased risk for deficits in speech/language, sensory/motor, attention, memory, and visual-spatial skills (Hovels-Gurich et al., 2002; Miatton et al., 2007a, 2007b; Bellinger et al., 2003; 2009; 2011; Brosig, Mussatto, Kuhn, & Tweddell, 2007; Calderon et al., 2010; Gaynor et al., 2010; Schaefer et al., 2013). Educational/academic achievement difficulties (Bellinger et al., 2003, 2011), social cognitive deficits (Bellinger et al., 2008, 2011; Calderon et al., 2010), and emotional/behavioral problems (Bellinger et al., 2009; Brosig et al., 2007) are also elevated.
Children and adolescents with CHD are also at risk for deficits in executive function. “Executive function” (EF) refers to a constellation of skills, mediated by densely interconnected neuroanatomical networks involving frontal/prefrontal (Robbins, 1996), parietal (Champod & Petrides, 2010), cerebellar (Stoodley & Schmahmann, 2010), and subcortical structures (Little et al., 2010; Provost, Petrides, & Monchi, 2010), that are necessary for effective regulation of behavior, emotion, cognition, and social adaptation (Diamond, 2013). Children with severe CHD exhibit problems with inhibitory control (Miatton et al., 2007a; Gaynor et al., 2010, 2014; Calderon et al., 2010, 2012), planning (Bellinger et al., 2003, 2011; Miatton et al., 2007a, 2007b; Calderon et al., 2010, 2012), cognitive flexibility (i.e., switching/shifting; Bellinger et al., 2003, 2011; Calderon et al., 2010, 2012), working memory (Calderon et al., 2010, 2012), and executive attention (Hovels-Gurich et al., 2007). Some also struggle with abstract problem-solving and inferential reasoning, and can have a hard time with efficient retrieval and generation of verbal output (Bellinger et al., 2003). Composite parent and teacher ratings of EF skills confirm self-regulatory and metacognitive difficulties at home and school (Bellinger et al., 2011, 2014a). In contrast, self-report ratings of global EF abilities have generally failed to document significant concerns (Bellinger et al., 2011, 2014a), leading investigators to suggest that “…relying solely on self-reports of patients with congenital heart disease might underestimate the severity of their challenges, at least in the domain of executive functions” (Bellinger et al., 2014a, p. 9).
The primary aim of the current investigation was to compare EF outcomes in four groups of children and adolescents: three with CHD (TGA, TOF, or single-ventricle cardiac conditions culminating in the Fontan procedure) and a group of typically-developing controls. Although prior studies have shown that children with severe CHD are at risk for EF deficits, none to date have been designed and/or adequately powered to determine whether distinct forms of CHD are associated with distinct patterns of EF vulnerabilities. In the present study, we operationalized the EF construct broadly, using a combination of well-validated laboratory tasks and parent, teacher, and self-report rating scales, within a large mixed-CHD sample of children and adolescents who participated in one of three cardiac neurodevelopmental studies at Boston Children’s Hospital.
We hypothesized that children/adolescents with CHD would perform worse on all laboratory EF tasks and would be rated by parents and teachers as having more real-world EF problems than controls. Self-report ratings were not expected to reflect the same degree of problem severity as parent and teacher reports; nonetheless, by examining perceived concerns across a wide range of specific EF domains, this study provides a more comprehensive test than previous investigations of whether children/adolescents with CHD self-identify EF problems in everyday life. Finally, because data contrasting neurodevelopmental outcomes on the basis of cardiac diagnosis are limited, we conducted exploratory comparisons across CHD groups.
Method
Recruitment and Procedure
Data were compiled from three large-scale, single-center studies: 1) the Boston Circulatory Arrest Study of children/adolescents with TGA (Bellinger et al., 2011); 2) a study of children/adolescents with TOF (Bellinger et al., 2014a); and 3) a study of children/adolescents with single-ventricle cardiac anatomy who underwent the Fontan operation (SVF; Bellinger et al., 2014b). All three studies included extensive neuropsychological evaluation (lasting approximately 4 hours). Psychological measures were administered in a fixed order by a licensed psychologist or supervised research assistant.
Studies were approved by the Institutional Review Board and conducted in accordance with the Helsinki Declaration. Informed consent was obtained from parents of participants; adolescents provided assent.
TGA group
The Boston Circulatory Arrest Study has been well-described previously (e.g., Newburger et al., 1993; Bellinger et al., 1995, 2003, 2011). Eligible participants included children/adolescents 14–16 years old with TGA who underwent the arterial switch operation by 3 months of age. Exclusion criteria included birth weight <2.5 kg, recognized genetic syndrome, prior heart surgery, or cardiovasculature requiring reconstruction of the aortic arch. Enrolled infants were randomly assigned to receive the arterial switch operation using a strategy of vital organ support of cardiopulmonary bypass with predominant deep hypothermic circulatory arrest or predominant low-flow bypass. Children were followed serially after surgery. Data from the most recent assessment were analyzed in the current study.
TOF group
Eligible participants included children/adolescents 13–16 years old with TOF (with or without pulmonary atresia) who underwent surgical repair at least 6 months prior to assessment. Exclusion criteria included diagnosis of trisomy 21 and/or presence of a disorder/device contraindicated for MRI.
SVF group
Eligible participants included children/adolescents 10–19 years old with single-ventricle cardiac anatomy and who underwent the Fontan procedure, Fontan re-do, or other open-heart surgical procedure at least 6 months before evaluation. Exclusion criteria included history of cardiac transplantation and/or presence of a disorder/device contraindicated for MRI.
Control group
A total of 111 typically-developing children/adolescents 10–19 years old were recruited (61 during the TOF study and 50 during the SVF study) in accordance with admission criteria for the NIH MRI Study of Normal Brain Development (Waber et al., 2007).
Participants
Among the 497 children/adolescents included in our cohort, 34 (23 TOF and 11 SVF) had identified genetic/syndromic conditions (e.g., 22q11) and were excluded from analyses. The final pooled sample for the current study included 463 children/adolescents (63.3% male; 139 TGA, 68 TOF, 145 SVF, and 111 controls) ranging in age from 10 to 19 years (M = 15.17, SD = 2.04). Table 1 presents sample demographic and medical/surgical characteristics.
Table 1.
Sample demographic characteristics by cardiac diagnosis
| CHD (n = 343–357) |
Control (n = 101–111) |
TGA (n = 138–139) |
TOF (n = 62–68) |
SVF (n = 141–145) |
|
|---|---|---|---|---|---|
| Family SES a | 47.88 (12.71) | 53.06 (9.55) | 45.81 (12.18) | 48.65 (11.95) | 49.50 (13.34) |
| Gestational age (weeks) | 39.34 (1.98) | 39.58 (1.30) | 39.75 (1.25) | 39.17 (2.49) | 39.00 (2.24) |
| Birth weight (kg) | 3.39 (0.58) | 3.48 (0.59) | 3.55 (0.45) | 3.21 (0.67) | 3.31 (0.62) |
| Sex: male n (%) | 234 (66.5) | 59 (53.2) | 106 (76.3) | 38 (55.9) | 90 (62.1) |
| Race/Ethnicity n (%) | |||||
| White/Caucasian/Non-Hispanic | 303 (86.1) | 89 (80.2) | 126 (90.6) | 59 (86.8) | 118 (81.4) |
| Nonwhite: | 49 (13.9) | 22 (19.8) | 13 (9.4) | 9 (13.2) | 27 (18.6) |
| White/Hispanic | 26 (7.4) | 3 (2.7) | 5 (3.6) | 5 (7.4) | 16 (11.0) |
| Asian | 6 (1.7) | 5 (4.5) | 2 (1.4) | 0 (0.0) | 4 (2.8) |
| Black | 9 (2.6) | 9 (8.1) | 2 (1.4) | 2 (2.9) | 5 (3.4) |
| Pacific Islander | 1 (0.3) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Biracial/Mixed Race | 7 (2.0) | 5 (4.5) | 3 (2.2) | 2 (2.9) | 2 (1.4) |
| Age at assessment (years) | 15.13 (2.11) | 15.30 (1.81) | 16.08 (0.51) | 14.67 (1.18) | 14.44 (2.91) |
| One-minute Apgar scoreb | 7.66 (1.37) | 8.97 (1.35) | 7.50 (1.35) | 7.68 (1.73) | 7.86 (1.30) |
| Five-minute Apgar scorec | 8.50 (0.84) | 9.45 (0.51) | 8.32 (0.90) | 8.83 (0.65) | 8.65 (0.75) |
| Age at first operation (days) | 62.17 (151.12) | -- | 9.89 (11.76) | 185.56 (233.41) | 54.42 (142.47) |
| Total cardiac operations Mdn (min-max) | 2 (1–7) | -- | 1 (1–4) | 2 (1–7) | 3 (1–5) |
Note. CHD = congenital heart disease; TGA = dextro-transposition of the great arteries; TOF = tetralogy of Fallot; SVF = single-ventricle children/adolescents who underwent the Fontan procedure; SES = family socioeconomic status according to Hollingshead Four Factor Index of Social Status (1975). The CHD category includes all participants in the TGA, TOF, and SVF groups. Some demographic data were missing; therefore, sample sizes, which are provided above as min-max, depict valid n’s by group. Unless otherwise specified, results are presented as mean (standard deviation).
Hollingshead, A. A. (1975). Four-factor index of social status. Unpublished manuscript, Yale University, New Haven, CT.
ns: CHD = 256; Control = 30; TGA = 127; TOF = 25; SVF = 104
ns: CHD = 252; Control = 29; TGA = 126; TOF = 23; SVF = 103
Measures
The present investigation used a common subset of laboratory data from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) and questionnaire data from the Behavior Rating Inventory of Executive Function (BRIEF; Gioia et al., 2000; Guy et al., 2004) to examine EF outcomes. Of note, broad neurobehavioral outcomes from the three larger studies, including a D-KEFS composite score and BRIEF General Executive Composite scores, have been described in previous reports (Bellinger et al., 2011, 2014a, 2014b). The present study provides a detailed analysis of EF outcomes, utilizing individual D-KEFS subtest scores and BRIEF subscale/index scores that have not been published elsewhere.
D-KEFS
The D-KEFS is a widely-used battery of laboratory EF tasks. Five subtests were included in the current study. The Verbal Fluency Test is a measure of verbal generativity and switching consisting of 3 conditions (Letter Fluency, Category Fluency, Category Switching). The Design Fluency Test is a measure of visual-spatial generativity and switching consisting of 3 conditions (Filled Dots, Empty Dots, Dot Switching). The Sorting Test is a measure of cognitive flexibility and problem-solving in which participants are asked to sort cards into as many 3-card groups as possible. The Word Context Test is a measure of verbal concept formation and hypothesis-testing requiring participants to determine the definitions of 10 nonsense “mystery” words using a series of context clues. The Tower Test is a measure of visual-spatial planning that requires participants to build a series of towers by arranging flat disks on a board with 3 vertical pegs. Age-referenced scaled scores (M = 10, SD = 3) were included in analyses.
BRIEF
The BRIEF is a questionnaire designed to solicit information about an individual’s use of EF skills in real-world settings. Parent, teacher, and self-report ratings were administered. Age-referenced T-scores (M = 50, SD = 10) were included in analyses. Scores ≥ 65 are considered “clinically significant;” self-report scores ≥ 60 may “warrant clinical interpretation” (Guy et al., 2004, p. 16; Gioia et al., 2000).
Data Analysis
Data analyses were conducted using IBM SPSS Statistics Version 21 and SAS Version 9.3. Variables were examined for normality and outliers; no concerning outliers were present. D-KEFS variables were normally distributed. BRIEF data were significantly positively skewed and could not be normalized adequately with transformation, thereby precluding them from analyses assuming normality. To establish comparability of groups on EF outcomes, six factors potentially related to EF development [socioeconomic status (SES), birth weight, gestational age, age at assessment, sex, and race (white/Caucasian/non-Hispanic vs. nonwhite)] were subjected to separate Multivariate Analysis of Covariance (MANCOVA) models and examined for between-group differences.
Profile analysis was used initially to assess D-KEFS score patterns across CHD (combined) and control groups, and subsequently to compare profiles across CHD subgroups (TGA, TOF, and SVF). This analysis was conducted using a general linear model (PROC GLM in SAS) with the D-KEFS subtests as the outcome variables and with group and other significant covariates included as predictors. Performance on D-KEFS subtests was compared across groups using contrasts from the profile analysis with a Bonferroni correction for pairwise comparisons. D-KEFS subtest scores were then dichotomized using a cutoff of 1.5 SD below population mean (scaled scores ≤ 6) to denote impairment. BRIEF data were also dichotomized using accepted cutoff scores. Logistic regression, controlling for significant covariates, was used to compare the odds of scoring within the impaired/elevated range between CHD groups and controls on D-KEFS/BRIEF measures. Paired t-tests (calculated separately for CHD and control groups) were performed to compare self-report vs. parent/teacher BRIEF ratings. We used Benjamini and Hochberg’s (1995) false discovery rate procedure to limit the chance of reporting a falsely significant result to be no more than 5%. We first determined significance of the overall group effect for each model and then, if significant, conducted pairwise comparisons between groups to identify significant group differences. Using this procedure, a p-value ≤ 0.031 was considered statistically significant. In an exploratory analysis, Spearman partial correlation coefficients were calculated to evaluate associations between D-KEFS and BRIEF variables.
Results
Comparability of Groups
SES, birth weight, gestational age, age at assessment, sex, and race were examined as potential covariates in four separate MANCOVA models: 1) all D-KEFS variables, 2) BRIEF Parent, 3) BRIEF Teacher, and 4) BRIEF Self-Report. Groups did not differ in gestational age or birth weight for any EF outcome. Significant findings were as follows: for D-KEFS: SES [F (11, 413) = 4.91, p < .001], age at assessment [F (11, 415) = 1.81, p = .05], sex [F (11, 415) = 2.84, p = .001], and race [F (11, 415) = 2.70, p = .002]; for BRIEF-Parent: SES [F (8, 439) = 5.91, p < .001] and sex [F (8, 441) = 2.66, p = .007]; for BRIEF-Teacher: sex [F (8, 235) = 2.86, p = .005] and race [F (8, 235) = 2.46, p = .01]; for BRIEF-Self: SES [F (8, 416) = 2.81, p = .005]. Significant factors for each respective EF outcome source were included as covariates in primary analyses. Consistent with the rationale proposed by Dennis et al. (2009), IQ was not included as a covariate in any analysis (see also Miller, Loya, & Hinshaw, 2013).
Primary Analyses
D-KEFS task performance
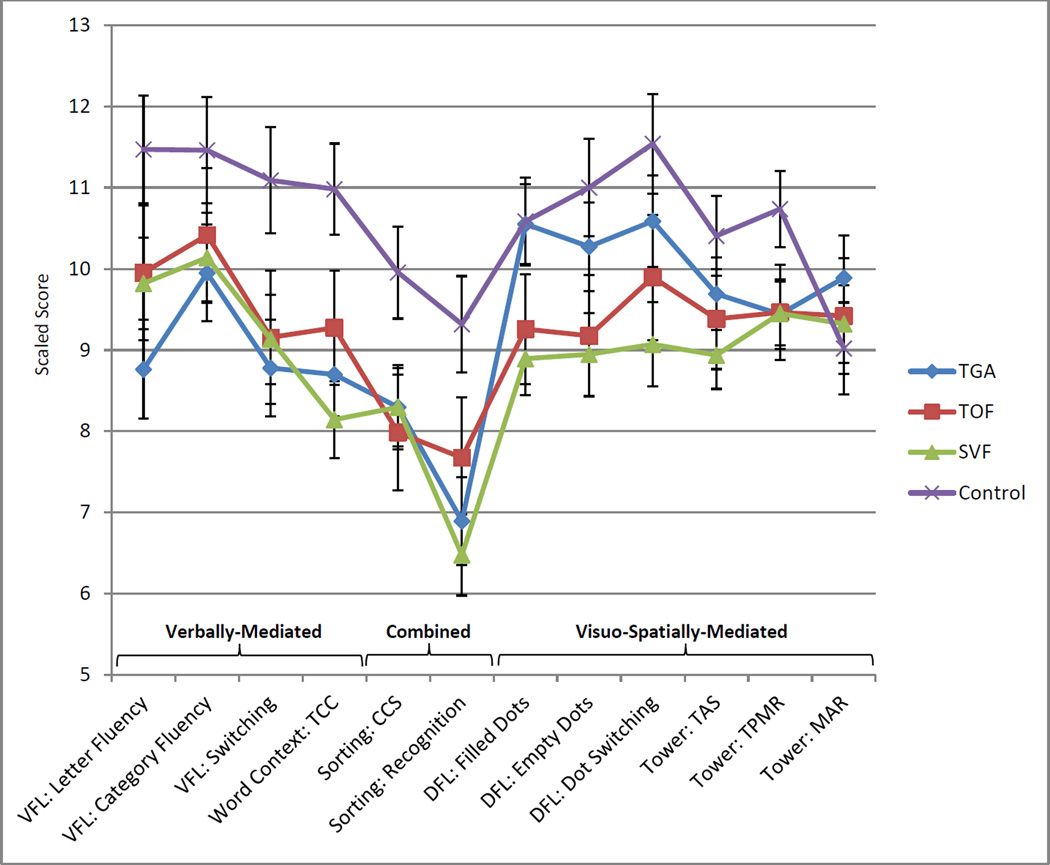
Most D-KEFS subtest scores were within the average range, except for the Sorting Recognition score, which was below average in some CHD groups (Table 2). Performance profiles are depicted graphically in Figure 1. Profile analysis indicated that D-KEFS score patterns between combined CHD and control participants were not parallel [F (11, 410) = 2.65, p < .001]. Looking specifically at score patterns across the TGA, TOF, and SVF groups, likewise revealed a lack of parallelism, F (22, 820) = 3.16, p < .001; the TGA profile differed significantly from the SVF [F (11, 410) = 4.53, p < .001] and TOF [F (11, 410) = 2.65, p = .003] profiles, and the TOF and SVF profiles were also significantly different [F (11, 410) = 2.01, p = .026].
Table 2.
D-KEFS subtest performance and impaired subtest score percentages by cardiac diagnosis
| D-KEFS Subtest | CHD | Control | TGA | TOF | SVF | CHD vs. Control | Pairwise Comparisons (Cohen’s d) | |
|---|---|---|---|---|---|---|---|---|
| n = 330–351 | n = 102–111 | n = 128–139 | n = 62–68 | n = 140–144 | p-value | Cohen’s d | ||
| VERBAL | ||||||||
| Verbal Fluency Test | ||||||||
| Letter Fluency | 9.25 (3.49) | 11.77 (3.40) | 8.63 (3.51) | 9.49 (3.56) | 9.73 (3.37) | <.001 | .60 | C > TGA*** (.79), SVF*** (.49) |
| Category Fluency | 9.94 (3.31) | 11.73 (3.34) | 9.80 (3.14) | 9.96 (3.18) | 10.08 (3.53) | .001 | .41 | C > TGA** (.45), SVF*(.40) |
| Cat. Switch Correct | 8.87 (3.35) | 11.20 (3.42) | 8.57 (3.26) | 8.87 (3.48) | 9.17 (3.37) | <.001 | .64 | C > TGA*** (.69), TOF** (.59), SVF*** (.59) |
| Word Context Test | ||||||||
| Total Consec. Correct | 8.42 (3.54) | 11.09 (2.42) | 8.66 (3.24) | 8.82 (3.91) | 8.01 (3.61) | <.001 | .78 | C > TGA*** (.72),TOF** (.55), SVF*** (.91) |
| VISUO-SPATIAL | ||||||||
| Design Fluency Test | ||||||||
| Filled Dots | 9.45 (2.99) | 10.67 (2.81) | 10.29 (2.94) | 8.87 (2.99) | 8.92 (2.86) | .002 | .35 | C > TOF* (.47), SVF*** (.59); TGA > TOF* (.45), SVF*** (.57) |
| Empty Dots | 9.37 (2.96) | 11.05 (2.90) | 9.96 (3.10) | 8.91 (3.07) | 9.02 (2.69) | <.001 | .53 | C > TOF*** (.64), SVF*** (.71); TGA > SVF** (.45) |
| Dot Switching | 9.65 (3.16) | 11.52 (2.88) | 10.38 (2.97) | 9.46 (3.71) | 9.03 (2.92) | <.001 | .58 | C > TOF** (.55), SVF*** (.82); TGA > SVF*** (.50) |
| Tower Test | ||||||||
| Total Ach. Score | 9.20 (2.74) | 10.32 (2.20) | 9.68 (2.46) | 8.91 (3.31) | 8.87 (2.65) | <.001 | .45 | C > SVF*** (.59) |
| TPMR | 9.45 (2.55) | 10.71 (1.29) | 9.48 (2.31) | 9.39 (2.73) | 9.45 (2.69) | <.001 | .55 | C > TGA*** (.54); TOF** (.54), SVF*** (.54) |
| MAR | 9.57 (2.85) | 9.19 (2.62) | 9.93 (2.50) | 9.52 (3.44) | 9.26 (2.87) | .112 | .18 | -- |
| COMBINED | ||||||||
| Sorting Test | ||||||||
| Conf. Correct Sorts | 8.10 (3.03) | 9.95 (2.37) | 7.88 (2.63) | 7.81 (3.56) | 8.44 (3.10) | <.001 | .64 | C > TGA*** (.60),TOF*** (.73), SVF*** (.61) |
| Sort Recognition | 6.75 (3.33) | 9.46 (2.82) | 6.76 (3.32) | 7.37 (3.84) | 6.47 (3.07) | <.001 | .81 | C > TGA*** (.78),TOF** (.55), SVF*** (.94) |
| % scoring ≥ 1.5 SDs below the population M on at least 1 subtest |
77.5 | 43.6 | 80.5 | 77.0 | 75.0 | |||
Note. CHD = congenital heart disease; TGA = dextro-transposition of the great arteries; TOF = tetralogy of Fallot; SVF = single-ventricle children/adolescents who underwent the Fontan procedure; C = control; Total Ach. Score = Tower Total Achievement score; TPMR = Tower Time-per-Move Ratio; MAR = Tower Move-Accuracy Ratio; Conf. Correct Sorts = Sorting Test Confirmed Correct Sorts. Unless otherwise specified, results are presented as mean (standard deviation). All p-values are derived using linear contrasts from the profile analysis described in the data analysis section; significant differences between CHD groups are bolded. Cohen’s d effect size estimates were calculated using estimated marginal means and adjusted SDs. Some D-KEFS data were missing; therefore, sample sizes, which are provided above as min-max, depict valid ns by group.
p ≤ .031,
p ≤ .01,
p ≤ .001.
Figure 1.
Estimated marginal means of D-KEFS tasks in cardiac and control groups, controlling for SES, age, sex, and race (error bars represent 95% confidence intervals). TGA = dextro-transposition of the great arteries; TOF = tetralogy of Fallot; SVF = single-ventricle children/adolescents who underwent the Fontan procedure; VFL = Verbal Fluency; TCC = Total Consecutively Correct; CCS = Confirmed Correct Sorts; DFL = Design Fluency; TAS = Total Achievement Score; TPMR = Time per Move Ratio; MAR = Move-Accuracy Ratio.
Linear contrasts comparing TGA, TOF, SVF, and controls identified significant group differences. At least one CHD group performed significantly worse than controls for all D-KEFS measures except the Tower MAR, which was not statistically different among the groups. See Table 2 for a summary of significant pairwise differences between groups. No significant differences between CHD groups were noted on verbally-mediated EF tasks (Verbal Fluency and Word Context Tests) or on tasks with combined verbal/visuo-spatial demands (Sorting Test). In contrast, TGA- (and, in some instances, TOF-) group performance was relatively secure on most visuo-spatial EF tasks. On the Design Fluency Test, TGA-group performance was not statistically different than controls - and better than the SVF group - across all three trials; the TGA group also outperformed the TOF group on Filled Dots trial. On the Tower Test, TGA- and TOF-group Total Achievement Scores were not statistically different than controls; however, all three CHD groups scored lower than controls on Time-per-Move Ratio, indicating greater efficiency in task-completion among healthy children/adolescents than those with CHD. Pairwise CHD group differences were moderate in effect size.
Table 3 presents results from the logistic regression models used to compare the odds of impaired D-KEFS task performance among the control and CHD groups. Looking first at verbal EF tasks, the odds of impairment on the Category Fluency task were no worse among CHD groups than controls. In contrast, TGA and TOF groups had greater odds of impaired Letter Fluency than controls; odds of impaired Letter Fluency in the SVF group were lower than in the TGA group. All three CHD groups had greater odds of impaired Category Switching compared to controls, arguably the most demanding of the verbal fluency tasks. The highest odds of impairment were on the Word Context Test, with the SVF group significantly higher than both control and TGA participants.
Table 3.
Odds of impairment on D-KEFS subtest by CHD group as compared to controls
| D-KEFS Subtest | TGA | TOF | SVF | CHD-Group Pairwise Comparisons |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | ||
| VERBAL | ||||||||||
| Verbal Fluency Test | ||||||||||
| Letter Fluency | <.001 | 5.36 | 2.27–12.66 | .004 | 4.02 | 1.54–10.49 | .07 | 2.33 | 0.95–5.72 | TGA > SVF** (OR = 1.15) |
| Category Fluency | .11 | 2.21 | 0.84–5.86 | .13 | 2.33 | 0.78–6.95 | .04 | 2.76 | 1.07–7.14 | -- |
| Cat. Switch Correct | .002 | 3.29 | 1.57–6.89 | .02 | 2.74 | 1.18–6.37 | .01 | 2.62 | 1.25–5.48 | -- |
| Word Context Test | ||||||||||
| TCC | .001 | 6.59 | 2.13–20.37 | <.001 | 12.37 | 3.81–40.19 | <.001 | 16.15 | 5.40–48.31 | SVF > TGA*** (OR = 1.18) |
| VISUO-SPATIAL | ||||||||||
| Design Fluency Test | ||||||||||
| Filled Dots | .35 | 1.76 | 0.54–5.73 | .003 | 5.90 | 1.81–19.26 | .001 | 6.72 | 2.26–19.99 | TOF > TGA* (OR = 1.13), SVF > TGA*** (OR = 1.16) |
| Empty Dots | .004 | 6.50 | 1.79–23.60 | .001 | 10.04 | 2.69–37.44 | <.001 | 9.07 | 2.63–31.29 | -- |
| Dot Switching | .26 | 1.79 | 0.66–4.87 | .002 | 4.91 | 1.80–13.37 | .006 | 3.64 | 1.44–9.22 | TOF > TGA* (OR = 1.14) |
| Tower Test | ||||||||||
| Total Achievement | .19 | 2.26 | 0.68–7.55 | .008 | 5.24 | 1.55–17.72 | .005 | 4.90 | 1.61–14.94 | -- |
| TPMR | .05 | 4.72 | 0.98–22.74 | .02 | 6.66 | 1.33–33.36 | .02 | 6.13 | 1.35–27.79 | -- |
| MAR | .07 | 0.48 | 0.22–1.05 | .92 | 1.05 | 0.46–2.39 | .65 | 0.85 | 0.43–1.69 | -- |
| COMBINED | ||||||||||
| Sorting Test | ||||||||||
| CCS | <.001 | 5.15 | 2.12–12.53 | <.001 | 7.58 | 2.93–19.57 | .009 | 3.30 | 1.35–8.05 | TOF > SVF* (OR = 1.16) |
| Sort Recognition | <.001 | 4.80 | 2.49–9.26 | <.001 | 4.09 | 1.94–8.62 | <.001 | 4.66 | 2.45–8.88 | -- |
Note. CHD = congenital heart disease; TGA = dextro-transposition of the great arteries; TOF = tetralogy of Fallot; SVF = single-ventricle children/adolescents who underwent the Fontan procedure; OR = odds ratio; CI = confidence interval for the OR; C = control; TCC = Total Consecutively Correct; TPMR = Time-per-Move Ratio; MAR = Move-Accuracy Ratio; CCS = Confirmed Correct Sorts. Reference group = controls. Variables were dichotomized using 1.5 SD below population mean as cutoff score. Boldface denotes significant odds ratios. Only significant CHD-group pairwise comparisons are presented.
p ≤ .031,
p ≤ .01,
p ≤ .001.
On visuo-spatial EF tasks, the odds of impairment on all three Design Fluency trials and two out of three Tower measures were statistically greater among TOF and SVF groups than controls; the TGA group was more likely than controls to score within the impaired range on Empty Dots, but was otherwise at no greater risk for impaired Design Fluency or Tower Test performance than controls. Moreover, the TGA group was at lower risk than TOF for impairment on the Dot Switching trial, and at lower risk than both TOF and SVF groups for impairment on Filled Dots trial.
Finally, the odds of impairment on the Sorting Test were higher in all three CHD groups than controls.
BRIEF reports
Group-level means were within normal limits (Table 4). Percentages of children/adolescents obtaining at least one elevated subscale differed markedly across CHD and control groups for parent and teacher ratings. Self-report ratings with at least one elevated score also differed from controls using a clinical cutoff score of ≥ 65, but more closely approximated parent/teacher percentages using a relaxed cutoff score of ≥ 60.
Table 4.
BRIEF descriptive results and elevated subscale score percentages by CHD diagnosis
| CHD | Control | TGA | TOF | SVF | |
|---|---|---|---|---|---|
| BRIEF Parent-Report | (n = 348) | (n = 105) | (n = 138) | (n = 67) | (n = 143) |
| Behavior Regulation Index | 52.88 (12.31) | 44.47 (7.21) | 51.50 (11.76) | 52.63 (13.23) | 54.32 (12.31) |
| Inhibit | 52.07 (12.04) | 46.22 (7.62) | 50.85 (10.80) | 52.18 (13.08) | 53.20 (12.62) |
| Shift | 53.74 (12.33) | 44.96 (8.09) | 52.41 (11.96) | 53.55 (13.14) | 55.11 (12.24) |
| Emotional Control | 52.03 (12.04) | 44.55 (6.76) | 51.01 (11.19) | 51.70 (12.69) | 53.18 (12.51) |
| Metacognition Index | 57.21 (12.17) | 45.73 (8.74) | 56.02 (12.04) | 57.07 (12.80) | 58.42 (11.94) |
| Initiate | 56.19 (12.33) | 45.87 (9.32) | 54.59 (12.42) | 55.79 (12.50) | 57.92 (12.02) |
| Working Memory | 57.15 (13.66) | 45.37 (8.74) | 55.92 (14.11) | 56.64 (14.16) | 58.57 (12.94) |
| Planning/Organization | 56.68 (12.36) | 45.56 (8.14) | 55.39 (12.05) | 56.09 (12.91) | 58.20 (12.31) |
| Organization of Materials | 54.52 (10.55) | 48.97 (9.73) | 54.21 (10.16) | 55.57 (10.49) | 54.32 (10.98) |
| Monitor | 56.20 (12.13) | 45.57 (9.00) | 56.06 (11.51) | 56.01 (13.13) | 56.43 (12.31) |
| % rated ≥ 1.5 SDs above the population mean on at least 1 subscale |
56.3 | 15.2 | 50.7 | 56.7 | 61.5 |
| BRIEF Teacher-Report | (n = 205–207) | (n = 42) | (n = 78–79) | (n = 44–45) | (n = 83) |
| Behavior Regulation Index | 56.77 (15.58) | 51.38 (9.31) | 55.21 (13.32) | 59.93 (19.93) | 56.55 (14.88) |
| Inhibit | 54.55 (13.93) | 50.52 (7.66) | 52.11 (10.38) | 57.16 (17.01) | 55.45 (14.81) |
| Shift | 59.72 (19.50) | 51.90 (13.40) | 60.22 (20.58) | 62.00 (22.30) | 58.05 (16.77) |
| Emotional Control | 54.75 (15.33) | 51.07 (10.37) | 52.96 (13.17) | 57.89 (19.39) | 54.75 (14.66) |
| Metacognition Index | 61.42 (16.71) | 52.33 (10.67) | 60.53 (16.40) | 61.76 (18.43) | 62.07 (16.17) |
| Initiate | 60.03 (15.88) | 51.02 (10.21) | 59.97 (16.62) | 58.67 (15.73) | 60.83 (15.38) |
| Working Memory | 61.90 (17.62) | 52.95 (11.09) | 60.57 (16.76) | 62.47 (19.88) | 62.86 (17.27) |
| Planning/Organization | 60.14 (15.45) | 51.83 (11.35) | 59.78 (16.03) | 61.22 (17.05) | 59.89 (14.09) |
| Organization of Materials | 59.21 (19.42) | 51.74 (11.59) | 57.89 (18.06) | 58.87 (20.78) | 60.66 (20.05) |
| Monitor | 59.36 (15.28) | 52.57 (9.97) | 57.71 (13.62) | 60.56 (17.56) | 60.29 (15.52) |
| % rated ≥ 1.5 SDs above the population mean on at least 1 subscale |
57.1 | 28.6 | 52.6 | 54.5 | 62.7 |
| BRIEF Self-Report | (n = 321) | (n = 109) | (n = 136) | (n = 65) | (n = 120) |
| Behavior Regulation Index | 49.32 (11.14) | 43.65 (9.28) | 49.79 (11.61) | 49.23 (11.17) | 48.83 (10.64) |
| Inhibit | 49.10 (10.99) | 45.23 (8.63) | 49.90 (11.48) | 49.20 (11.71) | 48.13 (9.98) |
| Shift | 49.81 (11.22) | 43.86 (10.10) | 49.55 (11.36) | 49.43 (10.88) | 50.32 (11.32) |
| Emotional Control | 49.14 (10.50) | 44.62 (8.62) | 49.24 (10.84) | 49.25 (10.71) | 48.98 (10.08) |
| Monitor | 50.04 (10.39) | 45.50 (9.55) | 50.68 (11.27) | 50.09 (10.60) | 49.29 (9.19) |
| Metacognition Index | 50.86 (11.33) | 46.18 (10.35) | 51.40 (11.55) | 50.37 (10.42) | 50.51 (11.62) |
| Working Memory | 51.38 (11.44) | 47.25 (11.44) | 51.56 (11.38) | 51.12 (12.20) | 51.32 (11.18) |
| Planning/Organization | 49.99 (10.75) | 45.45 (9.31) | 50.95 (11.34) | 49.08 (9.51) | 49.39 (10.71) |
| Organization of Materials | 49.78 (10.03) | 48.43 (10.01) | 50.80 (10.05) | 50.02 (9.74) | 48.49 (10.09) |
| Task Completion | 51.69 (11.52) | 46.33 (10.09) | 51.59 (11.52) | 51.12 (11.17) | 52.13 (11.79) |
| % rated ≥ 1.5 (1.0) SDs above the population mean on at least 1 subscale |
30.8 (49.5) | 15.6 (22.9) | 35.3 (52.9) | 27.7 (49.2) | 27.5 (45.8) |
Note. CHD = congenital heart disease; TGA = dextro-transposition of the great arteries; TOF = tetralogy of Fallot; SVF = single-ventricle children/adolescents who underwent the Fontan procedure. Unless otherwise specified, results are presented as mean (standard deviation). Some BRIEF Teacher-report data were missing; therefore, sample sizes, which are provided above as min-max, depict valid ns by group.
Results of logistic regression analyses and pairwise comparisons are presented in Table 5. On parent report, the odds of being rated as having clinically significant metacognitive and self-regulatory problems were statistically higher among CHD groups than controls for most domains. Inhibition was the only exception; odds of parent-rated inhibitory control problems were greater than controls for the SVF group, but no different for TGA and TOF groups. Children/adolescents in the SVF group were also statistically more likely than those in the TGA group to have parent-reported problems with initiation and working memory.
Table 5.
Odds of clinically significant problems on BRIEF subscale by CHD group as compared to controls
| TGA | TOF | SVF | CHD-Group Pairwise Comparisons |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | ||
| BRIEF Parent | ||||||||||
| Inhibit | .30 | 1.77 | 0.61–5.14 | .04 | 3.21 | 1.05–9.85 | .003 | 4.46 | 1.65–12.07 | -- |
| Shift | .008 | 5.43 | 1.56–18.87 | <.001 | 9.81 | 2.72–35.41 | .001 | 8.28 | 2.44–28.04 | -- |
| Emot. Control | .02 | 6.01 | 1.35–26.82 | .02 | 6.51 | 1.35–31.50 | .003 | 9.57 | 2.20–41.67 | -- |
| Initiate | .01 | 3.14 | 1.30–7.60 | .002 | 4.61 | 1.79–11.86 | <.001 | 6.87 | 2.95–15.99 | SVF > TGA** (OR = 1.16) |
| Working Memory | .001 | 8.33 | 2.45–28.32 | <.001 | 13.40 | 3.78–47.51 | <.001 | 17.80 | 5.35–59.22 | SVF > TGA** (OR = 1.16) |
| Plan/Organize | <.001 | 8.03 | 2.74–23.56 | <.001 | 10.30 | 3.33–31.87 | <.001 | 13.13 | 4.55–37.87 | -- |
| Org. of Materials | .005 | 3.09 | 1.40–6.82 | .01 | 3.08 | 1.26–7.51 | .005 | 3.08 | 1.40–6.76 | -- |
| Monitor | <.001 | 6.27 | 2.54–15.50 | <.001 | 7.02 | 2.65–18.64 | <.001 | 7.10 | 2.89–17.42 | -- |
| BRIEF Teacher | ||||||||||
| Inhibit | .84 | 1.17 | 0.27–5.00 | .01 | 5.81 | 1.49–22.68 | .13 | 2.80 | 0.75–10.43 | TOF > TGA** (OR = 1.23) |
| Shift | .07 | 2.53 | 0.92–6.91 | .08 | 2.64 | 0.89–7.85 | .07 | 2.54 | 0.94–6.86 | -- |
| Emot. Control | .07 | 3.31 | 0.89–12.34 | .05 | 3.95 | 0.99–15.72 | .12 | 2.80 | 0.76–10.38 | -- |
| Initiate | .01 | 3.52 | 1.31–9.47 | .06 | 2.83 | 0.96–8.33 | .004 | 4.14 | 1.56–10.98 | -- |
| Working Memory | .01 | 3.65 | 1.34–9.89 | .02 | 3.57 | 1.22–10.48 | .004 | 4.19 | 1.57–11.20 | -- |
| Plan/Organize | .002 | 5.04 | 1.79–14.20 | .008 | 4.49 | 1.48–13.65 | .009 | 3.97 | 1.41–11.21 | -- |
| Org. of Materials | .20 | 2.03 | 0.69–5.99 | .05 | 3.13 | 1.00–9.82 | .034 | 3.13 | 1.09–8.94 | -- |
| Monitor | .01 | 4.20 | 1.33–13.28 | .02 | 4.53 | 1.34–15.34 | .009 | 4.54 | 1.46–14.15 | -- |
| BRIEF Self (≥ 60) | ||||||||||
| Inhibit | .006 | 4.10 | 1.50–11.25 | .04 | 3.37 | 1.09–10.42 | .07 | 2.64 | 0.92–7.60 | -- |
| Shift | .003 | 3.78 | 1.58–9.03 | .01 | 3.64 | 1.37–9.68 | <.001 | 6.00 | 2.54–14.19 | -- |
| Emot. Control | .01 | 3.59 | 1.30–9.90 | .01 | 4.24 | 1.40–12.82 | .003 | 4.67 | 1.70–12.81 | -- |
| Monitor | .005 | 3.34 | 1.45–7.71 | .14 | 2.09 | 0.78–5.62 | .56 | 1.32 | 0.52–3.35 | TGA > SVF** (OR = 1.13) |
| Working Memory | .04 | 2.09 | 1.03–4.24 | .15 | 1.85 | 0.80–4.28 | .006 | 2.69a | 1.33–5.44 | -- |
| Plan/Organize | .006 | 2.92a | 1.36–6.27 | .08 | 2.24 | 0.91–5.53 | .10 | 1.98 | 0.88–4.44 | -- |
| Org. of Materials | .11 | 1.76 | 0.88–3.54 | .32 | 1.54 | 0.66–3.56 | .42 | 1.36 | 0.65–2.84 | -- |
| Task Completion | .08 | 1.81 | 0.93–3.52 | .25 | 1.58 | 0.72–3.49 | .08 | 1.85 | 0.94–3.64 | -- |
Note. CHD = congenital heart disease; TGA = dextro-transposition of the great arteries; TOF = tetralogy of Fallot; SVF = single-ventricle children/adolescents who underwent the Fontan procedure; OR = odds ratio; CI = confidence interval for OR. Variables were dichotomized using 1.5 SD above population mean for parents/teachers, 1.0 SD for self-report. Boldface denotes significant odds ratios. Only significant CHD-group pairwise comparisons are presented.
The overall group effect was not significant (p > 0.031). Pairwise differences were not assessed for statistical significance.
p ≤ .031,
p ≤ .01,
p ≤ .001.
On teacher report, the odds of being rated as having clinically significant metacognitive problems were statistically higher among CHD groups than controls for most domains. The TOF group had significantly greater odds of having problems with inhibition than both control and TGA groups. Otherwise, the odds of clinically significant self-regulatory problems were not statistically greater among children/adolescents with CHD than controls, according to teachers.
Logistic regression analyses of self-report ratings revealed no significant differences in odds of obtaining ratings ≥ 65 across groups. Using a cutoff score of ≥ 60, however, the odds of self-identifying problems with shifting and emotion regulation were statistically greater among all three CHD groups than controls. TGA participants were more likely than controls to endorse problems with inhibition and self-monitoring. On self-report, the odds of rating oneself as having clinically significant problems with metacognitive skills were statistically no greater among CHD than control participants.
Paired t-tests examining differences between BRIEF self-report and parent/teacher ratings showed that, for CHD participants, self-report ratings were lower than parent/teacher scores for all subscales; effect sizes ranged from small to medium (Table 6). Control self-report ratings were significantly lower than teacher ratings for inhibit, shift, emotion control, monitor, and plan/organize subscales, with small to medium effects. Control self- and parent-report ratings were not statistically different.
Table 6.
Paired t-test results comparing BRIEF self-report vs. parent/teacher ratings
| Mean (95% CI) | t-statistic | p-value | Cohen's d | |
|---|---|---|---|---|
| CHD (combined) | ||||
| Self-Report vs. Parent (n = 320) | ||||
| Inhibit | −2.57 (−3.80, −1.34) | −4.1 | <.001 | −0.23 |
| Shift | −3.49 (−4.93, −2.05) | −4.76 | <.001 | −0.27 |
| Emotion Control | −2.63 (−4.01, −1.25) | −3.75 | <.001 | −0.21 |
| Monitor | −5.96 (−7.36, −4.56) | −8.38 | <.001 | −0.47 |
| Working Memory | −5.71 (−7.15, −4.28) | −7.83 | <.001 | −0.44 |
| Plan/Organize | −6.22 (−7.55, −4.89) | −9.22 | <.001 | −0.52 |
| Org. of Materials | −4.76 (−5.95, −3.58) | −7.89 | <.001 | −0.44 |
| Self-Report vs. Teacher (n = 187) | ||||
| Inhibit | −4.65 (−6.55, −2.75) | −4.82 | <.001 | −0.35 |
| Shift | −8.03 (−10.80, −5.27) | −5.73 | <.001 | −0.42 |
| Emotion Control | −4.75 (−6.92, −2.59) | −4.32 | <.001 | −0.32 |
| Monitor | −8.39 (−10.64, −6.14) | −7.35 | <.001 | −0.54 |
| Working Memory | −9.83 (−12.33, −7.32) | −7.74 | <.001 | −0.57 |
| Plan/Organize | −9.77 (−12.11, −7.43) | −8.25 | <.001 | −0.60 |
| Org. of Materials | −9.05 (−11.70, −6.4) | −6.73 | <.001 | −0.49 |
| Control | ||||
| Self-Report vs. Parent (n = 103) | ||||
| Inhibit | −1.28 (−3.02, 0.46) | −1.46 | 0.15 | −0.14 |
| Shift | −1.40 (−3.50, 0.70) | −1.32 | 0.19 | −0.13 |
| Emotion Control | −0.38 (−2.09, 1.34) | −0.44 | 0.66 | −0.04 |
| Monitor | −0.28 (−2.50, 1.94) | −0.25 | 0.80 | −0.02 |
| Working Memory | 1.55 (−0.52, 3.63) | 1.48 | 0.14 | 0.15 |
| Plan/Organize | −0.42 (−2.41, 1.57) | −0.42 | 0.68 | −0.04 |
| Org. of Materials | −0.55 (−2.69, 1.58) | −0.51 | 0.61 | −0.05 |
| Self-Report vs. Teacher (n = 42) | ||||
| Inhibit | −4.31 (−7.01, −1.61) | −3.22 | 0.002 | −0.50 |
| Shift | −7.55 (−12.41, −2.69) | −3.14 | 0.003 | −0.48 |
| Emotion Control | −6.79 (−10.41, −3.16) | −3.78 | <.001 | −0.58 |
| Monitor | −5.76 (−10.28, −1.24) | −2.57 | 0.01 | −0.40 |
| Working Memory | −2.40 (−7.32, 2.51) | −0.99 | 0.33 | −0.15 |
| Plan/Organize | −4.81 (−8.83, −0.79) | −2.42 | 0.02 | −0.37 |
| Org. of Materials | −0.93 (−5.20, 3.35) | −0.44 | 0.66 | −0.07 |
BRIEF-D-KEFS correlations
Spearman partial correlation coefficients, controlling for SES, age, sex, and race, were calculated between BRIEF and D-KEFS variables for CHD and control groups separately. Given the large number of variables included, a p-value < .001 was considered statistically significant. Results are presented in Table 7. For CHD groups, correlations between parent and teacher ratings and several D-KEFS measures (most notably Sorting, Verbal Fluency, and Design Fluency) were statistically significant yet small in magnitude. For controls, BRIEF ratings did not correlate significantly with any D-KEFS variable.
Table 7.
Correlations between D-KEFS and BRIEF variables for CHD and control groups
| Verbal Fluency | Word Context | Sorting | Design Fluency | Tower | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Letter | Categ | Switch | TCC | CCS | Recog | Filled | Empty | Switch | TAS | TPMR | MAR | |
| CHD (combined) | ||||||||||||
| BRIEF Parent (n = 327) | ||||||||||||
| Inhibit | −0.07 | −0.11 | −0.19 | −0.12 | −0.18 | −0.21 | −0.06 | −0.06 | −0.12 | −0.13 | 0.02 | −0.08 |
| Shift | −0.10 | −0.23 | −0.13 | −0.22 | −0.15 | −0.25 | −0.15 | −0.21 | −0.26 | −0.10 | −0.15 | 0.02 |
| Emotion Control | −0.05 | −0.12 | −0.04 | −0.13 | −0.11 | −0.18 | −0.10 | −0.12 | −0.17 | −0.06 | −0.04 | −0.01 |
| Initiate | −0.07 | −0.13 | −0.18 | −0.12 | −0.13 | −0.21 | −0.13 | −0.13 | −0.19 | −0.15 | −0.14 | −0.05 |
| Working Memory | −0.14 | −0.21 | −0.24 | −0.26 | −0.20 | −0.27 | −0.15 | −0.17 | −0.24 | −0.17 | −0.15 | 0.00 |
| Plan/Organize | −0.11 | −0.19 | −0.19 | −0.14 | −0.16 | −0.22 | −0.13 | −0.12 | −0.19 | −0.17 | −0.10 | −0.04 |
| Org of Materials | 0.04 | 0.01 | −0.08 | 0.00 | 0.03 | −0.03 | 0.01 | 0.02 | 0.01 | −0.05 | 0.05 | −0.06 |
| Monitor | −0.13 | −0.18 | −0.24 | −0.20 | −0.16 | −0.22 | −0.16 | −0.14 | −0.21 | −0.15 | −0.04 | −0.04 |
| BRIEF Teacher (n = 197) | ||||||||||||
| Inhibit | −0.05 | −0.01 | −0.14 | −0.19 | −0.15 | −0.13 | −0.09 | −0.13 | −0.07 | −0.12 | −0.02 | −0.01 |
| Shift | −0.19 | −0.11 | −0.17 | −0.30 | −0.28 | −0.27 | −0.19 | −0.23 | −0.20 | −0.10 | −0.13 | 0.05 |
| Emotion Control | −0.05 | −0.05 | −0.14 | −0.17 | −0.13 | −0.11 | −0.07 | −0.12 | −0.13 | −0.12 | −0.04 | 0.02 |
| Initiate | −0.30 | −0.20 | −0.29 | −0.41 | −0.32 | −0.30 | −0.26 | −0.30 | −0.25 | −0.23 | −0.28 | 0.08 |
| Working Memory | −0.21 | −0.14 | −0.22 | −0.39 | −0.27 | −0.26 | −0.18 | −0.26 | −0.20 | −0.19 | −0.18 | 0.07 |
| Plan/Organize | −0.19 | −0.12 | −0.20 | −0.38 | −0.30 | −0.26 | −0.18 | −0.25 | −0.22 | −0.25 | −0.18 | 0.01 |
| Org of Materials | −0.10 | −0.05 | −0.14 | −0.21 | −0.10 | −0.10 | −0.04 | −0.12 | −0.07 | −0.08 | −0.08 | 0.06 |
| Monitor | −0.13 | −0.10 | −0.22 | −0.31 | −0.24 | −0.24 | −0.11 | −0.18 | −0.17 | −0.19 | −0.14 | −0.01 |
| BRIEF Self (n = 303) | ||||||||||||
| Inhibit | −0.05 | −0.06 | −0.10 | −0.03 | −0.08 | −0.08 | −0.01 | −0.02 | −0.02 | −0.03 | 0.01 | −0.08 |
| Shift | −0.09 | −0.20 | −0.18 | −0.17 | −0.16 | −0.16 | −0.12 | −0.11 | −0.10 | −0.06 | −0.11 | 0.00 |
| Emotion Control | −0.11 | −0.14 | −0.06 | −0.10 | −0.17 | −0.17 | −0.11 | −0.13 | −0.10 | −0.09 | −0.10 | −0.03 |
| Monitor | −0.03 | −0.15 | −0.13 | −0.09 | −0.11 | −0.13 | −0.06 | −0.08 | −0.03 | −0.11 | −0.05 | −0.11 |
| Working Memory | −0.08 | −0.13 | −0.15 | −0.12 | −0.17 | −0.20 | −0.07 | −0.08 | −0.08 | −0.09 | −0.09 | −0.02 |
| Plan/Organize | −0.09 | −0.17 | −0.13 | −0.09 | −0.14 | −0.13 | −0.04 | −0.04 | −0.07 | −0.07 | −0.06 | −0.03 |
| Org of Materials | −0.03 | −0.08 | −0.05 | 0.08 | 0.02 | 0.02 | 0.05 | 0.05 | 0.01 | 0.01 | 0.01 | −0.03 |
| Task Completion | −0.08 | −0.17 | −0.14 | −0.14 | −0.15 | −0.20 | −0.09 | −0.10 | −0.11 | −0.11 | −0.04 | −0.09 |
| Control | ||||||||||||
| BRIEF Parent (n = 94) | ||||||||||||
| Inhibit | −0.05 | 0.14 | 0.06 | −0.11 | −0.01 | −0.13 | 0.01 | 0.00 | 0.08 | 0.12 | −0.05 | 0.08 |
| Shift | −0.09 | 0.05 | 0.01 | −0.16 | 0.02 | −0.13 | −0.06 | −0.11 | 0.06 | −0.01 | −0.07 | 0.02 |
| Emotion Control | −0.01 | 0.14 | 0.07 | 0.01 | −0.07 | −0.18 | 0.04 | 0.01 | −0.07 | −0.10 | −0.26 | 0.01 |
| Initiate | −0.02 | −0.06 | −0.05 | −0.06 | −0.03 | −0.28 | −0.03 | −0.14 | 0.05 | 0.04 | −0.12 | −0.01 |
| Working Memory | −0.01 | −0.04 | −0.02 | −0.09 | 0.04 | −0.26 | 0.02 | −0.13 | −0.03 | 0.08 | −0.24 | −0.06 |
| Plan/Organize | −0.14 | 0.03 | −0.04 | −0.02 | 0.00 | −0.23 | −0.06 | −0.10 | 0.00 | 0.01 | −0.18 | −0.13 |
| Org of Materials | 0.03 | 0.12 | 0.10 | 0.12 | 0.01 | −0.09 | −0.16 | −0.16 | 0.00 | 0.10 | −0.02 | −0.11 |
| Monitor | −0.12 | 0.03 | −0.10 | −0.03 | −0.06 | −0.17 | 0.06 | 0.00 | 0.01 | 0.06 | −0.13 | −0.02 |
| BRIEF Teacher (n = 36) | ||||||||||||
| Inhibit | −0.12 | 0.20 | −0.13 | −0.15 | −0.18 | −0.50 | −0.15 | −0.04 | −0.23 | 0.05 | −0.31 | 0.14 |
| Shift | 0.04 | 0.19 | 0.02 | 0.10 | 0.09 | −0.33 | 0.18 | 0.02 | −0.28 | 0.18 | −0.38 | 0.35 |
| Emotion Control | 0.02 | 0.17 | 0.09 | 0.23 | −0.09 | −0.27 | −0.11 | −0.19 | −0.29 | −0.01 | −0.32 | 0.06 |
| Initiate | −0.17 | 0.04 | 0.08 | −0.19 | 0.07 | −0.49 | 0.00 | −0.18 | −0.34 | 0.06 | −0.15 | −0.01 |
| Working Memory | −0.23 | 0.05 | −0.13 | −0.10 | −0.13 | −0.40 | −0.33 | −0.33 | −0.30 | −0.10 | −0.33 | −0.05 |
| Plan/Organize | −0.21 | −0.01 | −0.06 | −0.26 | −0.04 | −0.32 | −0.37 | −0.42 | −0.31 | 0.10 | −0.11 | −0.07 |
| Org of Materials | −0.07 | −0.02 | −0.22 | −0.33 | −0.38 | −0.43 | −0.01 | −0.11 | −0.06 | −0.01 | −0.28 | −0.05 |
| Monitor | −0.35 | 0.00 | −0.14 | −0.30 | −0.16 | −0.46 | −0.17 | −0.24 | −0.44 | 0.05 | −0.22 | 0.04 |
| BRIEF Self (n = 97) | ||||||||||||
| Inhibit | −0.04 | 0.05 | 0.10 | −0.08 | −0.15 | −0.18 | −0.03 | 0.05 | 0.01 | 0.01 | −0.01 | −0.02 |
| Shift | −0.08 | −0.09 | 0.18 | −0.20 | −0.04 | −0.05 | −0.05 | −0.04 | 0.02 | −0.07 | −0.06 | −0.07 |
| Emotion Control | −0.03 | −0.01 | 0.16 | −0.12 | −0.11 | −0.17 | −0.15 | −0.06 | −0.04 | −0.04 | −0.11 | −0.02 |
| Monitor | −0.15 | −0.06 | 0.09 | −0.19 | −0.02 | −0.09 | −0.05 | −0.08 | 0.05 | 0.05 | −0.06 | 0.01 |
| Working Memory | −0.06 | −0.08 | 0.08 | −0.12 | 0.06 | −0.16 | −0.06 | −0.06 | −0.02 | −0.06 | −0.05 | −0.17 |
| Plan/Organize | −0.14 | −0.18 | 0.01 | −0.17 | −0.05 | −0.16 | −0.05 | −0.07 | 0.07 | −0.15 | −0.08 | −0.12 |
| Org of Materials | 0.01 | −0.06 | 0.03 | −0.07 | 0.10 | −0.16 | −0.15 | −0.09 | 0.07 | −0.06 | −0.03 | −0.14 |
| Task Completion | −0.21 | −0.21 | −0.09 | −0.09 | −0.04 | −0.30 | −0.15 | −0.23 | −0.17 | −0.08 | −0.27 | −0.12 |
Note. Results are Spearman partial correlation coefficients controlling for SES, age, sex, and race. TCC = Total Consecutively Correct; CCS = Confirmed Correct Sorts; TPMR = Time-per-Move Ratio; MAR = Move-Accuracy Ratio. Correlations significant at p < .001 are bolded.
Discussion
We found that, on average, CHD group means were within the expected age-range on most laboratory EF tasks and informant ratings. However, looking more specifically at areas of clinical impairment, the percentages of children and adolescents performing at least 1.5 SD below the population mean on at least one D-KEFS subtest were nearly twice as high for CHD groups (75–81%) than controls (43%). Percentages of children/adolescents with CHD obtaining clinically elevated scores on the BRIEF were four times higher than controls for parent ratings, and twice as high for teacher and self-report ratings.
Our prediction that controls would outperform CHD groups on all EF measures was partially confirmed: Tower Move-Accuracy Ratio demonstrated very poor sensitivity to differentiate between children with CHD and controls. Controls scored higher than CHD participants on all other D-KEFS measures.
Distinct EF profiles were documented between CHD groups on D-KEFS tasks. Comparing across groups, an interesting pattern emerged in relation to the predominant modality-specific demands of a given task. Controlling for SES, age, sex, and race, CHD was associated with relative deficits in cognitive flexibility/problem-solving and most verbally-mediated EF skills for TGA, TOF and SVF groups. Many visuo-spatially-mediated EF skills were also impaired relative to controls in TOF and SVF groups but were relatively preserved in the TGA group.
The etiology of this diagnosis-specific pattern of deficits is unclear but may stem from one or a combination of prenatal, postnatal, and surgical differences. One possibility is that brain regions critical for verbally- versus visually-mediated EF abilities may be differentially vulnerable to duration/extent of sub-optimal cerebral perfusion and/or oxygenation in prenatal development. Whereas fetuses with TGA experience reduced oxygenation but relatively normal cerebral perfusion prenatally and immediately after birth, single-ventricle conditions such as HLHS are associated with significant reductions in both oxygenation and perfusion (Licht et al., 2004). Absent in utero antegrade cerebral blood flow, which reduces cerebral perfusion and is common in HLHS (but not TGA), has been linked to reduced volumes of white matter, subcortical grey matter, and regional surface area in fetuses with HLHS (Sethi et al., 2013; Clouchoux et al., 2012) and, as such, may account for relatively greater risk for neural injury and associated functional impairment among single-ventricle participants.
Postnatal cerebral perfusion and oxygenation also differ dramatically across CHD groups. Infants with TGA typically undergo surgical correction within the first weeks of life, essentially normalizing the oxygen content and cerebral perfusion pressure. In contrast, there is much greater variability in age-at-first-surgery for infants with TOF and single-ventricle anatomy, with the majority experiencing ongoing alterations in perfusion and oxygenation for months or even years after birth. While children with TOF may undergo repair at a few months old, children with HLHS typically live for 2–3 years with chronic reductions in oxygenation and/or perfusion and continue to experience chronic circulatory changes even after palliation. Surgical management also differs greatly. Most children with TGA require a single postnatal corrective surgery with no additional cardiac operations. Those with TOF may undergo a single correction but typically not until a few months of age. On the other hand, children with single-ventricle conditions nearly always require more than one surgery, which not only exposes them to higher levels of general anesthetics (Jevtovic-Todorovic et al., 2013) and additional opportunities for surgical complications, but also necessitates that they endure a period of chronic hypoxemia while awaiting completion of staged palliation (Fenton, Lessman, Glogowski, & Duncan, 2007).
Finally, though patients were screened for syndromic findings, not all had genetic testing. Indeed, genetic/epigenetic factors influencing patterning of both heart and brain are more common in TOF and single-ventricle conditions than in TGA (Mahle et al., 2013; Newburger et al., 2012; Yi et al., 2014) and as-yet undescribed genetic factors could have contributed to some of the cognitive differences detected. Thus, more severe functional impairment may have been more common in participants with TOF or single-ventricle conditions than in those with TGA for several reasons, ranging from genetic and fetal cerebral hemodynamics to postnatal brain injury.
Regarding informant ratings, parents expressed the widest range of EF concerns, endorsing problems related to both regulatory and metacognitive functions. Teachers also recognized problems with metacognitive skills but in general did not rate children/adolescents with CHD as having more behavior or emotion regulation difficulties than controls. Understanding the nature of this discrepancy likely requires an appreciation of context. Teachers interact with students within the school environment, which is equipped with a range of external regulators (e.g., teachers, non-familial peers, strict scheduling) generally not available within the home that may help to mitigate self-control vulnerabilities. Schools may also present greater demands than home for organization, planning, and independent problem-solving, especially as students transition into middle and high school, which may increase the salience of a child’s metacognitive difficulties to his/her teachers. Managing metacognitive challenges at school may also be more effortful for children with CHD, taxing already vulnerable regulatory resources and making it harder for them to effectively modulate their behavior and emotions after school.
It has been suggested that “…relying solely on self-reports of patients with congenital heart disease might underestimate the severity of their challenges, at least in the domain of executive functions” (Bellinger et al., 2014a, p. 9). Consistent with this view, logistic regression analyses were indeed unable to distinguish CHD groups from controls using a cutoff score ≥ 65. However, application of a more relaxed cutoff score (≥ 60) revealed that children/adolescents with CHD do, in fact, rate themselves as having more problems than controls in select domains of EF. Self-identified concerns regarding cognitive flexibility/shifting, in particular, emerged among members of all three CHD groups, and were consistent with parent ratings and performance on select laboratory flexibility/switching tasks. The development of cognitive flexibility is protracted relative to other core EF abilities (Davidson et al., 2006) and is accompanied by increased capacity for switching fluidly between rules, accommodating unexpected changes in routine, and generating/entertaining less obvious perspectives than one’s own (Diamond, 2013). As such, cognitive flexibility facilitates not only cognitive and academic success but also social competence. Being able to accurately infer the mental states of others (i.e., theory of mind), for example, relies on the ability to toggle flexibly between self- and other-generated representations of the world (Müller, Zelazo, & Imrisek, 2005) and has been identified as an area of vulnerability among young children with TGA (Calderon et al., 2012). Interestingly, individual differences in EF have also recently been shown to predict benefit from theory-of-mind training in healthy preschool children (Benson, Sabbagh, Carlson, & Zelazo, 2013), suggesting that an understanding of the specific cognitive processes facilitating the development of social cognition in children with CHD may ultimately guide the development of effective prevention/intervention programs for this vulnerable population as well.
Self- and parent-report ratings also identified concerns regarding emotion regulation. These findings highlight the need for a broader conceptualization of EF in CHD, emphasizing not only decontextualized, “cool” EFs but also the range of “hot” EF skills involved in overcoming problems bearing greater emotional/motivational significance (Prencipe et al., 2011). Mediated by networks involving ventro-medial/orbitofrontal regions of the prefrontal cortex, as well as the amygdala and limbic structures, “hot” EF skills may be vulnerable to systemic perturbations affecting midline cardiac and neural development. Future studies should harness the power of sensitive behavioral tasks (see Crone & Van der Molen, 2004; Kerr & Zelazo, 2004) and neuroimaging techniques to elucidate the developmental course of “hot” EF skills in children with CHD.
Clinically, the self-report ratings collected in this study provide novel insight into how children and adolescents with severe CHD perceive themselves. To date, use of the BRIEF in cardiac neurodevelopmental research has generally been limited to composite variables that collapse across diverse EF skills. This study suggests that children with CHD may be more aware of their struggles than previously thought, particularly regarding problems with cognitive flexibility and emotion regulation. We recommend that clinicians working with the CHD population recognize BRIEF self-report scores ≥ 60 as potentially “warranting clinical attention” and provide recommendations to manage concerns. We also recommend that future research deconstruct multifaceted composite scores into component subscales to identify specific patterns of risk and protective factors among children with CHD.
This report outlines findings from the largest and most comprehensive study of EF in critical cyanotic CHD to date. There are, however, some limitations to be considered. First, in our effort to limit our sample to children without identified genetic conditions, 25% of the TOF group was excluded, rendering this group relatively underpowered to detect differences. Second, not all participants had genetic testing or evaluation by a geneticist, leaving open the possibility that some participants with undetected genetic/syndromic conditions may have been included in our sample. Third, it should be acknowledged that the controls in our study were carefully screened for conditions known or expected to adversely impact brain development and thus may be more representative of “super-normal” than “normal” population development (Waber et al., 2007). Our sample was also drawn from studies conducted at a single center and consisted largely of Caucasian participants. Although we attempted to mitigate these issues by controlling statistically for SES and other factors related to EF development, further research is necessary to determine the generalizability of our findings to the broader CHD population. Fourth, because of “task impurity” (Denckla, 1994), performance on tests purported to measure EF can be affected by a range of factors that cannot be adequately accounted for except in tightly controlled experimental paradigms that, unfortunately, were not included in the current study. Fifth, the surgical and postoperative management techniques used in infancy in our mostly adolescent samples might have changed over time in such a way as to produce better outcomes in patients who underwent cardiac surgery more recently. Finally, although the current investigation aimed to operationalize the EF construct broadly, it will be important for future studies to take this approach further, drawing from developmentally-informed models of EF (e.g., Lee, Bull, & Ho, 2013) to better understand how core EF abilities such as working memory, inhibitory control, and shifting are organized in children with CHD and how these putative core skills may be related to functional outcomes.
In conclusion, the current study demonstrates that children and adolescents developing within the context of critical cyanotic congenital heart disease are at increased risk for EF deficits. With greater understanding of the specific patterns of cognitive and self-regulatory vulnerabilities experienced by children with CHD, it may be possible to identify risks early and provide individualized supports necessary to promote optimal neurodevelopmental outcomes.
Acknowledgments
This research was supported in part by grants from the National Heart, Lung, and Blood Institute (HL77681, HL74734, and HL096825), the Farb Family Fund, and the National Center for Research Resources (RR02172). The authors have no conflicts of interest to declare. The authors thank the children and adolescents, parents, and teachers who participated in this study. The authors also wish to thank Jane Holmes Bernstein, PhD, Caitlin Rollins, MD, and Debbie Waber, PhD for their thoughtful comments on earlier versions of this manuscript, and Christian Stopp, MS, for his assistance with data management.
Abbreviations
- CHD
congenital heart disease
- TGA
dextro-transposition of the great arteries
- TOF
tetralogy of Fallot
- SVF
single-ventricle cardiac conditions requiring Fontan procedure
- HLHS
hypoplastic left heart syndrome
- D-KEFS
Delis-Kaplan Executive Function System
- BRIEF
Behavior Rating Inventory of Executive Function
References
- Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Strand RD. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. The New England Journal of Medicine. 1995;332(9):549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Are children with congenital cardiac malformations at increased risk of deficits in social cognition? Cardiology in the Young. 2008;18(1):3–9. doi: 10.1017/S104795110700176X. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Newburger JW, Wypij D, Kuban KCK, duPlesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiology in the Young. 2009;19(1):86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Rivkin MJ, DeMaso D, Robertson RL, Stopp C, Dunbar-Masterson C, Wypij D, Newburger JW. Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiology in the Young. 2014a;11:1–10. doi: 10.1017/S1047951114000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Rivkin MJ, DeMaso D, Robertson RL, Stopp C, Dunbar-Masterson C, Wypij D, Newburger JW. Neuropsychological and brain structure in adolescents with single ventricle lesions of the heart. 2014b Manuscript in preparation. [Google Scholar]
- Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. The Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Dunbar-Masterson C, Newburger JW. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Benson JE, Sabbagh MA, Carlson SM, Zelazo PD. Individual differences in executive functioning predict preschoolers’ improvement from theory-of-mind training. Developmental Psychology. 2013;49(9):1615–1627. doi: 10.1037/a0031056. [DOI] [PubMed] [Google Scholar]
- Brosig CL, Mussatto KA, Kuhn EM, Tweddell JS. Neurodevelopmental outcome in preschool survivors of complex congenital heart disease: implications for clinical practice. Journal of Pediatric Health Care: Official Publication of National Association of Pediatric Nurse Associates & Practitioners. 2007;21(1):3–12. doi: 10.1016/j.pedhc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Calderon J, Bonnet D, Courtin C, Concordet S, Plumet M-H, Angeard N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Developmental Medicine and Child Neurology. 2010;52(12):1139–1144. doi: 10.1111/j.1469-8749.2010.03735.x. [DOI] [PubMed] [Google Scholar]
- Calderon J, Angeard N, Moutier S, Plumet M-H, Jambaqué I, Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. The Journal of Pediatrics. 2012;161(1):94–98. doi: 10.1016/j.jpeds.2011.12.036. e1. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: A parametric functional magnetic resonance imaging study. Journal of Neuroscience. 2010;30(10):3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, Limperopoulos C. Delayed cortical development in fetuses with complex congenital heart disease. Cerebral Cortex. 2013;23(12):2932–2943. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- Crone EA, Van der Molen MW. Developmental changes in real life decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25(3):251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4–13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Denckla MB. Measurement of executive function. In: Lyon GR, editor. Frames of reference for the assessment of learning disabilities: New views on measurement issues. 1994. pp. 263–277. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton KN, Lessman K, Glogowski K, Fogg S, Duncan KF. Cerebral oxygen saturation does not normalize until after stage 2 single ventricle palliation. Annals of Thoracic Surgery. 2007;83(4):1431–1436. doi: 10.1016/j.athoracsur.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, Jarvik GP. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? The Journal of Thoracic and Cardiovascular Surgery. 2010;140(6):1230–1237. doi: 10.1016/j.jtcvs.2010.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor JW, Ittenbach RF, Gerdes M, Bernbaum J, Clancy RR, McDonald-McGinn DM, Spray TL. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. The Journal of Thoracic and Cardiovascular Surgery. 2014;147:1276–1283. doi: 10.1016/j.jtcvs.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G, Isquith P, Guy S, Kenworthy L. The Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- Guy SC, Isquith PK, Gioia G. Behavior Rating Inventory of Executive Function-Self Report Version. Odessa, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Hoffman JIE, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12084585. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. New Haven, CT: Yale University; 1975. Unpublished manuscript. [Google Scholar]
- Hovels-Gurich HH, Konrad K, Wiesner M, Minkenberg R, Herpertz-Dahlmann B, Messmer BJ, von Bernuth G. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Archives of Disease in Childhood. 2002;87:506–510. doi: 10.1136/adc.87.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövels-Gürich HH, Konrad K, Skorzenski D, Herpertz-Dahlmann B, Messmer BJ, Seghaye M-C. Attentional dysfunction in children after corrective cardiac surgery in infancy. The Annals of Thoracic Surgery. 2007;83(4):1425–1430. doi: 10.1016/j.athoracsur.2006.10.069. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Absalom AR, Blomgren K, Bambrink A, Crosby G, Culley DJ, Hemmings HC. Anaesthetic neurotoxicity and neuroplasticity: An expert group report and statement based on the BJA Salzburg Seminar. British Journal of Anaesthesia. 2013;111(2):143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdorp PA, Everaerd W, Kindt M, Mulder BJM. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. Journal of Pediatric Psychology. 2007;32(5):527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- Lee K, Bull R, Ho RMH. Developmental changes in executive functioning. Child Development. 2013;84(6):1933–1953. doi: 10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, Detre JA. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2004;128(6):841–849. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, Vossough A. Brain maturation is delayed in infants with complex congenital heart defects. The Journal of Thoracic and Cardiovascular Surgery. 2009;137(3):529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, du Plessis AJ. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121(1):26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little DM, Kraus MF, Joseph J, Geary EK, Susmaras T, Zhou XJ, Gorelick PB. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74(7):558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahle W, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(Suppl I):I109–I114. [PubMed] [Google Scholar]
- Mahle WT, Lu M, Ohye RG, Gaynor WJ, Goldberg CS, Sleeper LA, et al. A predictive model for neurodevelopmental outcome after the Norwood procedure. Pediatric Cardiology. 2013;34:327–333. doi: 10.1007/s00246-012-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mahle WT. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. The Journal of Pediatrics. 2007a;151(1):73–78. doi: 10.1016/j.jpeds.2007.02.020. 78.e1. [DOI] [PubMed] [Google Scholar]
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Intellectual, neuropsychological, and behavioral functioning in children with tetralogy of Fallot. The Journal of Thoracic and Cardiovascular Surgery. 2007b;133(2):449–455. doi: 10.1016/j.jtcvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Miller M, Loya F, Hinshaw SP. Executive functions in girls with and without childhood ADHD: Developmental trajectories and associations with symptom change. Journal of Child Psychology and Psychiatry. 2013;54(9):1005–1015. doi: 10.1111/jcpp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Zelazo PD, Imrisek S. Executive function and children’s understanding of false belief: How specific is the relation? Cognitive Development. 2005;20:173–189. [Google Scholar]
- Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Kuban KCK, Ware JH. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. The New England Journal of Medicine. 1993;329(15):1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Mussatto KA. Pediatric Heart Network Investigators. Circulation. 2012;125(17):2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M, Shevell M, Majnemer A, Limperopoulos C. Abnormal brain structure and function in newborns with complex congenital heart defects before open heart surgery: a review of the evidence. Journal of Child Neurology. 2011;26(6):743–755. doi: 10.1177/0883073811402073. [DOI] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD. Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology. 2011;108(3):621–637. doi: 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Provost JS, Petrides M, Monchi O. Dissociating the role of the caudate nucleus and dorsolateral prefrontal cortex in the monitoring of events within human working memory. European Journal of Neuroscience. 2010;32(5):873–880. doi: 10.1111/j.1460-9568.2010.07333.x. [DOI] [PubMed] [Google Scholar]
- Rivkin MJ, Watson CG, Scoppettuolo La, Wypij D, Vajapeyam S, Bellinger DC, Newburger JW. Adolescents with D-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. The Journal of Thoracic and Cardiovascular Surgery. 2013;146(3):543–549. doi: 10.1016/j.jtcvs.2012.12.006. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Schaefer C, von Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, Latal B. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Developmental Medicine and Child Neurology. 2013;55(12):1143–1149. doi: 10.1111/dmcn.12242. [DOI] [PubMed] [Google Scholar]
- Sethi V, Tabbutt S, Dimitropoulos A, Harris KC, Chau V, Poskitt K, McQuillen PS. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatric Research. 2013;73(5):661–667. doi: 10.1038/pr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G The Brain Development Cooperative Group. Journal of the International Neuropsychological Society. 2007;13:729–726. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Matsui M, Matsuzawa J, Tanaka C, Noguchi K, Yoshimura N, Gur RC. Impaired neuroanatomic development in infants with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery. 2009;137(1):146–153. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Tang SX, McDonald-McGinn DM, Calkins ME, Whinna DA, Souders MC, et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2014;165:137–147. doi: 10.1002/ajmg.b.32215. [DOI] [PMC free article] [PubMed] [Google Scholar]