Abstract
Recent studies suggest that bone marrow (BM)-derived stem cells have therapeutic efficacy in neonatal hyperoxia-induced lung injury (HILI). c-kit, a tyrosine kinase receptor that regulates angiogenesis, is expressed on several populations of BM-derived cells. Preterm infants exposed to hyperoxia have decreased lung angiogenesis. Here we tested the hypothesis that administration of BM-derived c-kit+ cells would improve angiogenesis in neonatal rats with HILI. To determine whether intratracheal (IT) administration of BM-derived c-kit+ cells attenuates neonatal HILI, rat pups exposed to either normobaric normoxia (21% O2) or hyperoxia (90% O2) from postnatal day (P) 2 to P15 were randomly assigned to receive either IT BM-derived green fluorescent protein (GFP)+ c-kit− cells (PL) or BM-derived GFP+ c-kit+ cells on P8. The effect of cell therapy on lung angiogenesis, alveolarization, pulmonary hypertension, vascular remodeling, cell proliferation, and apoptosis was determined at P15. Cell engraftment was determined by GFP immunostaining. Compared to PL, the IT administration of BM-derived c-kit+ cells to neonatal rodents with HILI improved alveolarization as evidenced by increased lung septation and decreased mean linear intercept. This was accompanied by an increase in lung vascular density, a decrease in lung apoptosis, and an increase in the secretion of proangiogenic factors. There was no difference in pulmonary vascular remodeling or the degree of pulmonary hypertension. Confocal microscopy demonstrated that 1% of total lung cells were GFP+ cells. IT administration of BM-derived c-kit+ cells improves lung alveolarization and angiogenesis in neonatal HILI, and this may be secondary to an improvement in the lung angiogenic milieu.
Keywords: c-kit, Hyperoxia, Stem cells, Angiogenesis, Bronchopulmonary dysplasia
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common chronic lung disease of infancy (14). It occurs as a consequence of two main insults: arrest of normal lung development (8) and abnormal repair of the preterm injured lung (22). Most preterm infants who develop BPD are in the late cannalicular or saccular stage of lung development (21). Furthermore, most preterm infants who acquire this disease have been exposed to relatively high oxygen concentrations (8). With the advent of use of gentler ventilation strategies, antenatal corticosteroids, and surfactant, the mortality of preterm infants has decreased, but this has been accompanied by a relative increase in the incidence of BPD (34). In fact, BPD occurs in approximately one in three very low birth weight premature infants, accounting for more than 12,000 new cases in the US annually. The economic impact remains tremendous (20). It is therefore apparent that new strategies to combat this disease are needed.
The bone marrow (BM) is a mesodermal-derived tissue consisting of a complex hematopoietic cellular component supported by a microenvironment composed of stromal cells embedded in a complex extracellular matrix (35). Albeit a now known rare occurrence, several studies have documented in multiple lung injury models the engraftment of unfractioned BM-derived cells as well as purified specific marrow-derived stem cell populations (1,9,10,17,18,24–27,36,39). These BM-derived stem cell populations include hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), and endothelial progenitor cells (EPCs). Although several studies have demonstrated the efficacy of BM-derived MSCs in regenerating the hyperoxic injured lung, the most efficacious population of BM-derived stem cells for neonatal lung repair is still unknown.
V-kit Hardy–Zuckerman 4 feline sarcoma viral oncogene homolog (c-kit) or cluster of differentiation 117 (CD117), a known marker of BM-derived and tissue stem cells, is a type III tyrosine kinase receptor known to be involved in vasculogenesis as well as angiogenesis (32,40). This receptor has been shown to be expressed on populations of EPCs, HSCs, and MSCs (12,33). The potential of BM-derived c-kit+ cells to regenerate the lung is unknown. However, previous studies have shown that BM-derived c-kit+ cells, and not MSCs, improved cardiac function following myocardial infarction by increasing endogenous cardiac progenitor cells (30). Moreover, local delivery of BM-derived c-kit+ cells increased perfusion following hindlimb ischemia by either incorporating into the vasculature or by increasing the secretion of angiogenesis-related factors (29). BM-derived c-kit+ cells also protected the heart from ischemic damage following infarction by enhancing the cardiac angiogenic milieu (15).
The present study sought to elucidate whether BM-derived c-kit+ cells would attenuate hyperoxia-induced lung injury (HILI) in neonatal rodents, an experimental model of BPD. We demonstrate that intratracheal (IT) administration of BM-derived c-kit+ cells improves angio-genesis and alveolarization in experimental BPD, and this is accompanied by increased secretion of proangiogenic factors and decreased lung apoptosis. These findings suggest a potent cell-derived therapeutic strategy to treat neonatal hyperoxia-injured lungs as seen in BPD.
MATERIALS AND METHODS
Animals
Adult male green fluorescent protein (GFP) transgenic Sprague–Dawley rats were purchased from The Rat Resource and Research Center (Columbia, MO, USA). Pregnant Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, USA).
All animal procedures were approved by the Animal Care Committee at the University of Miami.
Isolation of GFP+ BM-Derived c-kit+ Cells
Adult male GFP+ transgenic Sprague–Dawley rats were utilized for isolation of BM-derived c-kit+ cells as previously described (30). In brief, male GFP+ Sprague–Dawley rats were euthanized by CO2 asphyxiation. Tibiae and femurs were aseptically harvested and cleaned of adherent soft tissue. The proximal and distal ends of the femur were excised at the beginning of the marrow cavity. Whole bone marrow plugs were harvested by flushing the bone marrow cavity with a syringe connected to an 18-gauge needle (Monoject, St. Louis, MO, USA) filled with phosphate-buffered saline (PBS; Life Technologies, Grand Island, NY, USA) + 1% fetal bovine serum (FBS; Life Technologies). The c-kit+ population from among the bone marrow cells was then isolated by a magnetic-activated cell sorting (MACS) system (Miltenyi Biotec, Auburn, CA, USA). The yield and viability of cells was assessed by trypan blue (Life Technologies) exclusion and counted on a hemocytometer (Sigma-Aldrich, St. Louis, MO, USA). For injection, the cells were washed and resus-pended in PBS at a concentration of 5 × 104 cells/50 μl.
Therapeutic Efficacy of BM-Derived c-kit+ Cells in an Experimental Model of BPD
Within 48 h after birth, newborn Sprague–Dawley rat pups (n = 160; 16 litters; male to female ratio 1:1) received either normobaric normoxia (room air; RA) or hyper-oxia (90% O2). Mothers were rotated between normoxia and hyperoxia every 48 h to prevent oxygen toxicity to them. The rat pups were kept in their designated environment for a period of 1 week and randomly assigned to receive 5 × 104 BM-derived GFP+ c-kit− cells (50 μl) as placebo or BM-derived GFP+ c-kit+ cells on P8 in a single IT injection. This dosage was based on previous data showing efficacy in organ repair utilizing this dosage of BM-derived c-kit+ cells (19). Following anesthesia with intraperitoneal injections of ketamine (30 mg/kg; Bioniche Animal Health, Athens, GA, USA) and xylazine (4 mg/kg; LLOYD, Inc., Shenandoah, IA, USA), the trachea was exposed through a small incision in the midline of the neck, and BM-derived c-kit+ cells or c-kit− cells (5 × 104 in 50 μl) were delivered by tracheal puncture with a 30-gauge needle (Nipro Medical, Bridgewater, NJ, USA). The incision was closed with Vetbond™ tissue adhesive (3M, St. Paul, MN, USA), and the pups were allowed to recover within a warmed plastic chamber. After the injections, the animals were returned to their hyperoxic or normoxic environments for an additional period of 1 week. The animals were studied at P15. Lung alveolarization, vascular development, pulmonary hypertension, vascular remodeling, and epithelial cell apoptosis were evaluated at P15. Animals were sacrificed following measurements for pulmonary hypertension by CO2 asphyxiation.
Assessment of Lung Alveolarization
A 23-gauge catheter was introduced through the right ventricular wall and advanced into the pulmonary artery and fixed in this position by suturing to the ventricular wall. The catheter was connected to a reservoir containing 4% paraformaldehyde (Sigma-Aldrich). This solution was delivered at an air-driven pressure of 25 cmH2O for 5 min, and the atrium was punctured after distension. The airways were perfused through the trachea with 4% paraformaldehyde at a transpulmonary pressure of 20 cmH2O for 5 min. The lungs were excised and placed in 4% paraformaldehyde overnight at −4°C. After 24 h, they were serially dehydrated in ethanol and paraffin embedded. Serial paraffin-embedded lung sections 5 μm thick taken from the upper and lower lobes were stained by standard hematoxylin and eosin (H&E; Poly Scientific, Bayshore, NY, USA). Care was taken to exclude sections with large bronchioles or vessels. Mean linear intercept (MLI) was calculated by determining the average distance between intersects of alveolar septal tissue with a superimposed counting grid. Septal density was measured by counting the number of secondary septae per high power field (hpf). Images from six randomly selected, nonoverlapping parenchymal fields were acquired from lung sections of each animal (five to six per group) at 20× magnification (43).
Immunostaining
Lung sections were deparaffinized in xylene and rehydrated through graded ethanol. The sections were incubated with respective primary antibodies overnight at 4°C. For immunohistochemistry, the tissue sections were then incubated with biotinylated secondary IgG (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. The cell-bound biotinylated secondary antibody was detected with streptavidin–biotin–peroxidase complexes and diaminobenzidine substrates (Vector Laboratories). For immunofluorescence staining, the tissue sections were incubated with AlexaFluor 488- or AlexaFluor 594-labeled secondary antibodies (Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. After being washed with PBS, the tissue sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI; 100 ng/ml; Vector Laboratories) and mounted with glycerol.
Lung Vascular Density
Midlung sections 5 μm thick of the upper and lower lobes were deparaffinized, rehydrated, and stained with polyclonal rabbit anti-human Von Willebrand factor (vWF; 1:200; Dako Corp., Carpinteria, CA, USA), an endothelial cell marker. The number of blood vessels (20–50 μm in diameter) in each hpf was counted by a blinded observer. Five randomly selected, nonoverlapping parenchymal fields were evaluated from lung sections of each animal (five to six per group).
Pulmonary Hypertension (PH)
The degree of PH in neonatal rats was determined as previously described (47). Right ventricular systolic pressure (RVSP) was measured as a surrogate of pulmonary artery pressure.
Assessment of Pulmonary Vascular Remodeling
Paraffin-embedded sections were stained with polyclonal rabbit anti-human vWF (1:200) and monoclonal mouse anti-[.alpha]-smooth muscle actin (α-SMA: 1:500; Sigma-Aldrich). Medial wall thickness (MWT) of partially and fully muscular arteries (20–50 μm) was determined by using the formula: 2(MT)/ED, where MT is the distance between the internal and external elastic laminas and ED is the external diameter. Approximately 20 randomly chosen arteries were evaluated per slide (n=5–6/group), and all morphometric analyses were performed by a blinded observer.
Assessment of Lung Inflammation
Lung inflammation was assessed by immunostaining for Mac3 (1:200; BD Biosciences, San Jose, CA, USA), a macrophage-specific marker [which may be lysosomal-associated membrane protein 2 (LAMP2)]. The number of Mac3+ cells in the alveolar air spaces was counted from 10 random images taken with the 40× objective on each slide (n = 5–6/group).
Quantitative Real-Time PCR
RNA from lung tissue was extracted (RNeasy Midi Kit, Qiagen, Inc., Valencia, CA, USA) and reverse transcribed. The specific cDNA for angiopoietin-1 (Ang-1) and vascular endothelial growth factor (VEGF) was quantified by real time RT-PCR using SuperArray (Frederick, MD, USA) following the RT2 Real-Time Gene Expression Assay protocol. Primers for Ang-1, VEGF, and 18S (as an internal control) genes were predeveloped by SuperArray. The relative quantity of Ang-1 and VEGF was normalized to 18S expression.
VEGF ELISA
Lung VEGF concentration in rat lung homogenates (obtained from the right lower lobe of the rat pups) was evaluated by the Quantikine ELISA kit (R&D Systems) as per the manufacturer specifications.
Western Blot Analysis
The protein expression of cleaved caspase-3 (CC3) in lung homogenates was determined by Western blot analysis as previously described (47). The polyclonal antibody for CC3 (1:1,000) was obtained from Cell Signaling Technology (Danvers, MA, USA). The antibody for the normalization protein, β-actin (1:10,000), was obtained from Sigma-Aldrich.
TUNEL Assay
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining of lung sections was performed using a TUNEL kit (Roche Applied Science, Penzburg, Germany) as per manufacturer instructions. Five randomly selected fields per slide were photographed, and the number of apoptotic nuclei as well as the total number of nuclei was counted per hpf (n = 5–6/group). The apoptotic index was obtained by means of the formula: (number of apoptotic cells per field)/(total number of cells per field). Percent = apoptotic index × 100.
Cell Engraftment
Lung sections were immunostained with surfactant protein C (SP-C: 1:50; Millipore, Billerica, MA, USA) and vWF (1:200). Engrafted GFP+ BM-derived c-kit+ and c-kit− cells that express SP-C or vWF were manually counted in five random fields per section. Images were captured under a Zeiss Confocal Microscope (LSM-510; Carl Zeiss Microimaging, Inc., Thornwood, NY, USA).
Statistics
All results were reported as mean ± SD. Data were analyzed by two-way ANOVA followed by post hoc analysis (Holm-Sidak). Values of p < 0.05 were considered statistically significant. Statistical analysis was performed using SigmaStat software (SyStat Software, Inc., San Jose, CA, USA).
RESULTS
Bone Marrow-Derived c-kit+ Cells Improve Angiogenesis in Neonatal HILI
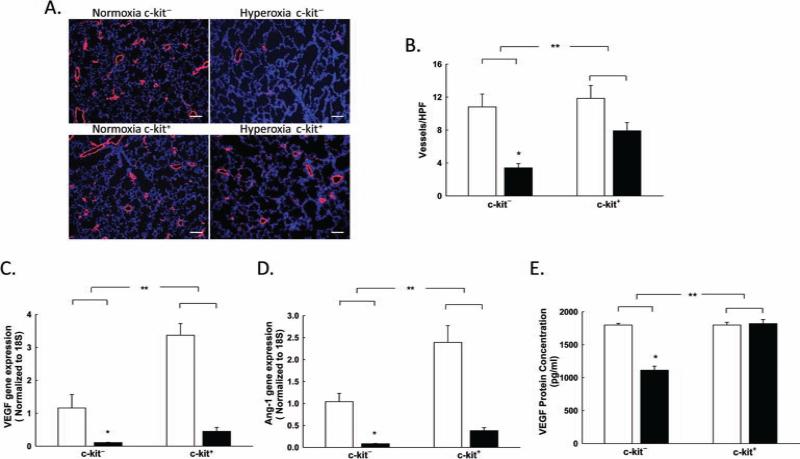
Neonatal hyperoxia exposure is associated with significant vascular pruning (46). Thus, we first questioned whether the administration of BM-derived c-kit+ cells to neonatal rats with HILI would improve lung vascular density. Exposure to hyperoxia resulted in marked vascular rarefaction in the group of rat pups treated with BM-derived c-kit− cells (10.8 ± 1.6 vs. 3.4 ± 0.5 vessels/hpf; RA BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit− cells; p < 0.001, n = 5–6/group) (Fig. 1A, B). In contrast, the administration of BM-derived c-kit+ cells significantly increased lung vascular density in the hyperoxia-exposed rats (3.4 ± 0.5 vs. 7.9 ± 1 vessels/hpf; hyperoxia BM-derived c-kit– cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.02, n = 5–6/group) (Fig. 1A, B). This improvement in lung angiogenesis was associated with a fourfold increase in lung VEGF gene expression (hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.001, n = 5–6/group) and a fivefold increase in lung Ang-1 gene expression (hyperoxia BM-derived c-kit− cells vs. hyper-oxia BM-derived c-kit+ cells; p < 0.001, n = 5–6/group) (Fig. 1C, D). Moreover, evaluation of the VEGF protein concentration in lung homogenates by ELISA revealed a marked increase in lung VEGF protein concentration in the hyperoxic rats treated with BM-derived c-kit+ cells (1,113 ± 61.4 vs. 1,820 ± 1.4 pg/ml; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.001, n = 5–6/group) (Fig. 1E).
Figure 1.
BM-derived c-kit+ cells improve lung vascular density in neonatal rats with HILI. (A) Lung sections stained with vWF (red) and 4′,6-diamidino-2-phenylindole (DAPI; blue) demonstrating increased vascular density/hpf in the hyperoxic pups treated with BM-derived c-kit+ cells. Original magnification: 100×. Scale bar: 100 μm. (B) Rarefaction of vessels in hyperoxia-exposed rats showing an increase in the number of vessels in the lungs of hyperoxic pups treated with BM-derived c-kit+ cells [*p < 0.001: normobaric normoxia (RA) c-kit− vs. hyperoxia c-kit−, **p < 0.02: hyperoxia c-kit− vs. hyperoxia c-kit+; n = 5–6/group]. White bars are RA, and black bars are hyperoxia. (C) Increased VEGF gene expression in hyperoxic pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyperoxia c-kit+; n=5–6/group). (D) Increased angiopoietin-1 (Ang-1) gene expression in hyperoxic pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyperoxia c-kit+; n = 5–6/group). (E) Increased lung VEGF protein concentration in hyperoxic pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyperoxia c-kit+; n = 5–6/group).
Bone Marrow-Derived c-kit+ Cells Improve Alveolarization in Neonatal HILI
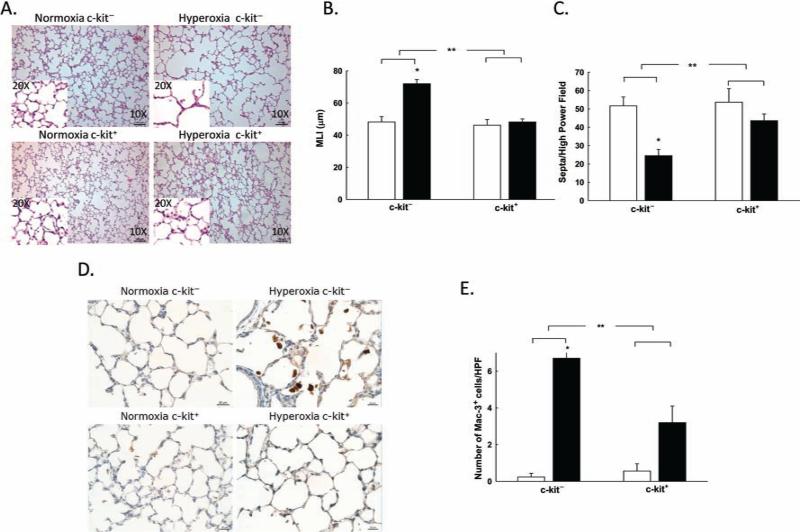
Since angiogenesis and alveolarization are interrelated processes (42), we next examined whether the administration of BM-derived c-kit+ cells would improve alveolarization in neonatal rats with HILI. Exposure to hyperoxia in rats treated with IT BM-derived c-kit− cells resulted in marked alveolar simplification (Fig. 2A). Moreover, morphometric analysis of these hyperoxic lungs revealed a marked increase in MLI (48.2 ± 3.3 vs. 72 ± 2.6 μm; RA BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit− cells; p < 0.001, n = 5–6/group) and a reduction in lung septal density (52 ± 5 vs. 25 ± 3 septa/hpf; RA BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit− cells; p < 0.001, n = 5–6/group) (Fig. 2A–C). In contrast, neonatal rats with HILI that were treated with IT BM-derived c-kit+ cells had a significant improvement in lung alveolarization as evidenced by a decrease in MLI (72 ± 2.6 vs. 48.2 ± 1.7 μm; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.001, n = 5–6/group) and increased lung septal density (25 ± 3 vs. 44 ± 4 septa/hpf; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.04, n = 5–6/ group) (Fig. 2A–C). These findings were accompanied by a marked decrease in Mac3+ cells in the alveolar spaces of rats that received IT BM-derived c-kit+ cells (6.7 ± 1.4 vs. 3.2 ± 0.9 Mac3+ cells/hpf; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.001, n = 5–6/group) (Fig. 2D, E).
Figure 2.
BM-derived c-kit+ cells improve alveolarization in neonatal rats with HILI. (A) H&E-stained lung sections demonstrating improved alveolarization in the lungs of hyperoxic pups treated with BM-derived c-kit+ cells. Original magnification: 100×. Scale bar: 100 μm. (B) Decreased MLI in the lungs of hyperoxic pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyperoxia c-kit+; n = 5–6/group). White bars are RA, and black bars are hyperoxia. (C) Increased septa/hpf in the lungs of hyperoxic pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.004: hyperoxia c-kit− vs. hyperoxia c-kit+; n = 5–6/group). (D) Decreased inflammation seen in lungs of rat pups treated with BM-derived c-kit+ cells as demonstrated by Mac3 (brown) immunostaining. (E) Decreased number of Mac3+ cells/HPF in lungs of hyperoxic pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyper-oxia c-kit+; n = 5–6/group). White bars are RA, and black bars are hyperoxia.
Bone Marrow-Derived c-kit+ Cells Do Not Affect PH in Neonatal HILI
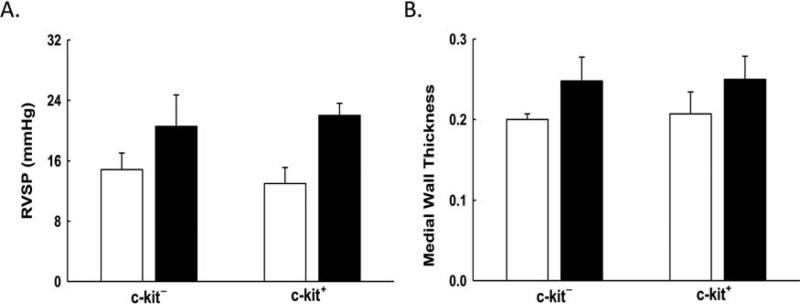
Exposure of the neonatal lung to hyperoxia also results in PH. This increase in pulmonary artery pressure is secondary to vascular pruning and pulmonary vascular remodeling (46). Thus, we next questioned whether the administration of IT BM-derived c-kit+ cells would also improve PH and vascular remodeling. Compared to RA rats, there was a significant increase in the RVSP, a surrogate measure of pulmonary artery pressure, in neonatal rats exposed to hyperoxia (Fig. 3A). There was no significant difference in the RVSP in the rats that received IT BM-derived c-kit+ cells or IT BM-derived c-kit− cells (21 ± 4 vs. 22 ± 1.6 mmHg; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM- derived c-kit+ cells; n = 5–6/group) (Fig. 3A). We also assessed the degree of pulmonary vascular remodeling by measuring the MWT of pulmonary arterioles (20–50 μm in diameter). Compared to RA, both hyperoxia-exposed groups had a significant increase in MWT, but there was no difference in the degree of vascular remodeling between the hyperoxia-exposed neonatal rats that received IT BM-derived c-kit+ or c-kit− cells (0.25 ± 0.03 vs. 0.25 ± 0.03 μm; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; n = 5–6/group) (Fig. 3B).
Figure 3.
BM-derived c-kit+ cells do not attenuate pulmonary hypertension. (A) No significant difference seen in RVSP between hyperoxic pups that received BM-derived c-kit+ cells or BM-derived c-kit− cells. (B) No significant difference seen in pulmonary vascular remodeling as evidenced by MWT between hyperoxic pups that received BM-derived c-kit+ cells or BM-derived c-kit− cells. White bars are RA, and black bars are hyperoxia.
Bone Marrow-Derived c-kit+ Cells Decrease Apoptosis in Neonatal HILI
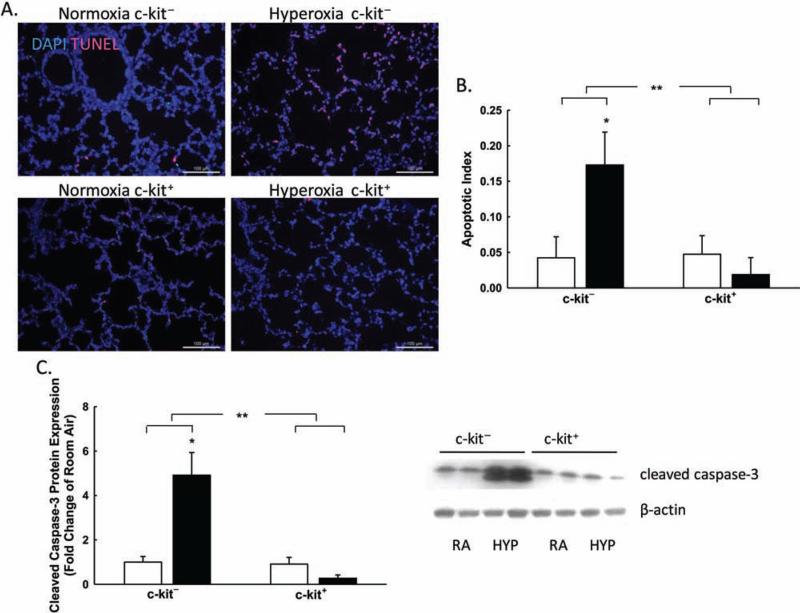
Hyperoxia-induced lung injury is known to induce epithelial cell apoptosis (41). Thus, we next asked whether the administration of IT BM-derived c-kit+ cells could decrease apoptosis in the lungs of hyperoxic pups treated with IT BM-derived c-kit+ cells. The degree of apoptosis was assessed by TUNEL staining and CC3 expression in lung homogenates obtained from all groups. Neonatal hyperoxic rats that received IT BM-derived c-kit− cells had a fourfold increase in the percentage of TUNEL+ cells or the apoptotic index (4.2 ± 2.9 vs. 17.3 ± 4.6%; RA BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit− cells; p < 0.001, n = 5–6/group) (Fig. 4A). In contrast, TUNEL staining of hyperoxia-exposed lungs that were treated with IT BM-derived c-kit+ cells demonstrated a significant decrease in the percentage of TUNEL+ cells (17.3 ± 4.6 vs. 1.8 ± 2.3%; hyperoxia BM-derived c-kit− cells vs. hyperoxia BM-derived c-kit+ cells; p < 0.001, n = 5–6/group) (Fig. 4A, B). Similarly, Western blot analysis revealed a fourfold decrease in the expression of CC3 in the lung homogenates of hyperoxic pups treated with IT BM-derived c-kit+ cells compared to those that received IT BM-derived c-kit− cells (p < 0.001, n = 5–6/ group) (Fig. 4C).
Figure 4.
BM-derived c-kit+ cells decrease apoptotic cell death. (A) Lung sections stained with DAPI (blue) and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL; red) demonstrating decreased TUNEL+ cells in the lungs of hyperoxia pups treated with BM-derived c-kit+ cells. Original magnification: 200×. Scale bar: 100 μm. (B) Decreased apoptotic index in the lungs of hyperoxia pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyper-oxia c-kit+; n = 5–6/group). (C) Decreased CC3 protein expression in the lungs of hyperoxia pups treated with BM-derived c-kit+ cells (*p < 0.001: RA c-kit− vs. hyperoxia c-kit−, **p < 0.001: hyperoxia c-kit− vs. hyperoxia c-kit+; n = 5–6/group) demonstrated graphically and in a Western blot with β-actin as loading control.
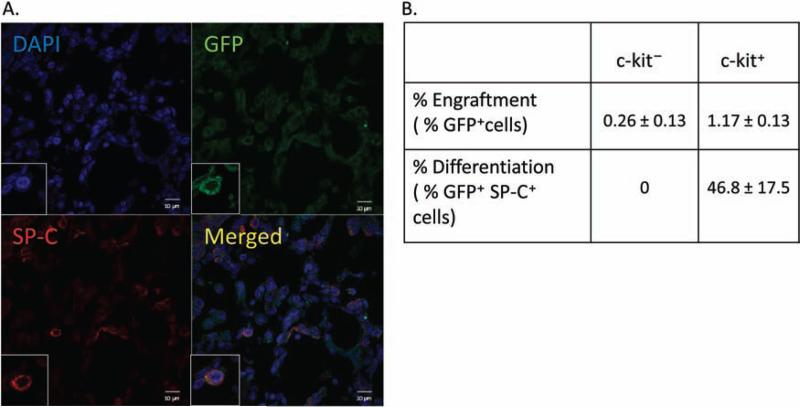
Engraftment of Bone Marrow-Derived c-kit+ Cells in Neonatal HILI
In order to ascertain whether the therapeutic effect of the BM-derived c-kit+ cells was secondary to cell engraftment, we evaluated the percentage of GFP+ cells that were present in the lungs of hyperoxic pups at P15. We demonstrated that only 1.17 ± 0.13% of total lung cells were GFP+ BM-derived c-kit+ cells, and of these engrafted cells, 47 ± 17% expressed SP-C (Fig. 5A, B). None of the retained GFP+ BM-derived c-kit− cells expressed SP-C (Fig. 5B). In addition, no GFP+ BM-derived c-kit+ or c-kit− cells expressed the endothelial cell marker vWF.
Figure 5.
Engraftment and differentiation of BM-derived c-kit+ cells. (A) Lung sections stained with surfactant protein C (SP-C; red) and DAPI (blue) demonstrating the engraftment and differentiation of green fluorescent protein-positive (GFP+) BM-derived c-kit+ cells (green) in hyperoxic pups treated with GFP+ BM-derived c-kit+ cells. Original magnification: 400×. Scale bar: 10 μm. (B) Sparse engraftment of GFP+ BM-derived c-kit+ or c-kit− cells in the lungs of hyperoxic pups 7 days post-IT injection with GFP+ cells expressing SP-C being evidence of differentiation.
DISCUSSION
Impaired alveolar development and dysmorphic vascular growth are the hallmarks of BPD or chronic lung disease of prematurity (22). Although several preventative and therapeutic strategies have been tried, BPD still affects 30–50% of infants with birth weights <1,000 g (38), and in a study of health care expenditure among children enrolled in the Washington State Medicaid Program, BPD ranked second for total payments of $17,481,047 annually (5). It is therefore apparent that innovative preventative and therapeutic options for this disease are urgently needed.
Recent preclinical studies suggest that cell-based therapies may be efficacious in attenuating neonatal HILI as evidenced in BPD (3,44,45). However, the exact population of cells that is most beneficial for neonatal lung repair remains unclear. The present study sought to ascertain whether BM-derived cell-based therapies targeting the c-kit population would be most effective in repairing the neonatal lung with phenotypic injury similar to that seen in BPD. We show that, compared to BM-derived c-kit− cells, the administration of IT BM-derived c-kit+ cells ameliorates neonatal HILI by improving angiogenesis, restoring alveolar structure, decreasing apoptosis, and increasing the secretion of angiogenesis-related factors. These findings have therapeutic implications as they suggest that the BM-derived c-kit+ cellular population may be most efficacious for neonatal lung regeneration.
c-kit is a type III tyrosine kinase receptor commonly utilized as a marker of BM-derived and tissue stem cells (23). Activation of this receptor by its ligand, stem cell factor, is known to promote cell migration, survival, and proliferation (2). Only 1–4% of all cells in the BM express c-kit (33). These include hemangioblasts, the presumptive common progenitor cell for HPCs and vascular endothelial cells (6), EPCs (12), and mast cells (11). Interestingly, within the peripheral blood, c-kit is evident in less than 0.1% of normal circulating peripheral blood cells as c-kit is downregulated following maturation of hematopoietic cellular lineages. In our present study, we compared the efficacy of BM-derived c-kit+ cells to BM-derived c-kit− cells in an experimental model of BPD. This cell population was targeted specifically as BPD is known to be characterized by defective angiogenesis, and prior studies had demonstrated the proangiogenic properties of BM-derived c-kit+ cells (29,42).
We demonstrate that the administration of BM-derived c-kit+ cells improves lung vascular density in newborn rodents with HILI. These findings are consistent with those of Fazel and colleagues who demonstrated that BM-derived c-kit+ cells promote myocardial repair following infarction by improving angiogenesis (15). Li et al. also showed that BM-derived c-kit+ cells injected into ischemic hindlimbs of mice increased perfusion by increasing VEGF secretion or by themselves differentiating into endothelial cells, which incorporated into vessels (29). These latter findings actually suggest that the proangiogenic effects of BM-derived c-kit+ cells may be both paracrine mediated or potentially by transdifferentiation. In our present study, while we demonstrated that the improvement in lung vascular density was accompanied by an increase in lung VEGF concentration, we did not show any incorporation of the cells in the vasculature, suggesting that the effects of the cells may be mainly paracrine-mediated. It is plausible that the disparity in our results may be due to the fact that in our study the cells were administered intratracheally rather than systemically. However, the IT route of administration of stem cells is particularly advantageous over the intravenous route as cells are delivered directly to the injured lung, and the propensity for systemic adverse effects is lessened.
In addition to improved lung angiogenesis, our present study also showed a significant improvement in alveolarization following administration of BM-derived c-kit+ cells to neonatal rodents with HILI. While this improvement in alveolarization may have been a direct consequence of the improvement in angiogenesis (42), parenchymal repair following BM-derived c-kit+ cell administration has been documented in several models of organ injury. Transplantation of BM-derived c-kit+ cells reduced hyper-glycemia in mice with streptozotocin-induced pancreatic injury (19). Additionally, in a mouse model of peritoneal fibrosis, BM-derived c-kit+ cells were shown to differentiate into mesothelial cells and to improve peritoneal repair (37). Interestingly, in our study, although the degree of retention of BM-derived c-kit+ cells as evidenced by the GFP+ population in the lung was limited, a significant percentage of retained cells did express surfactant protein-C, a type II alveolar epithelial cell marker. Whether this differentiation significantly enhanced lung repair is not presently clear. It should, however, be noted that the minimal degree of cell retention in our present study is similar to those of other investigators following IT administration of BM-derived stem cells in experimental models of BPD (44). Thus, our findings may suggest that similar to the paracrine-mediated improvement in angiogenesis, the restoration in alveolar development following BM-derived c-kit+ cells may also be due to an increase in VEGF secretion. This interpretation is consistent with prior studies, which have shown that VEGF is crucial for alveolar development (16) and moreover that recombinant VEGF restores alveolar structure in rodent models of neonatal HILI (28).
Yet another possible explanation for the improvement in alveolarization demonstrated in our study is the modest attenuation in epithelial cell apoptosis evidenced in the lungs of rodents that received BM-derived c-kit+ cells. Indeed, it has previously been shown that c-kit promotes cell survival by the activation of phosphoinositide 3-kinase and the subsequent protein kinase-B (Akt)-mediated phosphorylation of the B-cell CLL/lymphoma 2 (BCL2)-associated agonist of cell death (Bad) protein (7). Moreover, previous studies have demonstrated that c-kit dysfunction is associated with increased cardiomyocyte apoptosis following myocardial infarction (15) and, conversely, that augmentation of c-kit signaling decreased apoptosis in several models of injury (4,13).
Although this was a short-term study, an important consideration in cell-based therapies is the possibility of adverse effects. One main concern with the administration of BM-derived c-kit+ cells is the potential risk of worsening PH as prior studies had demonstrated that c-kit+ progenitor cells may contribute to pulmonary vascular remodeling (31). In our present study, we did not demonstrate any significant difference in RVSP or pulmonary vascular remodeling in the rodents that received BM-derived c-kit+ or BM-derived c-kit− cells, but long-term studies are warranted. Other investigations will also need to be performed to compare the relative therapeutic efficacy of BM-derived c-kit+ cells and other stem cell populations in neonatal HILI. Our present findings are, however, strengthened by the fact that we utilized BM-derived c-kit− cells as our placebo group, and thus any adverse effects specifically due to the c-kit+ cell population could be identified. Furthermore, compared to other cell therapies that require expansion of the cells in culture media prior to administration, BM-derived c-kit+ cells were administered directly following isolation.
In conclusion, the findings in our present study suggest that c-kit-targeted cell therapies may be efficacious in promoting neonatal lung repair. We demonstrate that IT administration of BM-derived c-kit+ cells upregulates angiogenesis-related genes, decreases apoptosis, and restores alveolar as well as vascular structure in an experimental model of BPD. We speculate that this may be a potent therapeutic strategy for lung regeneration in preterm infants with BPD.
ACKNOWLEDGMENTS
Supported by the National Institute of Health KO8 Award, Florida Biomedical Research Award, and Batchelor Research Foundation Award to Karen Young.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Aliotta JM, Keaney P, Passero M, Dooner MS, Pimentel J, Greer D, Demers D, Foster B, Peterson A, Dooner G, Theise ND, Abedi M, Colvin GA, Quesenberry PJ. Bone marrow production of lung cells: The impact of G-CSF, cardiotoxin, graded doses of irradiation, and sub-population phenotype. Exp. Hematol. 2006;34(2):230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashman LK. The biology of stem cell factor and its receptor C-kit. Int. J. Biochem. Cell Biol. 1999;31(10):1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 3.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 2009;180(11):1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J, Cukerman E, Stanford WL, Medin JA, Liu PP. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2006;103(7):2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancalari E, Wilson-Costello D, Iben SC. Management of infants with bronchopulmonary dysplasia in North America. Early Hum. Dev. 2005;81(2):171–179. doi: 10.1016/j.earlhumdev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122(10):3023–3033. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- 7.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 1998;8(13):779–785. doi: 10.1016/s0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 8.Bonikos DS, Bensch KG, Ludwin SK, Northway WH., Jr. Oxygen toxicity in the newborn. The effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab. Invest. 1975;32(5):619–635. [PubMed] [Google Scholar]
- 9.Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC, Egan ME, Krause DS. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc. Natl. Acad. Sci. USA. 2006;103(8):2965–2970. doi: 10.1073/pnas.0510758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24(10):2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- 11.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA. 2005;102(32):11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Falco E, Avitabile D, Totta P, Straino S, Spallotta F, Cencioni C, Torella AR, Rizzi R, Porcelli D, Zacheo A, Di Vito L, Pompilio G, Napolitano M, Melillo G, Capogrossi MC, Pesce M. Altered SDF-1-mediated differentiation of bone marrow-derived endothelial progenitor cells in diabetes mellitus. J. Cell. Mol. Med. 2009;13(9B):3405–3414. doi: 10.1111/j.1582-4934.2009.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhandapani KM, Wade FM, Wakade C, Mahesh VB, Brann DW. Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFκB. J. Neurochem. 2005;95(1):9–19. doi: 10.1111/j.1471-4159.2005.03319.x. [DOI] [PubMed] [Google Scholar]
- 14.Eber E, Zach MS. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax. 2001;56(4):317–323. doi: 10.1136/thorax.56.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 2006;116(7):1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 17.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305(5680):90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto N, Jin H, Liu T. Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Invest. 2004;113:243. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 2003;21(7):763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 20.Ireys HT, Anderson GF, Shaffer TJ, Neff JM. Expenditures for care of children with chronic illnesses enrolled in the Washington State Medicaid program, fiscal year 1993. Pediatrics. 1997;100(2 Pt 1):197–204. doi: 10.1542/peds.100.2.197. [DOI] [PubMed] [Google Scholar]
- 21.Jobe AJ, Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 22.Jobe AJ. The new BPD: An arrest of lung development. Pediatr. Res. 1999;46(6):641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Keshet E, Lyman SD, Williams DE, Anderson DM, Jenkins NA, Copeland NG, Parada LF. Embryonic RNA expression patterns of the c-kit receptor and its cog-nate ligand suggest multiple functional roles in mouse development. EMBO J. 1991;10(9):2425–2435. doi: 10.1002/j.1460-2075.1991.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, Lehmann U, Kreipe H. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am. J. Pathol. 2003;162(5):1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am. J. Respir. Cell Mol. Biol. 2005;33(4):328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128(24):5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 27.Krause DS. Engraftment of bone marrow-derived epithelial cells. Ann. N.Y. Acad. Sci. 2005;1044:117–124. doi: 10.1196/annals.1349.015. [DOI] [PubMed] [Google Scholar]
- 28.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289(4):L529–L535. doi: 10.1152/ajplung.00336.2004. [DOI] [PubMed] [Google Scholar]
- 29.Li TS, Hamano K, Nishida M, Hayashi M, Ito H, Mikamo A, Matsuzaki M. CD117+ stem cells play a key role in therapeutic angiogenesis induced by bone marrow cell implantation. Am. J. Physiol. Heart Circ. Physiol. 2003;285(3):H931–937. doi: 10.1152/ajpheart.01146.2002. [DOI] [PubMed] [Google Scholar]
- 30.Loffredo F, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8(4):389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, Huertas A, Hammad H, Lambrecht B, Simonneau G, Launay JM, Cohen-Kaminsky S, Humbert M. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2011;184(1):116–123. doi: 10.1164/rccm.201006-0905OC. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, Takakura N. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105(7):2757–2763. doi: 10.1182/blood-2004-08-3317. [DOI] [PubMed] [Google Scholar]
- 33.Papayannopoulou T, Brice M, Broudy VC, Zsebo KM. Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: Functional properties and composite antigenic profile. Blood. 1991;78(6):1403–1412. [PubMed] [Google Scholar]
- 34.Parker RA, Lindstrom DP, Cotton RB. Improved survival accounts for most, but not all, of the increase in bronchopulmonary dysplasia. Pediatrics. 1992;90(5):663–668. [PubMed] [Google Scholar]
- 35.Rafii S, Shapiro F, Rimarachin J, Nachman RL, Ferris B, Weksler B, Moore MA, Asch AS. Isolation and characterization of human bone marrow microvascular endothelial cells: Hematopoietic progenitor cell adhesion. Blood. 1994;84(1):10–19. [PubMed] [Google Scholar]
- 36.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005;33(2):145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiguchi Y, Hamada C, Ro Y, Nakamoto H, Inaba M, Shimaoka T, Io H, Koyanagi I, Aruga S, Inuma J, Kaneko K, Hotta Y, Margetts PJ, Mochizuki H, Horikoshi S, Tomino Y. Differentiation of bone marrow-derived cells into regenerated mesothelial cells in perito-neal remodeling using a peritoneal fibrosis mouse model. J. Artif. Organs. 2012;15(3):272–282. doi: 10.1007/s10047-012-0648-2. [DOI] [PubMed] [Google Scholar]
- 38.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD. Eunice Kennedy Shriver National Institute of Child, Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely pre-term infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am. J. Respir. Crit. Care Med. 2003;168(3):318–322. doi: 10.1164/rccm.200301-145OC. [DOI] [PubMed] [Google Scholar]
- 40.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102(2):199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 41.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: How many ways can cells die? Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294(4):L632–641. doi: 10.1152/ajplung.00262.2007. [DOI] [PubMed] [Google Scholar]
- 42.Thebaud B, Abman SH. Bronchopulmonary dysplasia: Where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 2007;175(10):978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurlbeck WM. Measurement of pulmonary emphysema. Am. Rev. Respir. Dis. 1967;95(5):752–764. doi: 10.1164/arrd.1967.95.5.752. [DOI] [PubMed] [Google Scholar]
- 44.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, Chen M, Hashimoto K, Abley D, Korbutt G, Archer SL, Thebaud B. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am. J. Respir. Crit. Care Med. 2009;180(11):1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Haaften T, Thebaud B. Adult bone marrow-derived stem cells for the lung: Implications for pediatric lung diseases. Pediatr. Res. 2006;59(4 Pt 2):94R–99R. doi: 10.1203/01.pdr.0000203550.50258.5a. [DOI] [PubMed] [Google Scholar]
- 46.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O'Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am. J. Pathol. 2011;178(6):2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young KC, Torres E, Hatzistergos KE, Hehre D, Suguihara C, Hare JM. Inhibition of the SDF-1/CXCR4 axis attenuates neonatal hypoxia-induced pulmonary hyper-tension. Circ. Res. 2009;104(11):1293–1301. doi: 10.1161/CIRCRESAHA.109.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]