Abstract
Prehypertension (BP 120–139/80–89 mmHg) is associated with an increased risk for future atherothrombotic events. Although the mechanisms underlying this elevated risk are not completely understood, one possibility is that prehypertension is associated with impaired endothelial fibrinolytic capacity. We tested the hypothesis that vascular endothelial release of t-PA is impaired in prehypertensive men. Net endothelial release of t-PA was determined, in vivo, in response to intrabrachial infusions of bradykinin (12.5, 25, 50 ng/100 mL tissue/min) and sodium nitroprusside at (1.0, 2.0, 4.0 µg/100 mL tissue/min) in 42 middle-age and older men: 16 normotensive (BP range: 100–119/57–79 mmHg); 16 prehypertensive (BP range: 120–139/76–89 mmHg); and 10 hypertensive (BP range: 140–150/74–100 mmHg). Net release of t-PA antigen was ~25% lower (P < 0.05) in the prehypertensive (−0.9 ± 0.8 to 42.4 ± 5.3 ng/100 mL tissue/min) compared with the normotensive (0.5 ± 1.0 to 53.9 ± 6.5 ng/100 mL tissue/min) men. There was no significant difference in t-PA release between the hypertensive (−1.8±1.6 to 40.8±6.6 ng/100 mL tissue/min) and prehypertensive groups. Sodium nitroprusside did not significantly alter t-PA release in any group. These data indicate that endothelial t-PA release is diminished in prehypertensive men. Further, the level of impairment in t-PA release seen with clinical hypertension is already apparent in the prehypertensive state. Impaired endothelial fibrinolytic function may underlie the increased atherothrombotic risk associated with blood pressure in the prehypertensive range.
Keywords: Endothelium, tissue-type plasminogen activator, blood pressure, hypertension
INTRODUCTION
Aside from age, elevated blood pressure (BP) is the most predominant cardiovascular disease (CVD) risk factor worldwide1. It is well-established that blood pressure-related CVD risk is not limited to values in the clinically hypertensive range (systolic BP > 140 mmHg and/or diastolic BP >90 mmHg), but also involves blood pressures in the prehypertensive range (systolic BP 120–139 mmHg systolic and/or diastolic BP 80–89 mmHg)2. Several epidemiological studies have demonstrated that prehypertension is linked not only with an increased risk for developing clinical hypertension but also increased prevalence of myocardial infarction, stroke and congestive heart failure3. Moreover, it is estimated that nearly 50% of blood pressure-related deaths occur in individuals with systolic blood pressure in the prehypertensive range4. Considering ~70 million adults in the United States are thought to have resting blood pressures in the prehypertensive range, prehypertension represents an important public health issue5.
Endothelial dysfunction plays a vital role in the initiation, development and progression of atherosclerosis6, 7. In addition to vasomotor regulation, a prominent thromboresistant property of the vascular endothelium is its modulatory influence on fibrinolysis. Endothelial cells are the principal site of synthesis and release of tissue-type plasminogen activator (t-PA), the primary activator of the fibrinolytic system8. Experimental and clinical data indicate that it is the capacity of the endothelium to release t-PA rapidly and acutely from intracellular storage pools that determines the efficacy of endogenous fibrinolytic activity9, 10. Diminished capacity of the endothelium to release t-PA is associated with the pathogenesis of coronary artery disease and associated atherothrombotic events11, 12 as well as a variety of CVD risk factors such as cigarette smoking13 and hypertension14. For example, Hrafnkelsdottir et al.14 reported that endothelial t-PA release is ~40% lower in hypertensive compared with normotensive middle-aged adults. Currently, it is unknown whether blunted endothelial t-PA release observed with clinical hypertension is evident in the prehypertensive state. If so, impaired endothelial fibrinolytic capacity may contribute to the increased vascular risk with prehypertension.
Accordingly, we tested the hypothesis that the capacity of the endothelium to acutely release t-PA is impaired with prehypertension. To address this hypothesis, we employed an isolated forearm model to assess endothelial t-PA release in vivo in normotensive, prehypertensive and hypertensive middle-aged and older men.
METHODS
Subjects
Forty-two adult men with normal and elevated blood pressure were studied: 16 normotensive (BP range: 100–119/57–79 mmHg); 16 prehypertensive (BP range: 120–139/76–89 mmHg); and 10 hypertensive (BP range: 140–150/74–100 mmHg). Blood pressure classification was based on JNC-7 guidelines15, determined by the average of two or more seated BP readings from two separate visits15. All subjects were free of overt cardiovascular and metabolic disease as assessed by medical history, physical examination, fasting blood chemistries, and electrocardiograms and blood pressure at rest and during incremental exercise performed to exhaustion. None of the subjects smoked, were taking medications (including vitamins), or performed regular physical exercise for at least 1 year before the start of the study. Prior to participation, all of the subjects had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder. All of the procedures were performed according to institutional guidelines.
Measurements
Body Composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance (Detecto, Webb City, MO). Percent body fat was determined by dual energy x-ray absorptiometry (Lunar Radiation Corporation, Madison, WI). Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to previously published guidelines16.
Treadmill Exercise Test
To assess aerobic fitness, subjects performed incremental treadmill exercise using a modified Balke protocol. Maximal oxygen consumption (O2max) was measured using on-line computer-assisted open circuit spirometry36.
Metabolic Measurements
Fasting plasma lipid, lipoprotein, glucose and insulin concentrations were determined using standard techniques by the clinical laboratory affiliated with the Clinical Translational Research Center at the University of Colorado at Boulder. Insulin resistance was estimated using the homeostasis model assessment (HOMA-IR) derived from fasting glucose and insulin concentrations17.
Intra-Arterial Fibrinolytic Protocol
All measurements were performed in a temperature-controlled room between 7 and 10 AM after an overnight fast as previously described by our laboratory8. Briefly, an intravenous catheter was placed in a deep antecubital vein of the non-dominant arm. Thereafter, a 5-cm, 20-gauge catheter was introduced into the brachial artery of the same arm under local anesthesia (1% lidocaine). Forearm blood flow (FBF) was measured using strain-gauge venous occlusion plethysmography (D.E. Hokanson, Bellevue, WA) and presented as mL/100 mL forearm volume/min. Following the measurement of resting blood flow for 5 minutes, bradykinin was infused intra-arterially at rates of 12.5, 25, 50 ng·100 ml tissue−1·min−1 and sodium nitroprusside at 1.0, 2.0, 4.0 µg·100 ml tissue−1·min−1 for 5 min at each dose as previously described8. To avoid an order effect, the sequence of drug administration was randomized.
Net endothelial release of t-PA antigen and plasminogen activator inhibitor (PAI)-1 antigen in response to bradykinin and sodium nitroprusside was calculated according to Jern et al.18 using the following equation:
where CV and CA represent the concentration in the vein and artery, respectively. For both t-PA and PAI-1, a positive difference indicated a net release and a negative difference, net uptake. Arterial and venous blood samples were collected simultaneously at baseline and the end of each drug dose. Enzyme immunoassay was used to determine t-PA and PAI-1 antigen concentrations. Hematocrit was measured in triplicate using the standard microhematocrit technique and corrected for trapped plasma volume within the erythrocytes19. The total amount of t-PA antigen released across the forearm in response to bradykinin was calculated as the incremental area under each curve using a trapezoidal model. In order to avoid confounding effects from potential infection or acute inflammation on fibrinolytic function, all subjects were free of recent infection/inflammation (> 2 weeks) as determined by questionnaire20.
Statistical Analysis
Differences in subject baseline characteristics and area under the curve data were determined by between-groups analysis of variance (ANOVA). Group differences in FBF and endothelial t-PA and PAI-1 antigen release in response to bradykinin and sodium nitroprusside were determined by repeated-measures ANOVA. When indicated by a significant F value, a post hoc test using the Newman-Keuls method was performed to identify differences between the groups. Relations between variables of interest were assessed by linear regression analysis. All data are expressed as mean ± SEM. Statistical significance was set a priori at P<0.05.
RESULTS
Selected subject characteristics are presented in Table 1. There were no differences in age, anthropometric or metabolic variables between the normotensive and prehypertensive groups. By design, systolic and diastolic blood pressures were greater (P<0.05) in the prehypertensive compared with the normotensive group. Aside from blood pressure, BMI, and plasma glucose and insulin concentrations were highest in the hypertensive men,
Table 1.
Selected Subject Characteristics
| Variable | Normotensive (n=16) |
Prehypertensive (n=16) |
Hypertensive (n=10) |
|---|---|---|---|
| Age (years) | 52±3 | 52±2 | 57±2 |
| Body mass (kg) | 82.5±2.9 | 84.8±2.4 | 89.6±2.9 |
| Body fat (%) | 23.1±1.9 | 26.6±1.4 | 30.2±1.5* |
| Waist Circumference (cm) | 91.7±2.3 | 95.4±2.1 | 100.6±2.0* |
| BMI (kg/m2) | 25.8±0.8 | 26.9±0.7 | 29.7±.0.9*† |
| Systolic BP (mmHg) | 114±2 | 128±2* | 145±2*† |
| Diastolic BP (mmHg) | 73±2 | 83±2* | 90±2*† |
| VO2 max (mL/kg/min) | 35.5±1.7 | 35.4±1.6 | 30.0±1.3*† |
| Total cholesterol (mmol/L) | 4.6±0.1 | 4.9±0.2 | 4.6±0.2 |
| HDL cholesterol (mmol/L) | 1.1±0.1 | 1.1±0.1 | 1.3±0.1 |
| LDL cholesterol (mmol/L) | 3.0±0.1 | 3.1±0.2 | 2.7±0.2 |
| Triglycerides (mmol/L) | 1.1±0.1 | 1.5±0.2 | 1.5±0.3 |
| Glucose (mmol/L) | 4.9±0.1 | 5.1±0.1 | 5.2±0.1* |
| Insulin (pmol/L) | 34.5±3.2 | 40.4±2.8 | 53.4±7.4*† |
| HOMA-IR | 1.3±0.1 | 1.5±0.1 | 2.2±0.3*† |
Values are mean ± SEM. BMI, body mass index; BP, blood pressure; VO2 max, maximal oxygen consumption; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostasis model assessment.
P<0.05 vs normotensive
P<0.05 vs prehypertensive
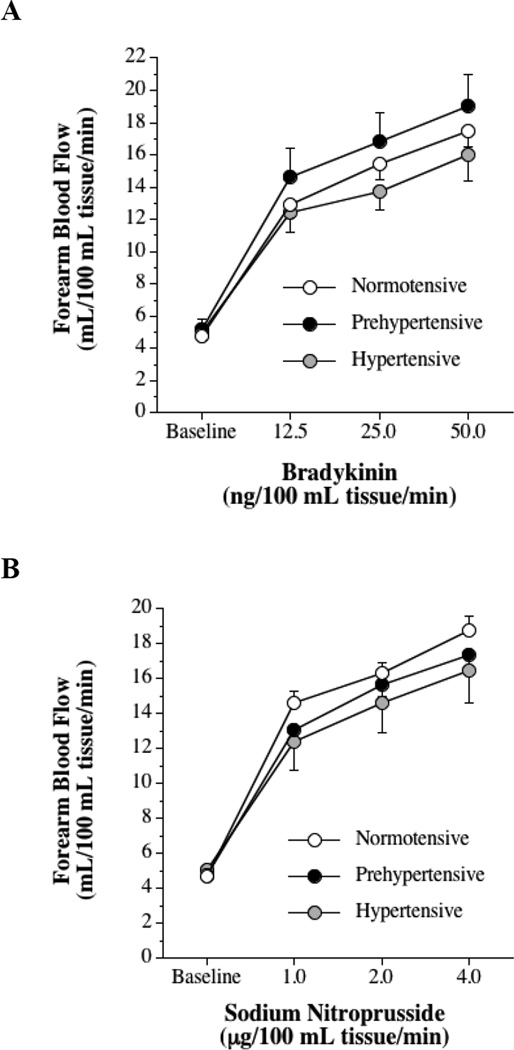
The FBF responses to bradykinin and sodium nitroprusside were not significantly different between groups (Figure 1). FBF in the non-infused arm remained constant throughout the infusion protocols and did not differ significantly between groups.
Figure 1.
Forearm blood flow responses to bradykinin (A) and sodium nitroprusside (B) in normotensive, prehypertensive and hypertensive men. Values are mean ± SEM.
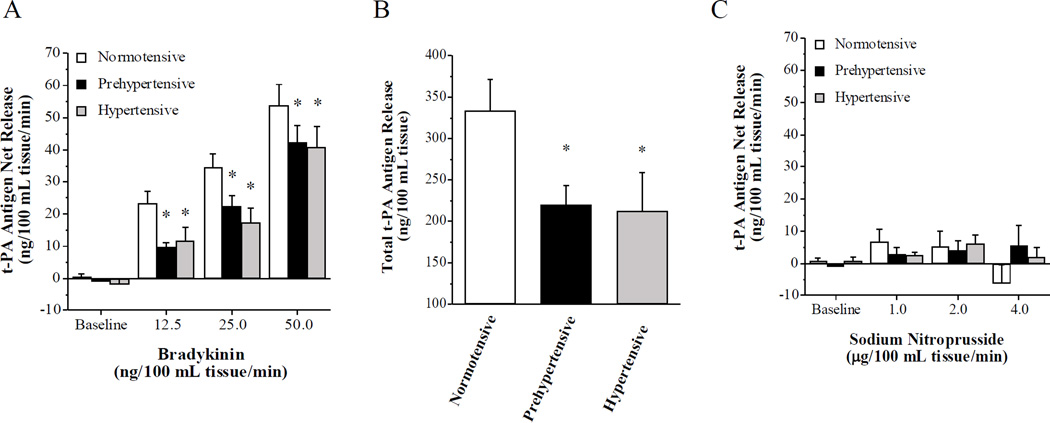
Basal endothelial t-PA antigen release was not significantly different amongst the groups. However, compared with the normotensive men, the capacity of the endothelium to release t-PA in response to bradykinin was significantly blunted in the prehypertensive and hypertensive men (Figure 2). Net release of t-PA antigen was ~25% lower (P < 0.05) in the prehypertensive (from −0.9 ± 0.8 to 42.4 ± 5.3 ng/100 mL tissue/min) compared with the normotensive (from 0.5 ± 1.0 to 53.9 ± 6.5 ng/100 mL tissue/min) men. As a result, the total amount of t-PA antigen released (area under the curve to all doses of bradykinin) was markedly lower (~35%; P < 0.05) in the prehypertensive (220 ± 24 ng/100 mL tissue) than normotensive (341 ± 34 ng/100 ml tissue) group (Figure 2). Interestingly, net release of t-PA antigen (from −1.8±1.6 to 40.8±6.6 ng/100 mL tissue/min) and total amount of t-PA antigen released (213±47 ng/100 mL tissue) in response to bradykinin in the hypertensive group, although lower (P<0.05) than normotenisve controls, was not significantly different from the prehypertensive group. There was an inverse relation between systolic BP and total t-PA release to bradykinin (r = −0.36, P<0.05). Infusion of sodium nitroprusside did not stimulate significant changes in t-PA release in the normotensive (from 0.4 ± 0.8 to −0.6 ± 3.1 ng/100 mL tissue/min), prehypertensive (from −0.5 ± 0.9 to 3.4 ± 5.7 ng/100 mL tissue/min) and hypertensive (from 0.8±1.3 to 2.1±3.0 ng/100 mL tissue/min) adults. Neither bradykinin nor sodium nitroprusside elicited significant changes in PAI-1 antigen release in any group (data not shown).
Figure 2.
Net release rate (A) and total amount (B) of tissue-type plasminogen activator (t-PA) antigen released across the forearm in response to bradykinin and sodium nitroprusside (C) in normotensive, prehypertensive and hypertensive men. Values are mean ± SEM; *P < 0.05 vs. normotensive.
DISCUSSION
The novel findings of the present study are as follows: 1) the capacity of the endothelium to release t-PA is diminished in middle-aged men with blood pressure in the prehypertensive range; and 2) the level of impairment in endothelial t-PA release in prehypertensive men is similar to that observed in hypertensive men. To our knowledge, this is the first study to examine the influence of blood pressure in the prehypertensive range on endothelial fibrinolytic function.
The vascular risks associated with prehypertension cannot be overlooked. Several epidemiological studies have demonstrated significantly increased risk of cardiovascular disease and associated acute vascular events in prehypertensive compared with normotensive adults2122232425. For example, multivariate-adjusted analyses of the original Framingham Heart Study cohort revealed that compared with normotension, prehypertension was associated with substantially greater risk of myocardial infarction (hazard ratio [HR]: 3.5)21. Moreover, a recently conducted meta analysis involving over one million individuals, spanning 20 prospective cohort studies, provided further confirmation of the elevated risk of CVD-related mortality with prehypertension2. The mechanisms for the increased vascular risk with blood pressure in the prehypertensive range are unclear. We26, 27 and others28 have reported that endothelial vasomotor function is markedly impaired in prehypertensive adults. Indeed, Weil et al.27 showed, in a similar population to that of the present study, that prehypertension is associated with a ~30% reduction in nitric oxide-mediated endothelium-dependent vasodilation. In addition to reduced nitric oxide bioavailability, the same authors, in a separate study, also reported that endothelin-1 vasoconstrictor tone is elevated (~20%) in the prehypertensive state26. The results of the present study complement and significantly extend these previous findings by demonstrating that the capacity of the endothelium to release t-PA in prehypertensive men is severely blunted (~35%). Although the potential clinical consequence of reduced t-PA release with prehypertension is outside the scope of this study, both animal29, 30 and clinical studies31 have highlighted the deleterious impact of diminished endothelial release of t-PA on cardiovascular health. Studies involving t-PA deficient mice, for example, demonstrated accelerated rates of atherosclerotic fibrin deposition and extensive myocardial tissue necrosis in these animals29, 30. In humans, reduced capacity of the endothelium to release t-PA has been linked to atheromatous plaque development and higher rates of myocardial infarction31. Thus, endothelial fibrinolytic dysfunction may be a contributing mechanism underlying the increased risk of thrombosis and acute cardiovascular events with prehypertension3.
Interestingly, in the current study there was no difference between the prehypertensive and hypertensive groups in endothelial t-PA release. Both groups demonstrated significantly lower (~35%) capacity of the endothelium to release t-PA than their normotensive counterparts. Of note, the impairment in endothelial t-PA release observed in the hypertensive group is in line with that previously described14. Hrafnkelsdottir and colleagues14 demonstrated that release of t-PA from the endothelium was significantly blunted in hypertensive compared with normotensive adults, independent of other risk factors. The results of the present study extend these findings by showing that the impairment in t-PA release with hypertension appears to be born in the prehypertensive state. This may account, in part, for the increase in vascular risk starting at blood pressure in the prehypertensive range3. Moreover, it provides further rationale for a more aggressive approach towards risk recognition and blood pressure control in the prehypertensive population.
The mechanisms underlying the impairment in endothelial t-PA release in adult males with blood pressure in the prehypertensive range are unclear. It has been demonstrated that elevations in blood pressure are associated with increased inflammation and oxidative stress32–35. Our group36, as well as others37, have shown that elevations in oxidative stress is associated with lower endothelial t-PA release. For example, Van Guilder et al.36 demonstrated that endothelium t-PA release was potentiated after acute intra-arterial vitamin C administration and chronic oral vitamin C supplementation in overweight and obese adults. While not assessed in this study, it is possible that oxidative stress may contribute to impaired endothelial t-PA release with prehypertension, and is worthy of future study. It is important to emphasize that aside from elevated blood pressure, subjects in the present study were free of clinically evident coronary artery disease and other cardiometabolic risk factors that have been shown to adversely influence endothelial t-PA release such as obesity and dyslipidemia. Thus, we believe that the observed impairment in t-PA is a primary consequence of chronic elevation in blood pressure.
There are a few experimental considerations regarding the present study that merit discussion. Firstly, the experimental design of our study was cross-sectional. Therefore, we cannot discount the possibility that variables such as genetics and/or lifestyle behaviors may have influenced our results. In the effort to minimize the influence of lifestyle behaviors, we studied sedentary adults who were non-smokers and not currently taking any medications or supplements that have been shown to influence endothelial fibrinolytic function. Secondly, it is important to emphasize that the present study involved men only. We have previously reported that postmenopausal women demonstrate markedly higher (~50%) endothelial t-PA release than middle-aged and older men38. It is possible that these gender differences may exist amongst prehypertensive adults. As such, studies are ongoing in our laboratory to examine the influence of prehypertension on endothelial t-PA release in middle-aged and older women. In addition, it is unknown whether prehypertension adversely affects endothelial fibrinolytic function in younger adults.
In conclusion, the results of this study indicate that the capacity of the endothelium to release t-PA is diminished in adult males with blood pressure in the prehypertensive range. Moreover, the level of impairment in t-PA release seen with clinical hypertension is already apparent in the prehypertensive state. Blunted endothelial fibrinolytic function may be an underlying factor contributing to the increased risk of cardiovascular disease and acute vascular events with prehypertension.
What is known about topic.
Nearly 50% of blood pressure-related deaths occur in individuals with systolic blood pressure in the prehypertensive range (systolic BP 120–139 mmHg systolic and/or diastolic BP 80–89 mmHg).
Mechanisms responsible for the increased cardiovascular risk in adults with blood pressure in the prehypertensive range are not fully understood.
What this study adds.
The capacity of the endothelium to release tissue-type plasminogen activator (t-PA), the primary activator of endogenous fibrinolysis, is markedly impaired in middle-aged men with blood pressure in the prehypertensive range.
The level of impairment in endothelial t-PA release in prehypertensive men is similar to that observed in hypertensive men.
Diminished endothelial fibrinolytic capacity may underlie the increased cardiovascular risk with prehypertension.
Acknowledgments
We would like to thank all of the subjects who participated in the study.
Support: This study was supported by National Institutes of Health awards HL088891, HL077450, HL076434 and RR00051 as well as American Heart Association awards 0840167N and 12PRE9280026.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics-2013 Update: A Report From the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, et al. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. American Heart Journal. 2014;167(2):160–168. e1. doi: 10.1016/j.ahj.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. The New England journal of medicine. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 4.Williams B. The year in hypertension. Journal of the American College of Cardiology. 2009;55(1):65–73. doi: 10.1016/j.jacc.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Elliott WJ, Black HR. Prehypertension. Nat Clin Pract Cardiovasc Med. 2007;4(10):538–548. doi: 10.1038/ncpcardio0989. [DOI] [PubMed] [Google Scholar]
- 6.Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11(11):1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 7.Quyyumi A. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. American Journal of Medicine. 1998;105:32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 8.Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, et al. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. American journal of physiology. Endocrinology and metabolism. 2005;289(5):E807–E813. doi: 10.1152/ajpendo.00072.2005. [DOI] [PubMed] [Google Scholar]
- 9.Parmer R, Mahata M, Mahata S, Sebald M, O'Connor D, Miles L. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Journal of Biological Chemistry. 1997;272:1976–1982. doi: 10.1074/jbc.272.3.1976. [DOI] [PubMed] [Google Scholar]
- 10.van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, Emeis JJ. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85(12):3510–3517. [PubMed] [Google Scholar]
- 11.Lucking AJ, Gibson KR, Paterson EE, Faratian D, Ludlam CA, Boon NA, et al. Endogenous tissue plasminogen activator enhances fibrinolysis and limits thrombus formation in a clinical model of thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(5):1105–1111. doi: 10.1161/ATVBAHA.112.300395. [DOI] [PubMed] [Google Scholar]
- 12.Robinson SD, Ludlam CA, Boon NA, Newby DE. Endothelial Fibrinolytic Capacity Predicts Future Adverse Cardiovascular Events in Patients With Coronary Heart Disease. 2007 doi: 10.1161/ATVBAHA.107.143248. [DOI] [PubMed] [Google Scholar]
- 13.Newby DE, McLeod AL, Uren NG, Flint L, Ludlam CA, Webb DJ, et al. Impaired Coronary Tissue Plasminogen Activator Release Is Associated With Coronary Atherosclerosis and Cigarette Smoking. Circulation. 2001 doi: 10.1161/01.cir.103.15.1936. [DOI] [PubMed] [Google Scholar]
- 14.Hrafnkelsdottir T, Wall U, Jern C, Jern S. Impaired capacity for endogenous fibrinolysis in essential hypertension. Lancet. 1998;352(9140):1597–1598. doi: 10.1016/S0140-6736(05)61044-6. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, et al. The seventh report of the joint national committe on prevention, detection, evaluation, and treatment of high blood pressure. The JNC 7 report. Journal of American Medical Association. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.Lohman T, Roche A, Mortorell R. Athropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Jern S, Wall U, Bergbrant A, Selin-Sjogren L, Jern C. Endothelium-dependent vasodilation and tissue-type plasminogen activator release in borderline hypertension. Arteriosclerosis, thrombosis, and vascular biology. 1997;17(12):3376–3383. doi: 10.1161/01.atv.17.12.3376. [DOI] [PubMed] [Google Scholar]
- 19.Chaplin H, Mollison P. Correction of plasma trapped in the red cell column of hematocrit. Blood. 1952;7:1227–1238. [PubMed] [Google Scholar]
- 20.Macko R, Ameriso S, Gruber A, Griffin J, Fernandez J, Brandt R, et al. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–2011. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 21.Qureshi AI, Suri MF, Kirmani JF, Divani AA, Mohammad Y. Is prehypertension a risk factor for cardiovascular diseases? Stroke. 2005;36(9):1859–1863. doi: 10.1161/01.STR.0000177495.45580.f1. [DOI] [PubMed] [Google Scholar]
- 22.Liszka HA, Mainous AG, 3rd, King DE, Everett CJ, Egan BM. Prehypertension and cardiovascular morbidity. Ann Fam Med. 2005;3(4):294–299. doi: 10.1370/afm.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, et al. Prehypertension and cardiovascular disease risk in the Women's Health Initiative. Circulation. 2007;115(7):855–860. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 24.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 25.Baldinger B, Schwarz C, Jaggy C. Cardiovascular risk factors, BMI and mortality in a cohort of Swiss males (1976–2001) with high-sum-assured life insurance cover. Journal of insurance medicine (New York, N.Y. 2006;38(1):44–53. [PubMed] [Google Scholar]
- 26.Weil BR, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Elevated endothelin-1 vasoconstrictor tone in prehypertensive adults. The Canadian journal of cardiology. 2012;28(3):347–353. doi: 10.1016/j.cjca.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Weil BR, Stauffer BL, Greiner JJ, DeSouza CA. Prehypertension Is Associated With Impaired Nitric Oxide-Mediated Endothelium-Dependent Vasodilation in Sedentary Adults. American journal of hypertension. 2009;24(9):976–981. doi: 10.1038/ajh.2011.88. [DOI] [PubMed] [Google Scholar]
- 28.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvàth T, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55(6):1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degan J, Bronson R, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 30.Christie P, Edelberg J, Picard M, Foulkes A, Mamuya W, Weiler-Guettler H, et al. A murine model of myocardial microvascular thrombosis. Journal of Clinical Investigation. 1999;10:533–539. doi: 10.1172/JCI7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KAA, Boon NA, et al. Endothelial Dysfunction, Impaired Endogenous Fibrinolysis, and Cigarette Smoking. Circulation. 1999 doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 32.Dikalov SI, Ungvari Z. Role of mitochondrial oxidative stress in hypertension. American journal of physiology. Heart and circulatory physiology. 2013;305(10):H1417–H1427. doi: 10.1152/ajpheart.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lip G, Edmunds E, Nuttall SL, Landray MJ, Blann AD, Beevers DG. Oxidative stress in malignant and non-malignant phase hypertension. Journal of human hypertension. 2002;16(5):333–336. doi: 10.1038/sj.jhh.1001386. [DOI] [PubMed] [Google Scholar]
- 34.Harrison DG, Gongora MC. Oxidative stress and hypertension. The Medical clinics of North America. 2009;93(3):621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Economou M, Papadimitriou L, et al. The association between pre-hypertension status and oxidative stress markers related to atherosclerotic disease: the ATTICA study. Atherosclerosis. 2007;192(1):169–176. doi: 10.1016/j.atherosclerosis.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Acute and chronic effects of vitamin C on endothelial fibrinolytic function in overweight and obese adult humans. The Journal of physiology. 2008;586(14):3525–3535. doi: 10.1113/jphysiol.2008.151555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaehler J, Koeke K, Karstens M, Schneppenheim R, Meinertz T, Heitzer T. Impaired capacity for acute endogenous fibrinolysis in smokers is restored by ascorbic acid. Free Radical Bio Med. 2008;44(3):315–321. doi: 10.1016/j.freeradbiomed.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Stauffer BL, Hoetzer GL, Van Guilder GP, Smith DT, DeSouza CA. Gender Differences in Endothelial Tissue-Type Plasminogen Activator Release in Middle-Aged Adults. Journal of the American College of Cardiology. 2005;45(9):1547–1549. doi: 10.1016/j.jacc.2005.02.025. [DOI] [PubMed] [Google Scholar]