Abstract
Objective
Mechanisms underlying the relationship between physical activity and quality of life (QOL) in breast cancer survivors are not well understood. The purpose of the present study was to longitudinally test a model examining self-efficacy and health status as potential mediators of this relationship.
Methods
At baseline and 6 months, breast cancer survivors (N= 1527) completed physical activity, self-efficacy, health status and QOL measures, and a subsample (n=370) wore an accelerometer. Panel analysis within a covariance modeling framework were used to test the hypothesis that physical activity indirectly influences QOL across time.
Results
The hypothesized model provided a good fit in the full sample (χ2 = 409.06; df = 91, p <. 001; CFI = 0.98; SRMR= 0.04) and the accelerometer subsample (χ2= 320.96, df= 134, p <.001; CFI = 0.95; SRMR=0.05) indicating physical activity indirectly, via self-efficacy and health status indicators, influences QOL across time.
Conclusions
Physical activity may influence QOL in breast cancer survivors through more proximal, modifiable factors.
Keywords: breast cancer survivors, physical activity, quality of life, self-efficacy, health status
INTRODUCTION
Side effects of breast cancer treatment (e.g. fatigue, lymphedema, nausea, weight gain, depression) [1, 2]) are associated with a number of deleterious consequences including poorer disease-related outcomes and reduced quality of life (QOL) [3]. Many of these side effects persist, or even increase, several years’ post-diagnosis [3] and some of may have a delayed onset or be irreversible [4, 5]. In addition, breast cancer survivors report significantly lower levels of health-related QOL than non-cancers survivors [6, 7], and health-related QOL has been associated with early treatment discontinuation [8], cancer recurrence [9] and overall survival [10]. Physical activity is one lifestyle factor that has been shown to have significant QOL benefits for breast cancer survivors and has also been associated with enhanced survival and reduced risk of recurrence and mortality [11-14]. Although there is a substantial body of literature supporting beneficial effects of regular physical activity on QOL in breast cancer survivors [14], the mechanisms underlying this relationship are not well understood.
One of the major reasons for a lack of knowledge regarding such mechanisms may be due to the conceptualization of QOL in the cancer literature. The majority of studies examining physical activity and QOL in the breast cancer literature have focused solely on health-related QOL (HRQOL) [15] adopting the traditional medical literature’s characterization of QOL as an “umbrella term” under which a broad array of health status and disease outcomes reside. Few studies have classified QOL as a global psychological construct reflecting a “conscious cognitive judgment of one’s current satisfaction with one’s life.” or life satisfaction [16]. While health status may be associated with global measures of QOL, these constructs are conceptually and theoretically independent [17, 18]. Limiting the focus on QOL to an umbrella term for multiple constructs limits theory development, understanding of variability and changes in QOL and knowledge about the meaning of HRQOL and health status for cancer patients and survivors [19, 20]. Thus, understanding not only the mechanisms underlying the relationship between physical activity and health status, but how health status relates to more global QOL may be particularly important in breast cancer survivors to identify important opportunities for intervention.
Research conducted in older adults adopting a social cognitive perspective has demonstrated that changes in physical activity and global QOL may be mediated by more proximal psychosocial outcomes of physical activity participation including exercise self-efficacy and physical and mental health status [17]. Further support for this model has been provided in community-dwelling older adults [21] and individuals with multiple sclerosis [22] indicating this model may be useful for understanding physical activity and QOL in breast cancer survivors, especially given that about 60% of breast cancer survivors are over the age of 65 [23]. However, simply examining physical or mental health status may be insufficient, particularly for breast cancer survivors, given the various side effects and life changes that may occur post-diagnosis. The present study was designed to explore the relationship between physical activity and QOL in a large geographically diverse sample of breast cancer survivors, by replicating and extending McAuley and colleagues’ [17] model to include multiple indicators of health status associated with breast cancer survivorship. Additionally, we extended previous research, by measuring physical activity by accelerometry in a subsample of participants. Specifically, we hypothesized that baseline physical activity would be indirectly associated with global QOL via self-efficacy and health status indicators (social, physical, functional and emotional). Self-efficacy was hypothesized to be indirectly associated with QOL through health status indicators, and each health status indicator was, in turn, proposed to have a direct effect on global QOL. Furthermore, these relationships were hypothesized to be invariable among changes in these constructs over a 6-month period while controlling for all other model variables and covariates including demographic, treatment and disease-related characteristics.
PARTICIPANTS AND METHODS
Participant Eligibility Criteria
Women over the age of 18, who had been diagnosed with breast cancer, were English-speaking and had access to a computer were eligible to participate in the present study.
Measures
Demographics
Self-reported marital status, age, race, ethnicity, occupation, income, and education were collected.
Health and cancer history
Participants’ were asked to indicate (yes or no) whether or not they have been diagnosed with a list of 18 comorbidities (i.e. diabetes, obesity, hypertension) and to self-report information regarding breast cancer and other cancer history including date of diagnosis, treatment, and stage of disease. Current height and weight were also self-reported to estimate body mass index (BMI).
Self-reported Physical Activity
The Godin Leisure Time Exercise Questionnaire [24] was assessed self-reported frequency of current participation in at least 10 minutes of strenuous (e.g., jogging), moderate (e.g., fast walking), and mild (e.g., easy walking) exercise over the past seven days. These frequencies were multiplied by 9, 5, and 3 metabolic equivalents, respectively, and summed to obtain a total leisure time activity score.
Objective Physical Activity
The Actigraph accelerometer (model GT1M version, Health One Technology, Fort Walton Beach, FL), a valid and reliable objective physical activity measure [25, 26], was used to measure physical activity in a subsample of the study population. These women were instructed to wear the monitor for seven consecutive days on the non-dominant hip during all waking hours, except for when bathing or swimming. Activity data was collected in one-minute intervals (epochs). For the purposes of this study, a valid day of data consisted of an individual wearing the monitor for at least 10 valid hours with a valid hour being defined as having no more than 30 minutes of consecutive zeros. The total number of counts for each valid day was summed and divided by the number of days of monitoring to arrive at an average number of activity counts. Only data for individuals with a minimum of 3 valid days of wear time at both time points were included in analyses [27].
Self-efficacy
The 6-iterm Exercise Self-Efficacy Scale [28] assessed participants’ beliefs in their ability to exercise five times per week, at a moderate intensity, for 30 or more minutes per session at two week increments over the next 12 weeks. The 15-item Barriers Self-Efficacy Scale [28] assessed participants’ perceived capabilities to exercise three times per week for 40 minutes over the next two months in the face of common barriers to participation. Items from each scale are scored on a 100-point percentage scale with 10-point increments, ranging from 0% (not at all confident) to 100% (highly confident). For each measure, total scores are calculated using the average confidence rating.
Health Status
The 27-item Functional Assessment of Cancer Therapy- General (FACT-G) [29] measured participants’ physical, social/family, emotional and functional well-being. Participants were asked to indicate how true each of the items had been for them over the last 7 days on a five-point Likert-scale ranging from 0 (not at all true) to 4 (very much true). Well-being subscale scores were calculated by multiplying the sum of the items from each subscale by the number of items in the subscale and then dividing by the number of items answered with higher scores indicating better QOL.
Global QOL
The 5-item The Satisfaction with Life Scale [30] assessed participants’ agreement with each statement on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). The items are summed to obtain a total score with higher scores representing greater life satisfaction.
Recruitment and Randomization
All study procedures and recruitment materials were approved by the University institutional review board. Participants were recruited using the Army of Women©, University e-mail, fliers, and on-line community groups and postings. After expressing initial interest, women (N = 2546) were e-mailed a link to a full study description. Those meeting eligibility criteria were extended an offer to participate and received an electronic version of the informed consent. Of those who qualified for participation and completed the informed consent (N = 1631), a subgroup of individuals (n=500) were completely randomly assigned using a pre-populated computer algorithm to wear an accelerometer for 7 days at baseline and 6 month follow-up. Women who completed at least half of the study survey at baseline were re-sent a link to the questionnaires at 6 months and women who had at least 3 valid days of accelerometer data at baseline were sent an accelerometer at 6 months. Thus, in the present study, 1527 women were included in the full sample analyses and 370 women were included in the accelerometer subsample analyses.
Data Collection
Women randomized to the survey only group were sent an individualized secure link to the study questionnaires. A study packet was prepared containing an accelerometer, related study materials and a self-addressed stamped envelope for those women randomized to wear the accelerometer. The study survey link was e-mailed to women in the accelerometer subgroup when their accelerometer packet was mailed. All participants were asked to return study materials within two weeks of receipt. Reminder e-mails to the accelerometer subgroup were continued until the accelerometer was returned to study investigators. The same procedures were followed for both groups at 6 months.
Data Analysis
To examine the hypothesized relationships, two separate panel analyses within a covariance framework were conducted in Mplus V6.0 [31]. Such an approach allowed us to examine the hypothesized relationships at baseline and those same relationships among changes in the constructs at 6 months controlling for all other variables in the model. The first panel analysis tested the hypothesized model in all participants whereas the second panel analysis tested the hypothesized model only in the subgroup of participants who had worn the accelerometer to determine whether physical activity measurement influenced model relationships. The following covariates were controlled for in each of the analyses: age, education, income, body mass index, number of comorbidities, time since diagnosis and stage of disease.
As a result of preliminary analyses indicating missing data were missing at random (MAR), full-information maximum likelihood (FIML), was used in the present study [32-34]. At baseline, the extent of missing data ranged from 9.5% (self-reported physical activity) to 15.1% (barriers self-efficacy). Missing data at 6-months ranged from 25.3% (self-reported physical activity) to 28.1% (physical well-being), and was largely the result of loss to follow-up.
The following hypothesized relationships were tested: (a) a direct path from physical activity to self-efficacy; (b) a direct path from self-efficacy to each health status indicator (functional, emotional, social and physical well-being); and (c) a direct path from each health status indicator to global quality of life. Self-efficacy was measured as a latent construct using the total scores from the Barrier Self-efficacy Scale and the Exercise Self-Efficacy Scale as indicators. In the accelerometer subsample, physical activity was modeled as a latent construct with the Godin Leisure Time Exercise Questionnaire total score and average accelerometer counts as indicators. Stability coefficients were calculated [35] to reflect correlations between the same variables measured in each model separately across time while controlling for the influence of other variables in the model.
The chi-square statistic assessed absolute fit of the model to the data [36]. The standardized root mean residual (SRMR) and Comparative Fit Index (CFI) were also used to determine the fit of the model. SRMR values approximating 0.08 or less demonstrate close fit of the model [37, 38] while CFI values of .90 indicate a minimally acceptable fit value and values approximating 0.95 or greater are indicative of a good fit [37].
RESULTS
Participant Characteristics
Prevalence of self-reported comorbidities and data regarding demographic, disease and treatment characteristics at baseline have been reported elsewhere [39]. Briefly, study participants were a nationwide sample of middle-aged (M age= 56.2, SD = 9.4), moderately overweight (M BMI = 26.6, SD = 5.74) breast cancer survivors. The sample consisted of mostly white (97.0%), non-Hispanic/Latino (98.5%) and highly educated women (67.0% had at least a college degree). The majority of women (86.0%) had an annual household income greater than or equal to $40,000 and about three-quarters of the sample reported having at least one comorbid condition (71.4%) with the most commonly reported comorbid conditions being arthritis (33.4%), osteoporosis (18.9%) and asthma (10.3%). Women were, on average, about 7 years post-diagnosis (M = 86.48 months, SD= 71.59) and most were diagnosed with early stage disease (83.6% diagnosed with DCIS, stage 1 or stage 2 disease). The majority of the sample (99.3%) underwent surgery and received chemotherapy (59.0%) and/or radiation therapy (67.7%). Only 3.2% of the sample was currently receiving chemotherapy or radiation, and 10.7% had been diagnosed with a breast cancer recurrence.
Physical Activity and QOL Associations Across Time
The means, standard deviations, and t-values for each of the variables included in the model are displayed in Table 1. Briefly, over the six month study, there was a modest but significant (p < 0.05) decline in exercise self-efficacy and self-reported physical activity in the full study sample. Additionally, physical activity measured by accelerometry also declined in the subsample who wore the monitors.
Table 1.
Descriptives of QOL Model Constructs at Baseline and 6 months
| Variable | Baseline | 6 months | t-value | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Self-efficacy | |||||
| Barriers Self-efficacy | 47.73 | 24.32 | 46.72 | 24.91 | 1.73 |
| Exercise Self-efficacy | 73.78 | 32.60 | 69.92 | 34.76 | 4.56* |
| Health Status | |||||
| Physical Well-being | 23.93 | 4.21 | 24.06 | 4.34 | -1.41 |
| Functional Well-being | 22.26 | 5.03 | 22.46 | 4.88 | -1.66 |
| Social Well-being | 21.65 | 5.42 | 21.68 | 5.73 | -0.22 |
| Emotional Well-being | 19.82 | 3.74 | 19.93 | 3.64 | -1.19 |
| Global Quality of Life | 25.83 | 6.68 | 26.04 | 6.74 | -1.57 |
| Self-reported Physical Activity | 31.72 | 21.21 | 30.41 | 21.97 | 2.17* |
| Objective Physical Activity§ | 25,2219.97 | 16,9805.00 | 21,2765.22 | 9,7755.46 | 4.50* |
Note: All values are for the full sample except for the accelerometer data which is only for the subset who wore the accelerometer;
significant at p=<.05;
Measured as Average Accelerometer Counts
Full sample
The proposed model provided an acceptable fit to the data (χ2 = 657.73; df = 93, p <. 001; CFI = 0.96; SRMR= 0.04). However, a specification search indicated that the model-data fit could be improved by adding a bidirectional correlation between barrier self-efficacy and exercise self-efficacy, independently, at each time point. The addition of these relationships is also consistent with Social Cognitive Theory [40] and previous work examining trajectories of exercise self-efficacy across time [41] and resulted in an improved model-data fit (χ2 = 409.06; df = 91, p <. 001; CFI = 0.98; SRMR= 0.04). This model is shown in Figure 1. The top panel illustrates the relationships among constructs at baseline, whereas the bottom panel reflects relationships among changes in these constructs over the 6-month period while controlling for covariates and all other variables in the model. Overall, the stability coefficients were acceptable and ranged from 0.58 (self-reported physical activity) to 0.66 (self-efficacy and social well-being). The stability coefficients and bi-directional correlations have been omitted from the figure for the sake of clarity.
Figure 1.
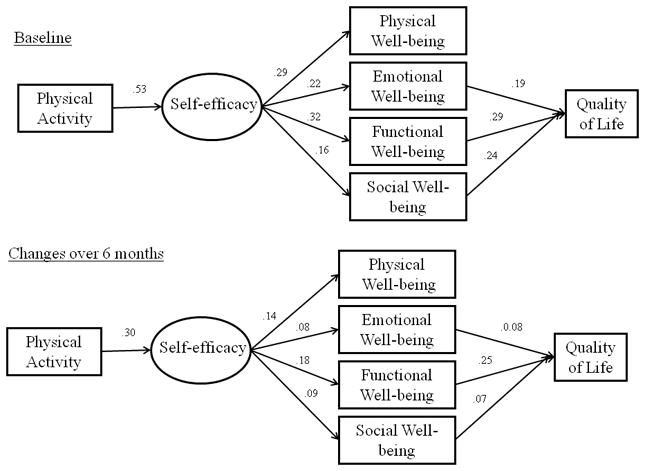
Physical Activity and Quality of Life in Breast Cancer Survivors Model in the Full Sample
Note: Significant paths are represented by solid lines. Non-significant paths and stability coefficients were not included for clarity purposes.
At baseline, more active breast cancer survivors reported significantly (p < 0.05) higher self-efficacy (β = 0.53). In turn, more efficacious women reported significantly higher physical (β = 0.29), emotional (β = 0.22), functional (β = 0.32), and social (β = 0.16) well-being. Finally, women reporting higher levels of emotional (β = 0.19), functional (β = 0.29), and social (β = 0.24) well-being also reported significantly higher global QOL. The direct path from physical well-being to global QOL was not significant. Furthermore, physical activity had statistically significant indirect effects on global QOL via self-efficacy and social, functional and emotional well-being at baseline.
At 6-month follow-up, changes in physical activity were significantly associated with residual changes in self-efficacy (β = 0.30). Changes in self-efficacy were, in turn, significantly related to residual changes in physical (β = 0.14), functional (β = 0.18), emotional (β = 0.08) and social (β = 0.09) well-being. Changes in functional (β = 0.25), emotional (β = 0.08) and social (β = 0.07) well-being were significantly associated with residual changes in global QOL. The indirect effect of changes in physical activity on global QOL via self-efficacy and changes in emotional and social well-being were also significant at follow-up. Overall, the model accounted for 40.7% and 65.7% of the variance in global QOL at baseline and follow-up, respectively.
Accelerometer Subgroup
Next, we tested the veracity of the model in the subsample with accelerometer data. This model also represented a good overall fit to the data (χ2 = 320.96, df = 134, p = <.001; CFI = 0.95; SRMR=0.05) and is shown in Figure 2. Compared to the full sample, the relationships among model constructs at baseline were identical in terms of significance and very similar in terms of magnitude in the accelerometer subgroup. In addition, the majority of the relationships among changes in model constructs were similar to those demonstrated in the total sample. However, the relationship between changes in self-efficacy and residual changes in emotional and social well-being were not significant while the relationship between physical well-being and global QOL became significant in the subsample. Overall, the model explained 49.7% and 64.3% of the variance in global QOL at baseline and follow-up, respectively.
Figure 2.
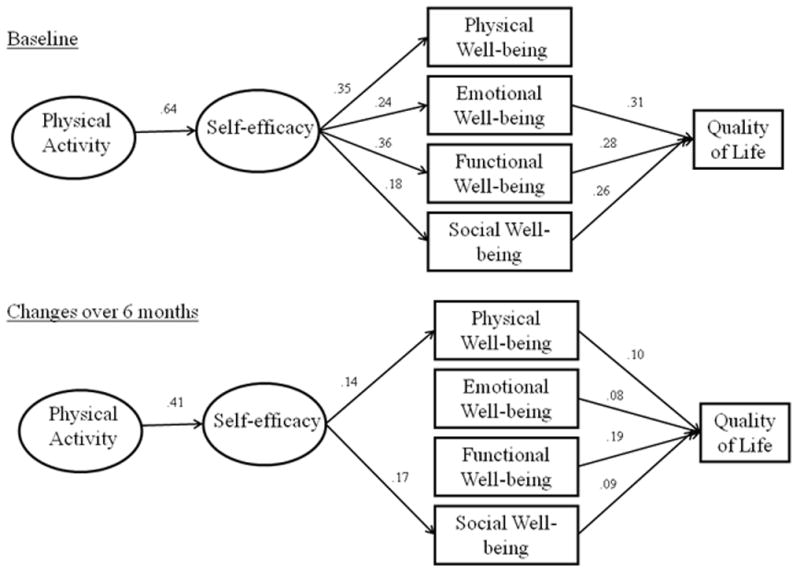
Physical Activity and Quality of Life Model in Breast Cancer Survivors in the Accelerometer subsample
Note: Significant paths are represented by solid lines. Non-significant paths and stability coefficients were not included for clarity purposes.
DISCUSSION
The purpose of this study was to examine the relationship between physical activity, health status and global QOL in a longitudinal sample of breast cancer survivors. McAuley and colleagues’ [17] model was extended to breast cancer survivors using an objective measure of physical activity and breast cancer-specific measures of health status as mediators of the relationship between physical activity and global QOL. Increases in physical activity were associated with increases in self-efficacy which, in turn, were associated with increases in health status indicators which were each associated with increases in global QOL. Thus, these findings provide support for the perspective that: (a) the relationship between physical activity and QOL across time in breast cancer survivors can be understood as incorporating more proximal, modifiable, and temporally sensitive factors (e.g., self-efficacy), as well as more stable and global constructs (e.g., satisfaction with life) and (b) multiple indicators of health status can be used as outcomes of physical activity and predictors of global QOL across time.
That physical well-being was not directly related to global QOL at baseline and did not act as a mediator between physical activity and global QOL at baseline in both groups or at follow-up in the full sample was unexpected. In previous studies, physical health status has mediated the relationship between physical activity and global QOL [17, 21]. One potential explanation for the findings relative to physical well-being is that those items on the FACT-G reflect how one’s “illness” is influencing their life. Because the average time since diagnosis was about 7 years, it is conceivable that this measure contains items that are neither relevant nor salient to these women as longer-term survivors. Consequently, these findings are to be interpreted with caution and other indicators of physical health should be included in future examinations of these relationships in breast cancer survivors. In addition, differences in the significant pathways in the accelerometer subsample may indicate a potential bias resulted as a function of how activity was measured. This may indicate that individuals who self-reported lower levels of health status may underreport their physical activity participation and vice versa. Objective measurement of physical activity reduces such a bias. Further testing of the hypothesized model using both self-report and objective physical activity measures is warranted.
Study findings represent an important first step in examining physical activity and global QOL of life in breast cancer survivors and determining potential mediators of this relationship. For example, it might be argued that health status and global QOL could be modified in breast cancer survivors if both physical activity and self-efficacy are targeted appropriately. Indeed, targeting self-efficacy as part of physical activity programs for breast cancer survivors may not only increase physical activity participation [42], but result in greater improvements in health status, and, in turn, global QOL. Such improvements have implications for the utilization of healthcare services, as well as serving as a motivating factor for the adoption, maintenance and adherence to physical activity programs in breast cancer survivors.
However, as with all studies, this study is not without limitations. First, a longitudinal observational design was used. Randomized controlled exercise trials will be needed to determine whether proposed relationships among changes in model constructs are sustained as a result of an intervention. Second, minimal changes in model constructs were observed over the 6 month period suggesting that either examining the mean values may not fully reflect variation in change over the 6-month study period or 6-months may not be a long enough period to observe mean-level changes in these constructs. Finally, the study sample was largely homogeneous and may not be entirely representative of this population at large. Thus, it is important to examine whether this model holds in other, more diverse samples of breast cancer survivors as well as within subgroups of this population. (e.g., survivors within 5 years of diagnosis versus longer term survivors, older versus younger survivors).
Nonetheless, this study does represent one of the largest, geographically diverse, longitudinal examinations of physical activity and QOL in breast cancer survivors to date. Moreover, we used an objective measure of physical activity. To the best of our knowledge, this is the only study that has examined the potential psychosocial pathways underlying the relationship between physical activity and global QOL in breast cancer survivors. These data underscore the importance of personal efficacy and health status in understanding global QOL in breast cancer survivors. The findings from this study can serve to inform future research and programming in regard to physical activity participation and outcomes in this population.
Future studies should replicate and refine the model presented, by the including additional potential psychosocial constructs associated with health status (e.g., self-esteem, depression, functional limitations, and cognitive function) as well as potential biological mediators of these relationships (e.g. inflammatory biomarkers, hormone levels). Additionally, the optimal time along the breast cancer continuum at which to intervene to maximize QOL benefits of a physical activity program has yet to be determined. Finally, as the demographic landscape of the country changes, it will be imperative to determine how physical activity influences health outcomes in the aging survivor population as older breast cancer survivors have been shown to have physical, social, and psychological health needs that may extend beyond those of the normal aging population [7, 43].
In conclusion, these data provide evidence for the role of self-efficacy and health status indicators in understanding the relationship between physical activity and global QOL in breast cancer survivors. As the population ages, and advances in early detection and treatment progress, the number of breast cancer survivors will continue to increase. Findings from this study indicate physical activity may play an important role in enhancing QOL in breast cancer survivors and can be used to inform future research and programs designed to enhance cancer survivorship.
Acknowledgments
This work was supported by the National Institute on Aging at the National Institutes of Health through award numbers F31AG034025 and AG020118 and the Shahid and Ann Carlson Khan endowed professorship.
Footnotes
Note: All work on this project was completed at the University of Illinois Urbana Champaign.
Conflict of Interest: Neither of the authors have any potential conflicts of interest.
References
- 1.Hewitt ME, Herdman R, Holland JC. Meeting psychosocial needs of women with breast cancer. National Academies Press; Washington D.C: 2004. [PubMed] [Google Scholar]
- 2.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 4.Aziz NM. Cancer survivorship research: State of knowledge, challenges and opportunities. Acta Oncol. 2007;46:417–432. doi: 10.1080/02841860701367878. [DOI] [PubMed] [Google Scholar]
- 5.Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: Challenge and opportunity. Semin Radiat Oncol. 2003;13:248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 6.Baker F, Denniston M, Haffer SC, Liberatos P. Change in health-related quality of life of newly diagnosed cancer patients, cancer survivors, and controls. Cancer. 2009;115:3024–3033. doi: 10.1002/cncr.24330. [DOI] [PubMed] [Google Scholar]
- 7.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97:674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 8.Richardson LC, Wang W, Hartzema AG, Wagner S. The role of health-related quality of life in early discontinuation of chemotherapy for breast cancer. Breast J. 2007;13:581–587. doi: 10.1111/j.1524-4741.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Kenne Sarenmalm E, Odén A, Öhlén J, et al. Changes in health-related quality of life may predict recurrent breast cancer. Eur J Oncol Nurs. 2009;13:323–329. doi: 10.1016/j.ejon.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 11.Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2006;16:104–121. doi: 10.1111/j.1365-2702.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 12.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–65. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 14.Speck RM, Courneya KS, Masse LCl. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 15.Lemieux J, Goodwin PJ, Bordeleau LJ, et al. Quality-of-life measurement in randomized clinical trials in breast cancer: An updated systematic review (2001–2009) J Natl Cancer Inst. 2011;103:178–231. doi: 10.1093/jnci/djq508. [DOI] [PubMed] [Google Scholar]
- 16.Diener E. Subjective well-being. Psychol Bull. 1984;95:542–575. [PubMed] [Google Scholar]
- 17.McAuley E, Konopack JF, Motl RW, et al. Physical activity and quality of life in older adults: Influence of health status and self-efficacy. Ann Behav Med. 2006;31:99–103. doi: 10.1207/s15324796abm3101_14. [DOI] [PubMed] [Google Scholar]
- 18.McAuley E, Doerksen SE, Morris KS. Pathways from physical activity to quality of life in older women. Ann Behav Med. 2008;36:13–20. doi: 10.1007/s12160-008-9036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol B Psychol Sci Soc Sci. 2001;56A:23–35. doi: 10.1093/gerona/56.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 20.Trask PC, Hsu MA, McQuellon R. Other paradigms: Health-related quality of life as a measure in cancer treatment: Its importance and relevance. Cancer J. 2009;15:435–440. doi: 10.1097/PPO.0b013e3181b9c5b9. [DOI] [PubMed] [Google Scholar]
- 21.White SM, Wójcicki TR, McAuley E. Physical activity and quality of life in community dwelling older adults. Health Qual Life Outcomes. 2009 doi: 10.1186/1477-7525-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry C, Kent EE, Mariotto AB, Alfano C, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motl RW, McAuley E, Snook EM, Gliottoni RC. Physical activity and quality of life in multiple sclerosis: Intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14:111–124. doi: 10.1080/13548500802241902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 25.Bassett DR, Ainsworth BE, Swartz AM, et al. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–S480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 26.Tudor-Locke C, Ainsworth BE, Thompson RW, Matthews CE. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc. 2002;34:2045–2051. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 27.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531–43. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 28.McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. J Behav Med. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- 29.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 30.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 31.Muthén LK, Muthén BO. Mplus (Version 6.0) Los Angeles, CA: Muthén & Muthén, Los Angeles 1998-2010; [Google Scholar]
- 32.Arbukle JL. Full information estimation in the presence of incomplete data. In: Marcoulide GA, Schumacker RE, editors. Advanced structural equation modeling: Issues and techniques. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 243–278. [Google Scholar]
- 33.Enders CK. The impact of nonnormality on full information maximum-likelihood estimation for structural equation models with missing data. Psychol Methods. 2001;6:352–70. [PubMed] [Google Scholar]
- 34.Enders C, Bandalos D. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. [Google Scholar]
- 35.Kessler RC, Greenberg DF. Linear panel analysis: Models of quantitative change. New York, NY: Academic Press; 1981. [Google Scholar]
- 36.Jöreskog KG, Sörbom D. LISREL 8: User’s reference guide. Chicago, IL: Scientific Software International; 1996. [Google Scholar]
- 37.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 38.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park: CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 39.Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long‐term breast cancer survivors. Psychooncology. 2013;22:783–91. doi: 10.1002/pon.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandura A. The explanatory and predictive scope of self-efficacy theory. J Soc Clin Psychol. 1986;4:359–4373. [Google Scholar]
- 41.McAuley E, Mailey EL, Mullen SP, Szabo AN, Wójcicki TR, White SM, Gothe N, Olson EA, Kramer AF. Growth trajectories of exercise self-efficacy in older adults: Influence of measures and initial status. Health Psychol. 2011;30:75–83. doi: 10.1037/a0021567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers LQ, Shah P, Dunnington G, et al. Social cognitive theory and physical activity during breast cancer treatment. Oncol Nurs Forum. 2005;32:807–815. doi: 10.1188/05.ONF.807-815. [DOI] [PubMed] [Google Scholar]
- 43.Yancik R. Population aging and cancer: A cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]


