Abstract
Purpose of the Study:
The purpose was to evaluate the effect of various surface treatments and sandblasting with different particle size on the bond strength of feldspathic porcelain with predominantly base metal alloys, using a universal testing machine.
Materials and Methods:
Totally, 40 specimen of nickel-chromium alloy were prepared in an induction casting machine. The groups divided were as follows: Group I-sandblasted with 50 μ Al2O3, Group II-sandblasted with 110 μ Al2O3, Group III-sandblasted with 250 μ Al2O3 and Group IV-sandblasted with 250 μ Al2O3, followed by oxidation and again sandblasted with 250 μ Al2O3. The dimensions of each specimen were adjusted so as to maintain the thickness of ceramic at 1 mm. The specimen were loaded on the assembly of the universal testing machine, and a cross head speed of 0.5 mm/min was used to apply a compressive force at the junction of metal and feldspathic porcelain. The force application continued until adhesive fracture occurred, and the readings of the load applied to that particular specimen were recorded.
Results:
The means for shear bond strength for Group I, II, III and IV were found to be (226.92 ± 1.67), (233.16 ± 3.85), (337.81 ± 16.97) and (237.08 ± 4.33), respectively. Means of shear bond strength among the groups were compared using one-way analysis of variance test. Comparison between individual groups were made with Tukey's Honestly Significant Difference post-hoc test.
Conclusion:
Different particle size and surface treatment have an important role on the bond strength of ceramic-metal interface. Greater particle size demonstrated higher bond strength.
Keywords: Metal-ceramic bond strength, metal-ceramic interface, nickel-chromium alloy, porcelain, surface treatments
INTRODUCTION
Despite extensive use of all ceramic systems today, metal-ceramic restorations are still considered the primary means of restoration due to their superior mechanical strength and their cost effectiveness. A considerable increase in the price of gold during the 1970s resulted in the development of alternative metallic systems. Two decades ago physical properties of nickel-chromium (Ni-Cr) and cobalt-chromium (Co-Cr) base metal alloys were discussed and compared to those found in noble metal alloys.[1] It was concluded that base metal alloys have higher melting temperatures, require more critical handling and care with the melting technique, and are more difficult to finish, when compared to noble metal alloys. Even though base metal alloys have superior mechanical properties including higher modulus of elasticity, sag resistance, longevity, and higher melting temperature, there appears to be an investigative controversy about the bond strength of base metal alloy systems to porcelain.
The metal substructure in a metal-ceramic restoration is ductile, bends under load, and has the ability to return to its original form.[2] The fracture resistance of the metal, in combination with the esthetic nature of porcelain, has provided dentists with both durable and esthetic restorations.[3] The bonding of porcelain to dental alloy occurs during porcelain firing, a process known as sintering. The metal-ceramic bond interface is critical in the functional and esthetic success of metal-ceramic restorations. Four factors contribute to the strength of the metal-ceramic bond which includes chemical bond, mechanical interlocking, vander Waals’ forces and compressive forces. Chemical bond is dictated by the oxide layer formed on the metal substrate that forms metallic, ionic, and covalent bonds with oxides in the opaque ceramic layer. Mechanical interlocking involves the ceramic physically engaging undercuts on the metal substrate surface. Van der Waals’ forces are based on molecular attraction between charges, and compressive forces are those based on the coefficient of thermal expansion.[4,5,6,7]
Once bonded the underlying metal substructure provides support to porcelain thereby increasing the strength of the ceramic and placing its innermost layer adjacent to the metal substructure in compression.[8] The most important of the four mechanisms is the formation of a chemical bond which results in a thermodynamic equilibrium between the metal and the porcelain by the formation of an intermediate oxide layer.[9] The metal-ceramic bond is further enhanced by an actual physical interlocking of the ceramic with the metal. Surface roughness can improve this phenomenon to a certain extent. However, it can also lead to the formation of voids at the interface, which can adversely affect bonding mechanism. To a lesser extent Van der Waals's forces contribute toward metal-ceramic bonding via inter-atomic forces.[10]
Failure of the metal-ceramic bond has been shown to be dependent on many variables, including firing temperature of veneering porcelain and surface textures of the alloy systems.[11] It is known that when a metal-ceramic system is loaded, failure occurs at the areas where bonding is the weakest, so that if the adhesive bond between the ceramic and metal is sufficient, the failure will be cohesive within the ceramic.[12] Factors such as impact and fatigue, occlusal forces, and incompatibility between physical properties of metal and porcelain may result in porcelain fracture, frequently of a cohesive nature.[13,14,15]
Oxidation heat treatment of the metal substructure is used to remove the entrapped gas, eliminate surface contaminants, and form the metallic oxide layer. During porcelain firing cycle before flow of the ceramic begins, the fusing ceramic comes into immediate contact with an oxide layer rather than with a metal surface.[16,17] The fusing ceramic dissolves the oxide originally formed and produces an interaction zone responsible for the formation of a bond.[18] A sandblasted surface may have higher surface energy that allows increased wetting of the metal during ceramic application. However, literature on whether particle size has an effect on the bond strength of porcelain to metal is scanty. Furthermore, it is known that the characteristic of the oxide layer is different before and after sandblasting.[19]
MATERIALS AND METHODS
This article compares shear bond strength of feldspathic porcelain to Ni-Cr alloy when subjected to various surface treatments. The study was conducted at the Department of Prosthodontics and Crown and Bridgework, Faculty of Dental Science, Dharmsinh Desai University, College Road, Nadiad, Gujarat. The testing of specimens was done at the Department of Civil Engineering, Charotar University of Science and Technology, Changa, Gujarat.
FABRICATION OF SPECIMENS
Totally, 40 specimens of Ni-Cr alloy (Bellabond, Bego, Germany) were fabricated with the dimensions 20 mm in length, 10 mm in width, and 5 mm in height. The measurements of specimens corresponded to the specifications of the universal testing machine (TUN 800, Miraj, India) which was subsequently used to test the specimens.
Totally, 40 blocks of inlay casting wax (Surana, India) were fabricated using a custom made metal mould. Heated inlay wax (27°C) was filled in the mould and allowed to cool for the subsequent hour. The set inlay wax pattern was retrieved from the mould, and 2.50 mm wide prefabricated sprue former was attached (Sigma Sprue Wax, Ambernath, India). The wax pattern was invested in a phosphate bonded investment material (Degudent, Dentslpy, Germany). Mixing ratio of the powder and liquid recommended by the manufacturer was followed (mixing ratio 100 g/15 ml). Wet asbestos ring liner (GC, Europe) was used to allow for symmetric expansion. After performing the programmed burnout (850°C) as per the manufacturer's instructions predominantly base metal alloy pellets in the form of Ni-Cr alloy (Bellabond, Bego, Germany) were used for casting process in an induction casting machine (Fornex T, Bego, Germany). The casting ring was allowed to stand for an hour and was retrieved. All forty specimens were prepared in a similar manner. Of these, three castings were found to be incomplete which were discarded, and the whole process was repeated for them.
A single operator carried out the above mentioned process to eliminate the bias and achieve standardization. The specimen was subjected to finishing procedures which involved high-speed rotatory equipment. The specimen were then steam cleaned (EV 1, Silfradent, Italy) and immersed in an ultrasonic cleaner (Zhengzhou Smile Dental Equipment CO., LTD. Henan, China) for 20 min. Specimens were then subjected to sandblasting (Dual Blaster, Germany) with different sized aluminum oxide (Al203) particles. The sandblasting was carried out for 10 s at a distance of 2 cm, under 2 bar pressure and an approximate angulation of 45°.
The specimen (n = 40) were divided into four groups. Each group (n = 10) was sandblasted with a different grit size of aluminum oxide (Bego, Germany).
Thus, the groups divided were as follows:
Group I - Sandblasted with 50 μ aluminum oxide
Group II - Sandblasted with 110 μ aluminum oxide
Group III - Sandblasted with 250 μ aluminum oxide
Group IV - Sandblasted with 250 μ aluminum oxide, followed by oxidation and again sandblasted with 250 μ aluminum oxide.
Group I, II and III were subjected to oxidation and the experimental surfaces were then coated with two thin layer of opaque feldspathic porcelain (Vita Master, Germany) using a brush and fired separately, in a calibrated porcelain vacuum furnace (Ivoclar Vivadent p300) to a temperature of 950°C. Body porcelain (Vita Master) was vibrated and condensed onto this fired opaque layer. The specimens were again fired under vacuum to 930°C, to achieve a thickness of 1 mm of body porcelain.
The thickness of the ceramic layer was measured with a digital vernier caliper (Insize, Japan) [Figure 1]. These measurements were again confirmed with the use of a stereomicroscope (Olympus, India). Glazing was carried out (Vita, Germany) at 910°C in the vacuum furnace (Ivoclar Vivadent p300). All steps and procedures were carried out as per the manufacturer's instructions.
Figure 1.

Digital vernier caliper
TESTING OF SPECIMENS
The universal testing machine [Figure 2] is considered standard equipment for evaluating shear bond strength of two dissimilar materials which are conjoined together. The specimen was loaded on the assembly, and a crosshead speed of 0.5 mm/min was used to apply a compressive force at the junction of metal and feldspathic porcelain. The force application continued until adhesive fracture occurred, and the readings of the load applied to that particular specimen were recorded. The same process was repeated for all the 40 specimens, and all the readings of the load applications were expressed in Megapascal.
Figure 2.

Universal testing machine with specimen
The shear bond strength values of the four groups were statistically analyzed by one-way analysis of variance (ANOVA) and Tukey's Honestly Significant Difference (HSD) post-hoc test.
RESULT
Tables 1 and 2 indicate that the highest mean value of shear bond strength was recorded in Group III (337.81 ± 16.97), followed by Group IV (237.08 ± 4.33), and Group II (233.16 ± 3.85). While the lowest mean value of shear bond strength was obtained for Group I (226.92 ± 1.67). This difference in mean values between groups is clearly demonstrated in Graph 1. The difference between shear bond strength among all groups was found to be statistically highly significant using one-way ANOVA test (P < 0.001) [Table 3]. In order to statistically verify the significance of difference between the individual groups, statistical analysis of the data (mean values) of the different groups using Tukey's HSD post-hoc test was performed in between groups [Table 4]. Here, Group III and Group I, Group III and Group II, and Group IV and Group III indicate that the difference is significant.
Table 1.
Shear bond strength and mean the value of all groups
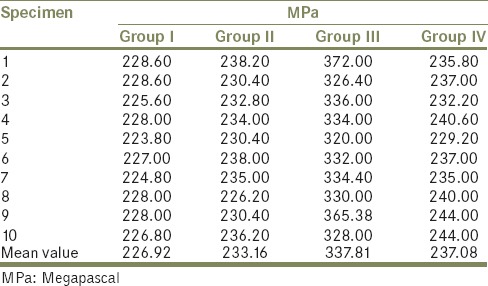
Table 2.
Mean, SD, SE, minimum and maximum load values for each groups
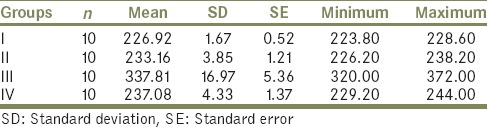
Table 3.
Comparison of shear bond strength among all groups using one-way ANOVA test (P<0.0001)
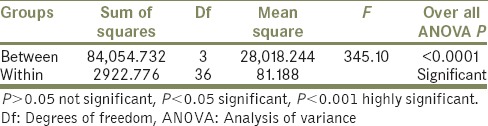
Table 4.
Comparison of shear bond strength between the individual test groups by Tukey's HSD post-hoc test
Graph 1.
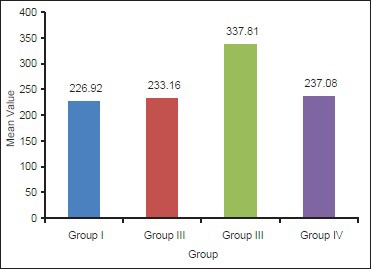
Mean shear bond strength for all groups
DISCUSSION
The purpose of conducting this study was to evaluate the shear bond strength of feldspathic porcelain to Ni-Cr alloy when subjected to various surface treatments which plays an important role in long-term success of metal-ceramic restorations. The study design gives in-depth understanding of the bonding mechanism which is essential for success of metal-ceramic restorations.
It is suggested that the oxide composition and amount of oxide formed are influenced by different surface finishing procedures.[20] An oxide layer forms on the surface of most dental porcelain fused to metal alloys when they were exposed to oxygen at high temperatures. Certain metallic element oxides such as Fe2O3, In2O3, Sn2O3 have been shown to accumulate at the porcelain-metal interface. The concentration of these elements does not necessarily indicate a discrete oxide layer and may merely represent a solution of their ions at the metal porcelain interface.
The fracture strength of repeatedly fired porcelain veneered to high noble and base metal alloy crowns were measured by Barghi et al. (1987). They found that as the number of firings increased, the fracture strength of porcelain veneered to high noble alloys decreased but fracture strength for porcelain veneered to base metal alloy did not change significantly.[18] Sandblasting the finished surface is thought to remove furrows, overlaps and flakes of metal created during the grinding process. A sandblasted surface may have higher surface energy which increases wetting of the metal during ceramic application. Evidence suggests that this roughened surface could also provide mechanical interlocking and increase the surface area for metal-ceramic bonding.[20]
In this study sandblasting with aluminum oxide 50 μ, 110 μ and 250 μ grit particle size was used as a metal surface treatment. The analysis of all the parameters used in assessing the bond strength between metal and porcelain has confirmed that the bond is strongest in the surface treated with sandblasting with 250 μ aluminum oxide particles. This is in contrast to the results obtained by Pietnicki et al. in their study which demonstrated highest bond strength between metal and porcelain achieved with 110 μ aluminum oxide particle sandblasting.[21] It would be noteworthy to mention that a study conducted by Yadav et al. demonstrated that abrasion conducted with particle size 250 μ on titanium castings produced some degree of marginal loss.[22] It should be noted that sandblasting with aluminum oxide particles followed by oxidation and again sandblasting significantly decreased the shear bond strength of the material. According to Brantley et al. sandblasting, has been shown to affect oxide layer thickness. The oxide layer formed before sandblasting differed from the one obtained after sandblasting.[19]
The most reliable evaluation of metal-ceramic bond strength should be based on minimal experimental variables and least residual stresses at metal-ceramic interfaces. Evaluation for types of metal-ceramic failure is critical. Failures can be either cohesive or adhesive. Cohesive failures occur within the layers of ceramic, even though, metal-ceramic bonding is adequate. In the present study, cohesive failures were considered as a success of metal-ceramic bonding mechanism even though it is clinically considered as failure of metal-ceramic restorations as only the bond strength was to be tested. Although laboratory studies offer predictable guidance to a comprehensive selection of materials, clinical longitudinal studies must be encouraged to complement laboratory results and enhance clinical standards of fixed partial denture treatment.[23]
CONCLUSION
Within the limitations of this research, it can be concluded that different surface treatments have an important role in determining bond strength of the metal-ceramic interface.
The different aluminum oxide grit sizes affect the shear bond strength of metal-ceramic alloys.
The shear bond strength is highest when metal alloy is sandblasted with 250 μ aluminum oxide particles.
Sandblasting of casting alloys, followed by oxidation and again sandblasting resulted in reduced bond strength as compared to conventional methods. It should be noted here that this comparison is between specimens sandblasted with 250 μ aluminum oxide particles only. Both Group III and Group IV specimens were sandblasted with 250 μ aluminum oxide particles. The results amply demonstrate that the bond strength reduced when sandblasting, followed by oxidation and again sandblasting was done.
Therefore to achieve adequate bond strength between Ni-Cr alloy and feldspathic porcelain, the alloy surface should be preferably sandblasted with aluminum oxide particles of 250 μ.
ACKNOWLEDGMENTS
I wish to express my sincere thanks to my respected Professor, Guide and Head of the Department, Faculty of Dental Science, Nadiad, Gujarat for providing me guidance throughout the course of this research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Johnson A, Winstanley RB. The evaluation of factors affecting the castability of metal ceramic alloy – investment combinations. Int J Prosthodont. 1996;9:74–8. [PubMed] [Google Scholar]
- 2.Campbell SD. A comparative strength study of metal ceramic and all-ceramic esthetic materials: Modulus of rupture. J Prosthet Dent. 1989;62:476–9. doi: 10.1016/0022-3913(89)90184-4. [DOI] [PubMed] [Google Scholar]
- 3.de Melo RM, Travassos AC, Neisser MP. Shear bond strengths of a ceramic system to alternative metal alloys. J Prosthet Dent. 2005;93:64–9. doi: 10.1016/j.prosdent.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Rosensteil SF, Land M, F. Fujimoto J. Contemporary Fixed Prosthodontics. 4th ed. St Louis, Missouri: Mosby, Elsevier; 2006. pp. 740–73. [Google Scholar]
- 5.Craig RG, Powers JM. Restorative Dental Materials. 2nd ed. St Louis: Mosby; 2002. pp. 576–81. [Google Scholar]
- 6.Shillingburg HT, Hobo S, Whitsett LD, Jacobi R, Brackett S.E. Fundamentals in Fixed Prosthodontics. 3rd ed. Chicago: Quintessence; 1997. pp. 456–7. [Google Scholar]
- 7.Murakami I, Schulman A. Aspects of metal-ceramic bonding. Dent Clin North Am. 1987;31:333–46. [PubMed] [Google Scholar]
- 8.Bagby M, Marshall SJ, Marshall GW., Jr Metal ceramic compatibility: A review of the literature. J Prosthet Dent. 1990;63:21–5. doi: 10.1016/0022-3913(90)90259-f. [DOI] [PubMed] [Google Scholar]
- 9.Pask JA, Fulrath RM. Fundamentals of glass-to-Metal bonding: VIII, Nature of wetting and adherence. J Am Ceram Soc. 1962;45:592–6. [Google Scholar]
- 10.Anusavice KJ. Phillips’ Science of Dental Materials. 10th ed. Philadelphia: Saunders; 1996. pp. 593–606. [Google Scholar]
- 11.Drummond JL, Randolph RG, Jekkals VJ, Lenke JW. Shear testing of the porcelain-metal bond. J Dent Res. 1984;63:1400–1. doi: 10.1177/00220345840630121201. [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer DM, Goldstein GR, Ricci JL, Silva NR, Hittelman EL. Comparison of bond strength of a pressed ceramic fused to metal versus feldspathic porcelain fused to metal. J Prosthodont. 2005;14:239–47. doi: 10.1111/j.1532-849X.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 13.Pameijer CH, Louw NP, Fischer D. Repairing fractured porcelain: How surface preparation affects shear force resistance. J Am Dent Assoc. 1996;127:203–9. doi: 10.14219/jada.archive.1996.0170. [DOI] [PubMed] [Google Scholar]
- 14.Chung KH, Hwang YC. Bonding strengths of porcelain repair systems with various surface treatments. J Prosthet Dent. 1997;78:267–74. doi: 10.1016/s0022-3913(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 15.Kelsey WP, 3rd, Latta MA, Stanislav CM, Shaddy RS. Comparison of composite resin-to-porcelain bond strength with three adhesives. Gen Dent. 2000;48:418–21. [PubMed] [Google Scholar]
- 16.Camacho GB, Vinha D, Panzeri H, Nonaka T, Goncalves M. Surface roughness of a dental ceramic after polishing with different vehicles and diamond pastes. Braz Dent J. 2006;17:145–9. doi: 10.1590/s0103-64402006000300003. [DOI] [PubMed] [Google Scholar]
- 17.Sarac D, Sarac YS, Yuzbasioglu E, Bal S. The effects of porcelain polishing systems on the color and surface texture of feldspathic porcelain. J Prosthet Dent. 2006;96:122–8. doi: 10.1016/j.prosdent.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Mackert JR, Jr, Parry EE, Hashinger DT, Fairhurst CW. Measurement of oxide adherence to PFM alloys. J Dent Res. 1984;63:1335–40. doi: 10.1177/00220345840630111701. [DOI] [PubMed] [Google Scholar]
- 19.Brantley WA, Cai Z, Papazoglou E, Mitchell JC, Kerber SJ, Mann GP, et al. X-ray diffraction studies of oxidized high-palladium alloys. Dent Mater. 1996;12:333–41. doi: 10.1016/s0109-5641(96)80043-1. [DOI] [PubMed] [Google Scholar]
- 20.Hofstede TM, Ercoli C, Graser GN, Tallents RH, Moss ME, Zero DT. Influence of metal surface finishing on porcelain porosity and beam failure loads at the metal-ceramic interface. J Prosthet Dent. 2000;84:309–17. doi: 10.1067/mpr.2000.109488. [DOI] [PubMed] [Google Scholar]
- 21.Pietnicki K, Wolowiec E, Klimek L. The effect of abrasive blasting on the strength of a joint between dental porcelain and metal base. Acta Bioeng Biomech. 2014;16:63–8. [PubMed] [Google Scholar]
- 22.Yadav B, Malhotra P, Nadiger R, Yadav H. Effect of air abrasion on the marginal configuration of titanium crowns after casting. Int J Prosthodont Restor Dent. 2013;3:131–5. [Google Scholar]
- 23.Hammad IA, Talic YF. Designs of bond strength tests for metal-ceramic complexes: Review of the literature. J Prosthet Dent. 1996;75:602–8. doi: 10.1016/s0022-3913(96)90244-9. [DOI] [PubMed] [Google Scholar]


