Abstract
Background
The aim of this study was to analyze the causes that lead to secondary damage of the radial nerve and to discuss the results of reconstructive treatment.
Material/Methods
The study group consisted of 33 patients treated for radial nerve palsy after humeral fractures. Patients were diagnosed based on clinical examinations, ultrasonography, electromyography, or nerve conduction velocity. During each operation, the location and type of nerve damage were analyzed. During the reconstructive treatment, neurolysis, direct neurorrhaphy, or reconstruction with a sural nerve graft was used. The outcomes were evaluated using the Medical Research Council (MRC) scales and the quick DASH score.
Results
Secondary radial nerve palsy occurs after open reduction and internal fixation (ORIF) by plate, as well as by closed reduction and internal fixation (CRIF) by nail. In the case of ORIF, it most often occurs when the lateral approach is used, as in the case of CRIF with an insertion interlocking screws. The results of the surgical treatment were statistically significant and depended on the time between nerve injury and revision (reconstruction) surgery, type of damage to the radial nerve, surgery treatment, and type of fixation. Treatment results were not statistically significant, depending on the type of fracture or location of the nerve injury.
Conclusions
The potential risk of radial nerve neurotmesis justifies an operative intervention to treat neurological complications after a humeral fracture. Adequate surgical treatment in many of these cases allows for functional recovery of the radial nerve.
MeSH Keywords: Peripheral Nerve Injuries, Radial Nerve, Suture Techniques
Background
Radial nerve injury is the most common damage to the peripheral nervous system associated with shaft fractures of long bones [1]. Approximately 1% to 3% of all fractures are fractures of the humerus [2]. Radial nerve palsy (RNP) occurs in 2% to 17% of the cases in this group [3].
RNP may be either partial or complete, and complete motor loss occurs in approximately 50% of cases [3,4]. Depending on the time of occurrence, radial nerve injuries can be divided into primary and secondary [4]. In primary nerve palsy, loss of function occurs at the time of injury and is associated with closed fractures. In secondary nerve palsy, loss of function appears during the course of treatment [5]. It occurs after conservative treatments, such as manipulation, impingement by or between fracture fragments, entrapment by a fracture callus, and scar tissue formation, as well as after surgery (iatrogenic nerve palsy) [6]. Iatrogenic radial nerve palsy may be a result of plate fixation or intramedullary nailing treatment. It is estimated that damage to the radial nerve occurs in 4% to 32% of patients who undergo surgical treatments to stabilize a fracture [7].
The high risk of radial nerve damage is associated with the very complicated anatomy in the area of the nerve. The radial nerve arises from the ventral branches of the nervi spinalis C7–Th1. It then creates the posterior cord of the brachial plexus [8]. Together with the accompanying vessels, it then proceeds from the medial side to the side of the posterior surface of the humerus in the groove of the radial nerve, and on the border of the middle and distal 1/3 of the humeral shaft turns to the side-arm front surface [9].
However, studies have shown a very large variability in the course of the nerve. The most-used landmarks are the acromion and lateral and medial epicondyle. The distance between the acromion and the point of entry to the groove of the radial nerve is estimated to be from 10 cm to 19 cm, whereas the distance between the exit point of the groove of the radial nerve and the lateral epicondyle is from 6 cm to 16 cm. [10]. Other studies have indicated a higher course for the radial nerve, from the proximal humerus (53% of the humeral length) at 12.0±2.3 cm (range, 7.4–16.6 cm) and from the olecranon fossa (36% of the humeral length) at 16.0±0.4 cm (range, 9.0–20.5 cm) [8,12].
The large anatomical variability in the course of the nerve, which increases the risk of damage to the radial nerve, affects both the surgical access and type of stabilization. This is why many different surgical approaches, including anterior, anterolateral, lateral, posterior, and modified approaches, have been used to expose the humerus and to complete fixation [8].
The aim of the present study was to analyze the causes that lead to secondary damage of the radial nerve and to discuss the results of reconstructive treatments.
Material and Methods
The study group consisted of 33 patients treated for radial nerve palsy after humerus fractures during 2007–2013. The ages of the patients ranged from 19 to 67 years (mean age 41). The study group included 12 (36%) women and 21 (64%) men. In 19 (57%) cases, surgery was performed on the right arm and in 14 (43%) cases on the left arm. Initially, the patients were treated outside our clinic and were sent to our clinic because it is a center for peripheral nerve surgery treatment.
Of the patients with a humeral fracture with an associated RNP, 11 patients had an injury as a result of a traffic accident, 10 from a sports injury, and 12 from a fall onto the same level.
The fractures were localized in the middle humerus, at the mid-third/distal-third junction, and at the proximal-third/mid-third junction. An analysis of X-ray images revealed spiral (12 A 1, B 1), oblique (12 A 2, B 2), transverse (12 A 3), and comminuted (12 C 3) fracture patterns according to AO classification.
Inclusion criteria for our study were a closed humerus fracture, confirmed proper function of the radial nerve after the fracture, and a complete palsy of radial nerve function after conservative treatment or surgery. Patients were diagnosed based on clinical examinations, ultrasonography (US), electromyography (EMG), or nerve conduction velocity (NCV). During each operation, the location and type of nerve damage were analyzed. We determined the location of the radial nerve based on where it leaves the spiral groove distally, which depends on the lateral and medial epicondyle. The following techniques were used as treatment: releasing the nerve (neurolysis), direct neurorrhaphy, and reconstruction using a nerve graft (sural nerve).
The strength of the muscles (triceps, wrist and digit extensors, and supinator) was evaluated using the Medical Research Council (MRC) scales, where grade 0 corresponds to no movement and grade 5 to normal muscle contraction against full resistance [13], and the quick DASH score. The mean follow-up time was 4.6 years (range, 2–7 years) after surgery. The results were analyzed according to the type of damage to the radial nerve, time from injury to second surgery, type of reconstructive injury, localization of the radial nerve injury, type of fracture, and type of stabilization during the first operation.
Results
Based on surgical reports from other centers, the radial nerve was exposed and protected in 10 cases. No cases of intraoperative injury were observed. The failure of the function of the radial nerve was observed for up to 6 h after the treatment. One superficial wound infection occurred, but it did not require any treatment.
During the revision operation, a lacerated nerve or entrapment in a callus was found (Figure 1A). In other cases, radial nerve neurotmesis with a gap of less than 1 cm (Figure 2A) or more than 1 cm (Figure 3A) was observed. Analysis of causes of damage to the radial nerve, type of injury, type of treatment, surgical approaches, visualized nerve, and stabilization methods during the first operation are presented in Table 1.
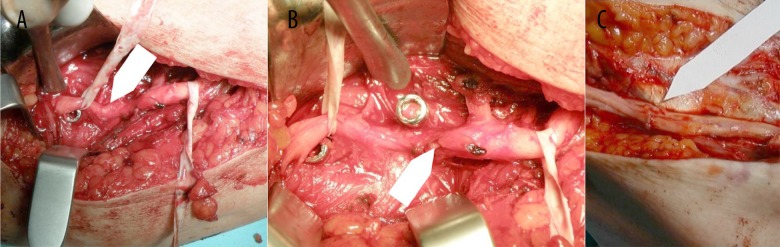
Figure 1.
Intraoperative image. (A) Radial nerve entrapment by a newly bony callus. (B) Radial nerve neurolysis.
Figure 2.
Intraoperative image. (A) Radial nerve neurotmesis using a locking screw. (B) Nerve rupture with a gap <1 cm. (C) Direct neurorrhaphy.
Figure 3.
Intraoperative image. (A) Radial nerve neurotmesis by plate. (B) Nerve rupture with a gap >1 cm, (C) Reconstruction with a sural nerve graft − 5 cm.
Table 1.
Analysis of causes damage to the radial nerve, type of injury, type of treatment, surgical approaches, visualized nerve and stabilization methods during first operation.
| Type of injury | The number of patients | Type of fracture treatment | Approach | Visualized nerve | Localization of injury | Probable cause of injury |
|---|---|---|---|---|---|---|
| Entrapment | 4 | Conservative treatment | No | At the level of fracture | Newly formed callus | |
| 2 | ORIF | Lateral | Yes | Under a plate | Compression by plate | |
| 2 | ORIF | Lateral | Yes | Between the bone fragments | Newly formed callus | |
| 1 | ORIF | Posterior | Yes | Under a plate | Compression by plate | |
| 2 | CRIF | Antegrade nail | Yes | At the level of the fracture | Newly formed callus | |
| Rupture with a gap <1 cm | 2 | ORIF | Lateral | Yes | Under a plate | Compression by plate |
| 1 | ORIF | Posterior | Yes | Under a plate | Compression by plate | |
| 4 | CRIF | Antegrade nail | No | Interlocking screw, 3–4 cm from lateral epicondyle | Insertion interlocking screw from lateral side | |
| 2 | CRIF | Antegrade nail | No | Interlocking screw, 4–5 cm from lateral epicondyle | Insertion interlocking screw from anterior side | |
| Rupture with a gap >1 cm | 6 | ORIF | Lateral | No | Under a plate, 2 cm deficit | Compression by plate |
| 1 | ORIF | Posterior | No | At the level of the fracture, 3 cm deficit | Reduction forceps | |
| 1 | ORIF | Lateral | No | At the level of the fracture, 2 cm deficit | Compression by fracture | |
| 1 | ORIF | Posterior | No | At the level of the fracture, 3 cm deficit | Compression by fracture | |
| 3 | CRIF | Antergrade nail | No | At the level of the fracture, 8 nerve deficit | Reaming of the medullary canal | |
| 2 | CRIF | Antergrade nail | No | At the level of the fracture, 4 cm deficit | Compression by fracture |
During surgery, neurolysis (Figure 1B), direct neurorrhaphy (Figure 2B, 2C), or reconstruction with a nerve graft was performed (Figure 3B, 3C).
A full return of function was observed in 18 patients and 11 patients achieved partial return of function. A response from the radial nerve was absent in 4 patients. In all 33 patients, a clinical and radiological union occurred at a mean of 8 weeks (range, 7–12 weeks). In the 4 patients who did not achieve satisfactory improvement in functional recovery of the radial nerve, a tendon transposition was performed at 12 months after the nerve graft. These patients had satisfactory results after 2 years. One of these patients was initially treated conservatively because the injury was in the middle third of the humerus. The 3 other patients had a mid-shaft spiral fracture with radial nerve neurotmesis treated by use of an intramedullary nail.
When we analyzed the time between nerve injury and reconstruction surgery, the results observed for operations performed with less than 6 weeks between the injury and second surgery were significantly better than those observed for operations performed after 12 weeks (MRC: median 5 vs. 1, p<0.001; DASH: median. 2.25 vs. 75.0, p=0.006) (Table 2). The best results were reported in groups treated less than 6 weeks after the radial nerve injury.
Table 2.
Post-treatment follow-up in MRC score and DASH scale with respect to the time between nerve injury and reconstruction surgery, type of damage to the radial nerve, surgery treatment, type of fracture, and type of fixation.
| Number of patients | MRC | DASH | ||||
|---|---|---|---|---|---|---|
| X±SD Me (range) |
p | X±SD Me (range) |
p | |||
| Time from injury to second operation | <6 weeks | 10 (30%) | 4.7±0.5 5 (4–5)a |
0.001** | 5.67±8.24 2.25 (0–25.0)a |
0.006** |
| Between 6–12 weeks | 12 (37%) | 3.7±1.2 4 (1–5) |
20.73±22.05 12.5 (0–75.0) |
|||
| Between 12–18 weeks | 11 (33%) | 2.0±1.8 1 (0–5)a |
51.10±32.89 75.0 (0–79.5)a |
|||
| Type of damage to the radial nerve | Entrapment | 11 (33%) | 4.7±0.5 5 (4–5)a |
<0.001** | 1.59±2.21 0 (0–4.5)a |
<0.001** |
| Rupture with a gap <1 cm | 8 (24%) | 3.9±1.0 4 (2–5) |
16.39±13.81 12.5 (0–42.5) |
|||
| Rupture with a gap >1 cm | 14 (43%) | 2.1±1.7 2 (0–5)a |
51.36±28.42 67.05 (3.0–79.5)a |
|||
| Type of fracture | Oblique | 9 (27%) | 3.8±1.3 4 (1–5) |
0.629** | 18.18±25.73 4.50 (0–75.0) |
0.587** |
| Transverse | 2 (6%) | 3.0±0 3 (3–3) |
34.10±0 34.1 (34.1–34.1) |
|||
| Spiral | 12 (37%) | 3.6±1.8 4 (0–5) |
27.28±33.27 10.25 (0–77.3) |
|||
| Comminuted | 10 (30%) | 3.0±2.1 4 (0–5) |
30.85±32.94 17.05 (0–79.5) |
|||
| Surgery treatment | Neurolysis | 11 (33%) | 4.7±0.5 5 (4–5)a |
<0.001** | 1.59±2.21 0 (0–4.5)a |
<0.001** |
| Direct neurorrhaphy | 7 (21%) | 4.0±0.6 4 (3–5) |
21.33±21.20 13.6 (0–59.1) |
|||
| Reconstruction with nerve graft | 15 (46%) | 2.2±1.8 2 (0–5)a |
46.72±29.62 34.1 (3.0–79.5)a |
|||
| Type of fixation | Plate | 16 (48%) | 3.7±1.5 4 (1–5) |
0.025* | 21.78±27.51 10.25 (0–75.0) |
0.022* |
| Intramedullary nail | 13 (39%) | 2.2±2.1 2 (0–5) |
44.52±35.74 59.1 (0–79.5) |
|||
X – mean; Me – median; SD – standard deviation;
Mann-Whitney U-test;
Kruskal-Wallis test;
pairwise comparison with p<0.005 following the Kruskal-Wallis test.
The outcome of the treatment depended on the type of damage to the radial nerve. The patients with entrapment of the radial nerve had significantly better results than those with radial nerve neurotmesis (MRC: median 5 vs. 2, p<0.001; DASH: median 0 vs. 67.05, p<0.001), but not when we compared the results of the groups with a lesion of less than 1 cm with the results of groups with lesions greater than 1 cm (Table 2).
The results of the surgical treatment were significantly different in patients with neurolysis compared to reconstruction with a sural nerve graft (MRC: median 5 vs. 2, p<0.001; DASH: median 0 vs. 34.1, p<0.001). The results after a direct neurorrhaphy were better than after neurorrhaphy with reconstruction. However, the difference was not statistically significant (Table 2).
Regarding the type of fracture, there were no statistically significant differences among the spiral, oblique, transverse, and comminuted groups. However, the best results were observed in the groups with oblique and spiral fractures (Table 2).
The difference between the ORIF and CRIF fixation methods were statistically significant (MRC: median 4 vs. 2, p<0.025; DASH: median 10.25 vs. 59.1, p<0.022) (Table 2). Complications after using intramedullary nails were more significant. Tenomyoplasty surgery was required in 3 cases in which intramedullary nails were used.
The location at which the radial nerve leaves the spiral groove distally depends on the lateral epicondyle, which was 11.5±3.5 cm, and on the lateral and medial epicondyle diameters, which were 2.5 ±1 times greater.
The mean time to initial radial nerve recovery after the revision operation was 8.3 weeks (range, 6 weeks to 6.6 months), and the mean time to recover full function was 6.1 months (range, 3.4–12 months).
Discussion
The problem of radial nerve palsy after the treatment of a humeral fracture is not uncommon. Treatments can involve either ORIF or CRIF. In cases of ORIF, nerve injury could exist at the level of the fracture, under and on a plate, as well as when a lateral or posterior approach is used. Newly formed callus, reduction techniques (e.g., use of clamps, forceps, or hooks), compression or nerve rupture by plate, or compression by fracture were the causes of this injuries. On the other hand, CRIF can occur at the level of the fracture or interlocking screw and may the cause of the newly formed callus, reaming of the medullary canal, compression by fracture, or insertion interlocking screw from lateral or anterior side.
Several studies have compared the incidence rates of radial nerve palsy between plate fixation and intramedullary nailing [14–18]. Because of the consistent results in the literature [19], a fixed-effects model was performed, which showed that the difference in radial nerve damage between these 2 groups was not significant [20,21]. However, the above work did not refer to the severity of damage. In our study the outcomes show that damage with the CRIF was more significant than with the ORIF. In contrast to those studies, we found statistically significant differences. On the other hand, we examined already damaged nerves without studying the population that had been treated or how the damage occurred (although treatment was performed by qualified people from various centers, no data on the number of complications in these centers were available). In our opinion, better results for ORIF are associated with less damage (i.e., more frequent entrapment and minor nerve deficit) and a faster decision on the revision of the nerve, compared to CRIF.
Some authors stressed that for cases using ORIF, surgeons should explore the nerve to avoid damage, while others emphasized that exposure and protection of the nerve does not guarantee avoidance of nerve injury and may cause fibrosis around the nerve in a small number of cases [22]. In our opinion, visualizing the nerve without separating it from the surrounding tissue significantly reduces the risk of damage. In a retrospective analysis of operational protocols from the first surgery, this technique was not routinely used. In our opinion, this is useful especially because of the wide range of variability in anatomic relationships.
As regards the type of damage to the radial nerve and surgery treatment, the best results were obtained when treated by entrapment neurolysis, in contrast to the damage of the gap >1 cm treated using the sural nerve. One explanation might be the type of nerve injury and nerve regeneration process. At the beginning of the regeneration nerve process directly after injury, chromatolysis and swelling take place in the cell body and nucleus [23,24], after which, Wallerian degeneration (axonal and myelin disintegration) proceeds both in a distal (antegrade) and proximal (retrograde) direction [23,24]. Antegrade Wallerian degeneration then continues with Schwann cells and macrophage infiltration to remove cell debris, leaving only the basement membrane for about 3–6 weeks [23,24]. In subsequent stages, Schwann cells start to proliferate and guide the axonal sprouts between the basement membranes of the 2 nerve ends [23,24]. The difference is that for entrapment, only the axon is affected and Wallerian degeneration appears in the distal part of the nerve (axonotmesis). In case of interruption (neurotmesis), Wallerian degeneration takes place in both antegrade and retrograde directions.
To analyze the variable course of the distal radial nerve, we compared our results with the most prominent landmark bone points (the lateral and medial epicondyle) [25,26]. The results were comparable with anatomic studies of these points in which the distal extent of the radial nerve in the spiral groove was 12.6±1.1 cm proximal to the lateral epicondyle of the humerus [27], and along the posterior aspect of the humerus from 20.7±1.2 cm proximal to the medial epicondyle [28]. In addition, we studied the position of the nerve in relation to the distance between the 2 epicondyles. Our results also emphasize considerable variability in nerve position, which may, in our opinion, cause damage.
In the case of intramedullary nails, the risk of damage to the radial nerve occurs during repositioning, drilling, and distal locking. Some authors have emphasized the advantages of locking from the side, while others have encouraged locking from the front. In cases of proximal interlocking in the frontal and sagittal planes, both branches of the axillary nerve can be damaged [29,30]. Screw insertion in the oblique position is considered potentially less hazardous. The theoretically high risk notwithstanding, only a few cases of iatrogenic injury to these nerves in anterograde and 1 case in retrograde interlocking IM nailing have been described to date [31]. Antero-posterior distal locking is considered as safer. However, the risk of injury to the musculocutaneous nerve is well recognized. Two cases of this type are described in the scientific literature [32]. Because of the risk of damage during locking, we recommend exposing the bone surface and locking under direct vision.
In the present study, the subsequent stages of the radial nerve treatment, from fracture to final results, were analyzed. It can be assumed that the type of fracture influences the type of fixation, the type of fixation influences the severity of the nerve lesion, the severity of the nerve injury influences the reconstruction technique, and the reconstruction technique influences the functional results.
Although the results of the secondary radial nerve palsy treatment were analyzed, we also analyzed the type of fracture because choice of fracture treatment method depends on it. However, the final results were not statistically significant, depending on type of fracture.
Another aspect is the diagnosis of radial nerve palsy after surgery. Neurophysiologic testing (electromyography and nerve conduction velocity) may be useful for characterizing both the level and the extent of nerve dysfunction. However, testing should be performed at a minimum of 4 weeks after an injury. These studies are more useful in assessing the return of nerve function. The brachioradialis and extensor carpi radialis are the first muscles to be reinnervated, and the extensor indicis proprius is the last muscle to recover. Complete recovery typically occurs within 6 to 12 months [33].
Diagnostics of radial nerve damage can complete an ultrasound examination, although the effectiveness of this protocol is debated. Some studies reported success using high-resolution ultrasound to evaluate the injured radial nerve, but others reported that the role of ultrasound has yet to be properly determined and cannot be used as part of an exemplary algorithm study [34,35].
Indications for further intervention after radial nerve palsy after a first operation are unclear. Nerve function often spontaneously recovers and a lack of clear markers of nerve damage makes the decision to re-explore difficult.
In the treatment of the radial nerve palsy there is no single algorithm for treatment.
The choices are no exploration, early exploration, or late exploration.
No exploration can be generally applied in closed fractures, where most often there is no interruption of the nerve because spontaneous recovery after such injuries is reported to occur in more than 70% of patients. Other studies show functional recovery but not full recovery in nearly 90% of patients. This has been confirmed by other studies that show radial nerve palsy is caused by a nerve contusion [36].
Early exploration has been advocated due to concerns, especially of iatrogenic nerve entrapment [37]. However, a review of published series demonstrated that the rate of spontaneous recovery is comparable to that of primary radial nerve palsy following humeral shaft fractures [38]. Although limited, the literature supports nonsurgical management of a patient with a humeral shaft fracture and secondary radial nerve palsy.
Early exploration may not be indicated in every case, but it allows for the assessment of the degree of damage apart from entrapment. Additionally, if the nerve is lacerated, quick repair after the injury allows tension reduction and promotes healing. Furthermore, if a nerve is ruptured with a large defect and reconstruction cannot be performed, nerve grafting or tendon transfer can be used at the beginning as a method of treatment [39]. It is evident that early exploration makes an operation easier and safer. Some studies also suggest that functional nerve recovery is more complete and consistent with this approach.
Late exploration is not the first-choice method of treatment and remains controversial. Entrapment during late exploration ranges from 6% to 25% [40] and nerve laceration in 20% to 42% of cases is observed; however, late exploration can allow for spontaneous return of function, thus avoiding an unnecessary operation. In addition, delayed surgery may allow the neurilemmal sheath to thicken, which facilitates repair if a neurorrhaphy is needed [41]. In contrast, delayed surgical intervention can include scarring, which can result in difficulty with nerve preparation [3].
Our study also showed better results in cases of early exploration. We believe that the risk of a bad result from the postponement of an operation justifies early exploration in cases of uncertain nerve damage.
We realize that the main limitation of this study is in the analysis of results of the EMG and NCV. The studies did not follow a set protocol, which did not allow us to carry out a statistical analysis. The most common description contains the conclusion “incomplete radial nerve palsy”. However, we believe that the most important is clinical examination; therefore, we have used quantitative (i.e., full return of function, partial improvement, no improvement), not qualitative, evaluation criteria of EMG and NCV.
Conclusions
Surgical techniques are associated with the risk of secondary radial nerve palsy, due in part to the large anatomical variability. The potential risk of radial nerve neurotmesis justifies an operational intervention in the treatment of neurological complications after a humeral fracture. Adequate surgical treatment in many of these cases allows for functional recovery of the radial nerve.
Acknowledgments
We thank Bartosz Witkowski who provided medical writing service on behalf of Wrocław Medical University.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
Source of support: Departmental sources
References
- 1.Li Y, Ning G, Wu Q, et al. Review of literature of radial nerve injuries associated with humeral fractures-an integrated management strategy. PLoS One. 2013;8:e78576. doi: 10.1371/journal.pone.0078576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekholm R, Adami J, Tidermark J, et al. Fractures of the shaft of the humerus. An epidemiological study of 401 fractures. J Bone Joint Surg Br. 2006;88:1469–73. doi: 10.1302/0301-620X.88B11.17634. [DOI] [PubMed] [Google Scholar]
- 3.DeFranco MJ, Lawton JN. Radial nerve injuries associated with humeral fractures. J Hand Surg Am. 2006;31:655–63. doi: 10.1016/j.jhsa.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Shah A, Jebson PJ. Current treatment of radial nerve palsy following fracture of the humeral shaft. J Hand Surg Am. 2008;33:1433–34. doi: 10.1016/j.jhsa.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Larsen LB, Barfred T. Radial nerve palsy after simple fracture of the humerus. Scand J Plast Reconstr Surg Hand Surg. 2000;34:363–66. doi: 10.1080/028443100750059156. [DOI] [PubMed] [Google Scholar]
- 6.Shao YC, Harwood P, Grotz MR, et al. Radial nerve palsy associated with fractures of the shaft of the humerus: A systematic review. J Bone Joint Surg Br. 2005;87:1647–52. doi: 10.1302/0301-620X.87B12.16132. [DOI] [PubMed] [Google Scholar]
- 7.Bumbasirević M, Lesić A, Bumbasirević V, et al. The management of humeral shaft fractures with associated radial nerve palsy: A review of 117 cases. Arch Orthop Trauma Surg. 2010;130:519–22. doi: 10.1007/s00402-009-0951-4. [DOI] [PubMed] [Google Scholar]
- 8.Arora S, Goel N, Cheema GS, et al. A method to localize the radial nerve using the ‘apex of triceps aponeurosis’ as a landmark. Clin Orthop Relat Res. 2011;469:2638–44. doi: 10.1007/s11999-011-1791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamineni S, Ankem H, Patten DK. Anatomic relationship of the radial nerve to the elbow joint: Clinical implications of safe pin placement. Clin Anat. 2009;22:684–88. doi: 10.1002/ca.20831. [DOI] [PubMed] [Google Scholar]
- 10.Fleming P, Lenehan B, Sankar R, et al. One-third, two-thirds: Relationship of the radial nerve to the lateral intermuscular septum in the arm. Clin Anat. 2004;17:26–29. doi: 10.1002/ca.10181. [DOI] [PubMed] [Google Scholar]
- 11.Bono CM, Grossman MG, Hochwald N, Tornetta P., III Radial and axillary nerves. Anatomic considerations for humeral fixation. Clin Orthop Relat Res. 2000;(373):259–64. [PubMed] [Google Scholar]
- 12.Claessen FM, Peters RM, Verbeek DO, et al. Factors associated with radial nerve palsy after operative treatment of diaphyseal humeral shaft fractures. J Shoulder Elbow Surg. 2015;24:e307–11. doi: 10.1016/j.jse.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Paternostro-Sluga T, Grim-Stieger M, Posch M, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40:665–71. doi: 10.2340/16501977-0235. [DOI] [PubMed] [Google Scholar]
- 14.Bichsel U, Nyffeler RW. Secondary radial nerve palsy after minimally invasive plate osteosynthesis of a distal humeral shaft fracture. Case Rep Orthop. 2015;2015:241968. doi: 10.1155/2015/241968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallucci GL, Boretto JG, Alfie VA, et al. Posterior minimally invasive plate osteosynthesis (MIPO) of distal third humeral shaft fractures with segmental isolation of the radial nerve. Chir Main. 2015;34:221–26. doi: 10.1016/j.main.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Pailhé R, Mesquida V, Rubens-Duval B, Saragaglia D. Plate osteosynthesis of humeral diaphyseal fractures associated with radial palsy: twenty cases. Int Orthop. 2015;39:1653–57. doi: 10.1007/s00264-015-2745-3. [DOI] [PubMed] [Google Scholar]
- 17.Liu GD, Zhang QG, Ou S, et al. Meta-analysis of the outcomes of intramedullary nailing and plate fixation of humeral shaft fractures. Int J Surg. 2013;11:864–68. doi: 10.1016/j.ijsu.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang H, Xiong J, Xiang P, et al. Plate versus intramedullary nail fixation in the treatment of humeral shaft fractures: An updated meta-analysis. J Shoulder Elbow Surg. 2013;22:387–95. doi: 10.1016/j.jse.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Putti AB, Uppin RB, Putti BB. Locked intramedullary nailing versus dynamic compression plating for humeral shaft fractures. J Orthop Surg (Hong Kong) 2009;17:139–41. doi: 10.1177/230949900901700202. [DOI] [PubMed] [Google Scholar]
- 20.Raghavendra S, Bhalodiya HP. Internal fixation of fractures of the shaft of the humerus by dynamic compression plate or intramedullary nail: A prospective study. Indian J Orthop. 2007;41:214–18. doi: 10.4103/0019-5413.33685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singisetti K, Ambedkar M. Nailing versus plating in humerus shaft fractures: a prospective comparative study. Int Orthop. 2010;34:571–76. doi: 10.1007/s00264-009-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Wang C, Wang M, et al. Postoperative malrotation of humeral shaft fracture after plating compared with intramedullary nailing. J Shoulder Elbow Surg. 2011;20:947–54. doi: 10.1016/j.jse.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Griffin MF, Malahias M, Hindocha S, Khan WS. Peripheral nerve injury: Principles for repair and regeneration. Open Orthop J. 2014;8:199–203. doi: 10.2174/1874325001408010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deumens R, Bozkurt A, Meek MF, et al. Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol. 2010;92:245–76. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Heineman DJ, Poolman RW, Nork SE, et al. Plate fixation or intramedullary fixation of humeral shaft fractures. Acta Orthop. 2010;81:216–23. doi: 10.3109/17453671003635884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zogbi DR, Terrivel AM, Mouraria GG, et al. Fracture of distal humerus: MIPO technique with visualization of the radial nerve. Acta Ortop Bras. 2014;22:300–3. doi: 10.1590/1413-78522014220601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Y, Li YW, Zhang HB, et al. Management of humeral shaft fractures with intramedullary interlocking nail versus locking compression plate. Orthopedics. 2015;38:e825–29. doi: 10.3928/01477447-20150902-62. [DOI] [PubMed] [Google Scholar]
- 28.Gerwin M, Hotchkiss RN, Weiland AJ. Alternative operative exposures of the posterior aspect of the humeral diaphysis with reference to the radial nerve. J Bone Joint Surg Am. 1996;78:1690–95. doi: 10.2106/00004623-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Baltov A, Mihail R, Dian E. Complications after interlocking intramedullary nailing of humeral shaft fractures. Injury. 2014;45:S9–S15. doi: 10.1016/j.injury.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Prince EJ, Breien KM, Fehringer EV, Mormino MA. The relationship of proximal locking screws to the axillary nerve during antegrade humeral nail insertion of four commercially available implants. J Orthop Trauma. 2004;18:585–88. doi: 10.1097/00005131-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Lögters TT, Wild M, Windolf J, Linhart W. Axillary nerve palsy after retrograde humeral nailing: clinical confirmation of an anatomical fear. Arch Orthop Trauma Surg. 2008;128:1431–35. doi: 10.1007/s00402-008-0607-9. [DOI] [PubMed] [Google Scholar]
- 32.Blyth MJ, Macleod CM, Asante DK, Kinninmonth AW. Iatrogenic nerve injury with the Russell-Taylor humeral nail. Injury. 2003;34:227–28. doi: 10.1016/s0020-1383(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen NO, Dahlin LB. Injury to the radial nerve caused by fracture of the humeral shaft: timing and neurobiological aspects related to treatment and diagnosis. Scand J Plast Reconstr Surg Hand Surg. 2007;41:153–57. doi: 10.1080/02844310701445586. [DOI] [PubMed] [Google Scholar]
- 34.Bodner G, Buchberger W, Schocke M, et al. Radial nerve palsy associated with humeral shaft fracture: evaluation with US – initial experience. Radiology. 2001;219:811–16. doi: 10.1148/radiology.219.3.r01jn09811. [DOI] [PubMed] [Google Scholar]
- 35.Wang JP, Shen WJ, Chen WM, et al. Iatrogenic radial nerve palsy after operative management of humeral shaft fractures. J Trauma. 2009;66:800–3. doi: 10.1097/TA.0b013e31816142cf. [DOI] [PubMed] [Google Scholar]
- 36.Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44:419–24. doi: 10.1016/j.ocl.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Ring D, Chin K, Jupiter JB. Radial nerve palsy associated with high-energy humeral shaft fractures. J Hand Surg Am. 2004;29:144–47. doi: 10.1016/j.jhsa.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Pidhorz L. Acute and chronic humeral shaft fractures in adults. Orthop Traumatol Surg Res. 2015;101:S41–49. doi: 10.1016/j.otsr.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Dabezies EJ, Banta CJ, II, Murphy CP, d’Ambrosia RD. Plate fixation of the humeral shaft for acute fractures, with and without radial nerve injuries. J Orthop Trauma. 1992;6:10–13. [PubMed] [Google Scholar]
- 40.Lowe JB, III, Sen SK, Mackinnon SE. Current approach to radial nerve paralysis. Plast Reconstr Surg. 2002;110:1099–113. doi: 10.1097/01.PRS.0000020996.11823.3F. [DOI] [PubMed] [Google Scholar]
- 41.Pollock FH, Drake D, Bovill EG, et al. Treatment of radial neuropathy associated with fractures of the humerus. J Bone Joint Surg Am. 1981;63:239–43. [PubMed] [Google Scholar]