Abstract
Objectives:
This study was planned to evaluate the dimensional stability of elastomers during cold sterilization or immersion disinfection and also to evaluate the same, along with acrylic resin trays which are used in clinical practice.
Materials and Methods:
A study mold according to revised American Dental Association. Specification no. 19 was used. Polyether, polyvinyl siloxane (PVS) (heavy body), PVS (regular body) and Hydrophilic addition reaction silicon (medium body) were selected for study. 2% glutaraldehyde and 0.525% sodium hypochlorite were the disinfectants used. The study was divided into group-I and group-II. In group-I study, 24 specimens of each impression material were prepared. Eight immersed in 2% glutaraldehyde, eight in 0.525% sodium hypochlorite and rest eight allowed to dry bench cure. After 16 h, the specimens measured under Leica WILD stereomicroscope and dimensions compared with master die. In group II study, 24 specimens of the material with the least dimensional changes were prepared and adhered to 24 acrylic resin disks using tray adhesive. Same immersion procedure was followed as in group I. The data were analyzed by one-way ANOVA and Tukey's multiple tests.
Results:
Of four impression materials used, PVS (heavy body) was the most dimensionally stable, and Polyether was the least dimensionally stable in both the groups.
Interpretation and Conclusion:
Within the limitation of the study, PVS (heavy body) was most stable, and polyether was least stable of all the impression materials.
Key Words: Acrylic resin trays, dimensionally stable, disinfectants, immersion, impression materials
INTRODUCTION
Dental impression materials are used to register the form and relation of the teeth and the surrounding oral tissues. Impression materials are subjected to several factors that can result in dimensional change. The accuracy also largely depends on the disinfection procedures.
Dental impressions are potential sources for cross-contamination and should be disinfected. Among the issues to be resolved are the composition, concentration of the ideal disinfectant, optimal exposure time, and the interaction between impression material and disinfectant solution.[1]
The most reliable method of disinfection is immersion of the impression. With this method, the disinfectant solution will come into contact with all surfaces of the impression material and tray.[2] Elastomers may be susceptible to dimensional change if immersed for a long time because few of them are hydrophilic in nature, and few are hydrophobic in nature.
Most commonly used disinfectants for dental impressions are glutaraldehyde and sodium hypochlorite.[3] The purpose of this study was to assess the dimensional accuracy of the elastomeric impression materials during cold sterilization in 2% glutaraldehyde and 0.525% sodium hypochlorite.
MATERIALS AND METHODS
Study was conducted to measure and compare the linear dimensional changes of four representative rubber elastomeric impression materials after their immersion in 2% glutaraldehyde solution and 0.525% sodium hypochlorite solution.
Materials
Polyether (Impregum™ Penta™ Soft, 3M ESPE, Germany)/Medium body
Polyvinyl siloxane (PVS) (Imprint TM, II Garant, 3M ESPE, Germany)/Heavy body
PVS (Imprint TM, II Garant, 3M ESPE, Germany)/Regular body
Hydrophilic addition reaction silicone (Aquasil Ultra Heavy, DENTSPLY/CAULK, USA)/Medium body
2% glutaraldehyde solution and
0.525% sodium hypochlorite solution
Tray adhesive (Vinyl Polysiloxane adhesive and Polyether adhesive 17 ml liquid. 3M ESPE, Germany)
Acrylic Resin Tray material (M.P Sai Enterprise, Mumbai – 53).
Group-I study
Preparation of mold
A study mold was prepared according to revised American Dental Association (ADA) specification no. 19.[4] [Figures 1 and 2].
Figure 1.
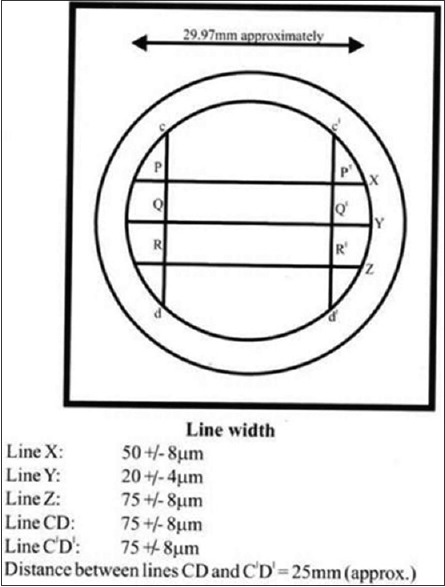
Schematic diagram showing ruled surface of die
Figure 2.
Specimens of four impression materials used in the study
Selection and manipulation of materials
The material was loaded into a fine-tipped impression syringe and applied to lined areas of the die. A polyethylene sheet followed by a flat glass plate (of weight 67 g) was placed on top of the mold.
After the manipulation of all the impression materials, the die was transferred to thermostatically controlled water bath at a temperature of 32°C ± 2°C (to simulate oral conditions) according to ADA specification No. 19.
The disinfectants used
25% glutaraldehyde was converted into 2% glutaraldehyde by diluting it with distilled water
4% sodium hypochlorite was converted into 0.525% sodium hypochlorite by adding buffers and adjusting the pH ranging 7–10.
Collection of specimens
The entire assembly was removed from the water bath after 13 min. The separated impression was numbered with a marker.
Measurement of scribed lines of impression material surface
Line reproduction on the specimen was evaluated following the criterion given by ADA specification No. 19. Each horizontal line was evaluated under stereomicroscope at × 10 magnification. The line reproduction was considered acceptable if two or three of the horizontal lines were reproduced continuously and well defined.
Thus prepared specimens were measuring 30 mm in diameter, 3 mm in thickness and had the lines X, Y, Z, CD, and C’D’ on it.
Selection and immersion of specimens in the disinfectants
Twenty-four samples of each impression material were used, eight were immersed in 2% glutaraldehyde, eight in 0.525% sodium hypochlorite solution, and another eight were allowed to dry bench cure [Figure 3 and Graphs 1, 2, 3]. After 16 h, specimens were measured under stereomicroscope at × 10 magnification.
Figure 3.

Samples of four groups kept in two disinfectants and control group each
Graph 1.
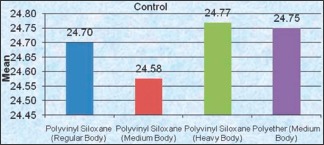
Comparison between impression materials for control group
Graph 2.
Comparison between impression materials for 2% Glutaraldehyde group
Graph 3.
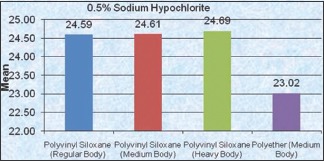
Comparison between impression materials for 0.525% Sodium Hypochlorite group
Comparison of linear dimensions of the impression material with those of the master die and the control group
The distance between the lines, CD and C’D’ reproduced on the samples, was measured at three different points PP’, QQ’, and RR’ using Leica 3MZ microscope with × 10 magnification. Three readings were obtained for each sample, and the overall mean was tabulated and subjected to statistical analysis for the comparison.
Statistical analysis
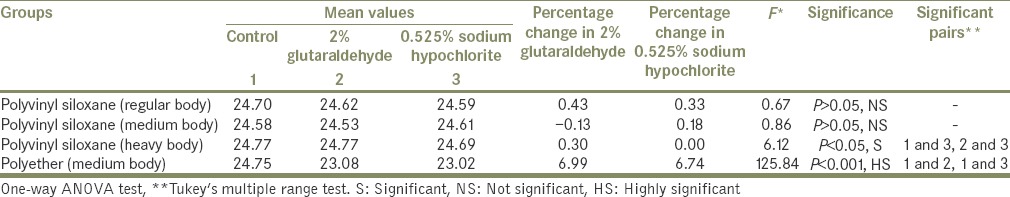
The data were analyzed by one-way ANOVA analyses. Significant differences were separated by Tukey's multiple comparison test [Table 1].
Table 1.
GROUP I STUDY. Intragroup comparison of four elastomeric impression materials for the Control group, 2% glutaraldehyde group, and 0.525% sodium hypochlorite group
The best impression material with the least dimensional changes was chosen for group-II study.
Group-II study
Preparation of acrylic resin disks
A total of 24 acrylic resin disks measuring 6 mm thick, 30 mm in diameter were prepared, and bench cured for 14 days, before use.
Selection and preparation of best impression material
Twenty-four samples of the best impression material were prepared. Each of them was cemented to the acrylic resin disc using respective tray adhesive [Figure 4 and Graph 4]. 8, out of 24 samples, were immersed in 2% glutaraldehyde, eight in 0.525% sodium hypochlorite and rest eight were allowed to bench cure. Same measuring procedures were utilized as done in group-I.
Figure 4.
Adhesion of specimen to the acrylic resin disk
Graph 4.
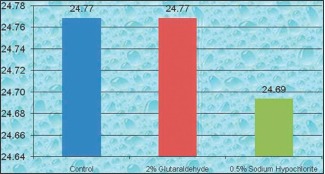
Comparison between Control group, 2% Glutaraldehyde group, 0.525% Sodium Hypochlorite group for polyvinyl siloxane (heavy body) impression material along with acrylic resin tray
Statistical analysis
The data were analyzed using the same tests as group I.
RESULTS
Group-I
Table 1 shows the intragroup comparison of four elastomeric impression materials for the Control group, 2% glutaraldehyde group, and 0.525% sodium hypochlorite group, using one-way ANOVA test as well as Tukey's multiple range test. This table also shows the dimensional changes in the specimens in percentage for 2% glutaraldehyde group and 0.525% sodium hypochlorite group.
Test for significance showed nonsignificant values for PVS regular body and medium body with P > 0.05. Values are significant for PVS (heavy body) with 0.525% sodium hypochlorite making significant pair with both control group and 2% glutaraldehyde group with P < 0.05. Highly significant values were shown for polyether (medium body).
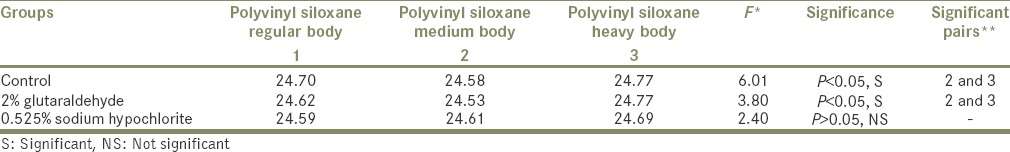
Table 2 shows statistical values comparing different consistencies of PVS impression material. In group-I study; PVS (heavy body) was very accurate with respect to Control group, 2% Glutaraldehyde group and 0.525% Sodium Hypochlorite group.
Table 2.
GROUP I STUDY Comparison of different consistencies of polyvinyl siloxane impression material
Group-II
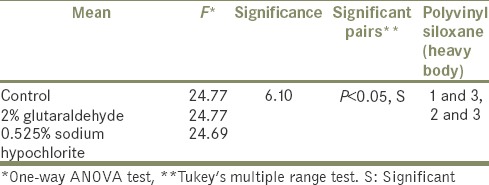
Of four impressions; PVS (heavy body) with least dimensional changes was used for Group-II study. It shows the mean values and F values of PVS (heavy body) specimens with acrylic resin tray, for each group showing the same results as in group-I study for PVS (heavy body) without tray [Table 3].
Table 3.
GROUP-II STUDY Polyvinyl siloxane (heavy body)
DISCUSSION
Dental professionals are exposed to a wide variety of pathogenic microorganisms in blood and saliva. Because of increasing frequency of acquired immune deficiency syndrome, disinfection and sterilization procedures are of importance. Immersion is the most recommended method.[5]

The procedure of disinfection differs depending on the type of impression material used and also on the chemical agent used.[6] The following methods could be utilized;
Evaluation of the accuracy of elastomers after immersion (polyether, vinyl polysiloxane and polysulfide) for 10 min in Neutral Glutaraldehyde, increased the distance between preparations and for polyether it is decreased 15–30 μm with disinfection which was acceptable.[7]
Some “disinfectant-impression” combinations proposed changes in the surface texture of the stone, so ideal disinfectant should be determined for each impression material.[8]
Most commonly used disinfectants for dental impressions are Glutaraldehyde and Sodium Hypochlorite.™ They are easily available and can be diluted easily to the recommended percentages.
For elastomers especially PVS, when immersed in glutaraldehyde has shown no significant changes. But, acid potentiated glutaraldehyde showed improved quality of surface of dies for the same.
Group-I study
Among the impression materials, if taken individually, PVS (regular body) when compared with the control group have shown no significant difference with P > 0.05 [Table 2].
Sixteen hour immersion in 2% Glutaraldehyde and 1% sodium hypochlorite disinfectants could be used for PVS without any dimensional changes in case of patients with positive reaction for hepatitis B surface antigen.[9]
However, when PVS (regular body) control group was compared with the Control groups of other impression materials, it has shown highly significant values.
30 min immersion of PVS in Glutaraldehyde solution, had no negative effects.[10] But the immersion for 60 min showed significant dimensional changes. Although dimensional changes were <0.03%, immersion in Glutaraldehyde solution, caused expansion of the impression material.[11] In the present study, the dimensional changes were up to 0.43% [Table 2].
However, it has been shown that there is a significant difference in dimensional (P ≤ 0.05) accuracy among 24 h 1-week and 2 weeks measurement times related to both disinfected and nondisinfected vinyl polysiloxane impressions exhibiting continuing shrinkage over time.[12]
According to Johnson, dimensional stability for addition silicone was unsatisfactory.[12] But studies have shown that various disinfectants and immersion periods for PVS have shown good compatibility and are dimensionally stable up to 18 h of the immersion period.[13,14]
In the present study, the second impression material, PVS (medium body) has shown no significant difference.
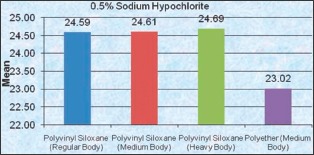
For 0.525% Sodium Hypochlorite disinfectant, there is a significant difference in the disinfected and nondisinfected specimen and 0.18% of dimensional change was seen after immersion for 16 h.
However, in contrast to above-mentioned statement, it is[15] observed that elastomeric impressions in higher concentration up to 5.25% sodium hypochlorite for 30 min caused expansion of impressions.
Polyvinyl siloxane (medium body) Glutaraldehyde group and Sodium Hypochlorite group was compared with the same groups of other impression materials. It was highly significant only with Polyether (PE) (medium body).
The third impression material in the present study, PVS (heavy body) specimens when compared with Control group have shown no change in the dimensions. Thus, silicones were insensitive to immersion in Glutaraldehyde.[11,16]
In the present study, when intragroup comparison was done for different consistencies of PVS (regular, medium, heavy) with each other, heavy consistency showed minimum dimensional changes with and without disinfection. This property of high dimensional stability of the heavy body could be attributed to higher filler content of the material.[17]
The fourth impression material that is polyether (medium body) showed the highest significant differences among the groups.
There was a very significant difference between the nondisinfected specimens and disinfected specimens. This could be attributed to the hydrophilicity of polyethers.[7,17]
PE (medium body) when disinfected by either 10 min immersion or 1 h immersion in 0.5% sodium hypochlorite solution, exhibited expansion with all disinfection time intervals,[12] which was in correlation with the present study.
All the solutions produced dimensional changes in polyether even after 15 min immersion and are not recommended for disinfection.[18] They are hydrophilic and can imbibe water and swell when immersed in disinfectant.[7]
Hence, the results of the present study show that PVS (Heavy body) was the most stable impression material and have claimed superior in accuracy and stability over polyethers.[19]
So when sterilization is imperative as in the case of HIV/hepatitis, the best option is to use PVS. This was also observed by Johansen and Stackhouse[20] that only addition reaction silicone remained stable after immersion in Glutaraldehyde solution after 16 h. So in the present study PVS (Heavy body) was chosen for the group-II study.
Group-II study
No significant difference was seen in the dimensional stability of the specimens adhered to trays (group-II), when compared to the specimens not adhered to trays as in group-I study. This shows that the acrylic tray material did not make any difference after disinfection.
In general, impression materials showed least significant changes for 2% Glutaraldehyde group when compared to 0.525% sodium hypochlorite group.
Whereas 0.525% sodium hypochlorite has shown to adversely affect the stability of all the impression materials and show highly significant differences among the nondisinfected and disinfected specimens, especially for PE (medium body).
One more observation of the present study was the polyether specimens immersed in 0.525% sodium hypochlorite were lighter in color and sticky compared to the Control group. The reason could be, as it is a bleaching agent in nature.[21] Polyether impression materials with 10-min and 1 h sodium hypochlorite immersion group, when compared with no disinfectant group showed a mottled and sticky surface,[12] which was also observed in the present study.
In the present study, PVS (heavy body) was found to be the most dimensionally stable and PE (medium body) was found to be the least dimensionally stable impression material. When intragroup comparison was done between different consistencies of PVS (regular, medium, and heavy consistency), PVS (heavy body) was most stable followed by PVS (medium body) and PVS (regular body).
This study suggests a possible method of disinfection for protecting a person, either a dentist or a technician who handle dental impressions and to choose a material of choice.
The trays did not affect the dimensional stability of PVS (heavy body).
The limitation of this in-vitro study was that all the measurements were linear, and the line criterion on the specimens was evaluated to check the dimensional stability.
CONCLUSION
Within the limitations of the study, following conclusion can be drawn from the present study after 16 h of immersion disinfection.
Both the disinfectants affect the dimensional stability of PVS and polyether impression material, after 16 h of immersion disinfection
2% Glutaraldehyde is a better disinfectant when compared to 0.525% Sodium Hypochlorite
PVS of different consistencies (regular, medium, heavy) used in this study, PVS (heavy body) is the most stable followed by PVS medium body and regular body
Dimensional changes shown by PVS are statistically nonsignificant making it a material of choice
The dimensional changes shown by the polyether are statistically significant which makes the material unfit for immersion disinfection
There is no difference in the dimensional changes of impressions when it is disinfected along with the acrylic custom tray for PVS (heavy body).
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.McCabe JF, Storer R. Elastomeric impression materials. The measurement of some properties relevant to clinical practice. Br Dent J. 1980;149:73–9. doi: 10.1038/sj.bdj.4804460. [DOI] [PubMed] [Google Scholar]
- 2.Berg JC, Johnson GH, Lepe X, Adán-Plaza S. Temperature effects on the rheological properties of current polyether and polysiloxane impression materials during setting. J Prosthet Dent. 2003;90:150–61. doi: 10.1016/s0022-3913(03)00297-x. [DOI] [PubMed] [Google Scholar]
- 3.New York: WHO; 1973. World Health Organisation. Technical Report Series 512, Viral Hepatitis. [PubMed] [Google Scholar]
- 4.Revised American Dental Association Specification no 19 for Non-aqueous, Elastomeric Dental Impression Materials. J Am Dent Assoc. 1977;94:733–41. doi: 10.14219/jada.archive.1977.0334. [DOI] [PubMed] [Google Scholar]
- 5.Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 6.Infection control recommendations for the dental office and the dental laboratory. Council on Dental Materials, Instruments, and Equipment. Council on Dental Practice. Council on Dental Therapeutics. J Am Dent Assoc. 1988;116:241–8. doi: 10.14219/jada.archive.1988.0341. [DOI] [PubMed] [Google Scholar]
- 7.Adabo GL, Zanarotti E, Fonseca RG, Cruz CA. Effect of disinfectant agents on dimensional stability of elastomeric impression materials. J Prosthet Dent. 1999;81:621–4. doi: 10.1016/s0022-3913(99)70219-2. [DOI] [PubMed] [Google Scholar]
- 8.Storer R, McCabe JF. An investigation of methods available for sterilising impressions. Br Dent J. 1981;151:217–9. doi: 10.1038/sj.bdj.4804658. [DOI] [PubMed] [Google Scholar]
- 9.Herrera SP, Merchant VA. Dimensional stability of dental impressions after immersion disinfection. J Am Dent Assoc. 1986;113:419–22. doi: 10.14219/jada.archive.1986.0214. [DOI] [PubMed] [Google Scholar]
- 10.Lepe X, Johnson GH. Accuracy of polyether and addition silicone after long-term immersion disinfection. J Prosthet Dent. 1997;78:245–9. doi: 10.1016/s0022-3913(97)70021-0. [DOI] [PubMed] [Google Scholar]
- 11.Bergman B. Disinfection of prosthodontic impression materials: A literature review. Int J Prosthodont. 1989;2:537–42. [PubMed] [Google Scholar]
- 12.Thouati A, Deveaux E, Iost A, Behin P. Dimensional stability of seven elastomeric impression materials immersed in disinfectants. J Prosthet Dent. 1996;76:8–14. doi: 10.1016/s0022-3913(96)90338-8. [DOI] [PubMed] [Google Scholar]
- 13.Minagi S, Kohada A, Akagawa Y, Tsuru H. Prevention of acquired immunodeficiency syndrome and hepatitis B. Part III: Disinfection of hydrophilic silicone rubber impression materials. J Prosthet Dent. 1990;64:463–5. doi: 10.1016/0022-3913(90)90044-d. [DOI] [PubMed] [Google Scholar]
- 14.Minagi S, Yano N, Yoshida K, Tsuru H. Prevention of acquired immunodeficiency syndrome and hepatitis B. II: Disinfection method for hydrophilic impression materials. J Prosthet Dent. 1987;58:462–5. doi: 10.1016/0022-3913(87)90277-0. [DOI] [PubMed] [Google Scholar]
- 15.Anusavice KJ. Phillips’ Science of Dental Materials. 11th ed. St. Louis, Missouri: Saunders, An imprint of Elsevier; 2003. [Google Scholar]
- 16.Johnson GH, Drennon DG, Powell GL. Accuracy of elastomeric impressions disinfected by immersion. J Am Dent Assoc. 1988;116:525–30. doi: 10.14219/jada.archive.1988.0307. [DOI] [PubMed] [Google Scholar]
- 17.Walker MP, Rondeau M, Petrie C, Tasca A, Williams K. Surface quality and long-term dimensional stability of current elastomeric impression materials after disinfection. J Prosthodont. 2007;16:343–51. doi: 10.1111/j.1532-849X.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 18.Langenwalter EM, Aquilino SA, Turner KA. The dimensional stability of elastomeric impression materials following disinfection. J Prosthet Dent. 1990;63:270–6. doi: 10.1016/0022-3913(90)90193-g. [DOI] [PubMed] [Google Scholar]
- 19.Lacy AM, Fukui H, Bellman T, Jendresen MD. Time-dependent accuracy of elastomer impression materials. Part II: Polyether, polysulfides, and polyvinylsiloxane. J Prosthet Dent. 1981;45:329–33. doi: 10.1016/0022-3913(81)90400-5. [DOI] [PubMed] [Google Scholar]
- 20.Johansen RE, Stackhouse JA., Jr Dimensional changes of elastomers during cold sterilization. J Prosthet Dent. 1987;57:233–6. doi: 10.1016/0022-3913(87)90152-1. [DOI] [PubMed] [Google Scholar]
- 21. Available from: http://www.en.wikipedia.org/wiki/sodium.hypochlorite .


