Abstract
Background:
Dentistry equipment are exposed to different types of pathogenic microorganisms. The aim of this study was to investigate the effect of spraying three different types of disinfectants on condensational silicones after 5 and 10 min.
Materials and Methods:
Totally, 66 circular samples of condensational silicone impression materials of 1 cm diameter and 2 mm thickness were contaminated by Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans fungus. Except for control samples, all of them were disinfected with sodium hypochlorite (NaOCl) 0.525%, Deconex and Epimax by spraying method. Afterward, they kept in plastic bags with humid rolled cotton for 5 and 10 min. In order to isolate microbiotas, the samples were immersed in 2% trypsin for 1 h and diluted with normal saline in a portion of 1, 1/2, and 1/4. The trypsin suspensions were transferred to culture plates for incubation and colony-forming unit assay. The data were analyzed by Mann–Whitney test and SPSS software version 16 at a significant level of 0.05.
Results:
There was a meaningful difference between disinfection effects of Epimax-Deconex for all mentioned microorganisms after 5 min (P = 0.034), and between disinfection effects of NaOCl 0.525%-Epimax for S. aureus (P = 0.043) and P. aeruginosa (P = 0.046) after 5 min. Furthermore, there was a meaningful difference between disinfection effects of Epimax-Deconex (P = 0.034) and NaOCl 0.525%-Epimax (P = 0.034) for P. aeruginosa after 10 min.
Conclusion:
Condensational silicone can be effectively disinfected by spraying tested three disinfecting agents. More specifically, Deconex showed the best results compared to the other agents.
Key Words: Condensational silicone, disinfection, impression materials, spray
INTRODUCTION
Dentist, dental materials, and dental laboratories are exposed to different types of pathogen microorganisms. The main source of cross-contamination is impression materials, impression trays, and poured stone casts.[1] New researches have shown that 67% of materials, which are sent to dentistry laboratories, are infected by various microorganisms.[2] The most frequently identified microorganisms are Streptococcus species, Staphylococcus species, Escherichia coli species, Actinomyces species, Antitratus species, Pseudomonas species, Enterobacter species, Klebsiella pneumonia, and Candida species.[3] Taking these facts in mind, we should make an effort to eliminate most of this microorganisms and cross-contamination. The International Dental Federation consequently insists on disinfecting all impressions from patients before sending them to laboratories.[4] Furthermore, the American Dental Association (ADA) has advised all dental workers to disinfect all patients’ impression trays.[5] In some studies, it has been declared that washing the impression materials with tap water; only removes 40% of bacteria. Even though, studies have reported that it has the capacity to reduce microorganisms by 90%.[6] The most common chemical disinfectants which are used by dentists are alcohols, aldehydes, chlorine combinations, phenols, biguanides, iodide combinations, and ammonium.[7] Based on the type of chemical disinfectants, there are two common methods to disinfect dental materials: (A) Immersion (B) spraying.[6] Immersing in chemical materials has proved to cover all surfaces in 1 time.[8] However, spraying is not capable of disinfecting all surfaces effectively specially all undercuts. In another view, immersing might cause amounts of distortion.[6]
Silicone impression materials are the first group of polymeric impression materials.[9] These materials have the best dimensional stability. Polyvinyls are the only impression materials which can be disinfected without any dimensional changes.[10] Also, different methods, such as soaking in glutaraldehyde for 30 min, have been suggested to disinfect these materials. Use of sodium hypochlorite (NaOCl) and phenol combinations with the soaking time less than pouring time have been suggested by some studies.[11] Bustos et al. investigated the effect of NaOCl 0.05% and glutaraldehyde after 5 and 10 min on silicone impressions. He declared that both of the materials can efficiently prevent the bacterial growth in impressions.[12] Also, Ghahramanloo et al. investigated the antimicrobial effect of NaOCl 0.525%, Deconex and Sanosil. They concluded that the use of NaOCl 0.525% sprayed onto the surface of alginate, effectively disinfected 96.6% of the samples.[13]
As none of the methods and materials above has been accepted as a standard gold for disinfecting dental materials, finding an appropriate method seems rational.
The aim of this study was to investigate the spraying disinfection effect of Deconex, NaOCl 0.525% and Epimax on condensational silicone impression material in 5 min and 10 min.
MATERIALS AND METHODS
This observational-analytical study was carried out with the cooperation of the School of Dentistry of the Isfahan University of Medical Sciences and the Department of Microbiology of the Medical School, aiming at evaluating the disinfection effect of: NaOCl 0.525% (Chloran, Tehran, Iran), Deconex (Borer chemie, Switzerland) and Epimax (Emad, Isfahan, Iran) on the condensational silicone impression material.
Sampling Methods
In order to prepare samples, the heavy body impression material (Putty) (Asia Chemi Teb Co; Tabriz, Iran, under the license of Coltene-Switzerland) was mixed with the catalyst according to manufacturer instructions. The mixture was placed in a syringe with 1 cm diameter and samples with 1.5 mm thickness were gained. Then the light body impression material (Wash) (Asia Chemi Teb Co; Tabriz, Iran, under the license of Coltene-Switzerland) was mixed with the catalyst on a paper pad with a sterile spatula and placed in the upper 0.5 mm of the syringe.
Eventually 66 samples, with 2 mm thickness and 1 cm diameter, were prepared. In order to ensure the samples were kept sterile during preparation, three samples were selected as negative controls (blank) and incubated on Tryptic soy broth (TSB) (Hi-media, Mumbai, Maharashtra, India) for 24–48 h; after which the bacterial growth was examined. For each bacterial type, 21 samples were used. NaOCl 0.525% was used to disinfect three of them for 5 min and three others for 10 min. The same sampling category was considered for Deconex and Epimax. Furthermore, three more samples were used as positive controls to check for any microbial pollution.
Preparation of Microbial Solution and Yeast
For many types of susceptibility testing, a standard inoculum of bacteria must be used. The standard inoculums were prepared according to 0.5 McFarland (1.5 × 108 CFU/ml) by transferring 1–2 colonies of 18–24 h cultures to TSB medium and incubate at 35°C until the turbidity of media were equal to 0.5 McFarland. For Candida albicans fungus, the sample was taken from 48 h Sabouraud and Dextrose Agar cultures (Hi-media, Mumbai, Maharashtra, India).
Contamination of Samples
To evaluate the disinfection effect of above mentioned three substances, samples were separately polluted with microbial suspensions of Staphylococcus aureus (ATCC29213), Pseudomonas aeruginosa (ATCC27853) and C. albicans fungus (PTCC5027). The impressions were put in sterile test tubes separately with 2 ml of microbial suspension for each one and were incubated at 35°C for 1 h.
Disinfection of Samples and Microbiological Surveys
After contamination, all samples were rinsed with sterile distilled water for 30 s. In order to disinfect all samples, except control, either NaOCl 0.525%, Deconex and Epimax was used on each sample, by applying spraying method, 10 puffs in 15 s. Then the samples were put into sterile plastic bags containing sterile cotton humidified with sterile distilled water to form a moisturized environment for 5 and 10 min.
Protease Trypsin (AG Scientific Inc., CA, USA), which is able to isolate the microbes from contaminated environments, was used. The ideal time and concentration for effective use of Trypsin are 60 min and 2%, respectively. This time and concentration are based on the maximum microorganisms isolated from the samples. After washing the samples with sterile distilled water during 30 s, they were put in Trypsin 2% solution for 60 min. The suspension of ½ and ¼ Trypsin solution were then prepared. Using 100 μl sampler, these samples were transferred to Muller Hinton Agar (Hi-media, Mumbai, Maharashtra, India) for the bacteria (P. aeruginosa and S. aureus), and Sabouraud and Dextrose Agar cultures was selected for C. albicans fungus. By using a Pasteur pipet, which was bent by heat at 90°, the samples were spread on cultures. After 24 and 48 h incubation, the grown bacterial colonies on culture were counted. The grown fungus colonies of C. albicans on Sabouraud culture were counted after 72 h.
Finally, the data were analyzed by Mann–Whitney test and SPSS software version 16 (IBM Corporation, NY, USA) at a significant level of 0.05.
RESULTS
Table 1 reveals the bacterial growth inhibition by different disinfectant agents in 5 and 10 min. Deconex completely eradicated all three kinds of microorganisms after 5 and 10 min. This was not true for NaOCl, as this material just eradicated S. aureus and P. aeruginosa after 10 min. After 5 min, Epimax could eradicate 95.78% of P. aeruginosa. This material could completely eradicate C. albicans and S. aureus after 10 min. By increasing time, from 5 to 10 min, disinfection ability of all agents increased, except for Deconex which was 100% for all microorganisms in both 5 min and 10 min.
Table 1.
Bacterial growth prevention (%) by different disinfectant agents in 5 and 10 min
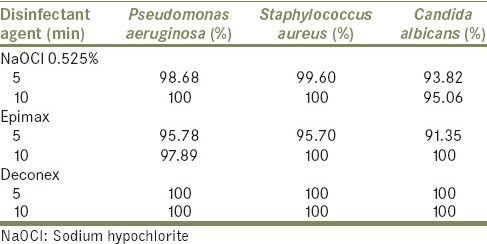
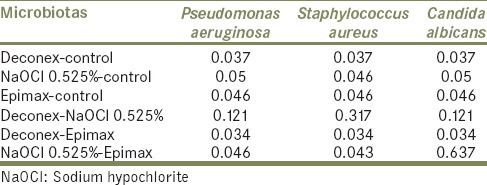
Table 2 represents pair-wise comparison of tested disinfectant agents in 5 min. Based on the analyzed data, there was a meaningful difference between disinfection efficacy of Deconex and Epimax in 5 min (P = 0.034). This difference was also meaningful for NaOCl and Epimax just for P. aeruginosa (P = 0.046) and S. aureus (P = 0.043). In other cases, there was not any significant difference in disinfection efficacy of materials after 5 min (P > 0.05).
Table 2.
Pair-wise comparison of tested disinfectant agents (P values) in 5 min
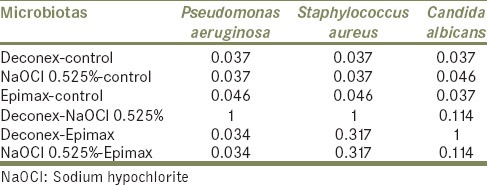
According to the Table 3, which manifests pair-wise comparison of tested disinfectant agents in 10 min, meaningful difference between disinfection efficacy of NaOCl – Epimax and Deconex-Epimax was seen after 10 min just for P. aeruginosa.
Table 3.
Pair-wise comparison of tested disinfectant agents (P values) in 10 min
DISCUSSION
Dental practitioners encounter potentially harmful microorganisms, and patients are the most common source of microorganisms.[14] Studies indicate the surface of impressions taken out of the mouth is polluted with bacteria.[15,16,17,18] As impressions and occlusal records cannot be sterilized by heat, chemical disinfection is still the common practicable method to eradicate microorganisms.[19,20] So far, there is no global way to disinfect impression materials.[21] The ADA recommends soaking impression materials in disinfectant solutions for <30 min.[22] Muller-Bolla et al. stated that the soaking method is applied in 63% of alginate impressions and in 73% of silicone impressions in European schools of dentistry. Furthermore, the approximate time of disinfection was 10.3 ± 6.3 min.[20]
Egusa et al. showed that impressions from patients’ mouth, contain hazardous microorganisms like Streptocci, S. aureus, Methicillin resistant Staphyloccocus, Candida, P. aeruginosa with rates of 100%, 55.6% 25.9%, 5.6%, and 5.6% respectively.[21] These are opportunist pathogens that spread and transfer through the oral cavity.[21] Candida causes common opportunist infections known as oral candidiasis, found in patients with immune deficiency.[22] P. aeruginosa is a deadly infectious agent that exists epidemically in hospital appliances and instruments.[21] Studies showed that the spreading rate of S. aureus to then as pharynx is only 10%. This rate for S. pneumonia is 20-32%, and it is 30% for S. aureus.[14] That is why three microorganisms, S. aureus, C. albicans, and P. aeruginosa were selected to investigate the disinfection ability of common disinfectant agents.
By the year 1991, washing the impression materials with running water was the common way to remove microorganisms.[21] This method could reduce about 90% of bacteria.[23] Running water can wash up saliva, blood, and debris. But recent studies indicate that such methods cannot eliminate microorganisms from impression materials completely. Therefore, washing the impression materials with running water, without disinfectants is not sufficient.[21]
In the present study, NaOCl 0.525% efficiently prevented microorganism's growth and disinfected the impression materials. In a study by Bustos et al.,[12] it was shown that silicone impressions immersion in NaOCl 0.5%, after 5 and 10 min, dramatically prevent the bacterial growth in comparison to the control group. Although spraying method was used in the present study, the results were similar to mentioned study. On the other hand, two studies showed that spraying NaOCl can effectively disinfect the impression materials.[24,25] Westerholm et al. showed that NaOCl could absolutely (99.99%) prevent the growth of S. aureus and this rate was about 99.60% after 5 min and 100% after 10 min for S. aureus.[24] In another study by Ghahramanloo et al., spraying NaOCl 0.525% could disinfect samples effectively (96.6%) after 10 min which is a good indicator of the high capacity of this agent.[13] In mentioned studies, the disinfection effect of these agents was assessed on irreversible hydrochloride (alginate), but in this survey, this effect has been assessed on condensational silicone. The results showed that there is no difference in disinfection capacity of NaOCl regardless of impression material, and this is a good proof for high penetration of this agent to impression materials porosities.
Decnex is a good alcoholic based disinfectant agent, which in this study could effectively disinfect impressions after 5 and 10 min (100%). But in Ghahramanloo et al. study, this agent could eradicate 70.4% of microorganisms.[13] May be the main reason for this difference is that they used irreversible hydrochloride, which has more porosities and cause deep penetration of microorganism into this impression material and can define the lesser capacity of disinfectant agent in eradicating microorganisms. The specific feature about this agent is that there is no difference in disinfection capacity of Deconex after 5 min and 10 min.
Epimax could not effectively eradicate microorganism after 5 min compared to two other agents, but after 10 min it completely (100%) eradicated C. albicans and S. aureus but this rate was 97.89 for P. aeruginosa which indicates the usage of this agent in longer time for best response.
In a study, by Izadi et al., the decontamination quality of NaOCl 0.5%, Sanosil 2% and 6% was observed after treating on alginate and condensational silicone impressions for 10 min. They stated that none of the tested disinfections were able to eradicate the microorganisms, completely.[26] However, in the present study, all of the microorganisms were successfully decontaminated after applying NaOCl 0.525%, Deconex (specially) and Epimax after 10 min. The different results might be due to different concentrations of NaOCl. Furthermore, the impression materials were contaminated by microorganisms’ standard strains in the present study, but they decontaminated impression materials obtained from oral flora of participants.
However, what we should consider in reviewing the results of this study is that they are not entirely comparable with the results of other studies, because of the different brands of impression materials and also different duration of usage of disinfecting agents. One of the limitations of our research is that our study was an in vitro experimental study which is different from clinical and in vivo situations. Usually, impression materials remain 3–5 min in patient's mouth, while in our study it took 60 min in order to attach all the bacterial types to the samples as 60 min is an effective time for bacterial adherence. Furthermore, pressure while taking an impression and saliva could alter bacterial adherence capacity. This survey investigated the effect of three common disinfectant agents on two types of bacteria and one fungus. As so many dentists are concerned about viruses such as HIV and HBV, further studies should conduct to find an effective way to eradicate this kind of pathogens.
CONCLUSION
The results of present in vitro study showed that NaOCl 0.525%, Deconex and Epimax could effectively disinfect condensational silicone contaminated by the tested microorganisms (C. albicans, S. aureus, and P. aeruginosa.). Nevertheless, Deconex demonstrated promising result in decontamination of tested microorganism, and it is recommended for disinfecting of condensational silicone impression materials by spraying method.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Orsi IA, Andrade VG. Effect of chemical disinfectants on the transverse strength of heat-polymerized acrylic resins submitted to mechanical and chemical polishing. J Prosthet Dent. 2004;92:382–8. doi: 10.1016/j.prosdent.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent. 1990;64:235–7. doi: 10.1016/0022-3913(90)90185-f. [DOI] [PubMed] [Google Scholar]
- 3.Pang SK, Millar BJ. Cross infection control of impressions: A questionnaire survey of practice among private dentists in Hong Kong. Hong Kong Dent J. 2006;3:89–93. [Google Scholar]
- 4.Recommendations for hygiene in dental practice, including treatment for the infectious patient. Fédération Dentaire Internationale. A revision of technical report no 10. Int Dent J. 1987;37:142–5. [PubMed] [Google Scholar]
- 5.Guidelines for infection control in the dental office and the commercial dental laboratory. Council on Dental Therapeutics. Council on Prosthetic Services and Dental Laboratory Relations. J Am Dent Assoc. 1985;110:969–72. doi: 10.14219/jada.archive.1985.0016. [DOI] [PubMed] [Google Scholar]
- 6.Al-Jabrah O, Al-Shumailan Y, Al-Rashdan M. Antimicrobial effect of 4 disinfectants on alginate, polyether, and polyvinyl siloxane impression materials. Int J Prosthodont. 2007;20:299–307. [PubMed] [Google Scholar]
- 7.Ahmad S, Tredwin CJ, Nesbit M, Moles DR. Effect of immersion disinfection with perform-ID on alginate, an alginate alternative, an addition-cured silicone and resultant type III gypsum casts. Br Dent J. 2007;202:E1. doi: 10.1038/bdj.2006.120. [DOI] [PubMed] [Google Scholar]
- 8.Merchant VA, Molinari JA. Infection control in prosthodontics: A choice no longer. Gen Dent. 1989;37:29–32. [PubMed] [Google Scholar]
- 9.Silicon Impression. Physical and Chemical Properties. Indication to Application Methods of Taking Combined Impression. [Last cited on 2010 Dec 15]. Available from: http://www.ortstom.odmu.edu.ua/en/methodical-work/practical/2nd-year-3rd-term/54-silikonovye-ottisknye-materialy-fiziko-himicheskie-svojstva-pokazanija-k-primeneniju-predstaviteli-metody-poluchenija-kombinirovannyh-ottiskov .
- 10.Deyton G. Impression Materials Used in Removable and Fixed Prosthodontics. [Last cited on 2008 May 20]. Available from: http://www.modental.org/docs/events/ce/ceimpression.pdf .
- 11.Shilinburg HT, Whitesett LD. Fundamentals of Fixed Prosthodontics. Chicago: Quintessence Publishing Co; 1981. [Google Scholar]
- 12.Bustos J, Herrera R, González U, Martínez A, Catalán A, González U, et al. Effect of inmersion desinfection with 0.5% sodium hypochlorite and 2% glutaraldehyde on alginate and silicone: Microbiology and SEM study. Int J Odontostomatology. 2010;4:169–77. [Google Scholar]
- 13.Ghahramanloo A, Sadeghian A, Sohrabi K, Bidi A. A microbiologic investigation following the disinfection of irreversible hydrocolloid materials using the spray method. J Calif Dent Assoc. 2009;37:471–7. [PubMed] [Google Scholar]
- 14.Szymanska J. Microbiological risk factors in dentistry. Current status of knowledge. Ann Agric Environ Med. 2005;12:157–63. [PubMed] [Google Scholar]
- 15.Rowe AH, Forrest JO. Dental impressions. The probability of contamination and a method of disinfection. Br Dent J. 1978;145:184–6. doi: 10.1038/sj.bdj.4804140. [DOI] [PubMed] [Google Scholar]
- 16.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–9. doi: 10.1016/0022-3913(91)90169-w. [DOI] [PubMed] [Google Scholar]
- 17.Hudson-Davies SC, Jones JH, Sarll DW. Cross-infection control in general dental practice: Dentists’ behaviour compared with their knowledge and opinions. Br Dent J. 1995;178:365–9. doi: 10.1038/sj.bdj.4808775. [DOI] [PubMed] [Google Scholar]
- 18.Jennings KJ, Samaranayake LP. The persistence of microorganisms on impression materials following disinfection. Int J Prosthodont. 1991;4:382–7. [PubMed] [Google Scholar]
- 19.Muller-Bolla M, Lupi-Pégurier L, Velly AM, Bolla M. A survey of disinfection of irreversible hydrocolloid and silicone impressions in European union dental schools: Epidemiologic study. Int J Prosthodont. 2004;17:165–71. [PubMed] [Google Scholar]
- 20.Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 21.Egusa H, Watamoto T, Matsumoto T, Abe K, Kobayashi M, Akashi Y, et al. Clinical evaluation of the efficacy of removing microorganisms to disinfect patient-derived dental impressions. Int J Prosthodont. 2008;21:531–8. [PubMed] [Google Scholar]
- 22.Egusa H, Soysa NS, Ellepola AN, Yatani H, Samaranayake LP. Oral candidosis in HIV-infected patients. Curr HIV Res. 2008;6:485–99. doi: 10.2174/157016208786501445. [DOI] [PubMed] [Google Scholar]
- 23.McNeill MR, Coulter WA, Hussey DL. Disinfection of irreversible hydrocolloid impressions: A comparative study. Int J Prosthodont. 1992;5:563–7. [PubMed] [Google Scholar]
- 24.Doddamani S, Patil RA, Gangadhar SA. Efficacy of various spray disinfectants on irreversible hydrocolloid impression materials: An in vitro study. Indian J Dent Res. 2011;22:764–9. doi: 10.4103/0970-9290.94662. [DOI] [PubMed] [Google Scholar]
- 25.Rueggeberg FA, Beall FE, Kelly MT, Schuster GS. Sodium hypochlorite disinfection of irreversible hydrocolloid impression material. J Prosthet Dent. 1992;67:628–31. doi: 10.1016/0022-3913(92)90160-c. [DOI] [PubMed] [Google Scholar]
- 26.Izadi A, Farnaz F, Soufiabadi S, Vafaee F, Kasraei S. Antibacterial effect of sanosil 2% and 6% and sodium hypochlorite 0.5% on impressions of irreversible hydrocolloid (alginate) and condensational silicone (speedex) Avicenna J Dent Res. 2013;5:e21107. [Google Scholar]


