Abstract
The stability of the implant at the time of placement and during the development of the osseointegration process are the two major issues governing the implant survival. Implant stability is a mechanical phenomenon related to local factors such as bone quality, quantity, type of placement technique and type of implant used. The application of a user-friendly, clinically reliable, non-invasive method to assess implant stability and the osseointegration process is considered highly desirable. Resonance frequency analysis (RFA) is one such method which shows almost perfect reproducibility and repeatability after statistical analysis. The aim of this paper is to review the various methods used to assess implant stability and on the currently used RFA method which is being highly accepted in the recent times.
Key Words: Implant stability, resonance frequency analysis, RFA
INTRODUCTION
Implant stability plays a critical role in successful osseointegration, which is the direct structural and functional connection between bone and the surface of a load-carrying dental implant.[1,2,3,4] Achieving and maintaining this implant stability is a prerequisite for a successful clinical outcome.[5,6] Therefore being able to measure this implant stability is important for evaluating the success of an implant.
Implant stability is achieved at two different stages: Primary and secondary. Primary stability comes from mechanical engagement of the implant with cortical bone whereas, the secondary stability is the eventual outcome from regeneration and remodeling of the bone and tissue around the implant.[3] Primary stability determines the secondary stability, which dictates the time of functional loading.[6,7]
The degree of implant stability may also depend on the condition of the surrounding tissues. It is, therefore, of utmost importance to be able to quantify implant stability at various points of time and to project a long-term prognosis based upon measured implant stability.[8] Hence, the need to measure implant stability.
METHODS TO MEASURE IMPLANT STABILITY
Various diagnostic methods, both invasive and non-invasive have been used for this purpose. Histomorphometric analysis, tensional test, push-out/pull-out test and removal torque test involve the disengagement of the implant following the waiting period to determine the extent of osseointegration and hence are limited to animal studies.[8,9] Percussion test, radiography, cutting torque test, periotest and resonance frequency analysis (RFA) being noninvasive are used in the clinical scenario.[10,11,12]
Percussing the implant with a mirror handle is unreliable and no longer used due to subjective variation.[12]
RADIOGRAPHIC ANALYSIS
Radiography, the most widely used diagnostic aid for evaluating the quantity and quality of bone in the area for an implant placement, is helpful in predicting implant stability by observing the process of osseointegration or peri-implant lesions.
However, there are many limitations with the conventional periapical and panoramic views. The facial bone loss which precedes the mesiodistal boneloss cannot be viewed, the limitation with image resolution making standardized X-rays difficult to achieve, distortion of images making quantitative measurements challenging and the difficulty in perceiving changes in the bone structure and morphology of the implant-bone interface unless over 30% bone loss has occurred are among the many few.[13]
Computer assisted measurements of crestal bone level may prove to be the most accurate way to use radiographic information as standard deviation between 0.1 mm (0.01 and 0.51 mm) has been reported. However, this method is not practical in clinical practice.[14]
Computed tomography and cone-beam computed tomography are widely used in the implant treatment as diagnostic aids for planning. They are used for determining the bone density, locating vital structures in the vicinity of the proposed implant site, determination of any pathology and for preplanning any augmentation procedures if required. They can also be used during the follow-up periods, following implant placement for studying the osseointegration of the implant.[15,16]
CUTTING TORQUE RESISTANCE ANALYSIS
Cutting resistance analysis (CRA) measures the energy required for current fed electric motor in cutting a unit volume of bone during implant surgery. This energy is significantly correlated to the bone density which is one of the important factors in implant stability. A torque gauge incorporated within the drilling unit is used in measuring the implant insertion torque value.
The major limitation of CRA is that it does not give information on bone quality till osteotomy site is prepared, and it also cannot identify the lower critical limit of cutting torque value.
Periotest
Dr. Schulte developed it to measure tooth mobility, and Teerlick was the first to use it to measure implant stability. Periotest evaluates the damping capacity and the stiffness of the natural tooth or implant by measuring the contact time of an electronically driven and electronically monitored rod upon percussing the test surface. Periotest value (PTV) ranges from −8 (low mobility) to +50 (high mobility) with a PTV of −8 to −6 is considered as good stability.
Periotest can measure all surfaces like the abutment or prosthesis, but the rod must make contact at a correct angle and distance. The perpendicular contact angle should not be more than 20° and the parallel contact angle not more than 4° in which case the measured value becomes invalid. Furthermore, the distance between the rod and the test surface must be maintained between 0.6 and 2.0 mm.[17,18] If the distance is over 5 mm, the measured value is insignificant.
The limitations of periotest are the inability of the instrument to measure the mesiodistal mobility, the possible effect of position and angle of the rods on the measured value and the most failing point is that the percussing force on a poor initial stability implant may further deteriorate the stability.
The need for a non-invasive, clinically applicable method which is user-friendly to measure implants stability lead to the development of RFA.
Resonance frequency analysis
Meredith et al. in 1996 reported the use of RFA to evaluate implant stability and proved in early in vitro the ability of the device in evaluating the stiffness change of the surface.
Resonance frequency analysis uses the principle of a vibrating fork that is, when a frequency of audibility range is repeatedly vibrated onto an implant, depending on the bone implant interface resonance occurs. The stronger interface, the higher the frequency.
Currently, there are two RFA instruments in clinical use: Ostell (Integration Diagnostics) and Implomates (Biotech One).
The first commercial product of the RFA, the first generation was Osstell™ introduced in 2001(Osstell AB, Goteborg, Sweden) which was, followed by second generation Osstell™ Mentor in 2004 and recently in 2009 Osstell™ implant stability quotient (ISQ) was introduced.
The first generation Ostell uses electronic technology and other devices (Osstell™ Mentor, Osstell™ ISQ) use magnetic technology.
First generation-electronic technology resonance frequency analyzer (Osstell™)
This early model Osstell™ produces alternating sine waves in a specific frequency range by uniform amplitude and makes the transducer connected to the implant or abutment vibrate under 1 mm like an electronic tuning fork. A cantilever small beam connected to the transducer has 2 piezo-ceramic elements attached. One of them receives the signal and vibrates the transducer and the other passes this vibration to the RFA [Figure 1]. Values are displayed on the monitor from 0 to 100. The value of 100 signifies the highest stability.[18,19]
Figure 1.
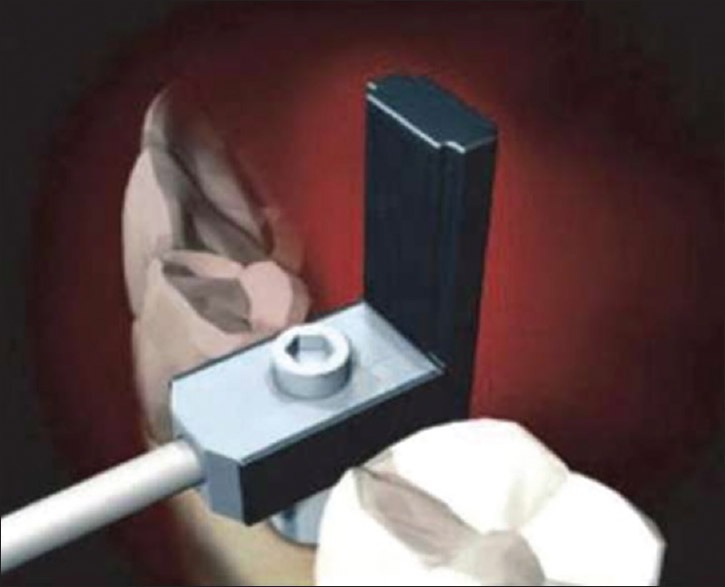
First Generation Osstell –Electronic technology Resonance Frequency Analyzer
The values are displayed by graphs on the computer monitor or expressed by values between 4500 and 8500 Hz. The obtained output is then calculated by the equation below.
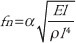
Where, fn is the resonance frequency of the beam, l is the effective vibration length of the beam, E is the young's modulus, I is the moment of inertia, ρ is the mass, α the constant that increases as peri-implant bone density increases. Therefore, when osseointegration is achieved, resonance frequency increases since α value increases. ‘l’ signifies the length of the implant above the bone. Hence, as bone is resorbed, this value increases and thus resonance frequency decreases. In other words, ISQ is affected by the effective implant length, type of bone at implant site and bone density.
Second generation and third generation-magnetic technology resonance frequency analyzer (Osstell™ Mentor, Osstell™ implant stability quotient)
Resonance frequency between 3.5 KHz and 8.5 KHz formed from the magnetic field is converted into ISQ values by Osstell Mentor™. Osstell Mentor™ has a magnetic peg which is fixed to the implant fixture or abutment by a screw below. When magnetic resonance frequency is released from the probe, the magnetic peg is activated. The activated peg starts to vibrate, and the magnet induces electric volt into the probe coil and the electric volt is sampled by the magnetic RFA.
After the osteotomy preparation and implant placement, prior to the placement of cover screw [Figure 2] the smart peg (respective for the implant system) is placed onto the implant with the help of the smart peg mount [Figure 3]. The mount is removed after securing the smart peg in the implant [Figure 4]. The RFA instrument is activated and the probe tip is placed maintaining a 1–3 mm distance from the smart peg, at an angle of 90°, and 3 mm above the soft tissue, [Figure 5] otherwise the measured value may be affected. The values are expressed as numbers between 1 and 100 in ISQ. Readings are taken in two directions-mesiodistal, and buccolingual directions since bone is not uniform all over. And the average of the two is recorded as the ISQ.[20] Two readings are taken in each direction [Figures 6 and 7].
Figure 2.
Implant placed
Figure 3.
Smart peg placed onto the implant with the help of the mount
Figure 4.
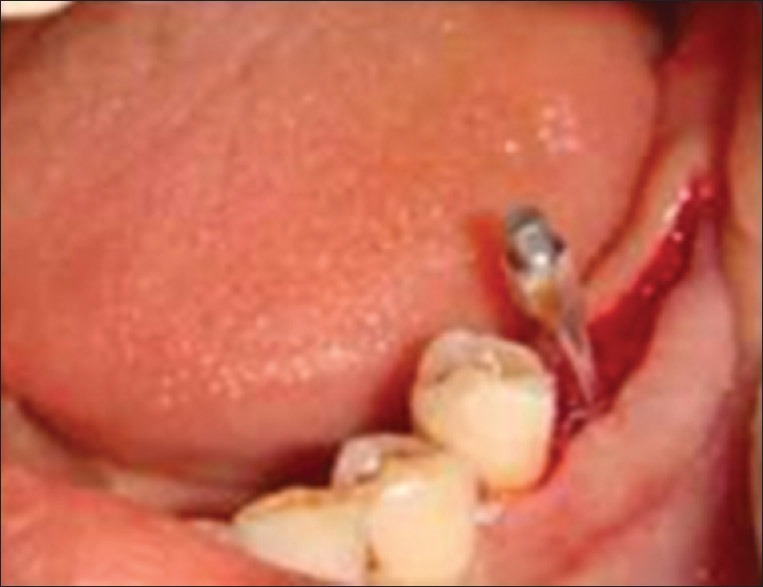
Smart peg in the implant
Figure 5.
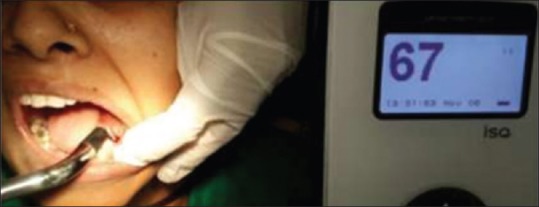
Activated probe tip gives the RFA reading
Figure 6.
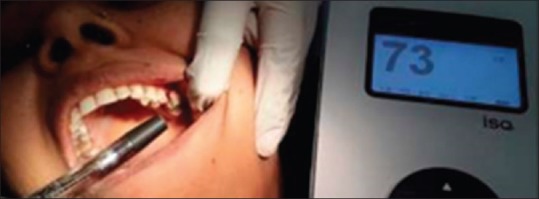
Buccolingual RFA reading
Figure 7.
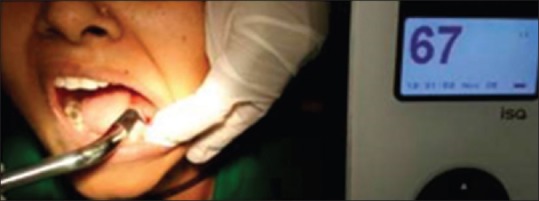
Mesiodistal RFA reading
Factors influencing implant stability quotient/resonance frequency analysis values
It has been reported that ISQ is affected by implant diameter, surface, form, bone contact ratio, implant site, implant system, surgical procedure, bone quality and bone height[8] (Table 1 shows the various studies conducted on factors affecting RFA and its reliability, reproducibility and repeatability). RFA is influenced by the changes in the interface stiffness, and is affected in three aspects.[2,3] First, bone-implant surface stiffness affects RFA, which increases through bone healing and remodeling. Second, the stiffness of bone itself and bone density as well as the ratio of cortical and cancelous bone affects RFA. Finally, the stiffness of implant components can act as a variable, and it is affected by the interlocking structures and the composing elements of the materials.
Table 1.
Studies on the reliability, repeatability, and reproducibility of RFA

Bone and implant surface stiffness may be affected using a small-diameter final drill, changes in surgical techniques such as bone compaction technique, self-tapping design implants, and wide tapered implants, but not by implant length.
Histomorphologic studies report that the RFA value has a high correlation with the bone-implant contact. On the contrary, other reports claim that there is no correlation between the bone density and ISQ. Therefore, RFA signifies the bone anchorage of implants but the relation of RFA and bone structure is not yet clear.[23,24] Such diverse results showed RFA value decreases during the first 2 weeks after implant placement, and this change can be related to early bone healing such as biological change and marginal alveolar bone resorption. Bone remodeling reduces primary bone contact and in the early stage after implant placement, the formation of bony callus and increasing lamellar bone in the cortical bone causes major changes in bone density. Thus, in the healing process, primary bone contact decreases and secondary bone contact increases.[25] Furthermore, the three-dimensional implant-bone contact is displayed two-dimensionally in the histological sample and BIC has possibility of inaccuracy to signify bone-implant contact.[26,27] The relationship of bone structure and RFA is not fully understood. Since primary stability is affected by bone volume or bone trabeculae structure, as well as cortical bone thickness and density, the effect of bone quality on implant stability, cannot be explained by bone.
Applications
Helps in making loading decisions: The prosthetic phase can be planned when an ISQ of 70 or more has been reached. However, a high initial stability does not necessarily mean the secondary stability will also be the same or even more since bone remodeling is variable. Furthermore, a lower initial stability does not indicate implant failure since following the waiting period of osseointegration there is an increase in bone-implant contact.[21,22] Hence, an ISQ of more than 70 achieved over the waiting period of osseointegration would be more valuable[2,3]
Warns of impeding failure: An ISQ of 55 or an ISQ which is gradually declining over the waiting period suggests of an impending failure and warns to take up necessary measures
Case documentation: Makes record maintenance and communication easy. It is of great assistance in medicolegal cases.
Limitations [Table 1]
The instrument is relatively expensive.
The smart pegs add to the additional cost.
The smart peg is respective for each implant system.
Cannot be used to record implant stability in a single piece implant (requires an additional attachment).
Cannot be used when the implant is subcrestally placed.
CONCLUSION
Resonance frequency analysis serves as a user-friendly and reliable, noninvasive method that can be used clinically as a diagnostic tool to measure implant stability during the healing stages, and the subsequent follow-up periods. However, further studies in the form of randomized clinical trials and longitudinal studies are required to establish the efficacy.[44]
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Branemark PI, Zarb G, Albrektsson T. Tissue-Integrated Prosthesis: Osseointegration in Clinical Dentistry. Chicago: Quintessance; 1985. [Google Scholar]
- 2.Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. J Periodontol. 2000;47:51–66. doi: 10.1111/j.1600-0757.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- 3.Sennerby L, Meredith N. Resonance frequency analysis: Measuring implant stability and osseointegration. Compend Contin Educ Dent. 1998;19:493–8. [PubMed] [Google Scholar]
- 4.Sennerby L, Roos J. Surgical determinants of clinical success of osseointegrated oral implants: A review of the literature. Int J Prosthodont. 2008;11:408–20. [PubMed] [Google Scholar]
- 5.Gapski R, Wang HL, Mascarenhas P, Lang NP. Critical review of immediate implant loading. Clin Oral Implants Res. 2003;14:515–27. doi: 10.1034/j.1600-0501.2003.00950.x. [DOI] [PubMed] [Google Scholar]
- 6.Lioubavina-Hack N, Lang NP, Karring T. Significance of primary stability for osseointegration of dental implants. Clin Oral Implants Res. 2006;17:244–50. doi: 10.1111/j.1600-0501.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- 7.Molly L. Bone density and primary stability in implant therapy. Clin Oral Implants Res. 2006;17(Suppl 2):124–35. doi: 10.1111/j.1600-0501.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 8.Atsumi M, Park SH, Wang HL. Methods used to assess implant stability: Current status. Int J Oral Maxillofac Implants. 2007;22:743–54. [PubMed] [Google Scholar]
- 9.Brunski J. Push out (pull), tensile and reverse torque tests of bone implant interfaces. Clin Oral Implant Res. 2006;1:33–40. [Google Scholar]
- 10.Friberg B, Sennerby L, Meredith N, Lekholm U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int J Oral Maxillofac Surg. 1999;28:297–303. [PubMed] [Google Scholar]
- 11.Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998;11:491–501. [PubMed] [Google Scholar]
- 12.Gupta RK, Padmanabhan TV. Resonance frequency analysis. Indian J Dent Res. 2011;22:567–73. doi: 10.4103/0970-9290.90300. [DOI] [PubMed] [Google Scholar]
- 13.Da Cunha HA, Francischone CE, Filho HN, de Oliveira RC. A comparison between cutting torque and resonance frequency in the assessment of primary stability and final torque capacity of standard and TiUnite single-tooth implants under immediate loading. Int J Oral Maxillofac Implants. 2004;19:578–85. [PubMed] [Google Scholar]
- 14.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 15.Ritter L, Elger MC, Rothamel D, Fienitz T, Zinser M, Schwarz F, et al. Accuracy of peri-implant bone evaluation using cone beam CT, digital intra-oral radiographs and histology. Dentomaxillofac Radiol. 2014;43:20130088. doi: 10.1259/dmfr.20130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Künzel A, Golubovic V, Mihatovic I, John G, Chen Z, et al. Accuracy of peri-implant bone thickness and validity of assessing bone augmentation material using cone beam computed tomography. Clin Oral Investig. 2013;17:1601–9. doi: 10.1007/s00784-012-0841-y. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Sato D, Yoneda S, Ito D, Kondo H, Kasugai S. Relevance of resonance frequency analysis to evaluate dental implant stability: Simulation and histomorphometrical animal experiments. Clin Oral Implants Res. 2008;19:9–14. doi: 10.1111/j.1600-0501.2007.01419.x. [DOI] [PubMed] [Google Scholar]
- 18.Schulte W. The new periotest method. J Compend Contin Dent. 1998;12:410–7. [Google Scholar]
- 19.Meredith N, Alleyne D, Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261–7. doi: 10.1034/j.1600-0501.1996.070308.x. [DOI] [PubMed] [Google Scholar]
- 20.Park JC, Lee JW, Kim SM, Lee JH. Implant stability - Measuring devices and randomized clinical trial for ISQ value change pattern measured from two different directions by magnetic RFA. J Implant Dent Rapidly Evolving Pract. 2011;5:111–30. [Google Scholar]
- 21.Huang HM, Chiu CL, Yeh CY, Lin CT, Lin LH, Lee SY. Early detection of implant healing process using resonance frequency analysis. Clin Oral Implants Res. 2003;14:437–43. doi: 10.1034/j.1600-0501.2003.00818.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang SM, Shin SY, Kye SB. Relationship between implant stability measured by resonance frequency analysis (RFA) and bone loss during early healing period. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e12–9. doi: 10.1016/j.tripleo.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Alsaadi G, Quirynen M, Michiels K, Jacobs R, van Steenberghe D. A biomechanical assessment of the relation between the oral implant stability at insertion and subjective bone quality assessment. J Clin Periodontol. 2007;34:359–66. doi: 10.1111/j.1600-051X.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 24.Huwiler MA, Pjetursson BE, Bosshardt DD, Salvi GE, Lang NP. Resonance frequency analysis in relation to jawbone characteristics and during early healing of implant installation. J Clin Oral Implants Res. 2007;18:275–80. doi: 10.1111/j.1600-0501.2007.01336.x. [DOI] [PubMed] [Google Scholar]
- 25.Perrotti V, Piattelli A, Iezzi G. Mineralized bone-implant contact and implant stability quotient in 16 human implants retrieved after early healing periods: A histologic and histomorphometric evaluation. Int J Oral Maxillofac Implants. 2010;25:45–8. [PubMed] [Google Scholar]
- 26.Zhou Y, Jiang T, Qian M, Zhang X, Wang J, Shi B, et al. Roles of bone scintigraphy and resonance frequency analysis in evaluating osseointegration of endosseous implant. Biomaterials. 2008;29:461–74. doi: 10.1016/j.biomaterials.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Ostman PO, Hellman M, Wendelhag I, Sennerby L. Resonance frequency analysis measurements of implants at placement surgery. Int J Prosthodont. 2006;19:77–83. [PubMed] [Google Scholar]
- 28.Gupta RK, Padmanabhan TV. An evaluation of the resonance frequency analysis device: Examiner reliability and repeatability of readings. J Oral Implantol. 2013;39:704–7. doi: 10.1563/AAID-JOI-D-11-00099. [DOI] [PubMed] [Google Scholar]
- 29.Monje A, Ortega-Oller I, Galindo-Moreno P, Catena A, Monje F, O’Valle F, et al. Sensitivity of resonance frequency analysis for detecting early implant failure: A case-control study. Int J Oral Maxillofac Implants. 2014;29:456–61. doi: 10.11607/jomi.3357. [DOI] [PubMed] [Google Scholar]
- 30.Bertl MH, Emshoff R, Celar A, Crismani AG. Inter- and intraobserver variability in resonance frequency analysis of palatal implants – a technical note. Int J Oral Maxillofac Implants. 2013;28:e215–9. doi: 10.11607/jomi.2831. [DOI] [PubMed] [Google Scholar]
- 31.Barikani H, Rashtak S, Akbari S, Badri S, Daneshparvar N, Rokn A. The effect of implant length and diameter on the primary stability in different bone types. J Dent (Tehran) 2013;10:449–55. [PMC free article] [PubMed] [Google Scholar]
- 32.Guler AU, Sumer M, Duran I, Sandikci EO, Telcioglu NT. Resonance frequency analysis of 208 Straumann dental implants during the healing period. J Oral Implantol. 2013;39:161–7. doi: 10.1563/AAID-JOI-D-11-00060. [DOI] [PubMed] [Google Scholar]
- 33.Nienkemper M, Wilmes B, Panayotidis A, Pauls A, Golubovic V, Schwarz F, et al. Measurement of mini-implant stability using resonance frequency analysis. Angle Orthod. 2013;83:230–8. doi: 10.2319/043012-354.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagliani L, Sennerby L, Petersson A, Verrocchi D, Volpe S, Andersson P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants: An in vitro study. J Oral Rehabil. 2013;40:221–7. doi: 10.1111/joor.12024. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad OK, Kelly JR. Assessment of the primary stability of dental implants in artificial bone using resonance frequency and percussion analyses. Int J Oral Maxillofac Implants. 2013;28:89–95. doi: 10.11607/jomi.2554. [DOI] [PubMed] [Google Scholar]
- 36.Hong J, Lim YJ, Park SO. Quantitative biomechanical analysis of the influence of the cortical bone and implant length on primary stability. Clin Oral Implants Res. 2012;23:1193–7. doi: 10.1111/j.1600-0501.2011.02285.x. [DOI] [PubMed] [Google Scholar]
- 37.Geckili O, Bilhan H, Cilingir A, Mumcu E, Bural C. A comparative in vitro evaluation of two different magnetic devices detecting the stability of osseo-integrated implants. J Periodontal Res. 2012;47:508–13. doi: 10.1111/j.1600-0765.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 38.Bertl MH, Weinberger T, Schwarz K, Gruber R, Crismani AG. Resonance frequency analysis: A new diagnostic tool for dental ankylosis. Eur J Oral Sci. 2012;120:255–8. doi: 10.1111/j.1600-0722.2012.00959.x. [DOI] [PubMed] [Google Scholar]
- 39.Simunek A, Kopecka D, Brazda T, Strnad I, Capek L, Slezak R. Development of implant stability during early healing of immediately loaded implants. Int J Oral Maxillofac Implants. 2012;27:619–27. [PubMed] [Google Scholar]
- 40.Karakoca-Nemli S, Aydin C, Yilmaz H, Sarisoy S. Stability measurements of craniofacial implants by means of resonance frequency analysis: 1-year clinical pilot study. Int J Oral Maxillofac Implants. 2012;27:187–93. [PubMed] [Google Scholar]
- 41.Stoker GT, Wismeijer D. Immediate loading of two implants with a mandibular implant-retained overdenture: A new treatment protocol. Clin Implant Dent Relat Res. 2011;13:255–61. doi: 10.1111/j.1708-8208.2009.00210.x. [DOI] [PubMed] [Google Scholar]
- 42.Turkyilmaz I, Company AM. Sensitivity of resonance frequency analysis method to assess implant stability. N Y State Dent J. 2011;77:44–9. [PubMed] [Google Scholar]
- 43.Ohta K, Takechi M, Minami M, Shigeishi H, Hiraoka M, Nishimura M, et al. Influence of factors related to implant stability detected by wireless resonance frequency analysis device. J Oral Rehabil. 2010;37:131–7. doi: 10.1111/j.1365-2842.2009.02032.x. [DOI] [PubMed] [Google Scholar]
- 44.Herrero-Climent M, Albertini M, Rios-Santos JV, Lázaro-Calvo P, Fernández-Palacín A, Bullon P. Resonance frequency analysis-reliability in third generation instruments: Osstell mentor®. Med Oral Patol Oral Cir Bucal. 2012;17:e801–6. doi: 10.4317/medoral.17861. [DOI] [PMC free article] [PubMed] [Google Scholar]


