Abstract
This article reviews the dental literature concerning the potential impact of the removable prosthesis (RP) on the health status of patients with certain systemic diseases. Literature was surveyed using Medline/PubMed database resources, as well as a manual search, up to 2015 to identify appropriate articles that addressed the aim of this review. The research used keywords about associations between RP and six pathologies: Human immunodeficiency virus infection, diabetes mellitus, pulmonary diseases, gastric-Helicobacter pylori, cancer, and cardiovascular diseases. Analysis of literature showed that in patients with dentures having one or more of the six general conditions listed, Candida albicans organism is more frequently found in the oral flora compared to healthy denture wearer. Although causality has not been established and pending further research on this topic, the hygienic practices necessary to minimize the risk of numerous pathologies should be strengthened in the case of these patients, all the more in the presence of physical or psychological disability. The relationship between the general diseases and increasing of oral candidiasis denture patients is not explained. Therefore, attention to oral hygiene and professional care for removing C. albicans may be beneficial in these medically compromised patients.
Key Words: Cardiovascular diseases, diabetes mellitus, gastrointestinal diseases, human immunodeficiency virus, pulmonary diseases, removable prosthesis
INTRODUCTION
In medically compromised patients, the knowledge of pathologies related or possibly induced by prosthetic microbial plaque is essential for maintaining the oral health and preventing possible complications.[1,2]
Denture plaque (DP) is defined as a dense microbial layer comprised of microorganisms and their metabolites.[3] It contains more than 1011 organisms per gram in wet weight and has essentially the same structure as dental plaque on natural teeth.[4,5] The composition of these microbial flora also resembles that of dental plaque, except for an increased number of Candida spp. Candida biofilm on dentures leading to a decrease in the bacterial diversity and then to a qualitative change in the composition of the oral microbiota.[6] DP containing Candida could give rise to denture-induced stomatitis, root caries, and periodontitis.[7,8,9] In addition, if the oral mucosa is weakened, the friction of the prosthesis can facilitate the breaking of the epithelial barrier and increase the risk of the passage of germs into the bloodstream.[10] Moreover, the continuous swallowing or respiration of microorganisms from DP exposes patients, particularly the immunocompromised host or polymedicated elderly, to the risk of unexpected infections.
Clinical studies are needed to confirm the existence of this risk and demonstrate the effectiveness of prosthetic biofilm control in these patients.[11,12]
The aim of this paper is to investigate the putative risks related to medically compromised patients wearing a removable prosthesis (RP).[13,14]
In this way, the purpose of our literature analysis is to review the knowledge about this subject with. An electronic database and manual searches were undertaken to identify the impact of prostheses on the oral flora of patients with one or more common six general pathologies. Association only between denture wearers (DWs) and in order “human immunodeficiency virus (HIV),” “diabetes mellitus,” “diabetic,” “pulmonary, pneumonia, diseases” “gastric-Helicobacter pylori,” “cancer,” and “cardiovascular diseases,” are conducted. Two reviewers independently analyzed the article selection. We found 232 references directly or indirectly concerned by the subject.
First, we will detail the literature data according to each of the systemic pathologies, and we will discuss these results in a second part.
MATERIALS AND METHODS
In this work, we chose to treat the most documented systemic diseases related to this issue. Moreover, it seems impractical to consider a comprehensive review of all systemic diseases. Based on recent publications, we limited our search to the main diseases that can be linked to a removable denture (RD). The six diseases considered are common diseases in prosthetic wearers and are a major risk factor for older patients about their survival.
An initial search was performed using PubMed© engine, limiting the search for papers published up to 2015. Multiple keyword combination was used: “RP,” “DW,” “HIV,” [Figure 1] “diabetes mellitus,” “diabetic, Type I and II,” “pulmonary diseases” “gastric-H. pylori,” “cancer,” “cardiovascular diseases,” and “pneumonia diseases.”
Figure 1.
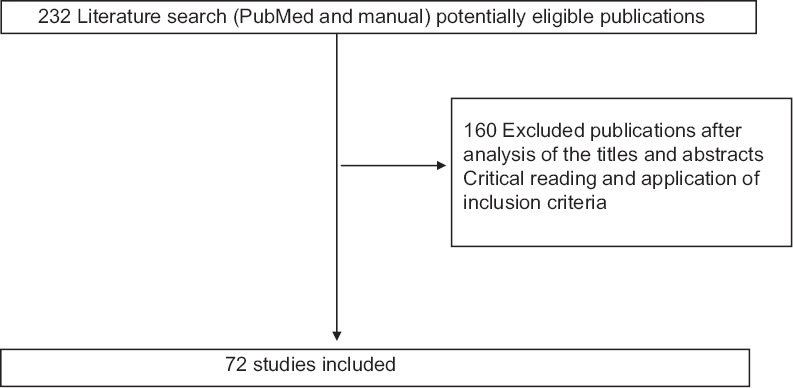
Selection of articles included in the review of the literature
RP and HIV or DW and HIV
RP and diabetes mellitus or DW and diabetes mellitus
RP and diabetic Type I and II or DR and diabetic Type I and II
RP and pulmonary diseases or DW and pulmonary diseases
RP and gastric-H. pylori or DW and gastric-H. pylori
RP and cardiovascular diseases or DW and cardiovascular diseases
RP and cancer or DW and cancer
RP and Pneumonia diseases or DW and pneumonia diseases.
Another manual search was done using Embase© and other electronic resources. Finally, all available papers were manually refined to exclude papers not pertinent to the review topic. All types of articles were included except for editorials, case reports, and statements of personal opinion.
Inclusion criteria
Articles written in English
Articles were included if they met the following criteria
Articles on studies of RD and systemic diseases.
Articles on studies of the relationship between the RD (resin denture) and (1) human immunodeficiency virus infection (HIV), (2) diabetes mellitus, diabetes type I and II, (3) “pulmonary diseases, (4) gastric-H. pylori, (5) cancer, (6) cardiovascular diseases, and (7) pneumonia diseases.”
In those reports, the materials investigated was the resin denture.
Exclusion criteria
Were excluded from this review:
Clinical case reports;
Articles written in languages other than English
Articles on the studies of denture other than the RD
Articles about healthy patients DWs.
Human immunodeficiency virus-infected denture wearer
Surface irregularities of acrylic resin in dentures make the entrapment of microorganisms, including Candida colonization easier. In HIV-infected patients who wear RPs, this fact takes on critical importance because of their immunosuppression.[15] For these patients, the influence of complete or partial removable dental prostheses (RDPs) increase the frequency of Candida albicans isolated from the mouth. The use of RDPs was an important factor in the isolation of C. albicans among HIV+ patients, and CD4 level seems to play a role in the presence of oral candidiasis.[16]
Diabetic denture wearer
Between the Types I and II, the prevalence of Type II diabetes (noninsulin-dependent diabetes mellitus) is the most common form of the disease concerning DW.[17] Detected most of the time at about 40–50 years, this disease is frequent. The levels of this disease are increasing worldwide, to rise to 5.4% of the population by 2025.[17] It can have serious consequences on the health if they are not correctly taken care, and unfortunately, since one-third of patients with diabetes are undiagnosed.[18] Dental professionals must play an important role in diagnosing and managing patients with diabetes.[19] Moreover, diabetes mellitus is positively correlated with edentulism.[20] Both diabetes Type II could be one factor that increase denture stomatitis (DS)[21] with periodontal diseases. (DS) is more common and more severe in patients with noninsulin-dependent diabetes mellitus compared to DWs with normal glucose metabolism.[22,23]
The first microbial etiological agent of DS was the yeast C. albicans, but nonalbicans species were also incriminate in the disease like Candida glabrata has dominating as a notable pathogenic agent in the oral mucosa[24,25,26] C. glabrata was currently find like the nonalbicans Candida species isolated in oral candidiasis in patients with diabetes, advanced cancer, and HIV infection.[27,28]
The occurrence rate of oral C. albicans in patients with dentures (diabetic and nondiabetic patients) was higher than in patients without dentures, while Staphylococcus spp. was isolated more frequently from the DP of diabetic patients, compared with nondiabetic patients.[29] In the case of Type II diabetes mellitus, there is a positive correlation between oral candidiasis in denture-bearing mucosa and elevated blood glucose levels in complete DWs. Thus, we have deduced that the elevated blood glucose induce more oral candidiasis. The oral hypoglycemic drug therapy has a positive effect in controlling oral Candida colonization in complete DWs.[30] Controlling the blood glucose level in uncontrolled Type II diabetic DWs with oral hypoglycemic drugs, can reduce increased colonization of Candida in denture-bearing mucosa and thus enhance improved health.[30] Therefore the role of the dentist is to educate patients in maintaining the hygiene of the prosthesis. In addition, patients should be advised of the importance of controlling their glucose level in the blood, to regularly clean their dentures and keep them dry overnight. This precaution seems appropriate to control the Candida proliferation.
Cardio-vascular diseases and denture wearer
The cardiovascular diseases include both, coronary heart disease identified by subjects who reported a history of coronary artery disease or myocardial infarction, and cardiovascular risk conditions (hyperlipidemia, hypertension, diabetes, and tobacco use). No evident relationship exists between cardiovascular disease survival and RD, but the number (quantity) of remaining teeth and their maintenance (quality) and the removal of potential inflammatory foci (e.g., pericoronitis or retained root tips) may positively impact cardiovascular survival.[31] In another study with about 400 subjects (between 29 and 70 years) with RDs, hypertension was the most common disease.[32] Among DWs, periodontitis is a bacterial infection common in dentate individuals, and denture stomatitis is a predominantly fungal infection.[33]
Both infections may increase a patient's risk for bacterial infection dissemination. This is may in turn increase the risk of chronic, inflammatory-based systemic diseases like atherosclerotic coronary disease[34] stroke, and hypertension.[35]
It appears that invasive oral pathogens trigger a systemic inflammatory response via mediators released by the cardiovascular system and liver, making the patient at increased risk for these diseases.[36] They may be introduced into wounds created by dental manipulation or treatment, and thus may adhere to heart valves and initiate subacute bacterial endocarditis.[37] In summary, the presence of cardiovascular disease with the DW have a life expectancy less than the partial DWs and less than dentate patients.[31] The determining factor seems to be the hygiene and maintenance, with the recommendations of oral healthcare programs.[31,38]
Cancer and denture wearer
The upper aerodigestive tract is an area at high risk of multiple primary cancers related inflammation.[39,40] The occurrence rate of oral C. albicans in patients with dentures (cancer and noncancer patients) was higher than in patients without dentures.[29] C. albicans occurs more frequently in patients taking antibiotics or immunosuppressant or patients who underwent radiotherapy and/or chemotherapy.[41] Staphylococcus spp. and Gram negative enteric bacilli were isolated more often from the DP, palate and tongue dorsa of cancer patients than from patients without cancer.[29]
In some cases, inflammatory conditions are present before a malignant change occurs, in other cases; an oncogenic change induces an inflammatory microenvironment that promotes the development of tumors.[42,43] In all these situations, the hygienic care is recommended.[44,45]
Pneumonia and denture wearer
Several studies show that lung infections may be related to oral bacteria[44,46] however, they emphasize the rarity of Candida pneumonia. This is an area of study that would benefit from the further investigation as it is known that respiratory pathogens can colonize DP.[47]
Denture wearing during sleep is associated not only with oral inflammation and microbial burden but also with incident pneumonia.[48] Oral care may be useful in preventing pneumonia in older patients in nursing homes, in this way Japan study of over 400 institutionalized patients, of whom 163 were wearing dentures, daily oral hygiene and denture hygiene performed by caregivers was reported to result in reduced pneumonia, febrile illness, and death from pneumonia over 2 years of monitoring.[49] It is important that oral management is performed by the caregivers for the prevention of pneumonia by development and maintenance of an oral hygiene program.[50]
Poor oral health can lead to denture colonization by respiratory pathogens and serve as a reservoir for these organisms;[47] inversely, reducing oral biofilms can reduce the risk of pneumonia in high risk populations.[51] Optimizing oral hygiene may prevent pneumonia in vulnerable patients.[51,52] Patients DW, with the chronic obstructive pulmonary disease, have the lower airways colonized with pathogenic bacteria in a stable period of the disease and during exacerbations. These patients showed a greater frequency of prosthetic stomatitis complicated by mucosal infections compared to healthy subjects.[53]
Indeed, the use of an RD, in the case of mucosal inflammation, promotes superficial candidiasis.[54] Conversely, we admit the possibility that oral microbial colonization, including prosthetic surfaces, can serve as a reservoir for swarming pulmonary circulation that is capable of penetrating the esophageal air channels. In this microbial population, commensal yeast proliferates, adheres to different receptors, develops, and promotes the installation of candidiasis.[55]
Gastric-Helicobacter pylori and denture wearer inflammation
Inflammation of the oral cavity caused by bacteria or fungi can be accompanied by gastric inflammation, this particularly in the presence of denture stomatitis, hyperplasia both fibrosa, as well as papillary showed in nearly 100% gastric-H. pylori infection, suggesting that gastric-H. pylori infection affects oral mucosa at a distance, as yet, unknown mechanism.[56]
H. pylori eradication from the oral cavity is more difficult than from the stomach, if the bacterium survives the antibacterial therapy in the oral cavity, it would be able to re-infect the stomach in a few weeks. Oral health and oral hygiene practices seem unlikely to increase the efficacy of H. pylori eradication from the stomach.[57] Contrary, long-term professional dental plaque control was associated with less gastric re-infection by H. pylori, suggesting that DP control may help to prevent H. pylori-induced gastric disease or re-infection.[58] Although after antibiotic therapy, H. pylori in dental plaque may represent a risk factor for gastrointestinal re-infection and ulcer relapse.[59]
DISCUSSION
Our choice of six systemic pathological disorders was dictated to us by the literature search. Indeed, by combining many diseases with wearing an RP we found that there are currently only a few publications on this subject. Thus, our investigation allowed us to retain the six mentioned pathologies; these are among the most common and represent a significant cause of death each year (WHO). Our aim is therefore just as for periodontal disease, to arouse the attention of health professionals on the need to take into account that wearing a removable device in patients with any of these six systemics diseases requires focus on hygiene and prosthetic followed. Many other pathologies such as malaria, asthma, are likely to be affected by association with the wearing of an RP, but did not result in any publications.
The microbiology of DP has received little attention in comparison with dental plaque, yet it differs in location and composition.[60] DP and poor denture hygiene are associated with stomatitis (Candida infection), and may also serve as a reservoir of potentially infectious pathogens.
Within the oral cavity, RP are a microbial vector that may affect the delicate balance of the ecosystem of vulnerable patients[61] [Figures 2 and 3], including patients with chronic diseases that are not considered infectious but whose pathophysiology involve microbiota (cancer, obesity, inflammation, diabetes, certain cardiovascular diseases, and neuropsychiatric disorders).[62,63,64,65,66] Denture related stomatitis, a periodontal disease with respiratory[51] and/or digestive infections[59] are susceptible to infectious disease and need to be monitored.
Figure 2.
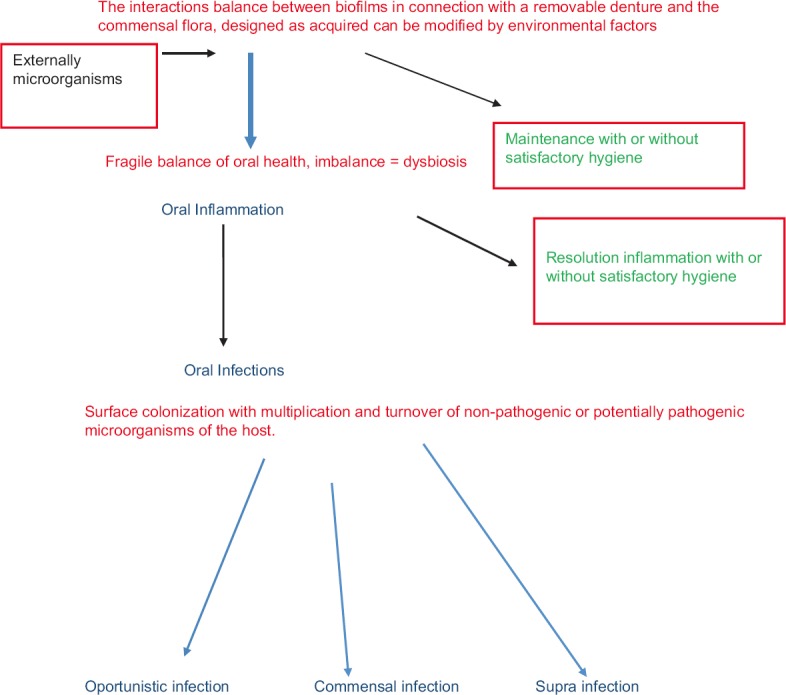
Schema of the influence of oral hygiene and microorganisms adhesion on removable denture
Figure 3.
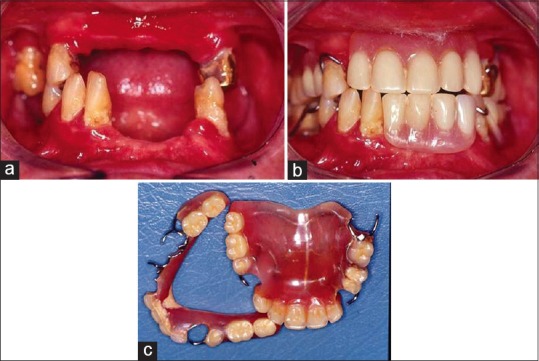
(a) Mouth of aging patient without hygiene, mucosal reactions (gingivitis, periodontal disease, residual ridge resorption, and caries). (b) Unsuitable removable prostheses in the mouth. (c) Removable prostheses up and down of patient
The systematic screening of at-risk subjects requires an examination and history of the disease. Once identified, these patients should receive special attention.[67]
Immunocompromised and polymedicated patients can see their quality of life affected by ingestion or aspiration of microorganisms from the prosthetic microbial plaque.[68] Therefore, we should be vigilant and monitor the flora while controlling it long-term by appropriate hygienic means.[69,70] Indeed biofilms, dental plaque, and DP cannot be eliminated.[71] Only the pathogenic nature of these biofilms can be controlled by the reduction of the total microbial load, with appropriate oral hygiene methods that include daily brushing, flossing, and rinsing with antimicrobial mouth rinses.[71] The prevention and management of the associated sequelae, including denture disorders, justify the possible impact on specific systemic disorders.[13]
Clinical studies have reported that disrupting the biofilm may be more important than the use of antifungal or antimicrobial agents in the prevention and treatment of denture stomatitis.[72,73]
Preventing the formation of a biofilm on the surface of the dentures is important to maintain hygiene compatible with the balance of oral health. Surface free energy, contact angle, and surface roughness control the initial adherence of bacteria to the denture.[71]
For preventing polishing techniques on the surface roughness of acrylic denture base resin was the most effective[74] and resin monomer has antimicrobial effects against oral bacteria.[75]
Basic hygiene is even more important for high risk patients as patients with xerostomia, those with a history of denture stomatitis, and those with motor disabilities, oral cancer, diabetes, immunosuppressant, and pulmonary and gastrointestinal diseases. Studies have similarly identified that removable partial dentures are susceptible to plaque accumulation,[76] and this has been attributed to a lack of awareness of the need for good denture hygiene by patients and the lack of a regular recall system.[77]
Many older vulnerable adults that wear dentures have oral microbial ecology determined by their diet, the latter is usually high in sugar. In these conditions, the local environment change. The interactions between the resident flora and the new arrivals, serve to promote or discourage the survival and growth of the individual species.[78] Among the nutrient sources, carbohydrates play an important role in the pathogenesis of fungal infections.[79,80] Dietary carbohydrates can modulate the development of C. albicans biofilms on the denture material surface.[81] During the early stages of biofilm development, the presence of glucose increased metabolic activity. For the mature biofilms, starch with glucose or sucrose showed the highest metabolic activity. Finally, diet can influence the proportions of different bacterial species later in biofilm development.[82]
CONCLUSION
The general condition of an individual throughout his or her life influences their oral ecosystem. Dentures that are initially compatible with the local and general environment can, over time, directly or indirectly cause tissue injury (inflammation, infection, ulceration, and/or hyperplasia). Two complementary therapeutic approaches exist to limit microbial accumulation in connection with RP. The first one is to limit the adhesion phenomenon on RP surfaces. The second consists of establishing and maintaining proper oral hygiene to eliminate regular deposits. We suggest better alertness in patients at-risk and maintenance particularly program in DWs. A quarterly monitoring is introduced with the reminder of the oral and prosthetic hygiene boards as well as the verification of the effectiveness of those ones.
Complications related to the use RD base resin require not only a functional but also a biological strategy (hygienic and analysis of bacterial and mycological screening) especially in at-risk patients (surveillance of immune, cardiovascular, metabolic, lung, and/or digestive diseases). Awareness of the responsibility of the oral health professional is necessary, as well as the obligation to inform patients. The health status of these patients and current literature encourage us to take all necessary measures to minimize the risk of inflammation associated with the use of RDs.
Finally, clinical studies are needed to better identification of the impact of the port of prostheses on the patient's health, especially the most vulnerable among them.
Dentures may present a putative risk of infection, especially for patients with lung and digestive diseases. It seems important to prevent this risk by means of information and patient education. Patient awareness of the nature of the risk and its consequences should improve the control of prosthetic biofilm and reduce infection rates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yang Y, Zhang H, Chai Z, Chen J, Zhang S. Multiple logistic regression analysis of risk factors associated with denture plaque and staining in Chinese removable denture wearers over 40 years old in Xi’an – A cross-sectional study. PLoS One. 2014;9:e87749. doi: 10.1371/journal.pone.0087749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preshaw PM, Walls AW, Jakubovics NS, Moynihan PJ, Jepson NJ, Loewy Z. Association of removable partial denture use with oral and systemic health. J Dent. 2011;39:711–9. doi: 10.1016/j.jdent.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Nikawa H, Hamada T, Yamamoto T. Denture plaque – Past and recent concerns. J Dent. 1998;26:299–304. doi: 10.1016/s0300-5712(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 4.Budtz-Jørgensen E. Oral mucosal lesions associated with the wearing of removable dentures. J Oral Pathol. 1981;10:65–80. doi: 10.1111/j.1600-0714.1981.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 5.Walter B, Frank RM. Ultrastructural relationship of denture surfaces, plaque and oral mucosa in denture stomatitis. J Biol Buccale. 1985;13:145–66. [PubMed] [Google Scholar]
- 6.Cho T, Nagao J, Imayoshi R, Tanaka Y. Importance of diversity in the oral microbiota including Candida species revealed by high-throughput technologies. Int J Dent 2014. 2014 doi: 10.1155/2014/454391. 454391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gimenez T, Braga MM, Raggio DP, Deery C, Ricketts DN, Mendes FM. Fluorescence-based methods for detecting caries lesions: Systematic review, meta-analysis and sources of heterogeneity. PLoS One. 2013;8:e60421. doi: 10.1371/journal.pone.0060421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Zhang Y, Wu W, Cheng M, Li Y, Cheng R. Prevalence and correlates of dental caries in an elderly population in northeast China. PLoS One. 2013;8:e78723. doi: 10.1371/journal.pone.0078723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X, Rodriguez R, Trinh M, Gunsolley J, Xu P. Oral microbiome of deep and shallow dental pockets in chronic periodontitis. PLoS One. 2013;8:e65520. doi: 10.1371/journal.pone.0065520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulthwaite L, Verran J. Potential pathogenic aspects of denture plaque. Br J Biomed Sci. 2007;64:180–9. doi: 10.1080/09674845.2007.11732784. [DOI] [PubMed] [Google Scholar]
- 11.Sumi Y, Kagami H, Ohtsuka Y, Kakinoki Y, Haruguchi Y, Miyamoto H. High correlation between the Bacterial species in denture plaque and pharyngeal microflora. Gerodontology. 2003;20:84–7. doi: 10.1111/j.1741-2358.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 12.Imsand M, Janssens JP, Auckenthaler R, Mojon P, Budtz-Jørgensen E. Bronchopneumonia and oral health in hospitalized older patients. A pilot study. Gerodontology. 2002;19:66–72. doi: 10.1111/j.1741-2358.2002.00066.x. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich T, Garcia RI. Associations between periodontal disease and systemic disease: Evaluating the strength of the evidence. J Periodontol. 2005;76(11 Suppl):2175–84. doi: 10.1902/jop.2005.76.11-S.2175. [DOI] [PubMed] [Google Scholar]
- 14.Senpuku H, Sogame A, Inoshita E, Tsuha Y, Miyazaki H, Hanada N. Systemic diseases in association with microbial species in oral biofilm from elderly requiring care. Gerontology. 2003;49:301–9. doi: 10.1159/000071711. [DOI] [PubMed] [Google Scholar]
- 15.Perezous LF, Flaitz CM, Goldschmidt ME, Engelmeier RL. Colonization of Candida species in denture wearers with emphasis on HIV infection: A literature review. J Prosthet Dent. 2005;93:288–93. doi: 10.1016/j.prosdent.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Witzel AL, Pires Mde F, de Carli ML, Rabelo GD, Nunes TB, da Silveira FR. Candida albicans isolation from buccal mucosa of patients with HIV wearing removable dental prostheses. Int J Prosthodont. 2012;25:127–31. [PubMed] [Google Scholar]
- 17.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Moore PA, Zgibor JC, Dasanayake AP. Diabetes: A growing epidemic of all ages. J Am Dent Assoc. 2003;134:11S–5S. [PubMed] [Google Scholar]
- 19.Yuen HK, Wolf BJ, Bandyopadhyay D, Magruder KM, Salinas CF, London SD. Oral health knowledge and behavior among adults with diabetes. Diabetes Res Clin Pract. 2009;86:239–46. doi: 10.1016/j.diabres.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felton DA. Edentulism and comorbid factors. J Prosthodont. 2009;18:88–96. doi: 10.1111/j.1532-849X.2009.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Phelan JA, Levin SM. A prevalence study of denture stomatitis in subjects with diabetes mellitus or elevated plasma glucose levels. Oral Surg Oral Med Oral Pathol. 1986;62:303–5. doi: 10.1016/0030-4220(86)90012-5. [DOI] [PubMed] [Google Scholar]
- 22.Farag YM, Gaballa MR. Diabesity: An overview of a rising epidemic. Nephrol Dial Transplant. 2011;26:28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 23.Dorocka-Bobkowska B, Budtz-Jörgensen E, Wloch S. Non-insulin-dependent diabetes mellitus as a risk factor for denture stomatitis. J Oral Pathol Med. 1996;25:411–5. doi: 10.1111/j.1600-0714.1996.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 24.Pereira-Cenci T, Del Bel Cury AA, Crielaard W, Ten Cate JM. Development of Candida-associated denture stomatitis: New insights. J Appl Oral Sci. 2008;16:86–94. doi: 10.1590/S1678-77572008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb BC, Thomas CJ, Whittle T. A 2-year study of Candida-associated denture stomatitis treatment in aged care subjects. Gerodontology. 2005;22:168–76. doi: 10.1111/j.1741-2358.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- 26.Dorocka-Bobkowska B, Konopka K. Susceptibility of Candida isolates from denture-related stomatitis to antifungal agents in vitro . Int J Prosthodont. 2007;20:504–6. [PubMed] [Google Scholar]
- 27.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93:2447–53. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Redding S, Dongari-Bagtzoglou A. Candida glabrata, an emerging oral opportunistic pathogen. Crit Rev Oral Biol Med. 2007;86:204–15. doi: 10.1177/154405910708600304. [DOI] [PubMed] [Google Scholar]
- 29.Daniluk T, Fiedoruk K, Sciepuk M, Zaremba ML, Rozkiewicz D, Cylwik-Rokicka D, et al. Aerobic bacteria in the oral cavity of patients with removable dentures. Adv Med Sci. 2006;51(Suppl 1):86–90. [PubMed] [Google Scholar]
- 30.Ganapathy DM, Joseph S, Ariga P, Selvaraj A. Evaluation of the influence of blood glucose level on oral candidal colonization in complete denture wearers with Type-II diabetes mellitus: An in vivo Study. Dent Res J (Isfahan) 2013;10:87–92. doi: 10.4103/1735-3327.111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janket SJ, Surakka M, Jones JA, Lam A, Schnell RA, Rose LM, et al. Removable dental prostheses and cardiovascular survival: A 15-year follow-up study. J Dent. 2013;41:740–6. doi: 10.1016/j.jdent.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Ercalik-Yalcinkaya S, Özcan M. Association between oral mucosal lesions and hygiene habits in a population of removable prosthesis wearers. J Prosthodont. 2015;24:271–8. doi: 10.1111/jopr.12208. [DOI] [PubMed] [Google Scholar]
- 33.Gendreau L, Loewy ZG. Epidemiology and etiology of denture stomatitis. J Prosthodont. 2011;20:251–60. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 34.Scannapieco FA, Genco RJ. Association of periodontal infections with atherosclerotic and pulmonary diseases. J Periodontal Res. 1999;34:340–5. doi: 10.1111/j.1600-0765.1999.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 35.Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: The heart of the matter. J Am Dent Assoc. 2006;137(Suppl):14S–20S. doi: 10.14219/jada.archive.2006.0402. [DOI] [PubMed] [Google Scholar]
- 36.Offenbacher S, Barros SP, Altarawneh S, Beck JD, Loewy ZG. Impact of tooth loss on oral and systemic health. Gen Dent. 2012;60:494–500. [PubMed] [Google Scholar]
- 37.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–58. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corsalini M, Rapone B, Grassi FR, Di Venere D. A study on oral rehabilitation in stroke patients: Analysis of a group of 33 patients. Gerodontology. 2010;27:178–82. doi: 10.1111/j.1741-2358.2009.00322.x. [DOI] [PubMed] [Google Scholar]
- 39.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 40.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Samaranayake LP, Hughes A, Weetman DA, MacFarlane TW. Growth and acid production of Candida species in human saliva supplemented with glucose. J Oral Pathol. 1986;15:251–4. doi: 10.1111/j.1600-0714.1986.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 42.Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb) 2011;21:264–75. doi: 10.11613/bm.2011.036. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence M, Aleid W, McKechnie A. Access to dental services for head and neck cancer patients. Br J Oral Maxillofac Surg. 2013;51:404–7. doi: 10.1016/j.bjoms.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Green SL. Anaerobic pleuro-pulmonary infections. (68-9).Postgrad Med. 1979;65:62–6. doi: 10.1080/00325481.1979.11715023. 72-4. [DOI] [PubMed] [Google Scholar]
- 45.Meurman JH, Grönroos L. Oral and dental health care of oral cancer patients: Hyposalivation, caries and infections. Oral Oncol. 2010;46:464–7. doi: 10.1016/j.oraloncology.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Martin BJ, Corlew MM, Wood H, Olson D, Golopol LA, Wingo M, et al. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia. 1994;9:1–6. doi: 10.1007/BF00262751. [DOI] [PubMed] [Google Scholar]
- 47.Sumi Y, Miura H, Sunakawa M, Michiwaki Y, Sakagami N. Colonization of denture plaque by respiratory pathogens in dependent elderly. Gerodontology. 2002;19:25–9. doi: 10.1111/j.1741-2358.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- 48.Iinuma T, Arai Y, Abe Y, Takayama M, Fukumoto M, Fukui Y, et al. Denture wearing during sleep doubles the risk of pneumonia in the very elderly. J Dent Res. 2015;94(3 Suppl):28S–36S. doi: 10.1177/0022034514552493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50:430–3. doi: 10.1046/j.1532-5415.2002.50106.x. [DOI] [PubMed] [Google Scholar]
- 50.El-Solh AA. Association between pneumonia and oral care in nursing home residents. Lung. 2011;189:173–80. doi: 10.1007/s00408-011-9297-0. [DOI] [PubMed] [Google Scholar]
- 51.Scannapieco FA. Pneumonia in nonambulatory patients. The role of oral bacteria and oral hygiene. J Am Dent Assoc. 2006;137(Suppl):21S–5S. doi: 10.14219/jada.archive.2006.0400. [DOI] [PubMed] [Google Scholar]
- 52.Sumi Y, Ozawa N, Michiwaki Y, Washimi Y, Toba K. Oral conditions and oral management approaches in mild dementia patients. Nihon Ronen Igakkai Zasshi. 2012;49:90–8. doi: 10.3143/geriatrics.49.90. [DOI] [PubMed] [Google Scholar]
- 53.Przybylowska D, Mierzwinska-Nastalska E, Rubinsztajn R, Chazan R, Rolski D, Swoboda-Kopec E. Influence of denture plaque biofilm on oral mucosal membrane in patients with chronic obstructive pulmonary disease. Adv Exp Med Biol. 2015;839:25–30. doi: 10.1007/5584_2014_42. [DOI] [PubMed] [Google Scholar]
- 54.Gümrü B, Kadir T, Uygun-Can B, Ozbayrak S. Distribution and phospholipase activity of Candida species in different denture stomatitis types. Mycopathologia. 2006;162:389–94. doi: 10.1007/s11046-006-0074-1. [DOI] [PubMed] [Google Scholar]
- 55.Gasparoto TH, de Oliveira CE, Vieira NA, Porto VC, Cunha FQ, Garlet GP, et al. Activation pattern of neutrophils from blood of elderly individuals with Candida-related denture stomatitis. Eur J Clin Microbiol Infect Dis. 2012;31:1271–7. doi: 10.1007/s10096-011-1439-z. [DOI] [PubMed] [Google Scholar]
- 56.Loster BW, Majewski SW, Czesnikiewicz-Guzik M, Bielanski W, Pierzchalski P, Konturek SJ. The relationship between the presence of Helicobacter pylori in the oral cavity and gastric in the stomach. J Physiol Pharmacol. 2006;57(Suppl 3):91–100. [PubMed] [Google Scholar]
- 57.Namiot DB, Namiot Z, Kemona A, Bucki R, Gotebiewska M. Oral health status and oral hygiene practices of patients with peptic ulcer and how these affect Helicobacter pylori eradication from the stomach. Helicobacter. 2007;12:63–7. doi: 10.1111/j.1523-5378.2007.00471.x. [DOI] [PubMed] [Google Scholar]
- 58.Jia CL, Jiang GS, Li CH, Li CR. Effect of dental plaque control on infection of Helicobacter pylori in gastric mucosa. Tex Dent J. 2012;129:1069–73. [PubMed] [Google Scholar]
- 59.Berroteran A, Perrone M, Correnti M, Cavazza ME, Tombazzi C, Goncalvez R, et al. Detection of Helicobacter pylori DNA in the oral cavity and gastroduodenal system of a Venezuelan population. J Med Microbiol. 2002;51:764–70. doi: 10.1099/0022-1317-51-9-764. [DOI] [PubMed] [Google Scholar]
- 60.Frank RM, Steuer P. Transmission electron microscopy of plaque accumulations in denture stomatitis. J Prosthet Dent. 1985;53:115–24. doi: 10.1016/0022-3913(85)90079-4. [DOI] [PubMed] [Google Scholar]
- 61.Pereira CA, Toledo BC, Santos CT, Pereira Costa AC, Back-Brito GN, Kaminagakura E, et al. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn Microbiol Infect Dis. 2013;76:419–24. doi: 10.1016/j.diagmicrobio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Landsman MJ, Sultan M, Stevens M, Charabaty A, Mattar MC. Diagnosis and management of common gastrointestinal tract infectious diseases in ulcerative colitis and Crohn's disease patients. Inflamm Bowel Dis. 2014;20:2503–10. doi: 10.1097/MIB.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 63.Sherrard LJ, Tunney MM, Elborn JS. Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. Lancet. 2014;384:703–13. doi: 10.1016/S0140-6736(14)61137-5. [DOI] [PubMed] [Google Scholar]
- 64.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384:691–702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt BL, Kuczynski J, Bhattacharya A, Huey B, Corby PM, Queiroz EL, et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS One. 2014;9:e98741. doi: 10.1371/journal.pone.0098741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He J, Li Y, Cao Y, Xue J, Zhou X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol (Praha) 2015;60:69–80. doi: 10.1007/s12223-014-0342-2. [DOI] [PubMed] [Google Scholar]
- 67.Sumi Y, Nakamura Y, Michiwaki Y. Development of a systematic oral care program for frail elderly persons. Spec Care Dentist. 2002;22:151–5. doi: 10.1111/j.1754-4505.2002.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 68.Samaranayake LP. Host factors and oral candidosis. In: Samaranayake MacFarlane TW., editor. Oral Candidosis. London: Butterworth; 1990. pp. 66–103. [Google Scholar]
- 69.Yang F, Zeng X, Ning K, Liu KL, Lo CC, Wang W, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012;6:1–10. doi: 10.1038/ismej.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lacoste-Ferré MH, Hermabessière S, Jézéquel F, Rolland Y. Oral ecosystem in elderly people. Geriatr Psychol Neuropsychiatr Vieil. 2013;11:144–50. doi: 10.1684/pnv.2013.0401. [DOI] [PubMed] [Google Scholar]
- 71.Thomas JG, Nakaishi LA. Managing the complexity of a dynamic biofilm. J Am Dent Assoc. 2006;137(Suppl):10S–5S. doi: 10.14219/jada.archive.2006.0409. [DOI] [PubMed] [Google Scholar]
- 72.Ramage G, Zalewska A, Cameron DA, Sherry L, Murray C, Finnegan MB, et al. A comparative in vitro study of two denture cleaning techniques as an effective strategy for inhibiting Candida albicans biofilms on denture surfaces and reducing inflammation. J Prosthodont. 2012;21:516–22. doi: 10.1111/j.1532-849X.2012.00865.x. [DOI] [PubMed] [Google Scholar]
- 73.Jose A, Coco BJ, Milligan S, Young B, Lappin DF, Bagg J, et al. Reducing the incidence of denture stomatitis: Are denture cleansers sufficient? J Prosthodont. 2010;19:252–7. doi: 10.1111/j.1532-849X.2009.00561.x. [DOI] [PubMed] [Google Scholar]
- 74.Al-Kheraif AA. The effect of mechanical and chemical polishing techniques on the surface roughness of heat-polymerized and visible light-polymerized acrylic denture base resins. Saudi Dent J. 2014;26:56–62. doi: 10.1016/j.sdentj.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu X, Wang S, Gu Y, Li X, Yan H, Yan H, et al. Possible variation of the human oral bacterial community after wearing removable partial dentures by DGGE. World J Microbiol Biotechnol. 2012;28:2229–36. doi: 10.1007/s11274-012-1030-5. [DOI] [PubMed] [Google Scholar]
- 76.Carlsson GE, Hedegård B, Koivumaa KK. Late results of treatment with partial dentures. An investigation by questionnaire and clinical examination 13 years after treatment. J Oral Rehabil. 1976;3:267–72. doi: 10.1111/j.1365-2842.1976.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 77.Wagner B, Kern M. Clinical evaluation of removable partial dentures 10 years after insertion: Success rates, hygienic problems, and technical failures. Clin Oral Investig. 2000;4:74–80. doi: 10.1007/s007840050119. [DOI] [PubMed] [Google Scholar]
- 78.Morhart RE, Fitzgerald RJ. Nutritional determinants of the ecology of the oral flora. Dent Clin North Am. 1976;20:473–89. [PubMed] [Google Scholar]
- 79.Ene IV, Heilmann CJ, Sorgo AG, Walker LA, de Koster CG, Munro CA, et al. Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans. Proteomics. 2012;12:3164–79. doi: 10.1002/pmic.201200228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NA, et al. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol. 2012;14:1319–35. doi: 10.1111/j.1462-5822.2012.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santana IL, Gonçalves LM, de Vasconcellos AA, da Silva WJ, Cury JA, Del Bel Cury AA. Dietary carbohydrates modulate Candida albicans biofilm development on the denture surface. PLoS One. 2013;8:e64645. doi: 10.1371/journal.pone.0064645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]


