Complex I (CI) is a large membranous mitochondrial enzyme that serves as the major entry point for electrons from NADH into the respiratory chain. The CI enzyme is considered to be conserved between different organisms, but plant CI includes an extra ‘egg-shaped’ module – the γ-carbonic anhydrase domain – and a paper by Córdoba et al. in this issue of Journal of Experimental Botany (pages 1589–1603) indicates that its functions are required during Arabidopsis embryogenesis.
The respiratory machinery of the mitochondrion is usually made up of four major protein complexes, CI to IV, embedded within the inner-mitochondrial membrane. Electron transfer through CI (NADH:ubiquinone oxidoreductase), CIII and CIV mediates the pumping of protons (H+) across the inner membrane, forming the chemical potential (ΔpH), which is utilized by ATP-synthases to generate ATP.
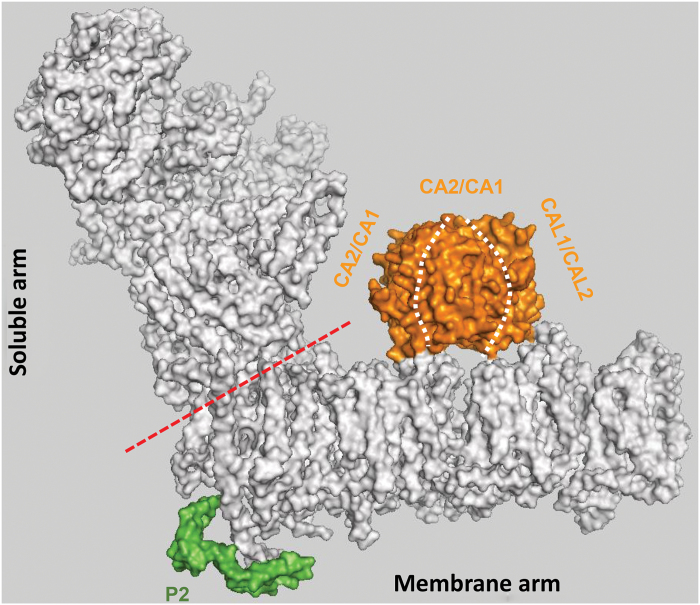
CI is present in many prokaryotes, including α-Proteobacteria, the proposed progenitors of mitochondria, and is thus considered to have arisen early in evolution. The bacterial and mammalian enzymes show an L-shaped structure composed of two major fragments, including an integral membrane-domain and a soluble arm (Baradaran et al., 2013; Shimada et al., 2014). The plant CI consists of ≥50 different subunits, encoded by both nuclear and mitochondrial loci, forming a ≥1.0 MDa structure that has several distinguishing features (Fig. 1) (Meyer, 2012; Braun et al., 2014). These include the presence of additional Nad subunits that are encoded in the mtDNA. The nad pre-RNAs in plant mitochondria undergo extensive maturation processes, including numerous RNA-editing events and the splicing of many group II-type introns that are removed posttranscriptionally from the coding region they interrupt (Brown et al., 2014).
Fig. 1.
A hypothetical 3D-structural model of Arabidopsis CI. The proton-pumping CI enzyme is a large bi-partite membranous protein assembly, which has a central role in the production of cellular energy in bacteria and mitochondria. CI has a characteristic L-shaped structure with the hydrophobic arm embedded in the membrane and the hydrophilic peripheral arm facing into the cytoplasm (bacteria) or the mitochondrial matrix. The bacterial enzyme is relatively simple, containing 14 different subunits, while the mitochondrial enzymes contain extra subunits. Sequence data for the different plant CI subunits is according to Meyer (2012). Homology models were constructed individually and fitted according to the electron density map of the bovine CI enzyme (Shimada et al., 2014). The approximate positions of the CA-related subunits were fitted based on the low-resolution negative stained map of Arabidopsis CI (Sunderhaus et al., 2006). The solvent accessible surface for the Arabidopsis CI enzyme was visualized in Pymol (v1.3). Different subunits that are shared between the plant CI and its related bacterial and mitochondrial enzymes are shown in grey; extra subcomplex I domains of the membrane arm, including the matrix-facing CA module (orange) and the P2-domain (green), are highlighted. The suggested CA module is an assembly with triplets containing either CA1-CA1-CAL1 (or CAL2) or CA2-CA2-CAL1 (or CAL2) subunits.
Another unique feature of plant CI, compared with those in animals and fungi, is the presence of many additional subunits (Meyer, 2012; Braun et al., 2014). These include several γ-carbonic anhydrase (CA)-related proteins, which form a matrix-exposed ‘egg-shaped’ module in the membrane arm (Fig. 1) (Sunderhaus et al., 2006; Braun and Zabaleta, 2007). However, the function of this CI-related module remains unclear, and this is where the data presented by Córdoba et al. (2016) are novel and important.
Mitochondrial biogenesis in germinating seeds
Respiration is key for successful germination and seedling development (Box 1). There are currently two models of mitochondrial biogenesis in germinating seeds: the ‘growth and division’ model and the ‘maturation’ hypothesis (Law et al., 2014). According to the first model, the development of mitochondria occurs from pre-existing mature and fully functional organelles, already present in the dry seed. However, the latter theory proposes that mitochondria are disassembled during seed maturation, and then reassembled from the immature ‘pro-mitochondrial’ organelles found in the embryonic cells upon imbibition (Logan et al., 2001).
Box 1. Germination and respiration
Mitochondria house the respiratory machinery and other metabolic processes essential for plant growth and development. Increased demands for metabolic energy are observed at different stages in the plant life cycle, but seem especially pronounced during the reproduction phase and germination. Upon fertilization, the seeds undergo a gradual maturation stage, in which the embryos develop and storage compounds (i.e. proteins, sugars or fatty acids) are synthesized in the maturing seeds. At maturity, the seeds are typically dispersed and enter the arrested mode (i.e. seed dormancy) where the embryo enters a ‘quiescent state’ (Bewley, 1997). Under these conditions seed respiration remains very low, most likely to preserve the stored energy reserves.
The timing of germination is critical for successful seedling development, and different environmental signals (e.g. water, temperature and light) are involved in breaking dormancy to enable germination. To achieve the high-energy demand during this critical stage, cellular respiration greatly increases shortly after imbibition, as the embryos develop. These processes may be accompanied by early biogenesis of the respiratory machinery.
This situation may resemble the differentiation of chloroplasts from non-photosynthesizing proplastids present in the embryos of mature seeds. In Arabidopsis, microarray data imply that mitochondrial biogenesis during embryo development involves the expression of numerous nuclear genes required in mitochondrial import and organellar gene expression, as well as many subunits of the respiratory machinery that are assembled with their related organelle-encoded counterparts upon their import into the organelle (Law et al., 2014). The paper by Córdoba et al. (2016) offers additional insights regarding the biogenesis of CI during early embryogenesis in plants.
The CAs are zinc-binding proteins that catalyse the reversible interconversion of CO2 and water into HCO3 – and H+ (Braun and Zabaleta, 2007; Braun et al., 2014). They fall into several distinct families (i.e. α, β, γ, δ and ε), which share only minor similarities in their sequences (possibly evolved independently though convergent evolution), and are mostly known with regard to CO2-concentrating mechanisms in the chloroplasts. However, their functions in other subcellular compartments, including mitochondria, are far less clear. Five different γ-CA subunits have been associated with the matrix-exposed CA-module in Arabidopsis mitochondria (Sunderhaus et al., 2006; Braun and Zabaleta, 2007) (see also Fig. 1). CA1, CA2 and CA3 share a conserved active site, while the two remaining subunits are less similar and were therefore denoted as CA-Like proteins 1 and 2 (CAL1 and CAL2) (Braun and Zabaleta, 2007).
The physiological roles of the γ-CA subunits of plant CI are under investigation. Gene expression data indicate that both ca1 and ca2 are downregulated under high CO2, supporting a role in mitochondrial carbon metabolism. It was also noted that the mitochondrial γ-CA subunits may play important roles in photorespiration, mainly for the release of CO2 under high-light conditions (Braun et al., 2014; Soto et al., 2015). Genetic studies of various ca mutants indicate that the expression of mitochondrial γ-CAs is a prerequisite for CI biogenesis, seemingly at the earliest stages of the assembly of the enzyme (Braun et al., 2014). Accordingly, CI exists only in residual amounts in Arabidopsis ca2 and ca3 mutants. Surprisingly, the phenotypes of the ca mutants are comparable with those of wild-type plants grown under similar conditions. Córdoba et al. (2016) indicate that while the functions of CA1 and CA2 are not essential during different stages in plant development, the double ca1ca2 knockout affects embryo development, indicating redundancy between the two CA subunits in the mitochondria. The delayed embryo phenotypes of the double ca1ca2 mutant are mainly associated with impaired mitochondrial membrane potential and increased levels of reactive oxygen species.
The importance of respiratory complex I
How essential is plant CI to embryo development? It is a matter of controversy. The phenotypes of individual ca (and a few other) mutants affected in CI biogenesis are barely distinguishable from those of wild-type plants. However, CI deficiencies in many other plants strongly affect cellular physiology and plant development (although the plants are typically able to germinate and develop under normal growth conditions; see, for example, Keren et al., 2012, and Cohen et al., 2014). Arabidopsis mutants that are completely lacking CI activity, due to inactivation of the catalytic core subunit, NDUFV1, demonstrated severe growth and development defective phenotypes, but could otherwise be maintained in a viable state under specific growth conditions on sugar-containing synthetic media (Kuhn et al., 2015). These data strongly suggest that CI functions are not essential in plants, probably due to the presence of alternative NAD(P)H-dehydrogenases that can bypass CI (Rasmusson et al., 2008).
What is triggering the delayed embryo development phenotypes of the ca1ca2 mutant? According to the model described by Córdoba et al. (2016), plant mitochondria may harbour different CI enzymes, according to the composition of their CA domains (i.e. the majority of CI, about 80%, is dependent on the presence of the CA2 subunit, while the remaining 20% are CA1-dependent forms). It was therefore suggested that no holo-CI could be formed in the absence of both CA1 and CA2 subunits. Interestingly, these may also coincide with the accumulation of various subcomplex I particles, observed in different plant CI mutants (Meyer et al., 2011; Cohen et al., 2014). Such CI assembly-intermediates may function incorrectly or interfere with normal respiratory activities. Alternatively, it remains possible that the phenotypes associated with the ca1ca2 double mutant are unrelated to those of CI enzyme. Reduced (or no) CA activity in the ca1ca2 mutant may affect the TCA cycle in the embryos due to altered CO2/HCO3 – ratios. However, any speculation regarding the roles of the CAs in mitochondrial carbon-metabolism needs to be supported experimentally.
Acknowledgements
The author is currently supported by grants from the German-Israeli Foundation (No. 1213/2012) and the Israeli Science Foundation (No. 741/15). I would also like to thank Dr Liron Klipcan (The Weizmann Institute) for his assistance in modelling the structure Arabidopsis complex I.
References
- Baradaran R, Berrisford JM, Minhas GS, Sazanov LA. 2013. Crystal structure of the entire respiratory complex I. Nature 494, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun H-P, Binder S, Brennicke A, et al. 2014. The life of plant mitochondrial complex I. Mitochondrion 19, Part B, 295–313. [DOI] [PubMed] [Google Scholar]
- Braun HP, Zabaleta E. 2007. Carbonic anhydrase subunits of the mitochondrial NADH dehydrogenase complex (complex I) in plants. Physiologia Plantarum 129, 114–122. [DOI] [PubMed] [Google Scholar]
- Brown GG, Colas des Francs-Small C, Ostersetzer-Biran O. 2014. Group II intron splicing factors in plant mitochondria. Frontiers in Plant Science 5, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Zmudjak M, Colas des Francs-Small C, et al. 2014. nMAT4, a maturase factor required for nad1 pre-mRNA processing and maturation, is essential for holocomplex I biogenesis in Arabidopsis mitochondria. The Plant Journal 78, 253–268. [DOI] [PubMed] [Google Scholar]
- Córdoba JP, Marchetti F, Soto D, Martin MV, Pagnussat GC, Zabaleta E. 2016. The CA domain of the respiratory complex I is required for normal embryogenesis in Arabidopsis thaliana . Journal of Experimental Botany 67, 1589–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Tal L, Colas des Francs-Small C, Araújo WL, Shevtsov S, Shaya F, Fernie AR, Small I, Ostersetzer-Biran O. 2012. nMAT1, a nuclear-encoded maturase involved in the trans-splicing of nad1 intron 1, is essential for mitochondrial complex I assembly and function. The Plant Journal 71, 413–426. [DOI] [PubMed] [Google Scholar]
- Kuhn K, Obata T, Feher K, Bock R, Fernie AR, Meyer EH. 2015. Complete mitochondrial complex I deficiency induces an up-regulation of respiratory fluxes that is abolished by traces of functional complex I. Plant Physiology 168, 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SR, Narsai R, Whelan J. 2014. Mitochondrial biogenesis in plants during seed germination. Mitochondrion 19, Part B, 214–221. [DOI] [PubMed] [Google Scholar]
- Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ. 2001. Mitochondrial biogenesis during germination in maize embryos. Plant Physiology 125, 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH. 2012. Proteomic investigations of complex I composition: how to define a subunit? Frontiers in Plant Science 3, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EH, Solheim C, Tanz SK, Bonnard G, Millar AH. 2011. Insights into the composition and assembly of the membrane arm of plant complex I through analysis of subcomplexes in Arabidopsis mutant lines. The Journal of Biological Chemistry 286, 26081–26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AG, Geisler DA, Møller IM. 2008. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria. Mitochondrion 8, 47–60. [DOI] [PubMed] [Google Scholar]
- Shimada S, Shinzawa-Itoh K, Amano S, Akira Y, Miyazawa A, Tsukihara T, Tani K, Gerle C, Yoshikawa S. 2014. Three-dimensional structure of bovine heart NADH: ubiquinone oxidoreductase (complex I) by electron microscopy of a single negatively stained two-dimensional crystal. Microscopy 63, 167–174. [DOI] [PubMed] [Google Scholar]
- Soto D, Córdoba JP, Villarreal F, Bartoli C, Schmitz J, Maurino VG, Braun HP, Pagnussat GC, Zabaleta E. 2015. Functional characterization of mutants affected in the carbonic anhydrase domain of the respiratory complex I in Arabidopsis thaliana . The Plant Journal 83, 831–844. [DOI] [PubMed] [Google Scholar]
- Sunderhaus S, Dudkina NV, Jansch L, Klodmann J, Heinemeyer J, Perales M, Zabaleta E, Boekema EJ, Braun HP. 2006. Carbonic anhydrase subunits form a matrix-exposed domain attached to the membrane arm of mitochondrial complex I in plants. The Journal of Biological Chemistry 281, 6482–6488. [DOI] [PubMed] [Google Scholar]