INTRODUCTION
Rates of angiographic recanalization following IV recombinant tissue plasminogen activator (rt-PA) vary from 23% to 56% depending upon the location of occlusion and time interval between administration of rt-PA and ascertainment of recanalization [1–3]. It remains unclear whether certain thrombus characteristics result in resistance to lysis with IV rt-PA. In animal models of embolic stroke and femoral artery thrombosis, white thrombi composed of platelets and fibrin displayed a relative resistance against thrombolysis, whereas erythrocytes-rich (red) thrombi were more vulnerable to lysis [4]. Platelet-rich thrombi were relatively resistant to rt-PA compared with in a rabbit model of femoral artery thrombosis [5]. Furthermore, thrombolytics exposure results in platelet activation, which increases the platelet content in thrombus and thus render thrombus resistant to additional thrombolytic activity [6].
We performed this study to evaluate and compare the histopathological characteristics of persistent in vivo thrombi retrieved from acute ischemic stroke patients who had received IV rt-PA prior to endovascular treatment.
METHODS
A retrospective study of consecutive patients with acute ischemic stroke who underwent endovascular treatment was performed. The institution maintained a prospective endovascular procedure database, which records information regarding the procedural components. All procedural records including devices used and intraprocedural medication with doses are archived into an electronic medical record system. The protocol for collecting data was reviewed and approved by the Institutional Review Board at the institution as part of a standardized database.
DATA COLLECTED
We recorded the presence of cardiovascular risk factors (active cigarette smoking, hypertension, atrial fibrillation, coronary artery disease, hyperlipidemia, diabetes mellitus, history of aspirin use, prior transient ischemic attack [TIA] or ischemic stroke), time interval between symptom onset and endovascular treatment, and use of IV rt-PA. IV rt-PA was administered using the protocol recommended by American Heart Association/American Stroke Association [7]. The times were identified individually by reviewing the angiographic images and recording the times of IV rt-PA initiation and deployment of retrieval system. We also recorded the time interval between symptom onset and deployment of retrieval system. We also recorded admission, 24-hour post-treatment, and discharge National Institutes of Health Stroke Scale (NIHSS) scores. Angiographic occlusion and recanalization were classified by interventional neurologists by using the previously validated grading scale [8,9]. Complete recanalization was defined as a grade of 0 and partial recanalization was defined by a reduction in severity of occlusion by 1 grade or greater.
Outcomes at the time of discharge were assessed by using the modified Rankin scale (mRS), ascertained from hospital discharge summaries and outpatient visit records by the vascular neurology team and occupational, speech, and physical therapists. The principal safety end points were intracerebral hemorrhage (ICH) and in-hospital mortality. Symptomatic ICH was defined as noncontrast CT–documented ICH resulting in neurologic deterioration (≥4-point worsening on an NIHSS score compared with previous clinical assessment). Favorable functional outcome at discharge was defined by a mRS score of 0–2.
Protocol for thrombus retrieval
6 Fr Envoy guiding catheter (Cordis, Miami Lake, FL USA) was introduced through a femoral sheath into the appropriate carotid artery or dominant, or navigable, vertebral artery over a 0.035-inch steerable guidewire using fluoroscopic guidance supplemented by road mapping technique. Infrequently, an 8 Fr or 9 Fr Merci balloon guiding catheter (Concentric Medical, Mountain View, CA, USA) was used. In a typical procedure, Prowler PLUS 0.021-inch two-tip microcatheter was advanced over the steerable 0.014-inch guidewire (SYNCHRO-II guidewire). Radiopaque marker bands located at distal tip of the catheter were used to position the microcatheter under fluoroscopic visualization across the site of occlusion. A microcatheter angiographic injection was then carried out in order to confirm and define the vasculature distal to the thrombus. The Solitaire FR revascularization device (EV3, Irvine, CA, USA) 4 × 20 mm was then introduced through the microcatheter via a 0.016-inch nitinol push wire deployed across the occluding thrombus. The Solitaire FR device was maintained in place for 7–10 min to allow device expansion and integration within the thrombus. Subsequently, the fully deployed Solitaire FR and the delivery microcatheter were retracted together and recovered through the guiding catheter. Infrequently, a 2.5 F MC18 Plus microcatheter (Concentric Medical Inc) was navigated over a 0.014 microwire across the occlusive thrombus. The Trevo device was then advanced into the microcatheter spanning the whole length of the occlusive thrombus. A similar procedure as described for Solitaire FR revascularization device was performed. Infrequently, Alteplase (1 mg/ml) was infused through manual injection using 3-cc syringe through the microcatheter in bolus doses of 2–3 mg at several intervals prior to and after stent retriever device deployment and retrieval. The status of recanalization was assessed at regular intervals through angiographic images acquired in both antereoposterior and lateral projections. Intravenous heparin was not administered in any patient. A continuous flush system of 0.9% sodium chloride with heparin (1000 U in 1 L) was used to irrigate the guide catheter, femoral introducer sheath, and microcatheter (when required). During the retrieval, manual aspiration with a 50-mL syringe was performed through the hemostatic valve during the retrieval in order to reverse the flow and to aspirate thrombus debris present in the lumen of the guide catheter.
PROCESSING OF EXTRACTED THROMBI
The thrombus was removed from the device or retrieval catheter by gentle disassociation without any dilution with any solvent. Subsequently, the thrombus was placed in a specimen cup with 10% neutral buffered formalin for fixation. The tissues selected were processed for paraffin section preparation. Specimens were carefully orientated to allow visualization of center of thrombus. Paraffin sections were cut at a thickness of 3–5 µm ensuring that only a single layer of cells makes up the section. The sections were deparaffinized and rehydrated. The sections were stained by hematoxylin and eosin (H/E) staining method which involved immersion into Harris hematoxylin solution for 8 minutes followed by counterstain in eosin–phloxine solution for 1 minute. The sections were mounted with xylene-based mounting medium. The thrombi sections were processed and slides were washed and counterstained with H/E stain (200x), observed under light microscopy. The erythrocytes were identifiable by discoid shape without any nuclei and fibrin was identifiable was pink amorphous deposits within the thrombus. The thrombi were further classified as erythrocytes aggregates, or erythrocytes nests with fibrin mesh in repeating patterns or in random patterns. The pattern was defined by alternating light and dark lines (lines of Zahn) which represent bands of fibrin (lighter) with entrapped white blood cells and platelets and erythrocytes (darker) [10].
QUANTITATIVE THROMBUS COMPOSITION ANALYSIS
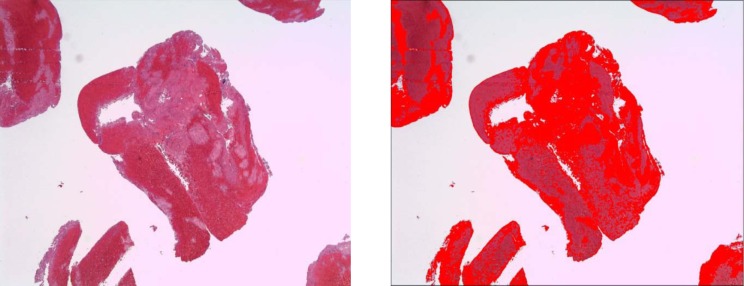
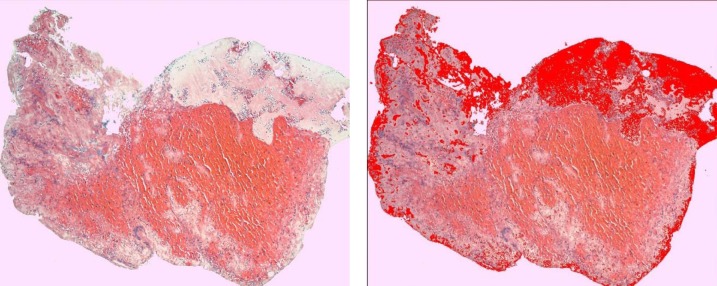
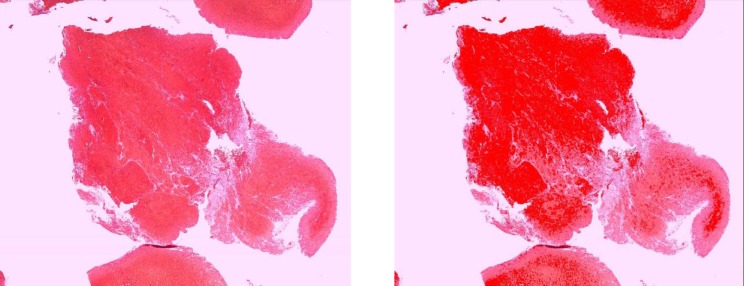
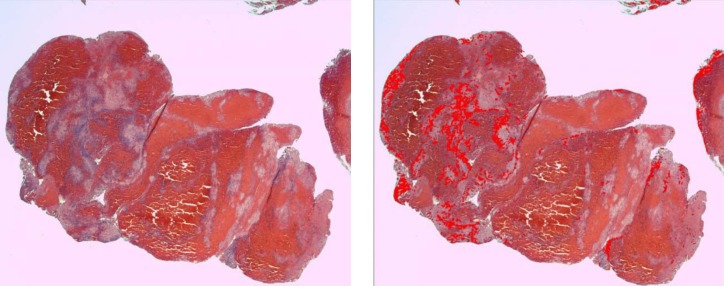
We identified the following four distinct regions within the thrombus: dense erythrocytes aggregates; mixed region consisting of dense eosinophilic amorphous material deposits with intermingled erythrocytes; eosinophilic amorphous material deposits; and basophilic staining cells in linear aggregates intermingled within the three above-mentioned regions. Thrombus type was defined as erythrocytes, fibrin, or mixed region dominant based on the component that occupied the largest proportion of thrombus. Hematoxylin and eosin-stained slides were scanned at 400x magnification using a LAS EZ (Leica microsystem, Bufflogrove, IL) digital scanner. Image J software (National Institutes of Health, Bethesda, MD) was used to quantify the four distinct areas as mentioned above based on automated thresholds to assign specific colors to imaging features of each thrombus component (Figs. 1 –4). The area of each region was expressed as percentage of total thrombus area in slide. In cases where multiple thrombus fragments were retrieved for analysis, the mean values across fragments were used for thrombus regions.
Figure 1. The mixed region in a rt-PA naïve thrombus (A) which is highlighted by automated thresholds to assign specific colors to clot component (B).
Figure 4. The fibrin deposition region in a rt-PA-resistant thrombus (A) which is highlighted by automated thresholds to assign specific colors to clot component (B).
STATISTICAL ANALYSIS
The analysis was predominantly descriptive with categorical and continuous variables expressed as frequencies and mean with standard deviations, respectively.
RESULTS
A total of 24 patients (mean age ± SD of 73.1 ± 14.4 years) underwent mechanical thrombectomy during the study period, out of them thrombi was retrieved from 18 patients. IV rt-PA was administered prior to endovascular treatment in seven of 18 patients. Thrombi were retrieved from the 18 patients using either solitaire (n = 16), trevo (n = 1), and penumbra (n = 1). The time interval (minutes ± SD) between symptom onset and thrombus retrieval was 363 ± 238 minutes and between IV rt-PA initiation and thrombus retrieval was 170 ± 122 minutes. The time interval (minutes ± SD) between symptom onset and thrombus retrieval was similar in patients who received or did not receive IV rt-PA prior to endovascular treatment (326 ± 83 minutes vs. 386 ± 301 minutes).
The baseline and clinical characteristics of patients in whom thrombi were extracted is presented in Tables 1 and 2. The proportion of thrombi according to predominant composition was as follows: erythrocytes dominant (n = 7), fibrin dominant (n = 5), and mixed region dominant (n = 6). The time interval between symptom onset and thrombus retrieval (mean ± SD) was 341 ± 106 min for erythrocytes dominant, 251 ± 67 minutes ± SD for fibrin dominant, and 482 ± 380 minutes for mixed region thrombi. The proportion of thrombi that were erythrocytes dominant was higher between those who did versus who did not receive IV rt-PA prior to endovascular treatment (57.1% vs. 27.3%). The proportion of mixed thrombi was lower in the patients who received or did not IV rt-PA (14.3% vs. 45.5%). The proportion of fibrin dominant thrombi was similar in those who did versus who did not receive IV rt-PA prior to endovascular treatment (28.6% vs. 27.3%). The erythrocytes aggregates were predominant topographic pattern in IV rt-PA-resistant thrombi compared with IV rt-PA naïve thrombi (57.1% vs. 36.4%). Random or repeated pattern of fibrin deposits with nest-like trapped erythrocytes clumps were more frequent in IV rt-PA naïve thrombi.
Table 1. Clinical and thrombus characteristics of acute ischemic stroke patients with IV rt-PA resistant thrombi.
| Age/gender | Time interval between symptom onset and thrombus retrieval (mins) | Initial NIHSS score | Location of occlusion | Cardiovascular risk factors | Thrombus type | Thrombus topography | Outcome at discharge (mRS) |
|---|---|---|---|---|---|---|---|
| 80/man | 383 | 19 | Middle cerebral artery | Hypertension, hyperlipidemia | Erythrocytes dominant | Erythrocyte aggregates | 1 |
| 63/man | 427 | 17 | Internal cerebral artery | Hyperlipidemia | Erythrocytes dominant | Random pattern | 2 |
| 70/man | 297 | 13 | Middle cerebral artery | Hypertension, diabetes mellitus | Erythrocytes dominant | Erythrocyte aggregates | 6 |
| 34/woman | 424 | 31 | Middle cerebral artery | Diabetes mellitus | Erythrocytes dominant | Erythrocyte aggregates | 2 |
| 70/woman | 253 | 20 | Middle cerebral artery | Hypertension, hyperlipidemia | Mixed region dominant | Repeated pattern | 5 |
| 76/woman | 279 | 19 | Middle cerebral artery | Hypertension, previous stroke | Fibrin dominant | Erythrocyte aggregates | 6 |
| 79/man | 225 | 19 | Middle cerebral artery | None | Mixed region dominant | Repeated pattern | 4 |
NIHSS – National institutes of Health stroke scale, mins – minutes, mRS-modified Rankin scale.
Random or repeated pattern of fibrin deposits with nest-like trapped erythrocytes.
Table 2. Clinical and thrombus characteristics of acute ischemic stroke patients with IV rt-PA naïve thrombi.
| Age/gender | Time interval between symptom onset and thrombus retrieval (min) | Initial NIHSS score | Location of occlusion | Cardiovascular risk factors | Thrombus type | Thrombus topography | Outcome at discharge (mRS) |
|---|---|---|---|---|---|---|---|
| 94/man | 340 | 19 | Middle cerebral artery | Hypertension, atrial fibrillation | Mixed region dominant | Erythrocyte aggregates | 4 |
| 64/man | 405 | 5 | Middle cerebral artery | Hyperlipidemia, atrial fibrillation | Erythrocytes dominant | Erythrocyte aggregates | 0 |
| 70/man | 327 | 7 | Vertebral artery | Hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation | Erythrocytes dominant | Random pattern† | 5 |
| 64/woman | 288 | 10 | Middle cerebral artery | None | Mixed region dominant | Repeat pattern† | 3 |
| 49/man | 1213 | 2 | Internal carotid artery | Hypertension, hyperlipidemia | Fibrin dominant | Repeat pattern† | 4 |
| 93/woman | 345 | 15 | Middle cerebral artery | Hypertension | Fibrin dominant | Repeat pattern† | 5 |
| 85/woman | 245 | 14 | Internal carotid artery | Hypertension, diabetes mellitus, hyperlipidemia | Mixed region dominant | Random pattern† | 4 |
| 88/woman | 586 | 10 | Middle cerebral artery | Hypertension, hyperlipidemia, atrial fibrillation | Mixed region dominant | Random pattern† | 2 |
| 68/man | 126 | 18 | Middle cerebral artery | Hypertension, hyperlipidemia, atrial fibrillation | Erythrocytes dominant | Random pattern† | 4 |
| 79/woman | 167 | 26 | Middle cerebral artery | Hypertension, hyperlipidemia, atrial fibrillation | Fibrin dominant | Erythrocyte aggregates | 6 |
| 49/man | 211 | 15 | Middle cerebral artery | Hypertension, hyperlipidemia | Fibrin dominant | Random pattern† | 4 |
NIHSS – National institutes of Health stroke scale, mins – minutes, mRS-modified Rankin scale.
Random or repeated pattern of fibrin deposits with nest-like trapped erythrocytes.
The quantitative analysis identified higher content of erythrocytes aggregates (39.2 ± 10.7% vs. 29.8 ± 15.5%) but lower eosinophilic amorphous material deposit content (21.2 ± 14.2% vs. 30.4 ± 23.4%) in IV rt-PA-resistant thrombi (Table 3). A time interval between symptom onset and thrombus retrieval ≥ 312 minutes was associated with lower content of eosinophilic amorphous material deposits (20.4 ± 14.5% vs. 33.3 ± 24.1%) but higher basophilic content (12.3 ± 5.5% vs. 8.3 ± 6.1%). In thrombi that were located in the middle cerebral artery, eosinophilic amorphous material deposit content was higher (32.5 ± 21.3% vs. 12.2 ± 5.1%) and mixed region content was less (24.3 ± 17.1% vs. 43.6 ± 10.1%) compared with thrombi in other locations.
Table 3. Proportion of thrombus components in various strata of patients.
| Erythrocytes aggregates (%) ± SD |
Eosinophilic deposits (%) ± SD |
Mixed region (%) ± SD |
Basophilic component (%) ± SD |
|
|---|---|---|---|---|
| Patients with IV rt-PA naïve thrombi | 29.8 ± 15.5 | 30.4 ± 23.4 | 30.3 ± 21.8 | 10.4 ± 6.6 |
| Patients with IV rt-PA-resistant thrombi | 39.2 ± 10.7 | 21.2 ± 14.2 | 28.5 ± 9.3 | 10.1 ± 5.3 |
| Time interval between symptom onset and mechanical thrombectomy < 312† mins | 31.4 ± 14.4 | 33.3 ± 24.1 | 28.8 ± 18.9 | 8.3 ± 6.1 |
| Time interval between symptom onset and mechanical thrombectomy ≥ 312 mins | 35.5 ± 14.7 | 20.4 ± 14.5 | 30.4 ± 17.3 | 12.3 ± 5.5 |
| Location in middle cerebral artery | 34.4 ± 16.0 | 32.5 ± 21.3 | 24.3 ± 17.1 | 9.9 ± 6.7 |
| Location in other arteries | 31.0 ± 9.8 | 12.2 ± 5.1 | 43.6 ± 10.1 | 11.4 ± 3.9 |
| Premorbid use of aspirin | 30.2 ± 11.5 | 27.6 ± 27.6 | 31.2 ± 19.0 | 11.0 ± 6.1 |
| No premorbid use of aspirin | 36.7 ± 16.7 | 26.1 ± 10.9 | 28.1 ± 17.1 | 9.6 ± 6.1 |
The time interval is dichotomized based on median value.
rt-PA, recombinant tissue plasminogen activator
DISCUSSION
Previous studies have recognized the heterogeneity of thrombus composition in the analysis of thrombi retrieved from patients with acute ischemic stroke [11–15]. There are three patterns of morphology seen in patients with acute ischemic stroke: fibrin-dominant, erythrocytes-dominant, and mixed. The largest proportions of thrombi are fibrin-dominant in previous studies [11–15].
Our results confirm that majority of thrombi are mixed with variable content of erythrocytes and fibrin but the relative ratio of fibrin and erythrocytes was different in IV rt-PA-resistant thrombi (compared with IV rt-PA naïve thrombi). IV rt-PA-resistant thrombi appeared as large erythrocytes aggregates and higher content of erythrocytes on quantitative analysis. In contrast, IV rt-PA naïve thrombi were fibrin-dominant with erythrocytes nests within fibrin meshwork in repeated or random patterns. Higher content of eosinophilic deposits on quantitative analysis in such thrombi provided additional support for higher fibrin composition. There were some effect of time elapsed between symptom onset thrombus retrieval with both qualitative and quantitative analyses, each demonstrating higher erythrocytes content with increasing time. A higher content of basophilic cell content was apparent in thrombi acquired at later intervals from symptom onset. It should be noted that there was no exclusive pattern of thrombus in IV rt-PA-resistant thrombi and IV rt-PA naïve thrombi. Therefore, it is likely that secondary and ongoing thrombogenesis may shift thrombi toward relative homogenization.
There are two other observations that provide support to “high erythrocytes and low fibrin content” in thrombi that were resistant to IV rt-PA. Previous studies have demonstrated that hyperattenuation (or density) of thrombus is higher on CT scan with higher erythrocytes content [11,13]. Lower rates of recanalization are observed in patients with increased density within the artery subsequent to IV rt-PA administration [1,16]. Puig et al. [17] reported that the positive and negative predictive values for lack of recanalization after IV rt-PA for high-density thrombus on CT scan (rHU ≤ 1.382) were 93.75% and 100%, respectively. Within our own cohort, “high erythrocytes and low fibrin content” thrombi were located in arteries other than middle cerebral artery. Previous studies have consistently demonstrated lower rates of recanalization with thrombolytics in occlusions located in arteries other than middle cerebral artery [18,19].
The “relatively high erythrocytes and low fibrin content” of thrombi that were resistant to IV rt-PA is supported by previous observations that higher erythrocytes content in thrombi may lead to resistance to thrombolytics [20,21]. The erythrocytes triggered variability in the fibrin network structure, individual fiber characteristics, and overall thrombus viscoelasticity in one study [22]. Through the extension of projections, erythrocytes become intertwined within a thrombus to stabilize and strengthen its structure [20]. Erythrocytes confer lytic resistance to fibrin resulting from modified fibrin structure and impaired plasminogen activation [21]. Lines of Zahn were less frequently found in thrombi resistant to IV rt-PA. This feature is usually seen in thrombus formed at the site of rapid arterial blood flow, with laminations produced by successive deposition of platelets and fibrin (pale layers) alternating with erythrocytes (dark layers) [10]. Thrombus lysis by erythrocytes bound plasminogen activator begins in focal segments of the thrombus and takes longer for lysis process to complete compared with free plasminogen activators [23]. The other possibility is that thrombus composition was independent of IV rt-PA administration and related to the time interval between symptom onset and thrombus extraction. Simon et al [11] proposed that different morphological appearances are based on four phases of thrombus formation: erythrocytes dominant, erythrocytes proportion equal to fibrin, fibrin-dominant, and organized fibrin. There is a stepwise increase in fibrin content among thrombi retrieved from acute coronary occlusion based on the delay between symptom onset and retrieval [24]. In our cohort of patients, the relationship between time interval elapsed between symptom onset and thrombus retrieval and thrombus morphology did not support such an explanation. The time interval was shorter in patients with higher fibrin content compared with those with higher erythrocytes content thrombi. The possibility that “relatively high erythrocytes and low fibrin content” of thrombi may be a consequence of IV rt-PA exposure should be considered. It is possible that lysis and dissolution of fibrin induced by IV rt-PA within an acute thrombus results in new aggregates of erythrocytes reforming the thrombus. The origin of the basophilic cells infiltrates within the thrombus is not well understood. The infiltrate may represent platelet aggregates within the thrombus. Platelets are either packed within fibrin-containing aggregates or seen as strings of nonaggregated platelets [25]. This inner zone of cytoplasm of the platelets contains bluish staining granules which are not individually visible and appear more or less homogeneously blue. The outer zone of platelet cytoplasm does not stain well enough for adequate visualization and visualization is further obscured by cellular contraction seen as part of thrombus retraction. The dense granules of platelets contain adenosine diphosphate (ADP) and ionized calcium, which are important contributors to thrombus formation. It is plausible that these cells represent basophilic granulocytes which have a two or three lobed nucleus that are trapped within the thrombus. The specific granules of basophils are stained deeply bluish or reddish-violet. The concentration in circulating blood of basophilic granulocytes is small and the only explanation for ≈8% thrombus content would be based on selective uptake of these cells into the thrombus. The granules within basophils contain heparin and histamine that may contribute to thrombus lysis. Basophilic stippling of erythrocytes may be a less plausible explanation secondary to degradation and abnormal collection of nucleic acid in dots throughout the cell.
There are some limitations that should be considered prior to the interpretation of our results. The sample size was small and any analysis assessing the relationship between thrombus compositions, angiographic recanalization, and clinical outcomes is subject to type II error. The heterogeneity of endovascular treatment in regards to administration of intravenous and/or intra- arterial thrombolytics and devices used adds an unmeasured bias on thrombus composition. However, such heterogeneity in endovascular treatment is consistent with current endovascular practices [26–31]. Retrieved thrombus may have undergone modifications during the retrieval process, such as loss of the intermediate zone, between the thrombus periphery and arterial wall [5]. Autopsy studies demonstrate well-organized thromboembolus and ingrowth of smooth muscle cells into the thromboembolus but are limited by the delay between acute stroke and observations of sample after death [32]. In another autopsy study, fibrin- and platelet-rich thrombi of various thicknesses develop at the atheromatous plaques of the cerebral arteries, followed by occlusion with fibrin- and red-cell-rich thrombi (red thrombi) [33]. The loss of peripheral zone and loosely attached components of thrombus is possible during the retrieval process. There may be components, such as endothelial cells present as isolated cells or clusters, that should not be necessarily components of initial thrombus but a secondary consequence of device retrieval [15]. We did not observe any calcification or endothelization as reported previously in anectdotal cases [34].
Although the observations are preliminary, we found “relatively high erythrocytes and low fibrin content” in thrombi that were resistant to IV rt-PA. It should be noted that there was no exclusive or pathognomic thrombus pattern seen in IV rt-PA-resistant thrombi and IV rt-PA naïve thrombi.
CONFLICT OF INTEREST
The authors report that no conflicts of interest.
Figure 2. The erythrocyte aggregates in a rt-PA-resistant thrombus (A) which is highlighted by automated thresholds to assign specific colors to clot component (B).
Figure 3. The basophilic cell aggregates in a rt-PA naïve thrombus (A) which is highlighted by automated thresholds to assign specific colors to clot component (B).
REFERENCES
- Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA acute stroke study group. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Han SW, Kim SH, Nam HS, Ahn SW, Kim DJ, Seo SH, Kim DI, Heo JH. Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke. 2007;38:192–193. doi: 10.1161/01.STR.0000251788.03914.00. [DOI] [PubMed] [Google Scholar]
- Demchuk AM, Goyal M, Yeatts SD, Carrozzella J, Foster LD, Qazi E, Hill MD, Jovin TG, Ribo M, Yan B, Zaidat OO, Frei D, von Kummer R, Cockroft KM, Khatri P, Liebeskind DS, Tomsick TA, Palesch YY, Broderick JP Investigators II. Recanalization and clinical outcome of occlusion sites at baseline CT angiography in the interventional management of stroke III trial. Radiology. 2014;273:202–210. doi: 10.1148/radiol.14132649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerup J, Kleinschnitz C. Visualization of clot composition in ischemic stroke: do we get what we see? Stroke. 2011;42:1193–1194. doi: 10.1161/STROKEAHA.110.612150. [DOI] [PubMed] [Google Scholar]
- Jang IK, Gold HK, Ziskind AA, Fallon JT, Holt RE, Leinbach RC, May JW, Collen D. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989;79:920–928. doi: 10.1161/01.cir.79.4.920. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ali Z, Ringer AJ, Boulos AS, Nakada MT, Alberico RA, Martin LB, Guterman LR, Hopkins LN. Intraarterial reteplase and intravenous abciximab for treatment of acute ischemic stroke. A preliminary feasibility and safety study in a non-human primate model. Neuroradiology. 2005;47:845–854. doi: 10.1007/s00234-003-1097-7. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H American Heart Association Stroke C, Council on Cardiovascular N, Council on Peripheral Vascular D, Council on Clinical C. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery. 2002;50:1405–1414. doi: 10.1097/00006123-200206000-00049. discussion 1414-1405. [DOI] [PubMed] [Google Scholar]
- Mohammad Y, Xavier AR, Christoforidis G, Bourekas E, Slivka A. Qureshi grading scheme for angiographic occlusions strongly correlates with the initial severity and in-hospital outcome of acute ischemic stroke. J Neuroimaging. 2004;14:235–241. doi: 10.1177/1051228404265716. [DOI] [PubMed] [Google Scholar]
- Lee R, Adlam D, Clelland CA, Channon KM. Lines of Zahn in coronary artery thrombus. Eur Heart J. 2012;33:1039. doi: 10.1093/eurheartj/ehs028. [DOI] [PubMed] [Google Scholar]
- Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2014. [DOI] [PubMed]
- Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, de Bruin PC, Mali WP, Velthuis BK. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:e88882. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, Shah SH, Towfighi A, Ovbiagele B, Kidwell CS, Tateshima S, Jahan R, Duckwiler GR, Vinuela F, Salamon N, Villablanca JP, Vinters HV, Marder VJ, Saver JL. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- Singh P, Doostkam S, Reinhard M, Ivanovas V, Taschner CA. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: does stent-retriever cause intimal damage? Stroke. 2013;44:1720–1722. doi: 10.1161/STROKEAHA.113.000964. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Janjua N, Divani AA. Is IV tissue plasminogen activator beneficial in patients with hyperdense artery sign? Neurology. 2006;66:1171–1174. doi: 10.1212/01.wnl.0000208407.69544.5a. [DOI] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Demchuk A, Daunis IEJ, Termes H, Blasco G, Soria G, Boada I, Remollo S, Banos J, Serena J, Castellanos M. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2012;33:90–96. doi: 10.3174/ajnr.A2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidat OO, Suarez JI, Santillan C, Sunshine JL, Tarr RW, Paras VH, Selman WR, Landis DM. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke. 2002;33:1821–1826. doi: 10.1161/01.str.0000020363.23725.67. [DOI] [PubMed] [Google Scholar]
- Linfante I, Llinas RH, Selim M, Chaves C, Kumar S, Parker RA, Caplan LR, Schlaug G. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke. 2002;33:2066–2071. doi: 10.1161/01.str.0000021001.18101.a5. [DOI] [PubMed] [Google Scholar]
- van der Spuy WJ, Pretorius E. Interaction of red blood cells adjacent to and within a thrombus in experimental cerebral ischaemia. Thromb Res. 2013;132:718–723. doi: 10.1016/j.thromres.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Wohner N, Sotonyi P, Machovich R, Szabo L, Tenekedjiev K, Silva MM, Longstaff C, Kolev K. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31:2306–2313. doi: 10.1161/ATVBAHA.111.229088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Minno G, Coppola A, Di Minno MN, Poon MC. Glanzmann’s thrombasthenia (defective platelet integrin alphaIIb-beta3): proposals for management between evidence and open issues. Thromb Haemost. 2009;102:1157–1164. doi: 10.1160/TH09-04-0225. [DOI] [PubMed] [Google Scholar]
- Gersh KC, Zaitsev S, Muzykantov V, Cines DB, Weisel JW. The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics. J Thromb Haemost. 2010;8:1066–1074. doi: 10.1111/j.1538-7836.2010.03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvain Johanne, Edmondson Katie, Bellemain-Appaix Anne, Pena Ana, Barthelemy Olivier, Beygui Farzin, Weisel John, Montalescot Gilles. Impact of time on thrombus composition in STEMI patients treated with Primary PCI. Archives of Cardiovascular Diseases. J-PC CN.
- Munnix IC, Cosemans JM, Auger JM, Heemskerk JW. Platelet response heterogeneity in thrombus formation. Thromb Haemost. 2009;102:1149–1156. doi: 10.1160/TH09-05-0289. [DOI] [PubMed] [Google Scholar]
- Georgiadis AL, Memon MZ, Shah QA, Vazquez G, Tariq NA, Suri MF, Taylor RA, Qureshi AI. Intra-arterial tenecteplase for treatment of acute ischemic stroke: feasibility and comparative outcomes. J Neuroimaging. 2012;22:249–254. doi: 10.1111/j.1552-6569.2011.00628.x. [DOI] [PubMed] [Google Scholar]
- Hassan AE, Chaudhry SA, Miley JT, Khatri R, Hassan SA, Suri MF, Qureshi AI. Microcatheter to recanalization (procedure time) predicts outcomes in endovascular treatment in patients with acute ischemic stroke: when do we stop? AJNR Am J Neuroradiol. 2013;34:354–359. doi: 10.3174/ajnr.A3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AE, Aman MM, Chauhdry SA, Grigoryan M, Tekle WG, Rodriguez GJ, Qureshi AI. Value of other endovascular techniques among patients with MERCI device failure during the treatment of acute ischemic stroke: what to do when MERCI fails? J Vasc Interv Neurol. 2013;5:9–13. [PMC free article] [PubMed] [Google Scholar]
- Yoon W, Jung MY, Jung SH, Park MS, Kim JT, Kang HK. Subarachnoid hemorrhage in a multimodal approach heavily weighted toward mechanical thrombectomy with solitaire stent in acute stroke. Stroke. 2013;44:414–419. doi: 10.1161/STROKEAHA.112.675546. [DOI] [PubMed] [Google Scholar]
- Shhadeh A, Garg A, Hassan AE, Hoover S, Saucedo S, Hassansad B, Cornett O, Tohidi V, Qureshi AI, Kirmani JF. Recanalization following various endovascular modalities for treatment of anterior circulation acute ischemic strokes. J Vasc Interv Neurol. 2012;5:10–16. [PMC free article] [PubMed] [Google Scholar]
- Brekenfeld C, Schroth G, Mordasini P, Fischer U, Mono ML, Weck A, Arnold M, El-Koussy M, Gralla J. Impact of retrievable stents on acute ischemic stroke treatment. AJNR Am J Neuroradiol. 2011;32:1269–1273. doi: 10.3174/ajnr.A2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin NS, Benavides S, Starkman S, Liebeskind DS, Saver JA, Salamon N, Jahan R, Duckwiler GR, Tateshima S, Vinuela F, Vespa PM, Chute DJ, Vinters HV. Autopsy findings after intracranial thrombectomy for acute ischemic stroke: a clinicopathologic study of 5 patients. Stroke. 2010;41:938–947. doi: 10.1161/STROKEAHA.109.576793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata J, Yutani C, Otsubo R, Yamanishi H, Naritomi H, Yamaguchi T, Minematsu K. Heart and vessel pathology underlying brain infarction in 142 stroke patients. Ann Neurol. 2008;63:770–781. doi: 10.1002/ana.21401. [DOI] [PubMed] [Google Scholar]
- Almekhlafi MA, Hu WY, Hill MD, Auer RN. Calcification and endothelialization of thrombi in acute stroke. Ann Neurol. 2008;64:344–348. doi: 10.1002/ana.21404. [DOI] [PubMed] [Google Scholar]