Abstract
Objective:
We tested whether abnormal arterial stiffness and blood pressure would be associated with progression of brain aging measured by brain MRI and neurocognitive testing.
Methods:
Framingham Offspring Cohort participants (n = 1,223, 61 ± 9 years, 56% women) without previous stroke or dementia underwent applanation tonometry, brain MRI, and neurocognitive testing at examination 7 (1998–2001). Follow-up brain MRI and neurocognitive testing was performed at examination 8 (2005–2008, mean interval 6.4 ± 1.3 years). We related examination 7 inverse-transformed carotid-femoral pulse wave velocity (iCFPWV), central pulse pressure (CPP), and mean arterial pressure to changes in the following variables between examinations 7 and 8: total cerebral brain volume, white matter hyperintensity volume, and performance on executive function and abstraction tasks, the Trail Making Test, Parts B and A (ΔTrails B-A), and Similarities tests.
Results:
Higher baseline iCFPWV and CPP were associated with greater progression of neurocognitive decline (iCFPWV and ΔTrails B-A association: SD unit change in outcome variable per SD change in tonometry variable [β] ± SE = 0.10 ± 0.04, p = 0.019; CPP and ΔSimilarities association: −0.08 ± 0.03, p = 0.013). Higher mean arterial pressure, but not iCFPWV or CPP, was associated with increase in white matter hyperintensity volume ([β ± SE] 0.07 ± 0.03, p = 0.017). No tonometry measures were associated with change in cerebral brain volume.
Conclusions:
In middle-aged and older adults without evidence of clinical stroke or dementia, elevated arterial stiffness and pressure pulsatility are associated with longitudinal progression of subclinical vascular brain injury and greater neurocognitive decline. Treatments to reduce arterial stiffness may potentially reduce the progression of neurovascular disease and cognitive decline.
Elevated arterial stiffness and pressure pulsatility, hallmarks of unfavorable aortic remodeling with age, result in transmission of potentially deleterious hemodynamic forces into the distal vasculature.1 Thus, the propagation of elevated pulsatile flow may damage conduit vessels and the microcirculation, at least partially accounting for the observed association of arterial stiffness with cardiovascular disease (CVD) events, including stroke.2–5 However, vascular disease and ultimate end-organ damage is a progressive process with a prolonged latent phase. Preclinical neurologic deficits are manifest on brain MRI, with lower brain volume, asymptomatic cerebral infarcts, and white matter hyperintensity lesions, and may also present in neurocognitive testing as mild cognitive impairment. Such deficits ultimately may lead to dementia, including Alzheimer type dementia.6,7 Cross-sectional investigations in several population studies suggest the association of higher arterial stiffness and abnormal vascular hemodynamics with subclinical neurovascular injury detected both by brain MRI and a standard neurocognitive battery.8–11
Given the progressive nature of CVD, we reasoned that arterial hemodynamics at any given time point may be associated with longitudinal progression of measures of brain vascular injury and neurocognitive function. We tested our hypothesis that tonometry measures of arterial stiffness and pressure pulsatility would be associated with progression of subclinical cerebrovascular disease in the community-based Framingham Offspring Study sample.
METHODS
Study participants.
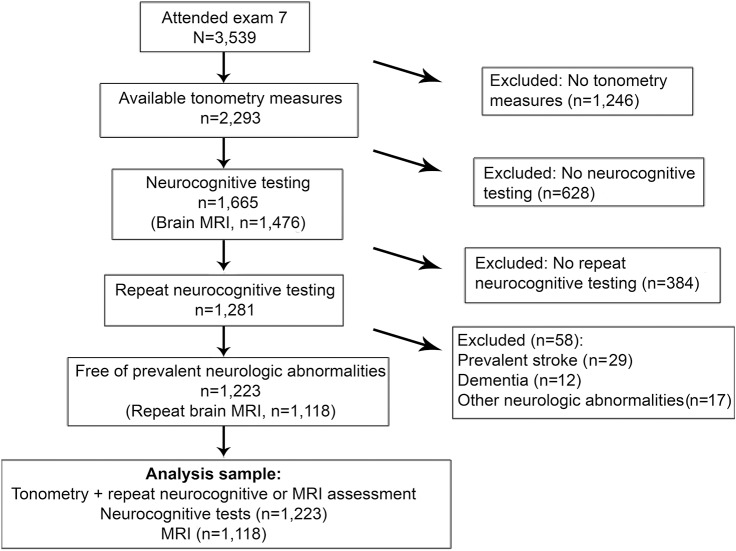
The Framingham Heart Study (FHS) Offspring Cohort is a community-based cohort with detailed phenotyping through serial examination cycles approximately every 4 years.12 At examination 7 (1998–2001), participants underwent arterial tonometry to assess carotid-femoral pulse wave velocity (CFPWV), central pulse pressure (CPP), and mean arterial pressure (MAP). We chose to study these measures to capture aortic stiffness (CFPWV), pressure pulsatility (CPP), and the steady component of pressure (MAP), as they may have differing relations with downstream organ damage.11 These measures are modestly, but not highly, correlated with the following r values: CFPWV-MAP, 0.38; CFPWV-CPP, 0.40; and CPP-MAP, 0.41, all p < 0.0001. Of the 3,539 participants who attended examination 7 (1998–2001), 2,293 participants had successful arterial tonometry measures. Characteristics of participants with and without tonometry measures are shown in table e-1 on the Neurology® Web site at Neurology.org. Of 2,293 participants who underwent tonometry, 1,665 individuals had neurocognitive measures and 1,476 had brain MRI. Of the participants with neurocognitive measures and brain MRI, 1,289 returned at examination 8 (2005–2008) for repeat neurocognitive testing and brain MRI. Participants were excluded for prevalent dementia (n = 12), stroke (n = 29), or other neurologic conditions (e.g., multiple sclerosis, craniectomy, severe head injury, or brain tumor, n = 17) that could confound the assessment of brain structure and function. Thus, our final sample size included 1,223 participants with tonometry and repeated neurocognitive measures, including 1,118 participants with repeated brain MRI measures. The characteristics of participants who underwent tonometry, with and without neurocognitive data, are shown in table e-2. Figure 1 shows the participant inclusion schema. The mean time between initial and subsequent brain MRI and neurovascular testing was 6.4 ± 1.3 years.
Figure 1. Inclusion of study participants.
All included participants had baseline tonometry measures and 2 assessments of brain MRI and neurocognitive function to analyze change in brain measures and neurocognitive function.
Arterial tonometry.
Data acquisition.
Under fasting conditions in the morning and following 5 minutes of rest, arterial tonometry was performed. Brachial systolic and diastolic blood pressures were obtained using an oscillometric device. All measures were obtained in the supine position. A commercially available tonometer (SPT-301; Millar Instruments, Houston, TX) was used for arterial tonometry with simultaneous ECG. The pulse wave transit distance was measured on the body surface as the distance from the suprasternal notch to the femoral artery. Tonometry and ECG data were digitized during the primary acquisition (1,000 Hz) and were analyzed blinded to clinical data (Cardiovascular Engineering, Inc., Norwood, MA).
Tonometry data analysis.
Arterial pressure waveforms were signal-averaged using the ECG R-wave as a fiducial point. The signal-averaged brachial pressure waveform was calibrated using the average systolic and diastolic cuff pressure. The peak and trough of the brachial waveform was then used to determine MAP. CPP was defined by carotid pressure tracings, which were calibrated using diastolic and integrated mean brachial pressures.13 CFPWV was calculated from tonometry waveforms and body surface measurements, which were adjusted for parallel transmission in the brachiocephalic artery and aortic arch, using the suprasternal notch as a fiducial point.14 To assess reproducibility of tonometry measures, the full visit examination (tonometry, echocardiography, and brachial artery flow–mediated dilation) was performed sequentially by 3 operators in each of 15 volunteers, and intraclass correlations were as follows: iCFPWV, 0.87 (95% confidence interval [CI] 0.72–0.95); CPP, 0.77 (95% CI 0.54–0.91); and MAP, 0.82 (95% CI 0.64–0.93). Because of the 2- to 3-hour duration of the full reproducibility examination, the correlations capture not only variability between operators but also physiologic variation in these measures. Thus, true intraclass correlations for simple repeated measures are likely greater.
Brain MRI.
The methods for brain MRI and blinded image analysis have been described previously15,16 and are consistent with consensus guidelines.17 We evaluated total cerebral brain volume (TCBV) and white matter hyperintensity volume (WMHV) as indices of general brain and vascular brain aging, respectively, where lower TCBV and greater WMHV are signs of aging. We computed TCBV as a ratio of intracranial volume to correct for differences in head size. Similarly, WMHV was also computed as a ratio of total cranial volume. The interrater reliabilities ranged between 0.90 to 0.94 for total cranial volume, TCBV, and WMHV.16 All measurements were performed using a custom-designed image analysis package, QUANTA 6.2, operating on a Sun Microsystems (Santa Clara, CA) Ultra 5 workstation.
Neurocognitive assessment.
Details of neurocognitive testing in the FHS Offspring Cohort have been reported elsewhere.18 A battery of neurocognitive tests targeting a range of domain-specific outcomes were conducted, from which we prespecified to study 2 in the realm of executive function and abstract reasoning. The difference score of Trail Making Test Part B minus Part A (Trails B-A) performance is a hallmark of vascular brain aging. The Trail Making Tests are measured in minutes required to accomplish the tasks, with greater score reflecting poorer executive function. We also studied the Similarities test, an index of abstract reasoning, where a greater number of similarities discerned reflect a better score.
Statistical analysis.
CFPWV was inverse-transformed to reduce heteroscedasticity and multiplied by −1,000 to restore directionality and to convert the units to milliseconds/meter (iCFPWV). CPP was natural logarithmically transformed to roughly normalize the skewed distribution. Baseline measures of WMHV and Trails B-A were logarithmically transformed to normalize the skewed distribution. The covariates plasma homocysteine and triglyceride levels were also logarithmically transformed to normalize their skewed distribution.
We examined multivariable-adjusted linear regression models for the analysis of continuous brain MRI and neurocognitive measures (dependent variables), with the arterial stiffness variables serving as the independent variables. We utilized annualized raw change in brain MRI and neurocognitive measures ([value at second MRI or neurocognitive examination − value at first MRI or neurocognitive examination] divided by the time interval between second and first evaluation in years) standardized to mean of 0, SD 1 to facilitate comparisons. A multivariable model adjusted for the following covariates: age, sex, time between tonometry and MRI or neurocognitive testing, diabetes mellitus, atrial fibrillation, current smoking, antihypertensive therapy, prevalent CVD, APOE genotype (presence or absence of at least one ε4 allele), log (plasma homocysteine), and the sex-specific fourth quartile of waist–hip ratio as an indicator of central obesity,19 and additionally adjusted for education and Center for Epidemiologic Studies Depression Scale score for cognitive variables. Covariates in the multivariable model were assessed at the time of examination 7 (baseline). Prevalent CVD was defined as the presence of coronary heart disease, clinical heart failure, or intermittent claudication. We also examined for the possibility of nonlinear relations between tonometry measures and neurocognitive outcomes. Quadratic terms of tonometry variables were entered into multivariable models and significant associations were further evaluated by quintile analysis to determine the nature of the relations. In secondary analyses, we included interaction terms to examine effect modification by age (<65 and ≥65 years) of the relations of arterial stiffness to brain MRI and cognitive outcomes. Lastly, we included sibling relationships as a covariate in multivariable models and mixed models to account for the lack of independence of observations introduced by sibling relationships.
Results are presented as the effect size in SD unit ± standard error (on the brain MRI or neurocognitive measure) per SD unit increment in tonometry measure. The level of statistical significance was set to a 2-sided p value of 0.05 and 0.10 for primary and interaction analyses, respectively. To increase interpretability of our results, we also calculated the effect of association of CFPWV with ΔTrails B-A as an example (e-Methods). All analyses were performed using SAS v.9.3 (SAS Institute, Cary, NC).
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the institutional review board at the Boston University Medical Center, and all participants gave written informed consent.
RESULTS
Study sample.
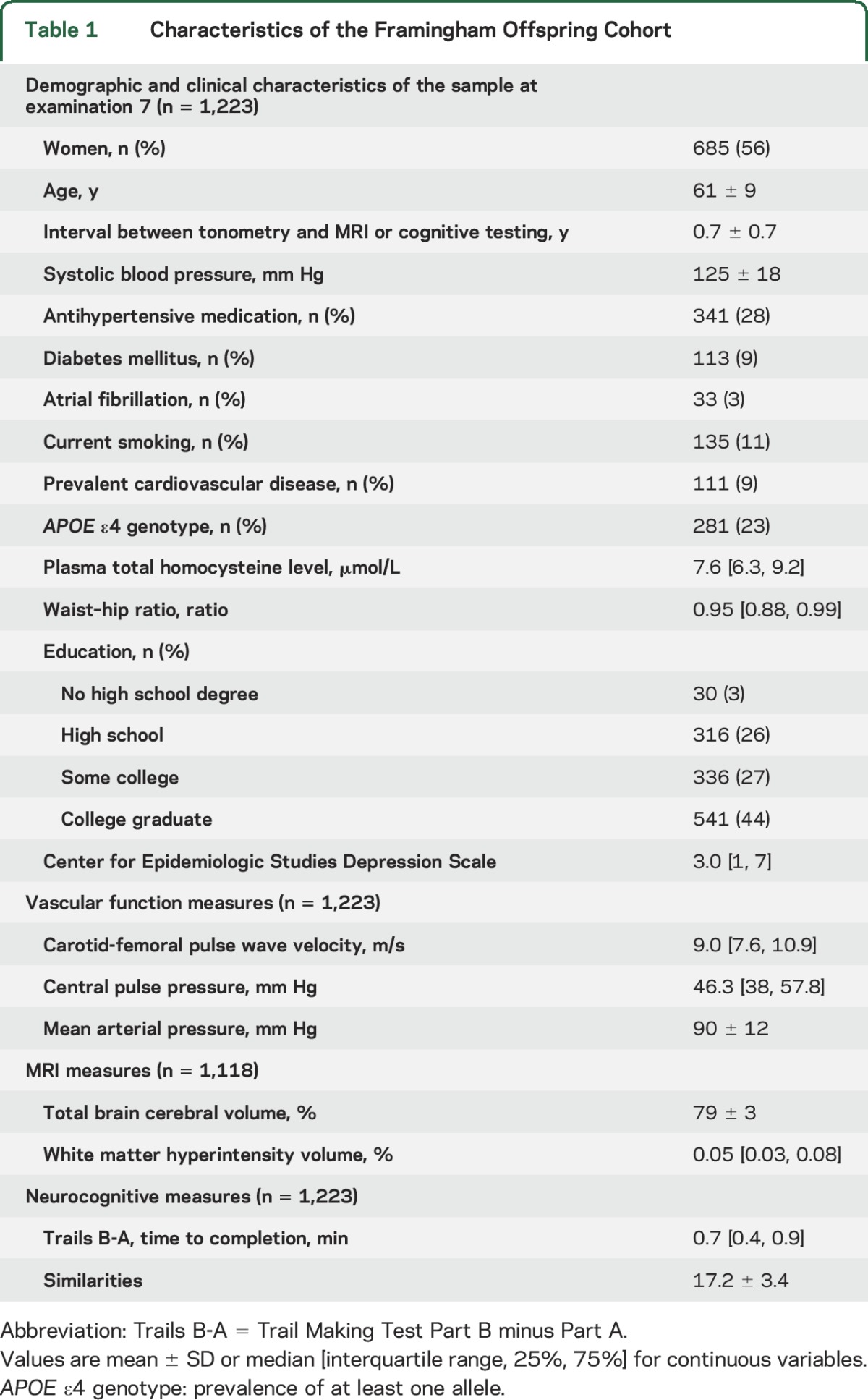
The characteristics of our study sample are shown in table 1. The FHS Offspring Cohort was middle-aged and older and had a relatively low CVD risk profile, with 28% taking antihypertensive medications, and a low prevalence of diabetes mellitus and CVD (9% for both).
Table 1.
Characteristics of the Framingham Offspring Cohort
Association of tonometry measures with change in brain MRI measures.
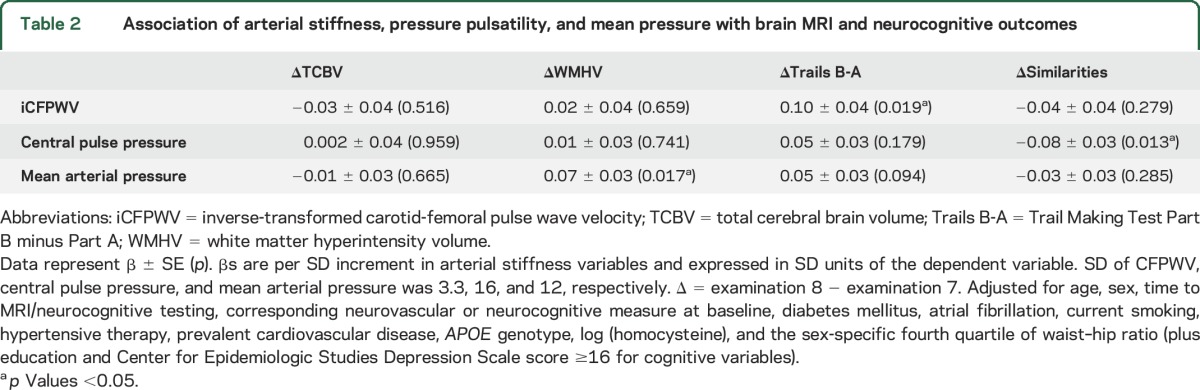
Higher MAP at examination 7 was associated with an increase in WMHV from examination 7 to 8 ([β ± SE] 0.07 ± 0.03, p = 0.017 in model 2; table 2). A similar association was seen in analyses restricted to sibling relationships (table e-3). There was no association between other tonometry measures and ΔWMHV or between tonometry measures and ΔTCBV. There was no age interaction observed of tonometry measures with ΔWMHV.
Table 2.
Association of arterial stiffness, pressure pulsatility, and mean pressure with brain MRI and neurocognitive outcomes
Association of tonometry measures with change in neurocognitive testing.
Higher iCFPWV at examination 7 was associated with an increase in ΔTrails B-A ([β ± SE] 0.10 ± 0.04, p = 0.019; table 2). A 1-SD increase in iCFPWV was associated with an increase of 1.2 seconds in Trails B-A, equivalent to nearly 1 year of brain aging. An increase in CFPWV from the median of the first to fourth quantile was associated with an increase in ΔTrails B-A of 2.9 seconds. This corresponds to more than 2 years of brain aging in a regression model of Trails B-A onto age at baseline examination 7, where the slope, or annual change of Trails B-A, is 1.3 seconds. We observed a relation of quadratic iCFPWV with ΔTrails B-A ([β ± SE] 0.07 ± 0.02, p = 0.003). There was a marked increase in β for ΔTrails B-A in the fifth quintile as compared with other groups (p = 0.0002) (figure 2A), suggesting greater decline in executive function at the highest CFPWV. The small decline seen in the relation of iCFPWV with ΔSimilarities was not statistically significant. However, effect modification by age was observed in the association of iCFPWV with ΔSimilarities, where individuals aged 65 years or older demonstrated a larger magnitude of effect ([β ± SE] −0.11 ± 0.07 in participants 65 years or older vs 0.02 ± 0.04 in participants younger than 65 years, p = 0.024). Higher CPP at examination 7 was associated with a greater decline in ΔSimilarities ([β ± SE] −0.08 ± 0.03, p = 0.013), reflecting poorer executive function. No age interactions were observed in the relations of CPP with any of the brain MRI or neurocognitive measures. MAP was not linearly associated with neurocognitive measures, but examination of quadratic MAP showed an association with ΔSimilarities ([β ± SE] 0.04 ± 0.02, p = 0.023). Analysis of the relation of MAP quintiles with ΔSimilarities suggested a reverse J-shaped relationship (figure 2B). If confirmed in future studies, the findings may indicate that lowest and highest groups of MAP are associated with better abstract reasoning.
Figure 2. Association of iCFPWV quintiles with ΔTrails B-A (A) and MAP quintiles with ΔSimilarities (B).
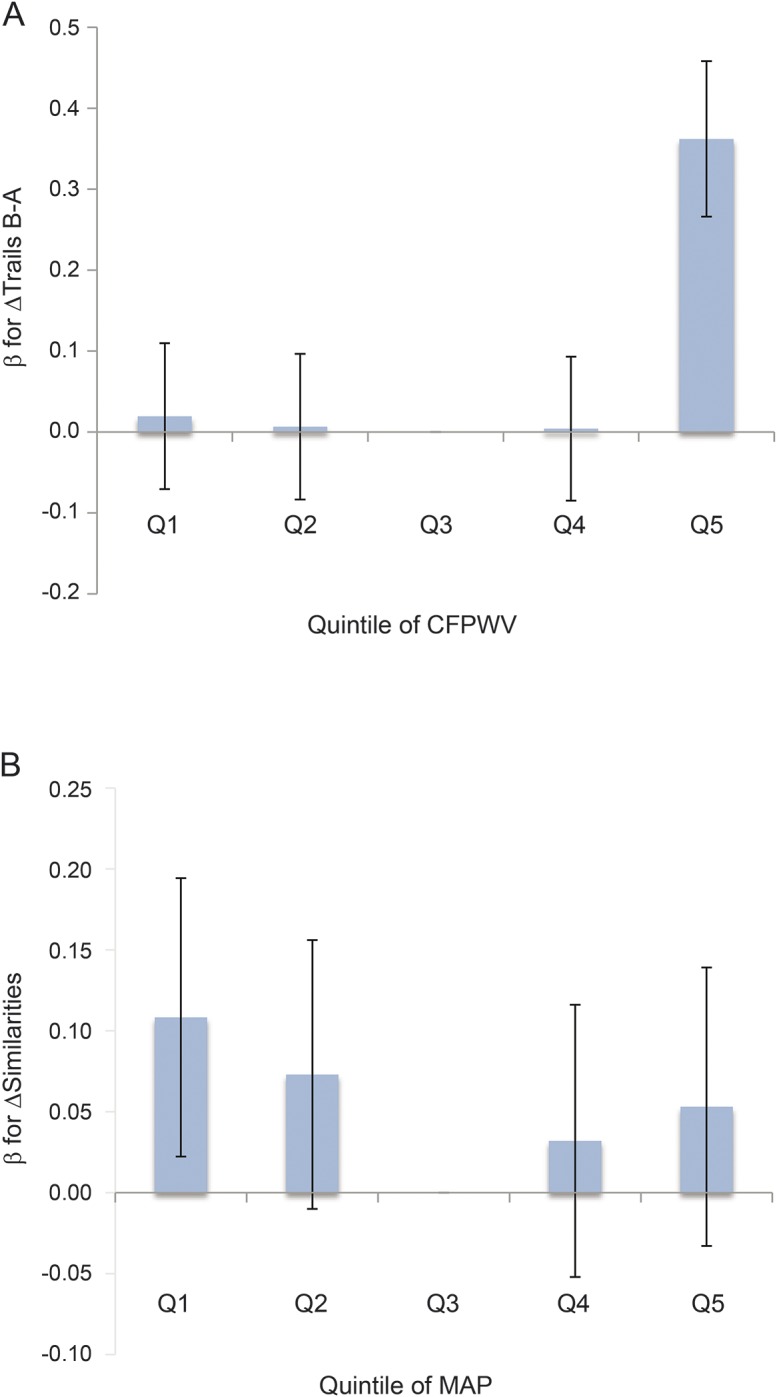
Q3 = referent quintile. The effect size of the association between iCFPWV and ∆Trails B-A was greatest at highest quintile of iCFPWV. The effect sizes of the association between MAP and ∆Similarities were greatest in the first and fifth quintiles. Bars represent standard errors. CFPWV = carotid-femoral pulse wave velocity; iCFPWV = inverse-transformed CFPWV; MAP = mean arterial pressure; Trails B-A = Trail Making Test Part B minus Part A.
DISCUSSION
In this longitudinal study of community-dwelling adults without prevalent stroke or dementia, we found that MAP was associated with a longitudinal increase in WMHV. Likewise, measures of arterial stiffness (CFPWV) and pressure pulsatility (CPP) were associated with a longitudinal decline in cognitive performance in executive function. These findings are consistent with models of vascular aging that emphasize white matter injury and frontal cognitive systems dysfunction.20 Older age was associated with a larger magnitude of effect in these associations. Our findings are consistent with our hypothesis of the deleterious effects of higher aortic stiffness on structural and cognitive neurologic deficits as well as on the progression of neurologic disease over time.
We and other groups, many from community samples with a low prevalence of CVD, have observed cross-sectional associations of elevated pulse wave velocity (PWV) and pulse pressure with brain aging detected by MRI, including lower brain volume, WMHV, and presence of silent cerebral infarcts.10,11,21–24 Consistent with these structural abnormalities, several studies have also shown PWV and CPP to be associated with cognitive function, including poorer memory and a worse performance on executive function tests.10,11,24–27 Much of the literature reflects cross-sectional studies, however, with fewer reports on the influence of arterial stiffness on neurovascular disease over time.
Our examination of the association of arterial stiffness and pulsatility with repeated measures of WMHV revealed that MAP was associated with a progression of WMHV over a 6-year period. In earlier cross-sectional analyses, baseline blood pressure and aortic PWV similarly predicted WMHV several years later in population-based cohorts.28–30 In our sample, CFPWV was associated with WMHV, predominantly in participants aged 65 years or older, in cross-sectional analyses,11 but was not associated with progression of WMHV. A possible explanation for this finding might be that our middle-aged cohort was relatively healthy and younger than other cohorts10,28 in which associations between arterial stiffness and WMH have been observed. Furthermore, unfavorable arterial hemodynamics may not affect all people or all areas of the brain equally: a recent study suggests a greater effect of aortic stiffness in black compared to white persons, and that elevated CFPWV may affect WMHV in certain brain regions but not others.28 A more diverse cohort or more sensitive measures of white matter injury, such as diffusion tensor imaging, could also increase the probability of finding an association between CFPWV and brain vascular injury.
Cognitive decline may be expected as the corollary of the progression of abnormalities on brain imaging. Indeed, we observed relations between higher aortic stiffness and worsening of cognitive function in vascular domains as assessed by the Trails B-A and the Similarities tests. Our findings are consistent with other longitudinal cohort studies that reported associations of greater arterial stiffness and pressure pulsatility with cognitive impairment, assessed by the Mini-Mental State Examination and tests of verbal learning, nonverbal and working memory, and delayed recall.31,32 Of note, we observed the most decline in executive function, measured by ΔTrails B-A, in the highest quintile of iCFPWV. Thus, the highest arterial stiffness is associated with greatest progression of cognitive decline. While this study evaluated relations of baseline measures of arterial stiffness to progression of neurocognitive disease, it would be of interest to investigate the relation of change in tonometry measures (e.g., in the lowest iCFPWV quintile) with neurocognitive decline.
A crucial role of the central vasculature is the buffering of the aortic pulse wave traveling to distal arteries. Advancing age is associated with vessel wall remodeling and a replacement of elastin by less compliant collagen fibers, resulting in greater vascular stiffness.33 A pulse wave traveling through a noncompliant central artery travels faster distally, resulting in microvascular damage. Associated manifestations of blood pressure dysregulation include greater systolic blood pressure, lower diastolic blood pressure, and resultant greater CPP.30,34 The elevated pulsatility propagated into the microcirculation is hypothesized to be responsible for microvascular and endothelial injury resulting in microhemorrhages and thrombosis.34,35 Conversely, episodes of relative hypotension in the setting of impaired compensatory microvascular vasodilation may also result in cerebral hypoperfusion and ischemia.36 The alternating high and low blood pressure suggests a mechanism by which progression of vascular disease begets further tissue injury. In addition, prior evidence has suggested that arterial stiffness, blood pressure, and vascular disease strongly influence amyloid deposition in the brain and clinical manifestations of Alzheimer disease.37–39
We also noted effect modification by age in our cohort, whereby larger effect sizes of the associations between abnormal arterial hemodynamics and neurocognitive dysfunction were seen in older individuals. Comparison of brain MRI data between our cohort and that of the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik), whose participants are on average 15 years older, reveals smaller brain volumes and greater WMHV in AGES-Reykjavik.10 While all measures tested did not overlap between our cohort11 and that of AGES-Reykjavik, effect sizes were generally greater in the latter study. The Baltimore Longitudinal Study of Aging also identified age interactions in the associations of both CFPWV and brachial pulse pressure with poorer cognitive performance.31 Collectively, the totality of evidence suggests that unfavorable central hemodynamics is the beginning of a detrimental path resulting in vascular injury in the brain and possibly contributing to cognitive decline.
The strengths of our study include the large, well-characterized cohort under continuous surveillance. The relatively healthy sample may have diminished some of the observed effect sizes and may have reduced our sensitivity to detect associations. Despite the relatively small effect sizes seen, the low prevalence of CVD in our sample supports the subclinical nature of our findings and suggests that effects of the observed relations may be stronger in the general population. In addition, these findings in a relatively healthy sample underscore the need for investigation regarding the benefits of modification of central arterial stiffness in primary prevention of CVD and neurocognitive decline. We did not account for multiple testing to avoid overcorrection and false-negative bias, but our results are consistent with those of other groups. Lastly, the FHS consists of predominantly white participants of Western European ancestry, potentially limiting the generalizability of our findings to people of other ethnicities. However, our results are consistent with studies incorporating other races and ethnicities, which have reported associations of arterial stiffness with future WMHV28,29 and with cognitive impairment by Mini-Mental State Examination.32 Future confirmation of our results in larger population samples and with longer follow-up is warranted.
In this community-based cohort free of stroke and dementia and with a low prevalence of CVD, we observed relations of higher arterial stiffness and greater pressure pulsatility with progression of neuroimaging traits and neurocognitive impairment. Our findings suggest that efforts should be directed toward investigating the effects of modulating central arterial hemodynamics to potentially reduce or prevent neurovascular brain injury and the resulting cognitive decline.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CPP
central pulse pressure
- CVD
cardiovascular disease
- FHS
Framingham Heart Study
- iCFPWV
inverse-transformed carotid-femoral pulse wave velocity
- MAP
mean arterial pressure
- PWV
pulse wave velocity
- TCBV
total cerebral brain volume
- Trails B-A
Trail Making Test Part B minus Part A
- WMHV
white matter hyperintensity volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Tsao: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. Dr. Himali: statistical analysis, technical, and material support. Dr. Beiser: analysis and interpretation of data, statistical analysis, critical revision of the manuscript for important intellectual content. Dr. DeCarli: acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Vasan: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content. Dr. Mitchell: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision. Dr. Seshadri: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (contracts HHSN268201500001I, N01-HC-25195, HL093029, HL060040, HL070100, HL080124, HL071039, HL077447, HL107385, and HL04334).
DISCLOSURE
C. Tsao is supported by grants from the NIH (K23HL118529) and American Heart Association (13SDG14250015), and Harvard Medical School Fellowship. J. Himali, A. Beiser, and M. Larson report no disclosures relevant to the manuscript. C. DeCarli is supported by the National Institute on Aging (AG010129). R. Vasan reports no disclosures relevant to the manuscript. G. Mitchell is the owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, and is funded by NIH HL094898, DK082447, HL107385, and HL104184. S. Seshadri is supported by the National Institute of Neurological Disorders and Stroke (NS17950) and the National Institute on Aging (AG008122, AG033193, AG16495). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008;105:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003;34:1203–1206. [DOI] [PubMed] [Google Scholar]
- 6.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol 2006;63:246–250. [DOI] [PubMed] [Google Scholar]
- 7.Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci 2012;322:141–147. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 2002;137:149–155. [DOI] [PubMed] [Google Scholar]
- 9.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik Study. Brain 2011;134:3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao CW, Seshadri S, Beiser AS, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 2013;81:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 13.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol 1992;20:952–963. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 15.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr 1992;16:274–284. [DOI] [PubMed] [Google Scholar]
- 16.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 17.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Au R, Seshadri S, Wolf P, et al. New norms for a new generation: cognitive performance in the Framingham Offspring Cohort. Exp Aging Res 2004;30:333–358. [DOI] [PubMed] [Google Scholar]
- 19.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension: the Framingham Heart Study. Curr Alzheimer Res 2007;4:111–116. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Kawas C, Morrison JH, Reuter-Lorenz PA, Sperling RA, Wright CB. Session II: mechanisms of age-related cognitive change and targets for intervention: neural circuits, networks, and plasticity. J Gerontol A Biol Sci Med Sci 2012;67:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging 2011;4:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CK, Lee SH, Kim BJ, Ryu WS, Yoon BW. Age-independent association of pulse pressure with cerebral white matter lesions in asymptomatic elderly individuals. J Hypertens 2011;29:325–329. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Inoue K, Moriki A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertens Res 2007;30:767–773. [DOI] [PubMed] [Google Scholar]
- 24.Poels MM, van Oijen M, Mattace-Raso FU, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam Study. Stroke 2007;38:888–892. [DOI] [PubMed] [Google Scholar]
- 25.Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension 2009;53:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pase MP, Pipingas A, Kras M, et al. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens 2010;28:1724–1729. [DOI] [PubMed] [Google Scholar]
- 27.Tarumi T, Gonzales MM, Fallow B, et al. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. J Hypertens 2013;31:2400–2409. [DOI] [PubMed] [Google Scholar]
- 28.Rosano C, Watson N, Chang Y, et al. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension 2013;61:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King KS, Chen KX, Hulsey KM, et al. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology 2013;267:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aribisala BS, Morris Z, Eadie E, et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension 2014;63:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008;51:99–104. [DOI] [PubMed] [Google Scholar]
- 32.Zeki Al Hazzouri A, Newman AB, Simonsick E, et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition Study. Stroke 2013;44:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother 2003;57:195–202. [DOI] [PubMed] [Google Scholar]
- 34.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007;50:1–13. [DOI] [PubMed] [Google Scholar]
- 35.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 2008;52:1120–1126. [DOI] [PubMed] [Google Scholar]
- 36.Ballard C, O'Brien J, Barber B, et al. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann NY Acad Sci 2000;903:442–445. [DOI] [PubMed] [Google Scholar]
- 37.Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension 2012;59:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA 1997;277:813–817. [PubMed] [Google Scholar]
- 39.Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology 2013;81:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.