Abstract
Objective:
To characterize the cognitive and neuropsychiatric symptoms of patients with behavioral variant frontotemporal dementia (bvFTD) over the natural course of the disease.
Methods:
We examined the initial and subsequent neuropsychological test performance and neuropsychiatric symptoms in a large cohort of patients with bvFTD (n = 204) across progressive stages of disease as measured by the Clinical Dementia Rating (CDR). We also compared cognitive and neuropsychiatric impairments of patients with bvFTD to those of an age-matched cohort with Alzheimer disease (AD) dementia (n = 674).
Results:
At the earliest stage (CDR = 0.5), patients with bvFTD had profound neuropsychiatric disturbances, insensitivity to errors, slower response times, and poor naming, with intact attention span, memory, and facial affect naming. Tests continuing to show progressive, statistically significant stepwise declines after the CDR = 1 stage included free recall, visuoconstruction, set-shifting, error insensitivity, semantic fluency, design fluency, emotion naming, calculations, confrontation naming, syntax comprehension, and verbal agility. At CDR = 0.5, patients with bvFTD significantly outperformed patients with AD in episodic memory and were faster in set-shifting, while scoring quantitatively worse in lexical fluency, emotion naming, and error sensitivity. The overall rate of disease progression in bvFTD was more rapid than in AD.
Conclusion:
There are distinct patterns of cognitive deficits differentiating the earlier and later disease stages in bvFTD, with the pattern of cognitive decline revealing in greater detail the natural history of the disease. These cognitive symptoms are readily apparent clinical markers of dysfunction in the principal brain networks known to undergo molecular and anatomical changes in bvFTD, thus are important indicators of the evolving pathology in individual patients.
Cognitive dysfunction, although usually overshadowed by prominent behavioral disturbances, is still an important symptom of behavioral variant frontotemporal dementia (bvFTD).1,2 Detection of the earliest cognitive deficits helps identify patients promptly and direct them to targeted therapeutic interventions, while precise understanding of the patterns of cognitive decline is essential for demonstrating effectiveness in clinical trials. However, despite a rich literature comparing the cognitive profiles of bvFTD with Alzheimer disease (AD) and other neurodegenerative dementias, a clear characterization of the progressive neuropsychological profile of bvFTD has not yet emerged. A wide variety of tests have been studied but have yielded inconsistent reports of the cognitive domains affected, and the best clinical tests to differentiate bvFTD from related disorders. Several factors contribute to such inconsistencies, including small sample sizes, tendency to group patients of varying disease severity together, lack of uniformity in patient classification, and heterogeneity of terminology.3 Given the imminence of novel therapeutic measures and clinical trials targeting bvFTD,4 it is critical that the field develops a systematic and comprehensive understanding of the cognitive deficits of bvFTD and their pattern of change with disease progression. To identify characteristic clinical profiles at different stages of disease severity, we used a mixed-model approach to evaluate the neuropsychological and neuropsychiatric deficits of a large cohort of patients with bvFTD, stratified by the Clinical Dementia Rating (CDR) scale. At each level of CDR, we compared the normalized scores of patients with bvFTD to an age-matched group of patients with AD dementia.
METHODS
Participants.
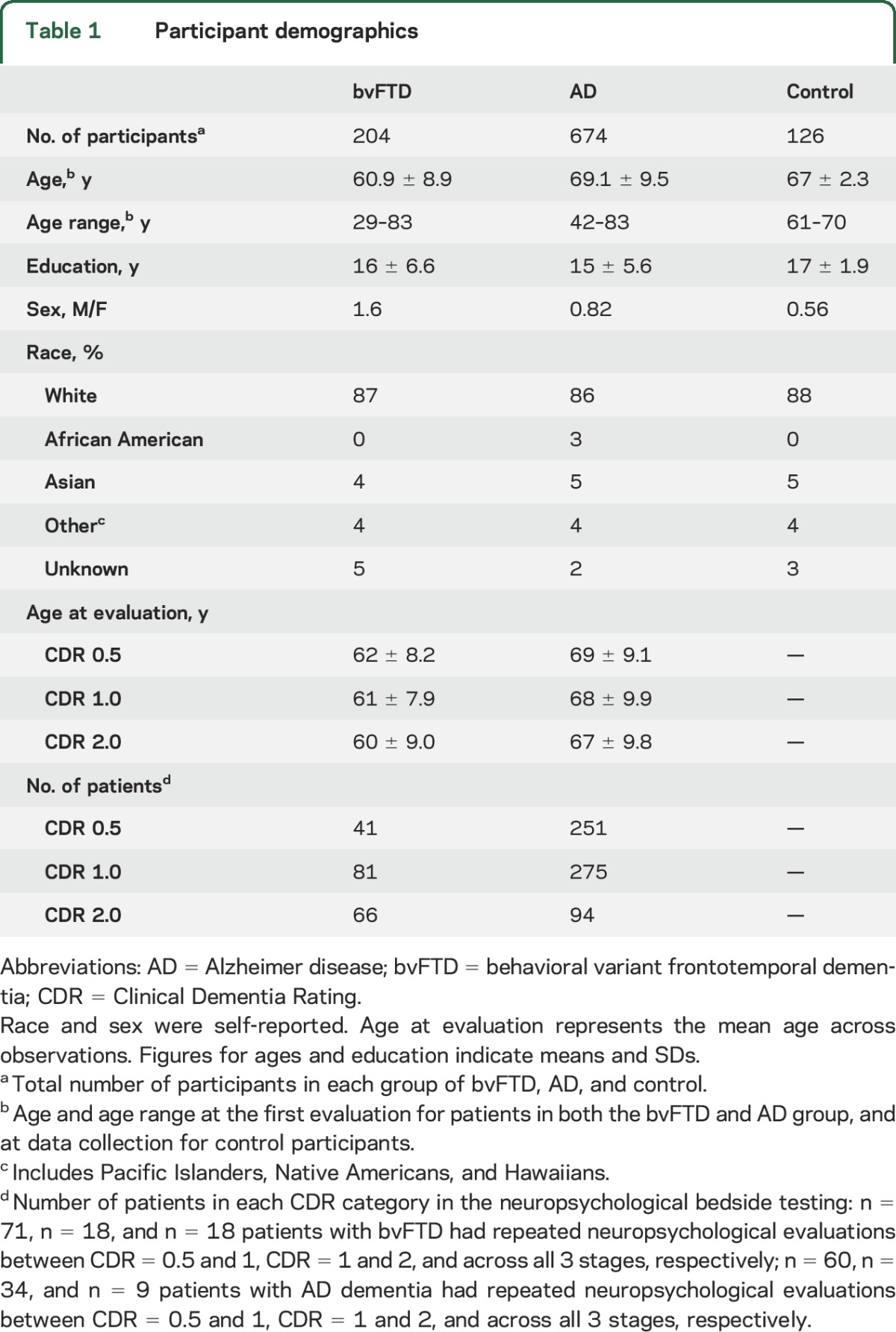
Two hundred four patients meeting the International bvFTD Consortium5 consensus research criteria for possible, probable, or definite bvFTD, and 674 patients meeting clinical criteria6 for possible, probable, or definite AD were evaluated at the University of California San Francisco (UCSF) Memory and Aging Center (MAC). All patients with bvFTD who had undergone cognitive testing at this center were included to represent the broadest possible sample of demographic and disease characteristics. Similarly, we began with a pool of all available patients with AD dementia, and then limited them to match the age range of patients with bvFTD. All patients underwent a complete clinical and cognitive evaluation. Diagnosis was made by consensus at a multidisciplinary meeting. We also examined 126 neurologically healthy control participants matched to the mean age of both patient groups (table 1). All participants of the study were fluent in English as an inclusion criterion, and 86% were Caucasian.
Table 1.
Participant demographics
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from all participants, and the study was approved by the UCSF institutional review boards for human research.
Neuropsychological and behavioral assessment.
We used a battery of neuropsychological tests detailed in previous reports1 (e-Methods on the Neurology® Web site at Neurology.org) to assess major cognitive domains. Based on a detailed caregiver interview, we recorded CDR7 and the frequency-by-severity and caregiver-distress scores of Neuropsychiatric Inventory (NPI) for all patients.8 The neuropsychological scores were standardized (z scores) based on age-matched healthy control performance. We used a cutoff score of z < −1.5 to designate clinical impairment.
Statistical analysis.
We examined the neuropsychological and NPI scores at each CDR stage (very mild, CDR = 0.5; mild, CDR = 1; moderate, CDR = 2; severe, CDR = 3). In patients who had multiple evaluations, we considered only the earliest presentation within a given CDR category. Our CDR categories, within each diagnostic group, had equal representation of patients within age range distributions.
The total number of neuropsychological observations per each patient group was 620 for AD dementia (n = 521) and 188 for bvFTD (n = 151). Seventy-one patients with bvFTD underwent repeated neuropsychological evaluations at CDR = 0.5 and CDR = 1, and 18 patients with bvFTD were evaluated between CDR = 1 and CDR = 2 as well as across all 3 stages of disease severity. Sixty patients with AD dementia underwent repeated neuropsychological evaluations at CDR = 0.5 and CDR = 1, and 34 patients with AD dementia were evaluated at both CDR = 1 and CDR = 2, while 9 patients with AD dementia were evaluated at all 3 stages. To avoid rejecting these valuable repeated data points, we used a longitudinal mixed model using SAS Proc Mixed. Mixed models using random coefficient matrixes are robust for analysis of data with variable numbers of observations and account for both within- and between-subject factors to provide a more accurate estimate of error.9,10 Unlike analysis of variance, mixed models successfully account for the repeated measures of unbalanced designs.11 Thus, this analytic approach might be best understood as a primarily cross-sectional analysis with observations stratified by disease severity, but which advantageously provides more precise estimates of error by allowing additional within-patient time points to be modeled. The analyses were adjusted for age and sex, and patient identity was entered into the model as a repeated factor. We excluded CDR = 3 in the neuropsychological analysis given that most patients were not able to complete neuropsychological testing at this advanced stage.
We examined the rate of disease progression in a subset of patients who were seen more than once and calculated the difference of CDR–Sum of Boxes (CDR-SOB) between the first and the last evaluation divided by time between the 2 assessments. Regression diagnostics using leverage and Cook's D measures determined 5 patients with bvFTD as outliers, and these were excluded from the regression analysis (e-Methods). We used Proc GLM and analysis of covariance procedures in SAS to generate and compare the slopes.
RESULTS
General cognitive dysfunction in bvFTD.
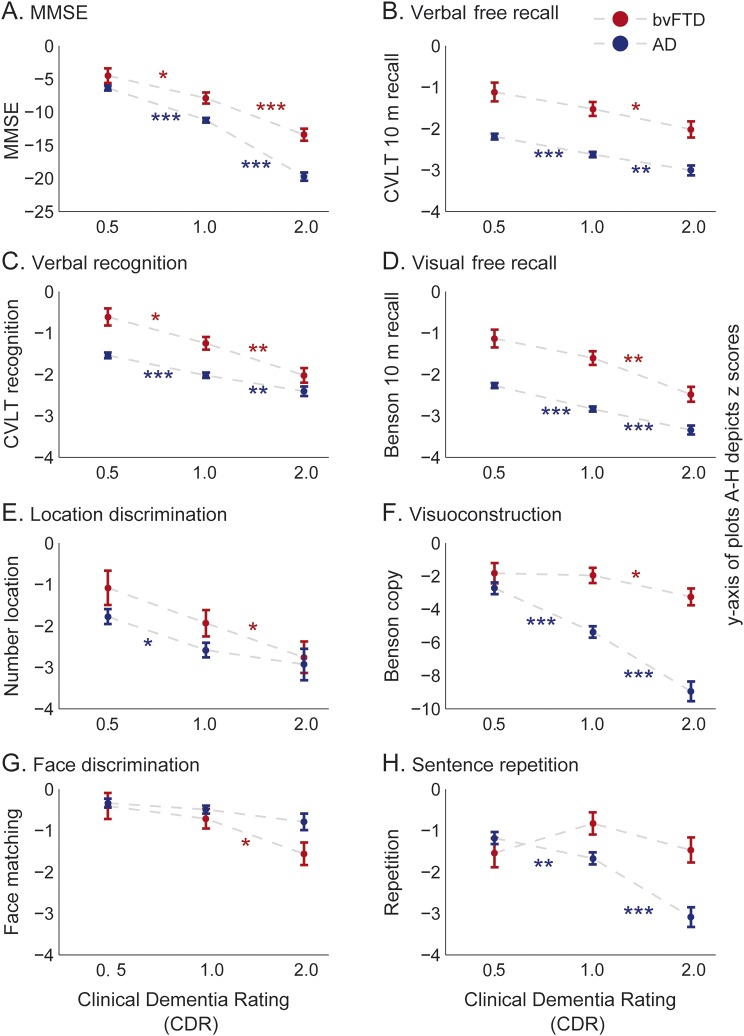
Mini-Mental State Examination (MMSE) showed cognitive deficits even at the very early stage of bvFTD (CDR = 0.5) (figure 1A, table 2). As dementia progressed, the MMSE scores of patients with bvFTD decreased significantly (figure 1A). The MMSE performance was consistently superior in patients with bvFTD than in patients with AD (figure 1A, table e-1).
Figure 1. MMSE, episodic memory, visuospatial, and language performance by patients with bvFTD.
Performance of patients with bvFTD is plotted against the stages of disease severity at 3 CDR stages (very mild, CDR = 0.5; mild, CDR = 1; moderate, CDR = 2). The plots depict the least square means corrected for age and sex, and the standard errors, derived from the mixed-model analysis, based on z-score estimates calculated using an age-matched control population. (A) MMSE. (B) Verbal free recall, depicted as the d′ of the number of words recalled from a 9-item CVLT word list after a 10-minute delay. (C) Verbal recognition, depicted by the d′ of the score achieved at recognizing the 9-item CVLT word list after 10 minutes. (D) Visual free recall, construction of Benson figure from memory after 10 minutes. (E) Location discrimination, as assessed by the number location task of the Visual Object and Space Perception Battery. (F) Visuoconstruction, as assessed by copy of the Benson figure. (G) Face recognition assessed by the face-matching subtest of the Comprehensive Affect Testing System involving 12 trials in which the participant determines whether 2 faces are the same or different. (H) Sentence repetition, assessed by having participants repeat 3 phonemically complex sentences following the examiner. Each subplot also illustrated the same measures for an AD patient group who were matched to the same age range of the bvFTD patient group. The d′ estimates of auditory free recall and auditory recognition for the control groups were calculated based on their performance on the CVLT long form (i.e., 16-item word list). Error bars indicate the standard errors derived from the mixed-model analysis. The asterisks indicate the significance from Tukey post hoc comparison between CDR stages within each patient group; *p < 0.05, **p < 0.01, ***p < 0.0001. AD = Alzheimer disease; bvFTD = behavioral variant frontotemporal dementia; CDR = Clinical Dementia Rating; CVLT = California Verbal Learning Test; MMSE = Mini-Mental State Examination.
Table 2.
Mixed-model analysis of neuropsychological test performance
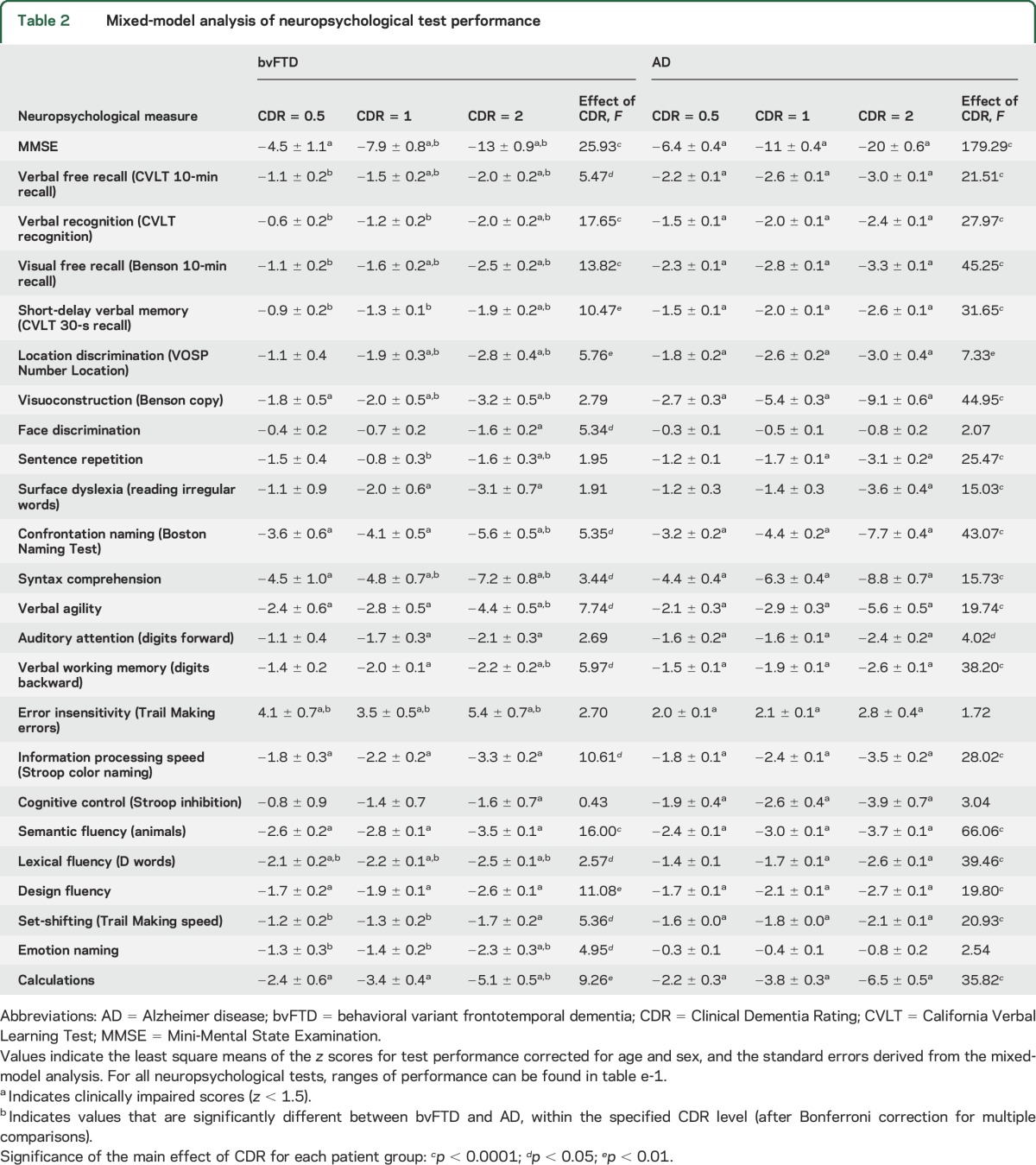
Domain-specific cognitive dysfunction in bvFTD.
Episodic memory.
Episodic memory performance was not impaired at the early stage of bvFTD but became significantly affected as the syndrome advanced. Verbal free recall, verbal recognition, visual free recall (figure 1, B–D), and short-delay verbal memory (figure e-1A) abilities were low-average/normal in bvFTD at CDR = 0.5 and became significantly impaired with advanced dementia (table 2). Both verbal and visual free recall of patients with AD were significantly below that of patients with bvFTD at all stages of CDR (figure 1, B and D; table 2; table e-1).
Visuospatial function.
At CDR = 0.5, patients with bvFTD showed a low-average/normal performance on location discrimination but already scored in the impaired range on visuoconstruction (figure 1, E and F; table 2). With advancing dementia, location discrimination also significantly declined (figure 1E, table 2). Patients with bvFTD showed intact performance on a face discrimination task during early disease, but reached impaired levels at CDR = 2 (figure 1G, table 2). Patients with AD dementia, in contrast, were significantly impaired at CDR = 0.5 in both location discrimination and visuoconstruction ability, and continued to decline (figure 1, E and F; table 2). Decline in visuoconstruction ability was more dramatic in patients with AD dementia compared to patients with bvFTD (figure 1F, table e-1).
Speech and language function.
At CDR = 0.5, patients with bvFTD showed low-average/normal performance on sentence repetition and irregular word reading, yet were already impaired on confrontation naming, verbal agility, and syntax comprehension (figure 1H, figure e-1, table 2). At CDR = 2, patients with bvFTD showed impairment on all language tests (table 2). At CDR = 0.5, both AD and bvFTD patient groups showed a similar pattern of language dysfunction (figure 1H; figure e-1, B–E; table 2). Patients with AD dementia showed a dramatic progressive decline in sentence repetition, as opposed to patients with bvFTD who did not show a significant decline (figure 1H, table e-1).
Attention and working memory.
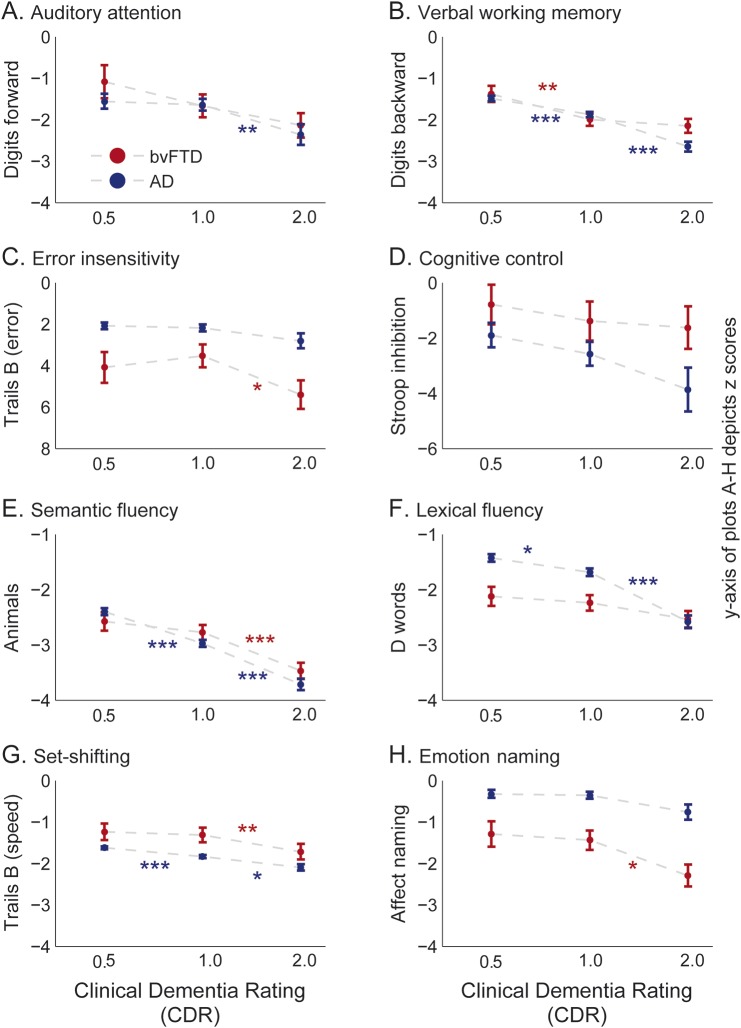
Both auditory attention and verbal working memory fell within low-average/normal performance at CDR = 0.5 of bvFTD (figure 2, A and B; table 2). Auditory attention ability did not show a statistically significant decrease across CDR stages of bvFTD, although verbal working memory declined significantly (figure 2, A and B; table 2). Both patient groups showed similar attention and working memory abilities except at CDR = 2 where patients with AD dementia showed significantly worse working memory than patients with bvFTD (figure 2, A and B; table 2).
Figure 2. Performance on attention and working memory, executive, and facial affect naming tasks by patients with bvFTD.
Performance by patients with bvFTD is plotted against the stages of disease severity (very mild, CDR = 0.5; mild, CDR = 1; moderate, CDR = 2). The plots depict the least square means corrected for age and sex, and the standard errors, derived from the mixed-model analysis, based on z-score estimates calculated using an age-matched control population. (A) Auditory attention, assessed by the number of digits correctly repeated in the same order from a list read by the examiner. (B) Verbal working memory, number of digits correctly repeated backwards from a list read by the examiner. (C) Error insensitivity, number of errors made during the modified Trail Making Test during 60 seconds. (D) Cognitive control, number of words correctly read in the Stroop color inhibition test within 60 seconds divided by the number of correct words in the Stroop color naming test. (E) Semantic fluency, the number of animals listed within 60 seconds. (F) Lexical fluency, the number of words starting from the letter D listed within 60 seconds. (G) Set-shifting, assessed by the number of correct lines drawn per second in the modified Trail Making Test, which requires the patient to serially alternate between numbers and days of the week. (H) Emotion naming as assessed by the affect matching subtest of the Comprehensive Affect Testing System containing 16 trials in which the participant is shown a photograph of an emotional face and required to select the correct label from a list (i.e., happy, sad, angry, frightened, surprised, disgusted, or neutral). The y-axis of the subplot (C) depicting the error insensitivity was inverted to make it more intuitive. Each subplot also illustrates the same measures for an AD patient group who were matched to the same age range of the bvFTD patient group. Error bars indicate the standard errors derived from the mixed-model analysis. The asterisks indicate the significance from Tukey post hoc comparison between CDR stages within each patient group; *p < 0.05, **p < 0.01, ***p < 0.0001. AD = Alzheimer disease; bvFTD = behavioral variant frontotemporal dementia; CDR = Clinical Dementia Rating.
Executive function.
Error insensitivity was the most profound dysfunction in the executive profile of early bvFTD (figure 2C) and showed the highest quantitative deviation from normal (table 2). Information processing speed was clinically impaired at CDR = 0.5 of bvFTD, while cognitive control and speed on a set-shifting task was low-average/normal (figure 2, D and G; figure e-1F; table 2). Performance on verbal (lexical and semantic) and visual (design) fluency also showed profound deficits at CDR = 0.5 of bvFTD, and also significantly declined with progression (figure 2, E and F; figure e-1G; table 2).
Patients with AD dementia showed impaired performance in all executive tasks other than lexical fluency at CDR = 0.5 (figure 2, D–G; figure e-1, F and G). Patients with AD dementia outperformed those with bvFTD on lexical fluency in early disease, yet progressed relatively rapidly compared to patients with bvFTD (figure 2F, table e-1). Semantic fluency performance by both patient groups were within similar ranges (figure 2E). Patients with AD dementia were also consistently better in monitoring errors than patients with bvFTD (figure 2C, table e-1).
Emotion naming.
Emotion naming remained at low-average/normal range at CDR = 0.5 and CDR = 1 of bvFTD, and became impaired at CDR = 2 (figure 2H, table 2). Patients with AD dementia, in contrast, retained their emotion naming ability throughout (figure 2H) and performed consistently better compared to patients with bvFTD (table e-1).
Neuropsychiatric symptoms of bvFTD.
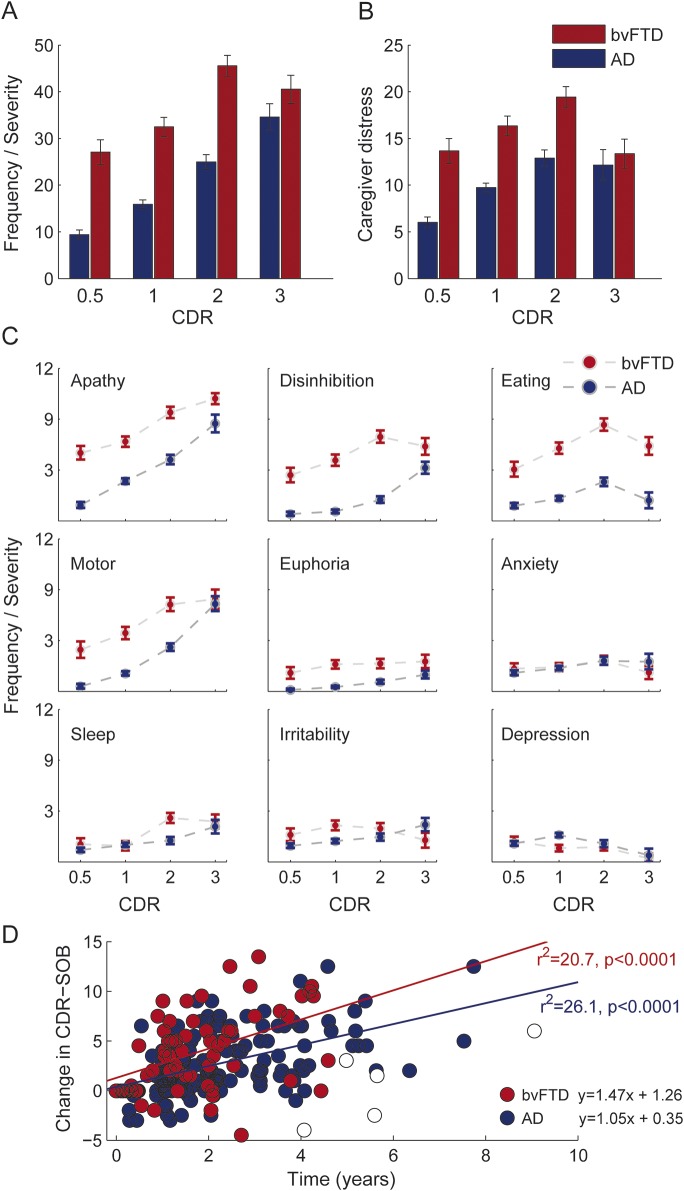
Patients with bvFTD reported high levels of neuropsychiatric disturbance and associated caregiver distress from the very early stage. The NPI scores and the caregiver distress increased significantly as dementia advanced (figure 3, A and B; effect of CDR: total NPI, F = 14.08, p < 0.0001; caregiver distress, F = 5.82, p < 0.01). At CDR = 0.5, apathy was the highest rated behavioral disturbance of bvFTD, followed by disinhibition, abnormal eating, and motor symptoms, all of which were significantly higher compared to AD (figure 3C, table e-2). Despite early high scores, apathy, disinhibition, eating, and motor symptoms continued to increase significantly (figure 3C, table e-2). Sleep disturbances rated low at CDR = 0.5 of bvFTD and showed a significant increase with progression (figure 3C, table e-2). While apathy continued to increase, all other NPI indices reached maximum levels at CDR = 2 in bvFTD (figure 3C).
Figure 3. Neuropsychiatric symptoms and progression of dementia.
Neuropsychiatric symptom battery listed in Neuropsychiatric Inventory used to assess the frequency of behavioral symptoms and their severity at the caregiver interview. The frequency-by-severity score denotes the product of the severity of any behavioral symptom graded out of 3, and the number of episodes. (A) Total of frequency-by-severity scores across all the behavioral symptoms, plotted against the disease severity denoted by 4 different CDR stages ranging from 0.5 to 3. (B) Total of caregiver distress calculated for each of the behavioral symptoms out of 5. (C) Frequency-by-severity scores at each CDR stage, for the top 9 neuropsychiatric symptoms (sorted according to score-ranks) of bvFTD and AD patient groups. CDR stages (very mild, CDR = 0.5; mild, CDR = 1; moderate, CDR = 2; severe, CDR = 3). Error bars indicate the standard errors derived from the mixed-model analysis. (D) Patients with bvFTD showed a faster progression of dementia compared to patients with AD as measured by CDR-SOB. The x-axis plots the difference of time between the first and the last evaluation and the y-axis shows the difference in CDR-SOB between the 2 evaluations. The analysis includes a subset of bvFTD and AD populations who have more than one evaluation. The open squares indicate patients with bvFTD whom we have identified as outliers and were not included in the regression equation. Number of patients in each group for subplots A–C: bvFTD (CDR 0.5, n = 47; CDR 1, n = 84; CDR 2, n = 66; CDR 3, n = 32); AD (CDR 0.5, n = 188; CDR 1, n = 229; CDR 2, n = 71; CDR 3, n = 20). Number of patients in each group for subplot D: AD, n = 162; bvFTD, n = 67. AD = Alzheimer disease; bvFTD = behavioral variant frontotemporal dementia; CDR = Clinical Dementia Rating; CDR-SOB = Clinical Dementia Rating–Sum of Boxes.
Sex differences.
MMSE, digit span backward, and visual free recall showed relatively higher degree of impairment in female patients with bvFTD (MMSE: men = 23.7, women = 21.2, p < 0.05; digit span backward: men = 3.9, women = 3.1, p < 0.05; visual free recall: men = 6.7, women = 5.0, p < 0.05). Female patients with bvFTD also showed elevated delusion scores, while apathy, sleep abnormalities, and caregiver distress were elevated in male patients (apathy: men = 8.2, women = 6.6, p < 0.01; sleep: men = 3.1, women = 1.5, p < 0.01; distress: men = 17.5, women = 13.8, p < 0.05; delusions: men = 0.5, women = 1.3, p < 0.05).
Rate of disease progression.
Progression of dementia (as measured by CDR-SOB, which is a broader dimensional measure of severity and is highly correlated with the nonordinal CDR total score) was significantly faster in bvFTD than in AD (figure 3D). Regression analysis after excluding outliers (see e-Methods) demonstrated that the slope of disease progression was significantly steeper in bvFTD than in AD (analysis of covariance, F = 20.5, p < 0.0001), with a projected rate of increase of 2.7 CDR-SOB points/year in bvFTD and 1.4 points/year in AD.
DISCUSSION
This study examined the largest single cohort of patients with bvFTD reported to date with an age-matched control group of patients with AD dementia, assessed using the same neuropsychological and neuropsychiatric measures. We demonstrate the cognitive and neuropsychiatric symptoms that appear earliest in bvFTD, and identify the neuropsychological tests that are most sensitive to progression of disease, as well as those most useful for differential diagnosis. These data may be used to improve the clinical detection of bvFTD as well as to design clinical trials in which markers of disease progression have a crucial role.
The patient with very early-stage bvFTD presents decreased error sensitivity and slower response times, with intact attention, cognitive control, memory, and facial affect naming. Poor scores on language tasks is common in early bvFTD, although performance on these tasks is susceptible to nonlanguage factors such as inattention, disorganization, slowed information processing speed, and lack of effort. Their visuoperceptual abilities are mostly spared despite subtle visuoconstruction deficits.
Error insensitivity (frequency of uncorrected errors) was a highly sensitive index of early bvFTD. Previous studies have shown that working memory and inhibitory control are mediated by relatively interdependent prefrontal cortex pathways, and although both right and left dorsolateral prefrontal cortices are involved in working memory, the right lateral prefrontal cortex may be preferentially engaged when response inhibition is a necessary component of task completion.12,13 Right lateral prefrontal cortex, particularly the inferior frontal gyrus, is implicated in rule violation errors following failure to suppress automatic behavior, likely in conjunction with other closely networked structures mediating top-down control, including the dorsal anterior cingulate.14–17 The tendency of patients with bvFTD to commit an unusually high number of errors implicates early involvement of these right lateral prefrontal networks.
Our results demonstrate that the testing modality in which the cognitive domain is probed is crucial in bvFTD. In early bvFTD, patients showed clear impairment of information processing speed and fluency tasks, yet maintained low-average/normal performance on attention and working memory tasks. Previous reports also found low-average/normal performance on attention and working memory1,18 alongside clearly impaired complex executive functions in bvFTD.1,18–22 Similarly, basic visuoperceptual processing was relatively preserved in bvFTD, although patients performed below expectation on more complex visuospatial tasks. These findings underscore the effect of executive organization (e.g., relative placement of details during visuoconstruction) or divided attention on test performance.23–27
Our study found that relative preservation of performance on all aspects of episodic memory tasks is characteristic of early bvFTD. Absence of early amnesia is one of the diagnostic criteria for bvFTD,5,28 thus to a certain degree this finding is circular; however, relative preservation of memory has previously been reported in autopsy-confirmed cases of bvFTD.29 In mild disease, patients with bvFTD demonstrated impaired free recall but maintained the capacity for recognition, suggesting that initial memory deficits occur as a result of inattentive or disorganized learning, rather than a hippocampally mediated consolidation deficit. Significant impairments in all components of memory at moderate bvFTD reflect the progression of pathologic changes to medial temporal lobes.1,23,30
High levels of neuropsychiatric disturbances were consistently found across our sample. This suggests that clinically significant elevations of neuropsychiatric symptoms are the rule, and caution should be exercised when diagnosing bvFTD while such symptoms are minimal or absent.28,31
We identified neuropsychological tests that show significant incremental changes in bvFTD without floor or ceiling effects as effective tools to monitor progression of disease. MMSE provided a sensitive index of disease progression throughout the entire disease course. The tests continuing to show significant progressive decline after the CDR = 1 stage included free recall, visuospatial function, set-shifting, error insensitivity, semantic fluency, design fluency, emotion naming, calculations, confrontation naming, syntax comprehension, and verbal agility. These tests provide useful markers for clinical trials focused on more advanced stages of the disease.4
Differential diagnosis of bvFTD from AD is a crucial step in patient management. At the very mild stage of the disease, patients with bvFTD significantly outperformed patients with AD dementia on episodic memory and were faster on a set-shifting task, while scoring quantitatively worse than the latter in lexical fluency, emotion naming, and error sensitivity, with the bvFTD group at CDR = 0.5 making more errors than the AD dementia group at CDR = 2. This pattern is consistent with the greater involvement of frontally mediated executive and socioemotional systems in bvFTD compared to AD. Patients with AD dementia showed significantly sharper decline compared to patients with bvFTD on the MMSE, visuoconstruction, working memory, sentence repetition, confrontation naming, and lexical fluency. These findings show distinct profiles of progressive decline in AD and bvFTD reflecting unique anatomical patterns of disease burden.2,32,33
Poor test performance of patients with bvFTD is not always attributable to cognitive deficits but rather to noncooperation with the testing procedures. The underlying behavioral disorder inherent to bvFTD causes many patients to demonstrate an early amotivational syndrome (sometimes called “denkfaulheit” or mental laziness)34 that reduces their attention and engagement in neuropsychological testing and makes them less concerned about accuracy.35 These deficits are likely direct consequences of the pathologic changes occurring early in bvFTD involving ventral salience network structures, which help patients identify personally salient stimuli,36,37 dorsal task control network structures, which aid in maintenance of task set and focused attention,17 as well as (in a subset of bvFTD cases) the orbitofrontal/limbic network that guides emotional evaluations.38,39 The profound increase of rule violation errors demonstrated by patients with bvFTD both in our study and in others12 suggests that these noncognitive performance factors likely contribute to neuropsychological scores starting from very mild stage of the disease.40
The main limitation of this study is the assignment of diagnosis based on the imperfect standards of clinical criteria. The UCSF MAC, however, holds an excellent record of diagnostic accuracy for bvFTD syndrome (MAC 93% vs international consensus sample 86%).5 Even if patients meeting clinical criteria for bvFTD were included who will eventually be determined not to have a frontotemporal lobar degeneration pathology, the large size of the cohort compensates for this error. Future studies involving pathologically proven samples of bvFTD patients will provide valuable input to corroborate current findings. The epidemiologic differences between bvFTD and AD introduced a few caveats into our study. First, as AD is more prevalent toward the older end of the age range and bvFTD is more prevalent toward the younger end, the normalized scores of AD may represent a slight underestimation bias while that of bvFTD may represent an overestimation bias. Based on this, the current results are more conservative in their detection of impairments in bvFTD and less conservative in contrasting bvFTD with AD. Second, the patients with bvFTD appeared to have more regularly undergone follow-up than patients with AD dementia, which may introduce a relative bias toward the number of repeated observations in each patient group. The current analysis, however, restricted to a single observation per subject within a CDR category, which minimized such bias to a certain degree. Third, our cohort included >80% white Caucasian participants who were fluent in English. Although this approach enabled us to generate a more unified sample reducing language bias on cognitive testing, it also limited generalization to other racial groups.
In conclusion, even at the earliest stages of the disease, patients with bvFTD demonstrate a number of quantitative deficits on traditional neuropsychological testing, many of which continue to show clinically significant declines with disease progression, and form a symptom profile that is significantly divergent from that seen in age-matched patients with AD dementia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and their caregivers for their participation in this research.
GLOSSARY
- AD
Alzheimer disease
- bvFTD
behavioral variant frontotemporal dementia
- CDR
Clinical Dementia Rating
- CDR-SOB
Clinical Dementia Rating–Sum of Boxes
- MAC
Memory and Aging Center
- MMSE
Mini-Mental State Examination
- NPI
Neuropsychiatric Inventory
- UCSF
University of California San Francisco
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.G.R. and K.P.R. designed the study, performed the analysis including the statistical analyses, and wrote the manuscript. I.V.L. supervised the statistical procedures and gave expert advice on statistics. J.H.K., V.E.S., B.M.B., K.P., S.C.Y., A.K.L., T.S.-U., and M.L.S. contributed to design and conduct of the neuropsychological and neuropsychiatric evaluations of the patients. K.A.V., M.L.G.-T., D.C.P., S.E.L., Z.A.M., H.J.R., A.B., W.W.S., G.D.R., and B.L.M. contributed to the design and conduct of the clinical and diagnostic evaluation of patients. All authors contributed in the diagnostic consensus panels involved in evaluating patient assessments. K.G.R. wrote the manuscript. K.P.R., K.A.V., G.D.R., and B.L.M. contributed to extensive editorial revisions of the manuscript.
STUDY FUNDING
This study was supported by the NIH grants P01AG019724, P50AG02350 (B.L.M.), R01AG029577, and K23AG021606 (K.P.R.), K23 AG038357 (K.A.V.), K23AG040127 (V.E.S.), K23 AG042492-01 (B.M.B.), K23 AG039414 (S.E.L.), K23AG045289 (D.C.P.), a grant from the Alzheimer's Association (PCTRB-13-288476) (K.A.V.) and was made possible by Part the Cloud, grants from the Larry L. Hillblom Foundation, Inc. 2013-A-029-SUP, 2007/2I, 2015-A-034-FEL (K.G.R.), and from the J.D. French Alzheimer's Foundation (K.A.V.).
DISCLOSURE
K. Ranasinghe, K. Rankin, I. Lobach, J. Kramer, V. Strum, B. Bettcher, K. Possin, S.C. You, A. Lamarre, T. Shany-Ur, M. Stephens, D. Perry, S. Lee, Z. Miller, M. Gorno-Tempini, H. Rosen, A. Boxer, W. Seeley, G. Rabinovici, and K. Vossel report no disclosures relevant to the manuscript. B. Miller has the following disclosures: he serves as medical director for the John Douglas French Foundation; scientific director for the Tau Consortium; director/medical advisory board member of the Larry L. Hillblom Foundation; and scientific advisory board member for the National Institute for Health Research Cambridge Biomedical Research Centre and the Biomedical Research Unit in Dementia and the Consortium for Frontotemporal Research. He receives support through the NIA and NINDS. He has consulted for Forum Pharmaceuticals. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003;16:211–218. [DOI] [PubMed] [Google Scholar]
- 2.Libon DJ, Xie SX, Wang X, et al. Neuropsychological decline in frontotemporal lobar degeneration: a longitudinal analysis. Neuropsychology 2009;23:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittenberg D, Possin KL, Rascovsky K, Rankin KP, Miller BL, Kramer JH. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol Rev 2008;18:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008;131:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 8.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 9.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models, 2nd ed Cary, NC: SAS Institute; 2006. [Google Scholar]
- 10.Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G. Longitudinal Data Analysis, 1st ed Boca Raton, FL: Chapman & Hall/CRC, Taylor & Francis Group; 2008. [Google Scholar]
- 11.McCulloch CE. Repeated measures ANOVA, R.I.P.? Chance 2013;18:29–33. [Google Scholar]
- 12.Possin KL, Brambati SM, Rosen HJ, et al. Rule violation errors are associated with right lateral prefrontal cortex atrophy in neurodegenerative disease. J Int Neuropsychol Soc 2009;15:354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain 2001;124:2074–2086. [DOI] [PubMed] [Google Scholar]
- 14.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 2014;18:177–185. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Hum Brain Mapp 2005;25:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci 2007;19:69–80. [DOI] [PubMed] [Google Scholar]
- 17.Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007;104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer's disease. Neurology 2000;54:2277–2284. [DOI] [PubMed] [Google Scholar]
- 19.Rosen HJ, Narvaez JM, Hallam B, et al. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord 2004;18:202–207. [PubMed] [Google Scholar]
- 20.Pasquier F, Lebert F, Grymonprez L, Petit H. Verbal fluency in dementia of frontal lobe type and dementia of Alzheimer type. J Neurol Neurosurg Psychiatry 1995;58:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges JR, Patterson K, Ward R, et al. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer's disease: a comparative neuropsychological study. Neuropsychology 1999;13:31–40. [DOI] [PubMed] [Google Scholar]
- 22.Johns EK, Phillips NA, Belleville S, et al. Executive functions in frontotemporal dementia and Lewy body dementia. Neuropsychology 2009;23:765–777. [DOI] [PubMed] [Google Scholar]
- 23.Diehl-Schmid J, Bornschein S, Pohl C, Forstl H, Kurz A, Jahn T. Cognitive decline in the behavioral variant of frontotemporal dementia. Int Psychogeriatr 2011;23:230–237. [DOI] [PubMed] [Google Scholar]
- 24.Pachana NA, Boone KB, Miller BL, Cummings JL, Berman N. Comparison of neuropsychological functioning in Alzheimer's disease and frontotemporal dementia. J Int Neuropsychol Soc 1996;2:505–510. [DOI] [PubMed] [Google Scholar]
- 25.Mendez MF, Cherrier M, Perryman KM, Pachana N, Miller BL, Cummings JL. Frontotemporal dementia versus Alzheimer's disease: differential cognitive features. Neurology 1996;47:1189–1194. [DOI] [PubMed] [Google Scholar]
- 26.Freeman RQ, Giovannetti T, Lamar M, et al. Visuoconstructional problems in dementia: contribution of executive systems functions. Neuropsychology 2000;14:415–426. [DOI] [PubMed] [Google Scholar]
- 27.Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase 2010;16:466–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 29.Rascovsky K, Salmon DP, Ho GJ, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology 2002;58:1801–1808. [DOI] [PubMed] [Google Scholar]
- 30.Whitwell JL, Anderson VM, Scahill RI, Rossor MN, Fox NC. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 2004;17:307–310. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs 2010;24:375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie SX, Libon DJ, Wang X, et al. Longitudinal patterns of semantic and episodic memory in frontotemporal lobar degeneration and Alzheimer's disease. J Int Neuropsychol Soc 2010;16:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman M, Xie SX, Libon DJ, et al. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology 2008;70:2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez MF, Shapira JS, Woods RJ, Licht EA, Saul RE. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement Geriatr Cogn Disord 2008;25:206–211. [DOI] [PubMed] [Google Scholar]
- 35.Mendez MF, Shapira JS. Loss of emotional insight in behavioral variant frontotemporal dementia or “frontal anosodiaphoria.” Conscious Cogn 2011;20:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009;62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012;73:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessup RK, O'Doherty JP. Distinguishing informational from value-related encoding of rewarding and punishing outcomes in the human brain. Eur J Neurosci 2014;39:2014–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson JC, Stopford CL, Snowden JS, Neary D. Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry 2005;76:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.