Abstract
Background
Inhaled antibiotics (ABs) are recommended for use in the therapy of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis (CF). The aim of this systematic literature review was to identify level of adherence to inhaled ABs and to determine predictors and consequences of nonadherence in CF.
Methods
A systematic literature search of English-language articles was conducted in April 2015 using Medline and Embase. No publication date limit was applied. The literature screening was conducted by two independent reviewers. All of the included studies were assessed for quality.
Results
The search yielded 193 publications, of which ten met the inclusion criteria and underwent data extraction. Seven studies focused on inhaled tobramycin, one on inhaled colistimethate, one on inhaled levofloxacin, and one on inhaled aztreonam lysine. Medication adherence to inhaled ABs was analyzed by pharmacy refill history, daily phone diary, parent and child self-reports, vials counting, or electronic monitoring. In randomized controlled trials (n=3), proportion of adherent patients (>75%–80% of required doses taken) ranged from 86% to 97%; in prospective cohort studies (n=3), adherence rates ranged between 36% and 92%, and in retrospective studies (n=4) it ranged between 60% and 70%. The adherence to inhaled ABs in CF was found to be associated with the complexity of treatment, time of drug administration, age of patients, treatment burden (adverse events, taste), and patient satisfaction.
Conclusion
The high diversity of adherence data was because of the different study designs (randomized controlled trials vs real-world studies) and the lack of a commonly accepted consensus on the definition of adherence in the reviewed articles. Routine adherence monitoring during CF care, discussing the possible reasons of suboptimal adherence with the patient, and changing treatment regimens on the basis of patient burden can individualize CF therapy for patients and may improve the level of adherence.
Keywords: cystic fibrosis, antibiotics, adherence, compliance, Pseudomonas aeruginosa
Introduction
Cystic fibrosis (CF) is an inherited disease that affects particularly the lungs, pancreas, hepatobiliar system, reproductive tract, and sweat glands.1,2 It is caused by mutations in the gene encoding the CF transmembrane conductance regulator protein.1,2
Chronic infection with Pseudomonas aeruginosa (PA) impacts the progress of lung disease, which determines the life expectancy in CF. PA is one of the most common gram-negative bacteria causing acute exacerbation and progress of the lung disease in CF.3 In the management of lung infection with PA, inhaled antibiotic (AB) therapy is recommended as maintenance therapy.4 Aerosol delivery of ABs yields high concentration directly to the airways, where the bacterium persists while minimizing systemic exposure and toxicity.5–7 Colistimethate inhalation has been widely used in Europe, while current CF guidelines recommend chronic use of tobramycin inhalation solution (TIS) in the USA to improve lung function and to decrease exacerbations in chronic PA infection.8 Use of inhaled aztreonam was approved by the US Food and Drug Administration in December 2009, and efficacy of inhaled levofloxacin has been studied in a clinical Phase III trial for chronic PA infection in CF patients.9,10
Medication adherence (compliance) “refers to the act of conforming to the recommendations made by the provider with respect of timing, dosage, and frequency of medication taking”.11 In chronic diseases, such as in CF, medication nonadherence poses a significant barrier to optimal disease management. The daily therapy of CF, including chest physiotherapy, numerous oral and inhaled medications, nutritional supplements, and vitamins, involves a complex and time-consuming regimen for the patient,12 which may be a significant predictor of nonadherence. In CF, previous studies reported somewhat higher adherence than for other chronic diseases (eg, asthma and allergic rhinitis).12–15 However, adherence is therapy-specific, and adherence rates may vary widely between different CF-related therapies. As it was identified in a cross-sectional study, higher treatment adherence to both respiratory (62%) and digestive treatment (88%), and lower adherence to physiotherapy (41%) and nutritional supplements (59%) were observed.16
Although many studies evaluated adherence to CF-related medications, only few of those investigated adherence to inhaled ABs.12,13,17 Nevertheless, evidence on prevalence and predictors of nonadherence to inhaled ABs would be highly valuable to the development of effective adherence-enhancing interventions and to improve disease management of CF patients with chronic PA infection. Therefore, the aim of this systemic review was to identify level of adherence to inhaled ABs for chronic PA infection in CF and to determine the possible predictors and consequences of nonadherence on the basis of the existing publications. To our knowledge, no systematic review encompassing this topic has been published to date.
Materials and methods
A systematic literature search of English-language articles was conducted in April 2015 using Medline and Embase (via Scopus) with the following search terms: (adheren* OR persisten* OR complian*) AND (“cystic fibrosis” OR mucoviscidosis) AND (“Pseudomonas aeruginosa”) AND (antibiotic*). No publication date limits were defined. The search results were processed in two steps: first, the titles and abstracts were screened; next, all the full text of all the potentially relevant articles were analyzed. The references of the included articles were screened for additional eligible studies. The literature screening was conducted by two independent reviewers; disagreements between reviewers were resolved by consensus.
The reasons for exclusion in both steps were the following: 1) the article reported no new evidence (ie, editorial, letter, case report, or review), 2) the article was a case study, 3) the article was not related to CF, and 4) the article had not assessed adherence to inhaled ABs for the treatment of chronic PA infection in CF. This review included only publications in which data on adherence to inhaled ABs were clearly separated from adherence data of other medications.
The data extraction was limited to findings relevant to the research topic. The following information was extracted from each included study: 1) the first author and year of publication, 2) the country, 3) the study design, 4) the study year, 5) study exclusion and inclusion criteria, 6) the study population characteristics, 7) the medication therapy, 8) the method of measuring and defining adherence, 9) adherence data, and 10) predictors and consequences of nonadherence to inhaled ABs in CF. In case of studies in which adherence rates were presented graphically only, WebPlotDigitizer 3.8 was used to extract numerical data accurately out of figures. The scope of this review was to give a systematic overview on the adherence to inhaled ABs in CF; therefore, results of adherence measures that were not applied to assess adherence to inhaled ABs were not abstracted from the included studies.
For the quality assessment of the included studies, the strengthening the reporting of observational studies in epidemiology (STROBE) checklist18 was used. All the studies were independently assessed by two review authors; disagreements were resolved by consensus. The results of the quality assessment were summarized as the percentage of the fulfilled criteria for each study (criteria that were not applicable to a study were excluded from the quality assessment), ranging from 0% (none of the applicable STROBE criteria fulfilled) to 100% (all applicable STROBE criteria fulfilled).
Results
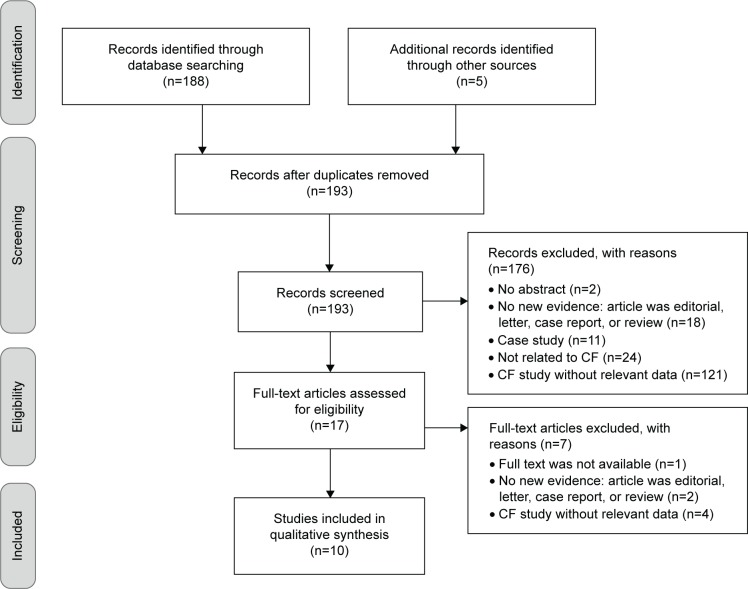
The literature search resulted in 188 hits, and five additional records were identified through hand search of the references of relevant articles. The screening of the titles and abstracts identified 17 potentially eligible studies. After the review of the relevant full texts, ten studies were finally included in the systematic review. The flow diagram of the systematic literature review process, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)19 template, is presented in Figure 1.
Figure 1.
The flow diagram of the systematic literature review process.
Abbreviation: CF, cystic fibrosis.
Three studies were randomized controlled trials (RCTs),20–22 three were prospective cohort studies,23–25 and four retrospective studies26–29 were also included. The population size varied between 2828 and 83229 subjects, with a mean age of 9.128 to 28.720 years. Seven studies focused on measuring adherence to inhaled tobramycin,21–24,26,27,29 one on inhaled colistimethate,28 one on inhaled levofloxacin,20 and one on inhaled aztreonam lysine.25 The general characteristics of the reviewed studies are summarized in Table 1.
Table 1.
General characteristics and adherence to inhaled antibiotics results of the included studies
| Study characteristics | Randomized controlled trials |
Real-world studies
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort studies
|
Retrospective studies
|
|||||||||
| Geller et al20 | Ramsey et al22 | Regnault et al21 | Harrison et al23 | Modi et al24 | Oermann et al25 | Briesacher et al26 | Eakin et al27 | McNamara et al28 | Wertz et al29 | |
| Study length | 28 daysa | 24 weeks | 24 weeks | 12 months | 3 months | 18 months | 12 months | 12 months | 12 months | 12 months |
| No of subjects, total (on inhaled ABs) | 151 (114) | 520 (258) | 454 (454) | 78 (TIP: 51, TIS: 78) | 37 (10) | 274 (BID: 89, TID: 189) | 804 (804) | 95 (65) | 28 (28) | 832 (388) |
| Age, years | 28.7 (9.0)b | • Tobramycin group: 20.8 (9.5)b • Placebo group: 20.6 (10.0)b |
25.6 (10.8)b | 26.3 (16–56)c | 10.1 (2.5)b | 28.5 (12.5)b | <6: 13.2% 6–10: 12.2% 11–17: 31.5% 18–25: 22.0% ≥26: 21.1% |
20.9 (11.9)b | 9.1 (3–15)d | TIS user group: 19e Nonuser group: 30e |
| Male, % | 56.3 | • Tobramycin group: 58 • Placebo group: 50 |
56.2 | 59 | 51 | 55.1 | 52.7 | 48.9 | 57 | TIS user group: 52e Nonuser group: 46e |
| FEV1, % predicted | 52.3 (15.3)b | • Tobramycin group: 49.9 (15.5)b • Placebo group: 51.2 (16.8)b |
52.8 (14.5)b | 63.5f | 79.6 (20.8)b | 55.6 (11.6)b | NR | 80.1 (24.8)b | 75 (54–99)c | NR |
| Inhaled ABs | Inhaled levofloxacin | Inhaled tobramycin | TIP, TIS | TIP, TIS | Inhaled tobramycin | Inhaled aztreonam lysine (BID, TID) | TIS | Inhaled tobramycin | Inhaled colistimethate | TIS |
| Adherence | ||||||||||
| Measurement | Vials counting | Vials counting | NR | Self-report adherence | Parent/child self-report, daily phone diary | Vials counting | Pharmacy refill records | Pharmacy refill records | Electronic monitoring | Pharmacy refill records |
| Variable type Defnition | Categorical Adherent patients: ≥80% of the required doses taken | Categorical Adherent patients: ≥75% of the ampoule dispensed | Continuous, categorical • Adherence: % of doses taken • Adherent patients: ≥80% of the required doses taken |
Categorical • Excellent: >80% adherence • Moderate: 50%–80% adherence • Poor: <50% adherence |
Continuous NR |
Continuous NR | Categorical • High: ≥4 cycles per 12 monthsg • Medium: >2 to <4 cycles per 12 monthsg • Low: ≤2 cycles per 12 monthsg |
Continuous MPR: defined as the sum of all days of medication supply received, divided by the number of days the medication was prescribed for chronic use during the study period |
Continuous The number of treatments taken divided by numbers of prescribed treatments ×100% |
Categorical • High: >4 fills per 12 months • Medium: 2–3 fills per 12 months • Low: 1 fill per 12 months |
| Results, %/mean (SD) %h | % of adherent subjects: 92.3%–97.3% | % of adherent subjects: 88% | • Adherence: ■ Cycle 1: 96.5 (10)%b,g ■ Cycle 2: 93.9 (15)%b,g ■ Cycle 3: 93.6 (14.5)%b,g • % of adherent subjects: ■ Cycle 1: 93.4%g ■ Cycle 2: 88.1%g ■ Cycle 3: 86.4%g |
• TIS at baseline: ■ Excellent: 44.9% ■ Moderate: 30.6% ■ Poor: 24.5% • TIP at 12-month: ■ Excellent: 77.5% ■ Moderate: 17.5% ■ Poor: 5.0% |
• Parent self-report: 85.0 (33.7)%b • Child self-report: 83.3 (25.8)%b • Diary: 36.1 (35.6)%b |
• BID: 92% • TID: 88% |
• High: 7% • Medium: 22% • Low: 71% |
MPR: 65%i | • Adherence at 6 months: 64 (35)%b • Overall monthly adherence at 12 months: 60%–70% |
• High: 29.64% • Medium: 34.54% • Low: 35.82% |
| Predictors/consequences | NR | NR | Higher patient satisfaction and lower perceived impact of side effects were found to be statistically significantly associated with better adherence | Adherence increased after transition from TIS to TIP | NR | • Dosing regimen was not statistically significantly associated with adherence • CFQ-R scores were comparable between BID and TID groups |
• High adherence was significantly associated with a decreased risk of hospitalization, but inpatient costs were similar between the three adherence groups • Outpatient costs were lower in high adherence groupj • Drug costs were higher among high usersj |
• Adherence was not correlated with number of medications prescribed • No statistically significantly difference in age was found in adherence • Adherence to inhaled ABs was not associated with the occurrence of pulmonary exacerbation, and change in FEV1% predicted slope |
• Evening adherence (75%) was significantly better than morning adherence (58%) • Tendency for better overall adherence in children, <12 years (71%) than in teenagers (50%)j • Changing the treatment regimens from twice daily to once daily improved adherence significantly (in the month pre- and post-change from 26% to 54%) |
• CF-related medication use was generally greater for the high adherence group compared with the medium and low adherence groups (P<0.0001) • Increase in adherence was associated with decreased health care resource utilization, increased drug costs, and decreased inpatient costs (P<0.05) |
Notes:
Treatment period;
mean (SD);
median (range);
mean (range);
mean;
median;
one cycle: 28 days on therapy followed by 28 days off therapy;
only adherence results for inhaled ABs are presented;
adherence rate was presented graphically only;
difference was not assessed with statistical methods.
Abbreviations: ABs, antibiotics; FEV1, forced expiratory volume in 1 second; BID, two times daily; CF, cystic fibrosis; CFQ-R, cystic fibrosis questionnaire-revised; TID, three times daily; NR, not reported; MPR, medication possession ratio; SD, standard deviation; TIP, tobramycin inhalation powder; TIS, tobramycin inhalation solution.
Medication adherence to inhaled ABs was analyzed by pharmacy refill history (30%),26,27,29 daily phone diary (10%),24 parent and/or child self-reports (20%),23,24 vials counting (30%),20,22,25 or electronic monitoring (10%).28 Regnault et al21 were the only authors who did not describe the method for measuring adherence, while Modi et al24 and Oermann et al25 did not report how adherence was defined in their studies. Six studies20–23,26,29 determined adherence as categorical variable only, five studies24,25,27,28 as continuous only, and one study as both.21 Measurement methods, results, and predictors/consequences of adherence to inhaled ABs are presented in Table 1.
Except for the studies conducted by Eakin et al27 and Modi et al,24 most studies assessed adherence to inhaled ABs only and did not evaluate adherence to other medications simultaneously (Table 2).
Table 2.
Comparison of adherence to inhaled antibiotics and other medications in patients with cystic fibrosis
| Adherence measurements | Inhaled tobramycin | Dornase alpha | Azithromycin | Hypertonic saline | Airway clearance | Combined nebulized medicationsa | Enzymes | Vitamins |
|---|---|---|---|---|---|---|---|---|
| Eakin et al27 | ||||||||
| MPR, %b,c | 65 | 71 | 76 | 49 | NA | NA | NA | NA |
| Modi et al24,d | ||||||||
| Parent self-report, mean (SD) % | 85.0 (33.7) | 90.4 (25.9) | NA | NA | 74.4 (35.3) | 82.4 (31.6) | 89.5 (21.7) | 88.4 (27.6) |
| Child self-report, mean (SD) % | 83.3 (25.8) | 77.8 (44.1) | 66.9 (30.2) | 80.0 (36.9) | 90.0 (25.5) | 93.8 (17.1) | ||
| Daily phone diary, mean (SD) % | 36.1 (35.6) | 56.7 (45.8) | 51.1 (40.2) | 47.6 (41.0) | 27.4 (22.9) | 22.2 (34.2) |
Notes:
Included dornase alpha, inhaled tobramycin, albuterol;
adherence rates were presented graphically only;
there were no statistically significant differences in MPRs among the different medications;
differences in adherence rates among different medications were not evaluated statistically.
Abbreviations: MPR, medication possession ratio; NA, not applicable; SD, standard deviation.
The included studies fulfilled the STROBE criteria between 52%28 and 73%.21,29 Details of the quality assessment are provided in Table S1. The majority of the studies described their scope, study design, settings, participants, variables, measurements, and main results adequately. All but one28 of the reviewed studies described their statistical methods in detail; however, none of them undertook a sensitivity analysis to examine the robustness of the results. Furthermore, no study provided information on missing data and how they dealt with the same.
Discussion
Ten studies were identified and included in this systematic review.20–29 The majority of the studies focused on measuring adherence to inhaled tobramycin (n=7).21–26,29 To our knowledge, this is the first systematic review of the adherence to inhaled ABs in CF. The results from the present study contribute to understanding the current status of adherence to inhaled ABs and for planning future research that can add to the global picture of adherence in CF.
Methodological issues: randomized controlled trials vs real-world studies
Most of the included publications were real-world studies (n=7)23–29 contrary to RCTs (n=3).20–22 From the viewpoint of health care professionals, real-world studies provide more reliable information about medication adherence in contrast with RCTs, which may overestimate adherence. As RCTs are mainly designed to assess safety and efficacy of pharmaceuticals, the study design emphasizes internal validity over generalizability. In these studies, because of protocol requirements, the data may not be applicable to the more heterogeneous patient group encountered in actual clinical practice. Effectiveness studies (eg, prospective cohort studies and retrospective studies), where treatment and/or disease management is studied under real-world conditions (eg, in unselected populations; patients are under routine care, taking open-label treatment, with no additional visits), remedy some of these limitations. This issue is especially relevant in case of adherence data. In RCTs, usually better treatment adherence is obtained due to the continuous, obligatory control visits or measurement of serum drug levels. Moreover, patients sometimes feel that they are in favored situation, because they are eligible to take part in the study and use a novel, not launched medication in CF. In addition, between regular visits, phone calls also remind patients to the use/administration of the drug, or they are asked about their experiences related to the new drug.
In the included RCTs,20–22 the proportion of adherent patients (>75%–80% of required doses taken) ranged between 86.4% (inhaled tobramycin, measurement method was not reported)21 and 97.3% (inhaled levofloxacin, measured by vials counting).20 In contrast, in the prospective cohort study conducted by Harrison et al,23 only 44.9% of patients reported greater than 80% adherence to TIS and 77.5% to tobramycin inhalation powder (TIP) (measured by patient self-report). In the included retrospective studies, the proportion of highly adherent patients (>4 fills per 12 months) was found to be in the range of 7% (inhaled tobramycin, measured by pharmacy refill records)26 to 29.6% (inhaled tobramycin, measured by pharmacy refill records)29 (Table 1).
Adherence rates to inhaled antibiotics
Determining an overall rate for adherence to inhaled ABs in CF was difficult because different adherence definitions and measurement methods were applied in the reviewed studies; furthermore, two of the included studies24,25 did not declare a clear definition for adherence. In prospective cohort studies, adherence rates ranged between 36% (inhaled tobramycin, measured by daily phone diary)24 and 92% (inhaled aztreonam, measured by vials counting),25 and in retrospective studies it ranged between 60% and 70% (inhaled colistimethate, measured by electronic monitoring)28 (Table 1). Adherence to inhaled ABs did not differ significantly from adherence rates to other CF-related medications (Table 2).27
Predictors of adherence
In the reviewed studies, adherence to inhaled ABs was found to be associated with the age of patients, patient’s satisfaction, treatment burden (ie, adverse events and taste), and time of drug administration (ie, morning vs evening inhalation).21,26,28 McNamara et al28 found that treatment adherence was better in those patients who were younger than 12 years of age (<12 years of age: 71% vs ≥12 years of age: 50%). A reasonable explanation may be the presence of parental supervision in this age group. This result is similar to the findings of Modi et al,24 who reported that parental supervision of CF medical treatments can improve children’s treatment adherence. Furthermore, preadolescents and adolescents who spent more of their treatment time supervised by mothers had better adherence. Interestingly, Eakin et al27 could not identify any relationships between age and treatment adherence. According to the findings of Regnault et al,21 patient satisfaction with inhalation AB therapy was linked to patient adherence, and side effects seemed to be the key drivers of patients’ behavior regarding their inhaled ABs in CF. Patients’ self-perception about the efficacy of the drug,30 clinical response,30 cumulative toxicity,30 drug intolerance,26 treatment burden, and the time and frequency of administration24 could determine adherence to therapy. McNamara et al28 found that evening adherence (75%) was significantly better than morning adherence (58%) to inhaled colistimethate.28 A possible explanation of this phenomenon could be that children have to wake up early in the morning for complete airway clearance, chest physiotherapy, and inhalation before going to school. Unpleasant taste of the inhaled AB, delay in the improvement of lung function (FEV1 [forced expiratory volume in 1 second]), and respiratory symptoms may also lead to nonadherence.20,26,31 Furthermore, adherence to a novel medication may be better than to an older one.20
Real-world studies enable better understanding of the predictors of nonadherence from patients’ viewpoint compared with RCTs. However, since measuring adherence to inhaled ABs as a phenomenon is relatively new, many of its potential predictors have not yet been evaluated, such as relationship between treatment adherence and health-related quality of life, relationship between clinical parameters (eg, FEV1) and adherence, or direct comparison of adherence between various type of inhaled ABs in CF.
Consequences of nonadherence
In RCTs, high adherence was observed between artificial conditions compared to real-world studies. Therefore this analysis made conclusions about consequences of nonadherence on the basis of results published by real-world studies.23–29 Most studies concentrated on the level of adherence, on the identification of nonadherence, and on how adherence can be improved. However, only few (n=4) researches dealt with the consequences.23,26,27,29 Further studies are needed to understand the clinical consequences of nonadherence in CF.
Generally, the examination of financial impact, not the clinical impact, of poor adherence dominated in the reviewed articles. However, it was difficult to clarify the real effect of nonadherence on clinical outcomes because of the natural progression of the disease. There are many factors (eg, microbiological agents and CF-related complications), not only treatment adherence, in CF that may have an impact on the progression of the disease. Our analysis suggests that better adherence to inhaled ABs in patients with CF is significantly associated with decreased health care utilization, median outpatient costs, excluding drug costs, and decreased risk of hospitalization.26,29 Moreover, reductions in CF-related hospitalizations translated to significant reductions in CF-related inpatient costs. That could mean that low-adherence users may have more frequent pulmonary exacerbations and CF-related complications.29 However, Eakin et al27 found that adherence to inhaled tobramycin was not significantly associated with the occurrence of pulmonary exacerbation and change in FEV1% predicted slope.
Enhancing adherence
Only few of the authors of the reviewed articles developed suggestions on how adherence to inhaled ABs can be improved. McNamara et al28 observed that changing treatment regimen from twice daily to once daily significantly improved adherence (26% vs 54%, in 1 month). In a real-world, prospective cohort study, Harrison et al23 demonstrated that the use of a more rapid delivery system could result in increased adherence to inhaled ABs. In their research, TIP was associated with better adherence compared with TIS (Table 1).
In the recent years, some useful tools have been developed to improve medication adherence in patients with CF. Internet-based adherence interventions and mobile phone applications became more and more popular.32 Besides telemedical patient management, it still seems important to improve patient education programs to provide home service programs in collaboration with social workers. However, the most important issue is to recognize the necessity of routine adherence monitoring during CF care. It is also important that physicians do not expect adherence to be the sole responsibility of patients, because improving treatment adherence is teamwork. Clinicians should understand their patients’ problems in relation to side effects or with time of administration and help patients to choose an inhaled AB that is the best for the patient. If adherence is routinely monitored and results are discussed openly with patients, then treatment regimens can be individualized for patients. This kind of interaction can result in sustainable levels of adherence during the CF care.
Limitations
The findings of this systematic review should be considered in light of the following limitations. The current systematic literature review involved searching only studies that were published in English and indexed in the selected databases; unpublished material and studies from other resources were precluded. The major limitation of the analysis is that because of the paucity of studies, small sample sizes, and diverse definitions of adherence, adherence measurement method results differ vastly, and it is difficult to make any generalizing statements regarding the general predictors of nonadherence. As a further effort, we screened the references of the included articles for additional eligible studies. STROBE checklist was developed for the quality assessment of observational studies. Specific issues related to research using routinely collected data are not addressed in STROBE; nevertheless, we also used this checklist for quality assessment of those studies in which retrospective analyses were performed.
Conclusion
Different adherence definitions and measurement methods made it difficult to determine an overall rate for adherence to inhaled ABs in CF based upon the reviewed studies. Our analysis suggests that probably because of their highly controlled conditions, RCTs provide higher adherence rate to inhaled ABs than real-world studies. Adherence to inhaled ABs was found to be associated with the complexity of treatment, time of drug administration, age of patients, treatment burden (ie, adverse events and taste), and patient satisfaction. Further research is needed to better understand the predictors of adherence to inhaled ABs, which would be critically important to the development of effective adherence-enhancing interventions. Routine adherence monitoring, discussing the possible reasons of nonadherence with the patient, and changing treatment regimens on the basis of patient burden may optimize patient management and hence improve adherence in patients with CF.
Supplementary materials
Table S1.
The quality assessment of the included studies
| Item (item number) | Briesacher et al26 | Eakin et al27 | Geller et al20 | Harrsion et al23 | McNamara et al28 | Modi et al24 | Oermann et al25 | Ramsey et al22 | Regnault et al21 | Wertz et al29 |
|---|---|---|---|---|---|---|---|---|---|---|
| Title and abstract | ||||||||||
| (la) | – | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | – |
| (lb) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Introduction | ||||||||||
| Background/rationale (2) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objectives (3) | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods | ||||||||||
| Study design (4) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Setting (5) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants (6a) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants (6b) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Variables (7) | ? | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| Data sources/measurement (8) | ? | ✓ | ✓ | ? | ? | – | ✓ | ✓ | ✓ | ✓ |
| Bias (9) | – | – | – | – | – | – | ? | – | ? | – |
| Study size (10) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Quantitative variables (11) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods (12a) | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods (12b) | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods (12c) | – | – | – | – | – | – | – | – | ✓ | – |
| Statistical methods (12d) | – | – | – | – | – | – | – | – | – | – |
| Statistical methods (12e) | – | – | ? | – | – | – | – | – | – | – |
| Results | ||||||||||
| Participants (13a) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants (13b) | ? | ✓ | – | ✓ | ? | ✓ | – | – | – | ? |
| Participants (13c) | – | – | ✓ | ✓ | – | – | – | – | – | ✓ |
| Descriptive data (14a) | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Descriptive data (14b) | – | – | ? | ? | – | ? | ✓ | – | ✓ | – |
| Descriptive data (14c) | – | – | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ |
| Outcome data (15) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Main results (16a) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Main results (16b) | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ? | ✓ | ✓ | ✓ |
| Main results (16c) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Other analyses (17) | ✓ | – | ✓ | – | – | – | ✓ | ✓ | ? | ? |
| Discussion | ||||||||||
| Key results (18) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Limitations (19) | ✓ | ✓ | – | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ |
| Interpretation (20) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ |
| Generalizability (21) | ? | ✓ | ✓ | ✓ | ? | ✓ | ? | ? | ? | ? |
| Other information | ||||||||||
| Funding (22) | ✓ | ✓ | ? | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| The proportion of adequately reported items (“✓” responses) of all applicable item (%) | 63% | 70% | 70% | 70% | 52% | 67% | 67% | 70% | 73% | 73% |
Notes: ✓, yes; –, no; ?, partially.
Abbreviation: NA, not applicable.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Knowles MR, Durie PR. What is cystic fibrosis? N Engl J Med. 2002;347(6):439–442. doi: 10.1056/NEJMe020070. [DOI] [PubMed] [Google Scholar]
- 2.Santis G. Basic molecular genetics. In: Hodson ME, Geddes DM, editors. Cystic Fibrosis. London, UK: Chapman & Hall; 1995. pp. 15–34. [Google Scholar]
- 3.Raidt L, Idelevich EA, Dubbers A, et al. Increased prevalence and resistance of important pathogens recovered from respiratory specimens of cystic fibrosis patients during a decade. Pediatr Infect Dis J. 2015;34(7):700–705. doi: 10.1097/INF.0000000000000714. [DOI] [PubMed] [Google Scholar]
- 4.Doring G, Conway SP, Heijerman HG, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J. 2000;16(4):749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 5.Doring G, Flume P, Heijerman H, Elborn JS. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11(6):461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Cooney GF, Lum BL, Tomaselli M, Fiel SB. Absolute bioavailability and absorption characteristics of aerosolized tobramycin in adults with cystic fibrosis. J Clin Pharmacol. 1994;34(3):255–259. doi: 10.1002/j.1552-4604.1994.tb03995.x. [DOI] [PubMed] [Google Scholar]
- 7.Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122(1):219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 8.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 9.FDA Briefing Document for Anti-Infective Drugs Advisory Committee Meeting Aztreonam for inhalation solution (NDA 50-814) for improvement of respiratory symptoms in cystic fibrosis patients 2009. [Accessed June 1, 2015]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM193023.pdf.
- 10.Cystic Fibrosis Foundation Drug Development Pipeline. 2015. [Accessed June 1, 2015]. Available from: http://www.cff.org/research/DrugDevelopmentPipeline/
- 11.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 12.Abbott J, Dodd M, Bilton D, Webb AK. Treatment compliance in adults with cystic fibrosis. Thorax. 1994;49(2):115–120. doi: 10.1136/thx.49.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway SP, Pond MN, Hamnett T, Watson A. Compliance with treatment in adult patients with cystic fibrosis. Thorax. 1996;51(1):29–33. doi: 10.1136/thx.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender BG. Motivating patient adherence to allergic rhinitis treatments. Curr Allergy Asthma Rep. 2015;15(3):10. doi: 10.1007/s11882-014-0507-8. [DOI] [PubMed] [Google Scholar]
- 15.Chan AH, Stewart AW, Harrison J, Camargo CA, Jr, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med. 2015;3(3):210–219. doi: 10.1016/S2213-2600(15)00008-9. [DOI] [PubMed] [Google Scholar]
- 16.Arias Llorente RP, Bousono Garcia C, Diaz Martin JJ. Treatment compliance in children and adults with cystic fibrosis. J Cyst Fibros. 2008;7(5):359–367. doi: 10.1016/j.jcf.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.White T, Miller J, Smith GL, McMahon WM. Adherence and psychopathology in children and adolescents with cystic fibrosis. Eur Child Adolesc Psychiatry. 2009;18(2):96–104. doi: 10.1007/s00787-008-0709-5. [DOI] [PubMed] [Google Scholar]
- 18.White RG, Hakim AJ, Salganik MJ, et al. Strengthening the reporting of observational studies in epidemiology for respondent-driven sampling studies: “STROBE-RDS” statement. J Clin Epidemiol. 2015;68(12):1463–1471. doi: 10.1016/j.jclinepi.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W264. [DOI] [PubMed] [Google Scholar]
- 20.Geller DE, Flume PA, Staab D, et al. Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;183(11):1510–1516. doi: 10.1164/rccm.201008-1293OC. [DOI] [PubMed] [Google Scholar]
- 21.Regnault A, Balp MM, Kulich K, Viala-Danten M. Validation of the treatment satisfaction questionnaire for medication in patients with cystic fibrosis. J Cyst Fibros. 2012;11(6):494–501. doi: 10.1016/j.jcf.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 23.Harrison MJ, McCarthy M, Fleming C, et al. Inhaled versus nebulised tobramycin: a real world comparison in adult cystic fibrosis (CF) J Cyst Fibros. 2014;13(6):692–698. doi: 10.1016/j.jcf.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5(3):177–185. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Oermann CM, Retsch-Bogart GZ, Quittner AL, et al. An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr Pulmonol. 2010;45(11):1121–1134. doi: 10.1002/ppul.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briesacher BA, Quittner AL, Saiman L, Sacco P, Fouayzi H, Quittell LM. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med. 2011;11:5. doi: 10.1186/1471-2466-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eakin MN, Bilderback A, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros. 2011;10(4):258–264. doi: 10.1016/j.jcf.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNamara PS, McCormack P, McDonald AJ, Heaf L, Southern KW. Open adherence monitoring using routine data download from an adaptive aerosol delivery nebuliser in children with cystic fibrosis. J Cyst Fibros. 2009;8(4):258–263. doi: 10.1016/j.jcf.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Wertz DA, Chang CL, Stephenson JJ, Zhang J, Kuhn RJ. Economic impact of tobramycin in patients with cystic fibrosis in a managed care population. J Med Econ. 2011;14(6):759–768. doi: 10.3111/13696998.2011.621004. [DOI] [PubMed] [Google Scholar]
- 30.Appel GB. Aminoglycoside nephrotoxicity. Am J Med. 1990;88:16S–20S. doi: 10.1016/0002-9343(90)90082-o. discussion 38S–42S. [DOI] [PubMed] [Google Scholar]
- 31.Chuchalin A, Amelina E, Bianco F. Tobramycin for inhalation in cystic fibrosis: beyond respiratory improvements. Pulm Pharmacol Ther. 2009;22(6):526–532. doi: 10.1016/j.pupt.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Hilliard ME, Hahn A, Ridge AK, Eakin MN, Riekert KA. User preferences and design recommendations for an mHealth app to promote cystic fibrosis self-management. JMIR Mhealth Uhealth. 2014;2(4):e44. doi: 10.2196/mhealth.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
The quality assessment of the included studies
| Item (item number) | Briesacher et al26 | Eakin et al27 | Geller et al20 | Harrsion et al23 | McNamara et al28 | Modi et al24 | Oermann et al25 | Ramsey et al22 | Regnault et al21 | Wertz et al29 |
|---|---|---|---|---|---|---|---|---|---|---|
| Title and abstract | ||||||||||
| (la) | – | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | – |
| (lb) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Introduction | ||||||||||
| Background/rationale (2) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Objectives (3) | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Methods | ||||||||||
| Study design (4) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Setting (5) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants (6a) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants (6b) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Variables (7) | ? | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| Data sources/measurement (8) | ? | ✓ | ✓ | ? | ? | – | ✓ | ✓ | ✓ | ✓ |
| Bias (9) | – | – | – | – | – | – | ? | – | ? | – |
| Study size (10) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Quantitative variables (11) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods (12a) | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods (12b) | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical methods (12c) | – | – | – | – | – | – | – | – | ✓ | – |
| Statistical methods (12d) | – | – | – | – | – | – | – | – | – | – |
| Statistical methods (12e) | – | – | ? | – | – | – | – | – | – | – |
| Results | ||||||||||
| Participants (13a) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Participants (13b) | ? | ✓ | – | ✓ | ? | ✓ | – | – | – | ? |
| Participants (13c) | – | – | ✓ | ✓ | – | – | – | – | – | ✓ |
| Descriptive data (14a) | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Descriptive data (14b) | – | – | ? | ? | – | ? | ✓ | – | ✓ | – |
| Descriptive data (14c) | – | – | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ |
| Outcome data (15) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Main results (16a) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Main results (16b) | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ? | ✓ | ✓ | ✓ |
| Main results (16c) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Other analyses (17) | ✓ | – | ✓ | – | – | – | ✓ | ✓ | ? | ? |
| Discussion | ||||||||||
| Key results (18) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Limitations (19) | ✓ | ✓ | – | ✓ | ✓ | ✓ | ? | ? | ✓ | ✓ |
| Interpretation (20) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ? | ✓ |
| Generalizability (21) | ? | ✓ | ✓ | ✓ | ? | ✓ | ? | ? | ? | ? |
| Other information | ||||||||||
| Funding (22) | ✓ | ✓ | ? | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| The proportion of adequately reported items (“✓” responses) of all applicable item (%) | 63% | 70% | 70% | 70% | 52% | 67% | 67% | 70% | 73% | 73% |
Notes: ✓, yes; –, no; ?, partially.
Abbreviation: NA, not applicable.