Abstract
INTRODUCTION
This study aims to describe the perceptions of orthopedic surgeons on the efficacy of intra-articular hyaluronic acid (IA-HA), the influence of IA-HA product characteristics on its efficacy, and to identify patterns and factors related to the use of IA-HA. Additionally, this study examines factors that influence IA-HA brand selection, focusing on Euflexxa® (1% sodium hyaluronate).
METHODS
We developed survey questions by reviewing the current literature and consulting with experts on the use of IA-HA in the management of knee osteoarthritis (OA). The survey included questions on demographics, previous experience with knee OA treatment, opinions on different treatment methods, and where information regarding treatments is obtained. Additionally, questions specific to opinions regarding IA-HA and the reasoning behind these opinions were asked.
RESULTS
A total of 117 orthopedic surgeons and physicians completed the survey. IA-HA is most frequently prescribed to patients with early-stage (82%) or mid-stage (82.8%) OA, while fewer orthopedic surgeons and physicians use IA-HA for patients with late-stage OA (57.4%). Respondents were generally uncertain of the effects that intrinsic characteristics, such as molecular weight, cross-linking, and production process, had on patient outcomes. Respondents typically use their own clinical experience and results as a deciding factor in utilizing IA-HA treatment, as well as in choosing an IA-HA brand.
CONCLUSION
Uncertainty regarding the efficacy of IA-HA treatments is likely due to inconsistency within clinical guidelines and the current literature. Additional research investigating the efficacy of IA-HA treatment and how product characteristics affect outcome and safety is required to provide clarity to the controversy surrounding IA-HA treatment for knee OA.
Keywords: knee, osteoarthritis, hyaluronic acid, survey
Introduction
As one of the leading causes of pain and disability in the Western countries, osteoarthritis (OA) has a significant impact on the quality of life for patients suffering from the disease, as well as a substantial direct and indirect financial burden to society.1,2 A number of options for the treatment of OA in the knee are available, including conservative treatments (eg, medications and physiotherapy) and surgical management (eg, joint replacement).3
Intra-articular hyaluronic acid (IA-HA) injections are used to treat OA-related symptoms in patients who are not ready to proceed with surgical intervention. Many clinical trials have been conducted evaluating the efficacy of IA-HA in the management of knee OA. These studies have reported conflicting results, and there is variability in methodological quality among them.4–7 Consequently, several professional organizations have prepared clinical practice guidelines that have also provided conflicting recommendations with respect to the use of IA-HA injections.1–3,8–14
Research on IA-HA treatments has not provided clear answers on the impact of the different characteristics of the IA-HA treatment (eg, production process and molecular weight) on patient outcomes.15,16 While IA-HA can be produced through the extraction of avian-derived molecules (AD-HA) or through a biological process of microbial fermentation (Bio-HA), studies have failed to show the impact that production process has with respect to outcome measures.15 Studies examining differences in the molecular weight and cross-linking of IA-HA products have shown inconsistencies as well, as some studies show benefit of high-molecular-weight products while others find no conclusive difference in efficacy attributed to molecular weight or cross-linking.16–19
While the current literature provides conflicting results regarding IA-HA, it is not known how this information affects surgeon’s perceptions or the use of IA-HA treatments. This study aims to describe the current perceptions of orthopedic surgeons on the efficacy of IA-HA and the influence of IA-HA product characteristics on its efficacy, as well as to identify patterns and factors related to the use of IA-HA. Additionally, this study examines factors that influence IA-HA brand selection, focusing on Euflexxa (1% sodium hyaluronate). This study was conducted under IRB approval. This research complied with the principles of the Declaration of Helsinki.
Methods
Survey development
A survey was used to obtain surgeon perceptions on IA-HA treatment in knee OA and specifically Euflexxa. We developed the survey questions by reviewing the current literature and consulting with experts on the use of IA-HA treatments in their management of knee OA. The survey was reviewed by five additional experts who were either orthopedic surgeons or research methodologists to ensure that no vital information was missed and that the wording of the questions was clear and precise.
Survey description
A total of 43 questions were included in the survey, comprising Likert scale questions, checkboxes, and brief open-ended questions. All the questions were straight forward and used clear and widely recognized terminology to enhance the validity of results. The survey length was kept to a minimum to maximize response rate and limit barriers that would adversely affect its proper completion. The survey included questions about demographics, previous experience with knee OA treatment methods, opinions of different treatment methods available, and where information regarding treatments is obtained. Additionally, the survey included questions specific to opinions regarding IA-HA and the reasoning behind these opinions.
Pretesting and validity assessments
The survey was pretested by having five orthopedic surgeons with experience in clinical research and the treatment of knee OA by evaluating the following: (1) Did the questionnaire, as a whole, appear to adequately address the question (face validity)? and (2) Did the individual questions adequately address the objectives of the current study (content validity)? The orthopedic surgeons who assisted with the pretesting were all practicing surgeons from different clinical centers in North America. They all had a strong understanding of research methodology and prior experience with survey design. The questions of the survey were then revised based on the recommendations of their independent review.
Survey administration
The revised survey was distributed by email to orthopedic surgeons and physicians throughout North America. The mailing list was created by using “HealthLink Dimensions” (http://www.healthlinkdimensions.com/). The survey was distributed to 2947 individuals at clinical sites across North America. At two, four, and six weeks following the initial email, the survey invitation was sent again to all nonresponders. Individual responses were anonymous and confidential, and questionnaire completion was voluntary.
Statistical analysis
A research associate processed all survey response data in aggregate and developed summary tables of the results. We summarized all categorical and dichotomous variables with frequencies and percentages. All analyses were conducted using the JMP statistical software, Version 11.
Results
Characteristics of the respondents
A total of 117 orthopedic surgeons and physicians from clinical sites across North America completed the survey. The number of responses for each survey item varied, as it was possible for respondents to skip questions. The 117 respondents included 61 (52.1%) practitioners from the United States and 32 (27.4%) from Canada, while 24 (20.5%) did not provide the location in which they reside. Participating clinicians were primarily orthopedic surgeons (87.2%), as well as eight rheumatologists (6.8%) and five sports medicine physicians (4.3%). The majority of the respondents (67.5%) had over 10 years of experience in treating patients with knee OA. A total of 48.7% reported treating over 200 patients with knee OA over the previous 12 months, while 23.9% treated between 100 and 200 patients and 27.3% treated between 1 and 100 patients with knee OA (Table 1).
Table 1.
Characteristics of the participants.
| CHARACTERISTIC | NUMBER OF RESPONSES N (%) N=117 |
|---|---|
| Gender | |
| Male | 107 (91.5%) |
| Female | 8 (6.8%) |
| No response | 2 (1.7%) |
| Age (Years) | |
| 30–40 | 29 (24.8%) |
| 41–50 | 34 (29.1%) |
| 51–60 | 34 (29.1%) |
| >60 | 20 (17.1%) |
| Location | |
| USA | 61 (52.1%) |
| Canada | 32 (27.4%) |
| No response | 24 (20.5%) |
| Type of practice | |
| Academic teaching hospital | 55 (47.0%) |
| Private practice | 37 (31.6%) |
| Community hospital | 17 (14.5%) |
| Large group practice | 7 (6.0%) |
| No response | 1 (0.9%) |
| Supervise residents | |
| Yes | 83 (70.9%) |
| No | 33 (28.2%) |
| No response | 1 (0.9%) |
| Specialty | |
| Orthopaedic surgeon | 102 (87.2%) |
| Rheumatologist | 8 (6.8%) |
| Sports medicine | 5 (4.3%) |
| Physiatrist | 1 (0.9%) |
| No response | 1 (0.9%) |
| Experience in treating knee OA | |
| <10 years | 34 (29.1%) |
| >10 years | 79 (67.5%) |
| No response | 4 (3.4%) |
| Number of knee OA patients treated in the past year: | |
| 1–25 | 10 (8.5%) |
| 26–50 | 5 (4.3%) |
| 51–100 | 17 (14.5%) |
| 101–200 | 28 (23.9%) |
| >200 | 57 (48.7%) |
| Proportion of treated patients with Early-stage OA (Kellgren-Lawrence (K-L) Grade I to II): | |
| 0–20% | 44 (37.6%) |
| 21–40% | 50 (42.7%) |
| 41–60% | 17 (14.5%) |
| 61–80% | 3 (2.6%) |
| 81–100% | 0 (0.0%) |
| Unsure | 1 (0.9%) |
| No response | 2 (1.7%) |
| Proportion of treated patients with Mid-stage OA (K-L grade III): | |
| 0–20% | 12 (10.3%) |
| 21–40% | 68 (58.1%) |
| 41–60% | 32 (27.4%) |
| 61–80% | 4 (3.4%) |
| 81–100% | 0 (0.0%) |
| Unsure | 1 (0.9%) |
| Proportion of treated patients with Late-stage OA (K-L grade IV) | |
| 0–20% | 34 (29.1%) |
| 21–40% | 43 (36.7%) |
| 41–60% | 23 (19.7%) |
| 61–80% | 12 (10.3%) |
| 81–100% | 2 (1.7%) |
| Unsure | 1 (0.9%) |
| No response | 2 (1.7%) |
Use of IA-HA
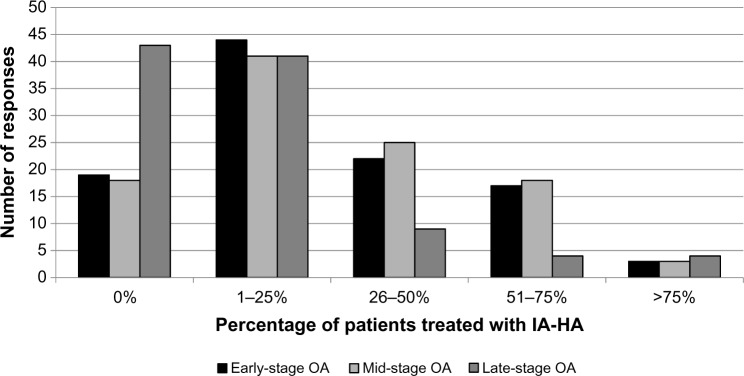
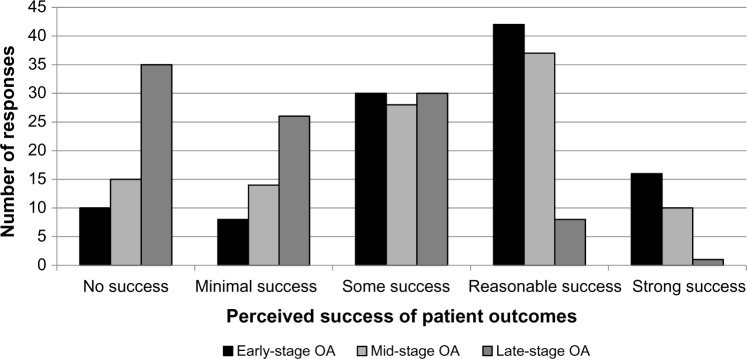
A total of 85.2% of respondents use IA-HA as a treatment at least once a year. IA-HA is most frequently prescribed to patients with early stage (82%) or mid-stage (82.8%) OA, while fewer orthopedic surgeons and physicians use IA-HA for patients with late-stage OA (57.4%; Fig. 1). The majority of respondents (83%) believe they have had at least some perceived success when using IA-HA for patients with early stage OA, while 72.1% and 39% of respondents felt they had at least some success using IA-HA for mid-and late-stage OA, respectively (Fig. 2). When considering the injection regimen, the majority (60.8%) of respondents prefer a one-shot regimen. Despite the high use of IA-HA, 90.6% of respondents believe there is some controversy regarding the efficacy of IA-HA as a treatment option for knee OA.
Figure 1.
Proportion of patients at different stages of OA treated with IA-HA per year.
Figure 2.
Perceived success of IA-HA treatment for different stages of knee OA.
Perceived influence of product characteristics
When asked if molecular weight of an HA product affects the success of the treatment in terms of outcome measures and safety, 41.9% believed that molecular weight does have some effect, while 15.4% felt molecular weight does not have impact on product efficacy and 29.1% were unsure of the role that molecular weight has on outcome measures and safety (Table 2). With respect to chain length and cross-linking, 43.6% of respondents were unsure of its effect on outcome measures and safety, while 27.4% believe that chain length and cross-linking do effect outcome and safety and 17.1% do not believe that these factors have any influence. When asked if the process in which an IA-HA treatment is produced (Bio-HA vs. AD-HA) has an effect on outcome measures and safety, 34.2% were unsure, 24.8% did not believe the production process has any effect, 23.9% believe Bio-HA provides better outcomes and safety, and 2.6% believe AD-HA provides better outcomes and safety. The majority of respondents (53.8%) do not consider anti-inflammatory effects when selecting an IA-HA treatment brand.
Table 2.
Perceived effect of product characteristics on outcome measures and safety.
| PRODUCT CHARACTERISTIC | NUMBER OF RESPONSES N (%) N=117 |
|---|---|
| Molecular weight | |
| Does affect outcome and safety | 49 (41.9%) |
| Unsure | 34 (29.1%) |
| Does not affect outcome and safety | 18 (15.4%) |
| No response | 16 (13.7%) |
| Chain length and cross-linking | |
| Unsure | 51 (43.6%) |
| Does affect outcome and safety | 32 (27.4%) |
| Does not affect outcome and safety | 20 (17.1%) |
| No response | 14 (12.0%) |
| Production process | |
| Unsure | 40 (34.2%) |
| Does not affect outcome and safety | 29 (24.8%) |
| Biologically produced has better outcome and safety | 28 (23.9%) |
| Avian-derived has better outcome and safety | 3 (2.6%) |
| No response | 17 (14.5%) |
| Anti-inflammatory effects | |
| Do not consider anti-inflammatory effects | 63 (53.8%) |
| Consider anti-inflammatory effects | 24 (20.5%) |
| Unsure | 14 (12.0%) |
| No response | 16 (13.7%) |
Factors influencing the use of IA-HA and brand selection
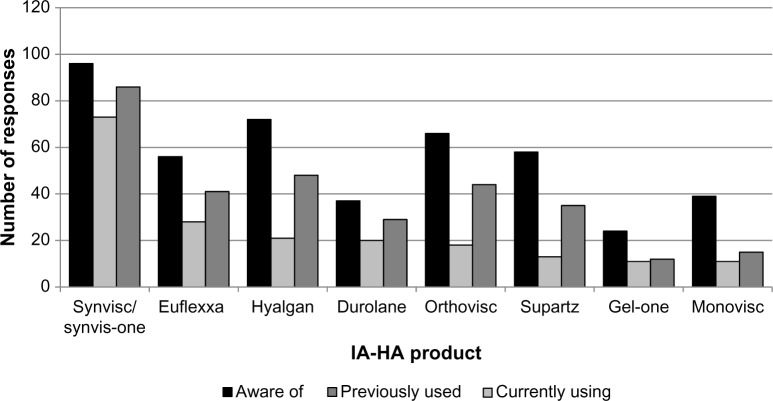
Factors that commonly influence respondents’ decisions to prescribe IA-HA include personal experience and clinical results (71.8%), that the patient is unsuitable or unwilling to undergo surgical intervention (52.1%), and sufficient evidence that supports the use of IA-HA (43.6%; Table 3). The brands of IA-HA that respondents (>50%) were most familiar with included Synvisc (96.0%), Hyalgan (72.0%), Orthovisc (66.0%), Supartz (58.0%), Euflexxa (56.0%), Monovisc (39.0%), Durolane (37.0%), and Gel-One (24.0%; Fig. 3). The most commonly used brands were Synvisc (78.5%) and Euflexxa (30.1%). Brand selection was subjectively attributed by respondents to personal experience (48.7%), sufficient scientific evidence (40.2%), and cost (40.2%).
Table 3.
Factors influencing the use of IA-HA and brand selection.
| NUMBER OF RESPONSES* N (%) N=117 |
|
|---|---|
| Factors influencing the use of IA-HA | |
| Personal experience and clinical results | 84 (71.8%) |
| Patient not suitable/unwilling to undergo operative intervention | 61 (52.1%) |
| Sufficient evidence that supports decision to use IA-HA | 51 (43.6%) |
| Failed previous treatments | 49 (41.9%) |
| Age of patient | 38 (32.5%) |
| Guideline recommendations provided by organizations | 32 (27.4%) |
| A viable mechanism of action presented in the literature | 31 (26.5%) |
| Overall cost of treatment | 30 (25.6%) |
| Inclusion of ha treatments in the formulary | 12 (10.3%) |
| No response/do not use IA-HA | 15 (12.8%) |
| Factors influencing ia-ha brand selection | |
| Personal experience and clinical outcomes | 57 (48.7%) |
| Sufficient evidence that supports your chosen brand | 47 (40.2%) |
| Overall cost of treatment | 47 (40.2%) |
| Injection regimen of your preferred brand | 46 (40.2%) |
| Molecular weight of the hyaluronic acid product | 38 (32.5%) |
| Product method used to create product (Bio-HA vs AD-HA) | 25 (21.4%) |
| Reputation of company | 25 (21.4%) |
| Cross-linking of product | 23 (19.7%) |
| Guideline recommendations provided by organizations | 14 (12.0%) |
| The information for brands in the formulary | 7 (6.0%) |
| No response/do not use IA-HA | 24 (20.5%) |
Note:
Multiple response options were permitted.
Figure 3.
IA-HA product awareness and use.
Use patterns and driving factors behind the use of Euflexxa
Of the responding clinicians, 65.7% have previously heard of the IA-HA brand Euflexxa and 42.1% of respondents have prescribed Euflexxa to patients. Those who prescribed Euflexxa indicated personal experience and clinical outcomes (46.5%), fewer serious joint effusions (32.6%), and the overall cost of Euflexxa (30.2%) as the reasons for using it (Table 4). Of those surgeons who have not prescribed Euflexxa, a lack of knowledge of Ferring Pharmaceuticals Inc., the manufacturers of Euflexxa (30.6%), and a lack of sufficient evidence that supports the use of Euflexxa (22.6%) were the reasons for not using it.
Table 4.
Reasons for prescribing Euflexxa.
| NUMBER OF RESPONSES* N (%) | |
|---|---|
| Reasons for prescribing Euflexxa | |
| Personal experience and clinical results | 20 (46.5%) |
| Fewer serious joint effusions when using Euflexxa | 14 (32.6%) |
| Overall cost of treatment | 13 (30.2%) |
| Sufficient evidence that supports Euflexxa | 12 (27.9%) |
| Formulary information regarding Euflexxa | 12 (27.9%) |
| Guideline recommendations provided by organizations | 6 (14.0%) |
| Failed previous treatments | 5 (11.6%) |
| Belief that Euflexxa is superior to other HA brands | 3 (7.0%) |
| Reasons for not prescribing Euflexxa | |
| Lack of knowledge of Ferring Inc, makers of Euflexxa | 19 (30.6%) |
| Not applicable, I prescribe Euflexxa | 14 (22.6%) |
| Lack of sufficient evidence that supports Euflexxa | 14 (22.6%) |
| Personal experience and clinical results | 12 (19.4%) |
| Overall cost of treatment | 11 (17.7%) |
| Guideline recommendations provided by organizations | 5 (8.1%) |
| Belief that there is a superior HA brand than Euflexxa | 5 (8.1%) |
| Advantages of Euflexxa over 1-shot treatments | |
| Unsure | 65 (55.6%) |
| Fewer number of serious joint effusions | 14 (12.0%) |
| Less irritation/adverse reactions at injection site | 14 (12.0%) |
| Greater effect size regarding primary outcome | 8 (6.8%) |
| Less acetaminophen use during follow-up | 5 (4.3%) |
| No response | 11 (9.4%) |
Note:
Multiple response options were permitted.
When respondents were asked how Euflexxa compares to other IA-HA products, 33.3% believe it is equal to or superior to other IA-HA products with respect to efficacy and safety. In order to demonstrate the superiority of Euflexxa to other treatment methods, respondents stated that they would like to see a trial directly comparing Euflexxa to other IA-HA brands (52.8%), specifically Synvisc (66.2%), and to corticosteroid injections (21.3%).
Discussion
The current study found that controversy and uncertainties continue to exist among clinicians regarding the efficacy of IA-HA as a treatment for knee OA. The vast majority of respondents (90.6%) to the survey indicated that there is controversy regarding the efficacy of IA-HA treatment. Conflicting information from various sources, including professional society treatment guidelines, also contributes to the uncertainty that surgeons and physicians are experiencing on the topic. Recent AAOS recommendations, which 91.6% of respondents are aware of, stated they cannot recommend the use of IA-HA for symptomatic OA of the knee due to a lack of demonstrated efficacy.8 However, recent reviews have proposed that these recommendations may have substantial methodological flaws that lead to potentially inaccurate suggestions on the efficacy of IA-HA as a treatment for knee OA.3,14,20 The recommendation against the use of IA-HA by the AAOS may also increase the perception of controversy due to the substantial body of literature showing positive effects of the use of IA-HA as a treatment for knee OA.21–27 The inconsistency between clinical guidelines and published research may have a large impact on the ability to prescribe appropriate treatments to patients, as guidelines have at least some influence on many surgeons’ opinions regarding treatments (49.6%) and on their decision-making when treating patients (55%).
We also found that substantial controversy exists regarding the effect of different product characteristics on outcome measures and safety. Studies investigating how product characteristics affect outcomes and safety have been conducted; however, the small number of these studies and their conflicting results may lead to clinician’s uncertainty regarding the effects of molecular weight, chain length, cross-linking, production process, and anti-inflammatory properties.15,17–19,28–42 Research studies examining differences between Bio-HA and AD-HA have generally shown that Bio-HA products have better safety profiles with respect to fewer injection site adverse events, although very few studies have been conducted in this area.15,41,42 The anti-inflammatory effects of IA-HA have been previously demonstrated; however, this factor is largely overlooked by surgeons when selecting IA-HA as a treatment modality.28–31 Additional high-quality studies may help to dispel uncertainty among clinicians regarding product characteristics by building an understanding of their effects on patient outcomes and safety.
The majority of respondents have used IA-HA to treat patients with knee OA and are aware of the products available. Synvisc and Euflexxa are the most commonly used IA-HA brands by survey participants. Respondents’ opinions regarding Euflexxa remain uncertain, and the respondents would like to see a randomized controlled trial demonstrating the efficacy of Euflexxa, although this was shown in a clinical trial and extension trial published by Altman et al.4,43 Additionally, respondents would like to see a trial comparing Euflexxa directly to Synvisc. Although a study published by Kirchner and Marshall demonstrates similar efficacy and fewer injection site adverse events for Euflexxa when compared to Synvisc,15 it appears to have been largely overlooked. The findings of these studies may need to be disseminated to the orthopedic community to a greater extent, as this discrepancy suggests a gap in clinician’s knowledge of these studies that are available in the published literature.
To our knowledge, this is the first paper addressing clinician’s perceptions regarding the use of IA-HA for the treatment of knee OA. The limitations of this study include that the majority of clinicians (87.2%) were orthopedic surgeons, which may affect generalizability across different professions, and 79.5% of surveyed practitioners were from North America, which may affect generalizability outside of Canada and the United States. Additionally, the small response rate by clinicians to the survey may indicate that only surgeons with an interest in IA-HA participated in the survey, which creates another potential limitation to the present study. Additionally, the inclusion of brand-specific information for Euflexxa provides useful information regarding opinions on product-specific characteristics; however, these results are not generalizable to other IA-HA products. The study population was comprised of nearly 30% of individuals who treated fewer than 100 OA patients a year, as well as nearly 30% of individuals with <10 years of experience in treating OA. This may provide results from individuals with limited experience in treating OA; however, we aimed to include the opinions of the orthopedic community as a whole and thus did not solely focus on individuals with a large amount of experience in treating knee OA.
In conclusion, uncertainty and controversy regarding the efficacy of IA-HA treatments are likely due to inconsistency within clinical guidelines and the current literature. Additional research to investigate the efficacy of IA-HA treatment and how product characteristics affect outcome and safety is required in order to provide clarity to the controversy surrounding IA-HA treatment for knee OA.
Acknowledgments
The coauthors would like to thank Mark Phillips and Global Research Solutions Inc. for their medical writing assistance, administration of the survey, and preparation of the manuscript for submission.
Footnotes
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 686 words, excluding any confidential comments to the academic editor.
FUNDING: This study was funded by Ferring Pharmaceuticals Inc.
COMPETING INTERESTS: Ferring Pharmaceuticals Inc is the manufacturer of Euflexxa®. Jeffrey Rosen is a consultant, a speaker, and a member of scientific advisory board for Ferring Pharmaceuticals Inc. Victoria Avram and Parag Sancheti have no disclosures or conflicts of interest. Faizan Niazi and Anke Fierlinger are paid employees of Ferring Pharmaceuticals Inc. Asheesh Bedi has received personal fees from Arthrex Inc. and A3 Surgical, outside of the submitted work.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: JR, VA, AF, FN, PS, AB. Analyzed the data: JR, VA, AF, FN, PS, AB. Wrote the first draft of the manuscript: Global Research Solutions Inc. Contributed to the writing of the manuscript: JR, VA, AF, FN, PS, AB. Agree with manuscript results and conclusions: JR, VA, AF, FN, PS, AB. Jointly developed the structure and arguments for the paper: JR, VA, AF, FN, PS, AB. Made critical revisions and approved final version: JR, VA, AF, FN, PS, AB. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Samson DJ, Grant MD, Ratko TA, et al. Treatment of Primary and Secondary Osteoarthritis of the Knee. Rockville, MD: AHRQ; 2007. [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence . Osteoarthritis Care and Management in Adults. London: NICE; 2014. p. CG177. [PubMed] [Google Scholar]
- 3.Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 4.Altman RD, Rosen JE, Bloch DA, et al. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthritis Cartilage. 2011;19(10):1169–75. doi: 10.1016/j.joca.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Lo GH, Lavalley M, Mcalindon T, et al. Intra-articular hyaluronic acid in treatment of knee osteoarthritis. J Am Med Assoc. 2012;290(23):3115–21. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 6.Lundsgaard C, Dufour N, Fallentin E, et al. Intra-articular sodium hyaluronate 2 mL versus physiological saline 20 mL versus physiological saline 2 mL for painful knee osteoarthritis: a randomized clinical trial. Scand J Rheumatol. 2008;37(17):142–50. doi: 10.1080/03009740701813103. [DOI] [PubMed] [Google Scholar]
- 7.Puhl W, Bernau A, Greiling H, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage. 1993;1:233–41. doi: 10.1016/s1063-4584(05)80329-2. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Orthopaedic Surgeons . Treatment of Osteoarthritis of the Knee: Evidence Based Guideline. 2nd ed. Washington, DC: AAOS; 2013. [DOI] [PubMed] [Google Scholar]
- 9.McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann Rheum Dis. 2003;62(12):1145–55. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Royal Australian College of General Practitioners . Guideline for the Non-Surgical Management of Hip and Knee Osteoarthritis. East Melbourne, VIC: RACGP; 2009. [Google Scholar]
- 12.National Collaborating Centre for Chronic Conditions . Guideline for the Non-Surgical Management of Hip and Knee Osteoarthritis. Folsom, CA: NCC-CC; 2008. [Google Scholar]
- 13.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85(1):49–56. [PubMed] [Google Scholar]
- 14.Altman R, Schemitsch E, Bedi A. Assessment of clinical practice guide-line methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Semin Arthritis Rheum. 2015;45(2):132–9. doi: 10.1016/j.semarthrit.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154–62. doi: 10.1016/j.joca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Elmorsy S, Funakoshi T, Sasazawa F, et al. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis Cartilage. 2014;22(1):121–7. doi: 10.1016/j.joca.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Sjögren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology. 2002;41:1240–8. doi: 10.1093/rheumatology/41.11.1240. [DOI] [PubMed] [Google Scholar]
- 18.Kotevoglu N, Iyibozkurt PC, Hiz O, et al. A prospective randomised controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26:325–30. doi: 10.1007/s00296-005-0611-0. [DOI] [PubMed] [Google Scholar]
- 19.Wobig M, Bach G, Beks P, et al. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999;21(9):1549–62. doi: 10.1016/s0149-2918(00)80010-7. [DOI] [PubMed] [Google Scholar]
- 20.Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86–9. doi: 10.1016/j.arthro.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Wobig M, Dickhut A, Maier R, et al. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20(3):410–23. doi: 10.1016/s0149-2918(98)80052-0. [DOI] [PubMed] [Google Scholar]
- 22.Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro-Sarabia F, Coronel P, Collantes E, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70(11):1957–62. doi: 10.1136/ard.2011.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859–66. doi: 10.1016/j.joca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Huang TL, Chang CC, Lee CH, et al. Intra-articular injections of sodium hyaluronate (Hyalgan®) in osteoarthritis of the knee. a randomized, controlled, double-blind, multicenter trial in the asian population. BMC Musculoskelet Disord. 2011;12(1):221. doi: 10.1186/1471-2474-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo con-trolled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33(5):951–6. [PubMed] [Google Scholar]
- 27.Wang Y, Hall S, Hanna F, et al. Effects of hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12(1):195. doi: 10.1186/1471-2474-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg DD, Stoker A, Kane S, et al. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage. 2006;14(8):814–22. doi: 10.1016/j.joca.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Waddell DD, Kolomytkin OV, Dunn S, et al. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res. 2007;465:241–8. doi: 10.1097/BLO.0b013e31815873f9. [DOI] [PubMed] [Google Scholar]
- 30.Yatabe T, Mochizuki S, Takizawa M, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68(6):1051–8. doi: 10.1136/ard.2007.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sezgin M, Demirel AÇ, Karaca C, et al. Does hyaluronan affect inflammatory cytokines in knee osteoarthritis? Rheumatol Int. 2005;25(4):264–9. doi: 10.1007/s00296-003-0428-7. [DOI] [PubMed] [Google Scholar]
- 32.Petrella RJ, Cogliano A, Decaria J. Combining two hyaluronic acids in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Clin Rheumatol. 2008;27(8):975–81. doi: 10.1007/s10067-007-0834-4. [DOI] [PubMed] [Google Scholar]
- 33.Raman R, Dutta A, Day N, et al. Efficacy of hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee – a prospective randomized clinical trial. Knee. 2008;15(4):318–24. doi: 10.1016/j.knee.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71:1454–60. doi: 10.1136/annrheumdis-2011-200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karatosun V, Unver B, Gocen Z, et al. Comparison of two hyaluronan drugs in patients with advanced osteoarthritis of the knee. A prospective, randomized, double-blind study with long term follow-up. Clin Exp Rheumatol. 2005;23(2):213–8. [PubMed] [Google Scholar]
- 36.Bayramoğlu M, Karataş M, Çetin N, et al. Comparison of two different visco-supplements in knee osteoarthritis – A pilot study. Clin Rheumatol. 2003;22(2):118–22. doi: 10.1007/s10067-002-0691-0. [DOI] [PubMed] [Google Scholar]
- 37.Maheu E, Zaïm M, Appelboom T, et al. Comparative efficacy and safety of two different molecular weight (MW) hyaluronans F60027 and hylan G-F20 in knee osteoarthritis (KOA): a non-inferiority randomised controlled trial. Osteoarthritis Cartilage. 2009;17:527–35. [PubMed] [Google Scholar]
- 38.Atamaz F, Kirazli Y, Akkoc Y. A comparison of two different intra-articular hyaluronan drugs and physical therapy in the management of knee osteoarthritis. Rheumatol Int. 2006;26(10):873–8. doi: 10.1007/s00296-005-0096-x. [DOI] [PubMed] [Google Scholar]
- 39.Karatay S, Kiziltunc A, Yildirim K, et al. Effects of different hyaluronic acid products on synovial fluid levels of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in knee osteoarthritis. Ann Clin Lab Sci. 2004;34(3):330–5. [PubMed] [Google Scholar]
- 40.Karatay S, Kiziltunc A, Yildirim K, et al. Effects of different hyaluronic acid products on synovial fluid NO levels in knee osteoarthritis. Clin Rheumatol. 2005;24(5):497–501. doi: 10.1007/s10067-004-1077-2. [DOI] [PubMed] [Google Scholar]
- 41.McGrath AM, McGrath AF. A comparison of intra-articular hyaluronic acid competitors in the treatment of mild to moderate knee osteoarthritis. J Arthritis. 2013;02(01):1–5. [Google Scholar]
- 42.Román JA, Chismol J, Morales M, et al. Intra-articular treatment with hyaluronic acid. Comparative study of Hyalgan and Adant. Clin Rheumatol. 2000;19(3):204–6. doi: 10.1007/s100670050157. [DOI] [PubMed] [Google Scholar]
- 43.Altman RD, Rosen JE, Bloch DA, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (The FLEXX Trial) Semin Arthritis Rheum. 2009;39(1):1–9. doi: 10.1016/j.semarthrit.2009.04.001. [DOI] [PubMed] [Google Scholar]