Abstract
Endometriosis is a benign gynecologic disease characterized by the presence of endometrial tissue outside the uterine cavity. The complexity of the disease results from its multiple clinical presentations, the multifocal pattern of distribution of the lesions, the presence of extra pelvic sites of the disease (mainly affecting the urinary and the intestinal tracts), and the difficulty in the preoperative diagnosis (by means of imaging studies) and in the surgical treatment. The preoperative mapping of the lesions, either by ultrasound or by magnetic resonance imaging, allows for an adequate surgical planning and a better preoperative patient counseling, especially in those women with deep infiltrating endometriosis affecting the bowel. Also, the choice of the surgical team that is going to perform the procedure may be based on the preoperative workup. In this paper, we highlight the important findings that should be described in the imaging examination reports for the preoperative workup of patients with deep infiltrating endometriosis of the intestine.
Keywords: deep infiltrating endometriosis, ultrasound, magnetic resonance imaging, laparoscopy, bowel
Introduction
Endometriosis is a benign gynecologic disease characterized by the presence of endometrial tissue outside the uterine cavity.1 The complexity of the disease is determined by the variety of clinical presentations, the multifocality, the involvement of nongynecological sites (mainly urinary and/or intestinal tracts), and the difficulty in the diagnosis by preoperative imaging examinations and in the definition of the proper surgical treatment.
The disease mainly affects young women of reproductive age, and its prevalence in the female population is estimated at about 10%.2,3 Three clinical presentations can occur alone or coexist: peritoneal endometriosis, ovarian endometrioma, and deep infiltrating endometriosis (DIE).4 DIE appears to affect approximately 20% of women suffering from the disease5,6 and is classically defined by the presence of endometriosis infiltrating deeper than 5 mm under the peritoneum.7
DIE has a multifocal pattern of distribution in the pelvis,8–10 affecting the urinary tract in about 14% and the gastrointestinal tract in 45.4% of cases.9 Intestinal DIE is defined as the lesion infiltrating at least the muscular layer of the bowel wall11 and usually affects the rectum and the sigmoid. Its prevalence is estimated at 45%–56% of women with DIE9,12 and 57.1% of women with ovarian endometrioma.8
Adequate surgical planning can be very well defined when there is a proper preoperative mapping of the disease by imaging studies, either ultrasound or magnetic resonance imaging (MRI).9,10,12–14 The definition of some aspects of the intestinal DIE lesions is essential to predefine the surgical steps of the surgical intervention13,14 as well as to provide accurate information for preoperative counseling of patients contemplating surgical intervention for DIE. In this article, we present the rationale for the preoperative imaging assessment of intestinal DIE.
Rationale for the Preoperative Imaging Examinations
Several imaging examinations have been used to evaluate the DIE lesions in the preoperative setting, including transvaginal ultrasound (TVUS), MRI of the pelvis, and transrectal ultrasound.10,12,15–19 Regardless of the imaging examination, the goal of the preoperative evaluation is to detect the presence of ovarian endometrioma, deep endometriosis lesions, and indirect signs of adhesions.
In 2007, Abrao et al19 showed that in women with DIE lesions affecting the rectosigmoid or the retrocervical area, TVUS had sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy superior to pelvic MRI and gynecological examination (digital examination). Similarly, Bazot et al18 compared the value of the physical examination, the TVUS, the transrectal ultrasound, and the MRI in the evaluation of different locations of DIE lesions, concluding that MRI has similar results to TVUS and transrectal ultrasound for the diagnosis of intestinal endometriosis but has higher sensitivity and higher likelihood ratios for the diagnosis of endometriosis in the uterosacral ligaments and vagina.
In the presence of intestinal lesions, the rectosigmoid is involved in more than 90% of the cases; however, one should not forget that the ileum, cecum, and appendix may also be infiltrated by DIE lesions.12 In their series of 100 cases of DIE affecting the midlow rectum (up to 8 cm from the anal verge), Chapron et al20 observed a high incidence of associated involvement of the ileocecal region (12% of lesions of the ileum, 8% of lesions of the appendix, and 6% of lesions of the cecum).
Furthermore, there are two basic characteristics of intestinal DIE lesions: multifocality and multicentricity. The former is defined by the presence of other intestinal DIE lesions within a 2-cm area to the main lesion, and the latter is defined by the presence of other DIE intestinal lesions beyond 2 cm from the main lesion. They seem to occur in 62% and 38% of surgical specimens, respectively.21
The success of the surgical treatment is related to the complete excision of the endometriosis lesions.22,23 Therefore, accurate preoperative diagnosis of the location and extent of endometriosis lesions is important for the surgical planning of patients with DIE, especially in those with intestinal involvement. This allows for an adequate preoperative patient counseling and planning of the surgical strategy.
Imaging Patterns of Intestinal DIE Lesions in Imaging Studies
Our standard imaging protocols for TVUS and pelvic/abdominal MRI have already been previously described in the literature10 and they are summarized below.
Imaging protocols
MRI protocol
Pelvic/abdominal MRI is performed using a 1.5-T MR imaging system (Achieva—Philips Medical System). Patients are requested to fast for six hours before the examination and to maintain moderate repletion of the bladder. Ultrasound gel is injected into the vagina (60 mL) and the rectum (120 mL) to enable better visualization of the dome and the fornices of the vagina, the rectovaginal septum, and the spaces of the posterior compartment of the pelvis (Fig. 1). Scopolamine-N-butyl bromide, an antispasmodic agent, is administered intravenously immediately before the examination to attenuate uterine contractions and to reduce motion artifacts caused by peristalsis.
Figure 1.
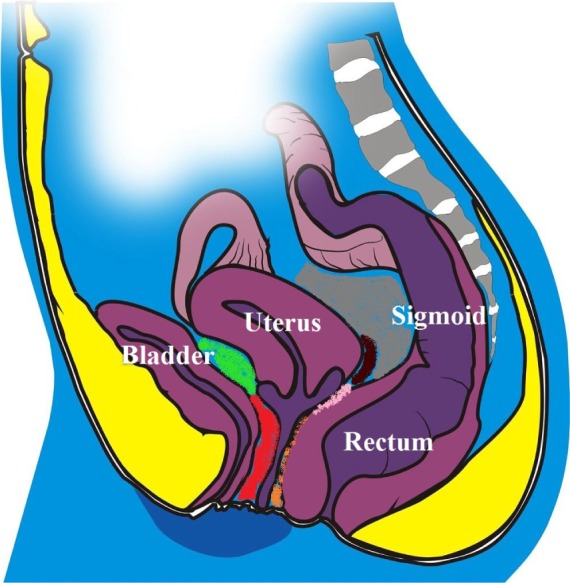
Schematic drawing of the pelvis demonstrating the bladder, the uterus, the sigmoid, the rectum, the posterior cul-de-sac (in grey), the retrocervical area (in brown), the posterior vaginal fornix (in pink), the rectovaginal septum (in orange), the vesicovaginal septum (in red), and the vesicouterine fold (in green).
Precontrast imaging: Our standard MR imaging protocol includes the acquisition of axial, sagittal, and coronal T2-weighted fast spin-echo images (for assessment of the entire pelvic anatomy and the pathologic changes) and axial T1-weighted gradient echo images in and out of phase and with fat suppression (for differentiation between blood and fat).
Postcontrast imaging: Gadolinium chelate (Dotarem) is administered intravenously followed by the dynamic acquisition of axial and sagittal volumetric fat-saturated T1-weighted sequences.
TVUS protocol
Bowel preparation was routinely performed in our patients to ensure a better visualization of the DIE lesions. On the day before the TVUS, the patient is requested to have a low-residue diet and administered a mild oral laxative. One hour prior to the examination, a rectal enema consisting of 120 mL of sodium diphosphate is performed.
Ultrasound gel is injected vaginally (60 mL) to distend the dome of the vagina. The transvaginal examination is performed using a 5–9 MHz transducer in order to assess the ovarian and adnexal regions, the uterus, all the structures and spaces of the anterior pelvic compartment (bladder, vesicovaginal septum, prevesical space, and vesicouterine pouch), and the posterior pelvic compartment (rectosigmoid, rectovaginal septum, posterior vaginal fornix, retrocervical space, and pouch of Douglas) (Fig. 1).
The rectosigmoid is usually assessed transvaginally; however, another possibility is ultrasonographic assessment using an endoanal probe.
Imaging findings
DIE lesions may have the following morphological characteristics in both ultrasound and MRI10,15–17:
- Nodular lesion
- Retractile or nonretractile
- Regular or irregular contours
- With or without endometriosis glands
- Plaque-like lesion
- Retractile
- Infiltrative
- Ill-defined margins
- With or without endometriosis glands
Transvaginal ultrasound
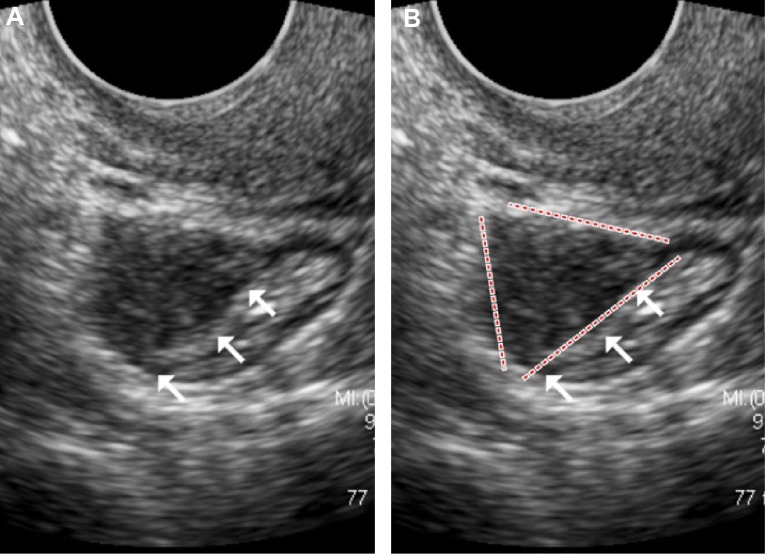
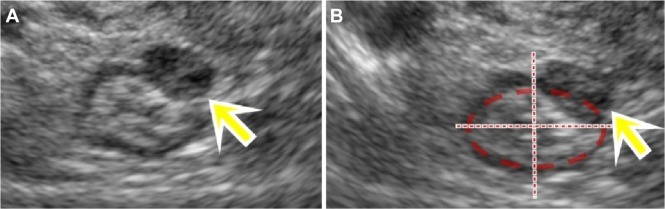
The typical image of intestinal DIE lesion is a solid hypoechoic retractile lesion with irregular contours, affecting the intestinal wall and promoting infiltration of it. Most often it affects the anterior wall of the rectosigmoid between the 10 and 2 o’clock position in the intestinal wall, often showing a pyramidal shape, in which the base of the pyramid is attached to the anterior rectal wall and the apex is oriented to the retrocervical region (Fig. 2). It may often involve other structures of the posterior compartment of the pelvis such as the uterosacral ligaments, the posterior vaginal fornix, and the posterior uterine serosa, extending or not to the adnexal regions. Usually, the lesion is restricted to the muscular layer of the intestinal wall. If the lesion involves a deeper layer, such as the submucosa, one can observe areas of disruption of this layer (hyperechoic) of the intestinal wall.
Figure 2.
(A) TVUS demonstrating an irregular and retractile hypoechoic nodule of intestinal DIE, (B) with the base of the pyramid adhering to the anterior rectosigmoid wall and the apex oriented toward the retrocervical area.
Pelvic/abdominal MRI
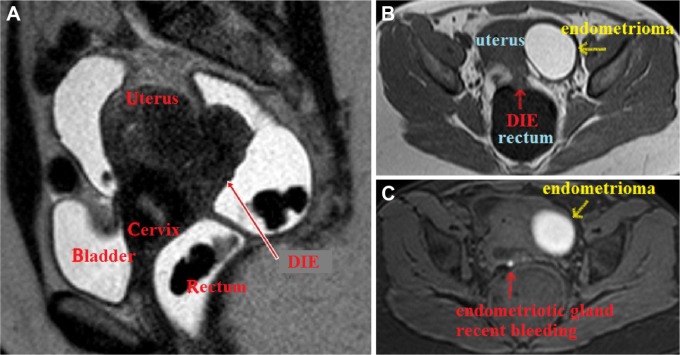
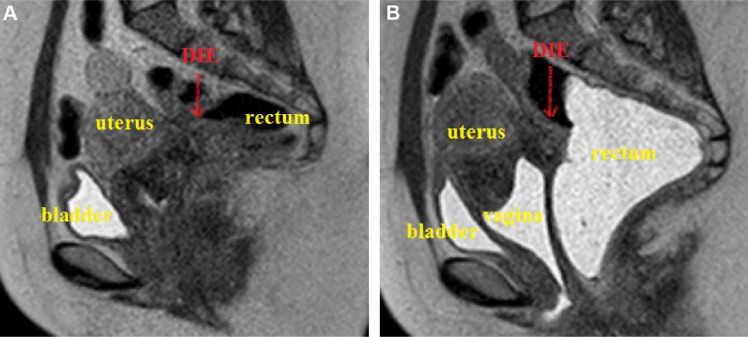
On pelvic/abdominal MRI, the DIE lesion appears isointense to the myometrium on T1-weighted images and hypointense on T2-weighted images. When present, the endometriosis glands appear hyperintense on fat-saturated T1-weighted gradient echo images, depending on the presence or not of recent bleeding (Fig. 3). The introduction of ultrasound gel inside the rectum improves the anatomical definition of the pelvis on MRI,24 increasing the possibility of detecting small intestinal lesions (Fig. 4); however, it is difficult to define which layer of the intestinal wall is affected by the disease by MRI.
Figure 3.
(A) Extensive DIE lesion at the posterior cul-de-sac with infiltration of the rectum, as well as the posterior aspect of the uterus and the uterine cervix, leading to retroflexion of the uterus. One may observe the hypointense aspect of the lesion on T2-weighted image. (B) T1-weighted TSE sequence demonstrating iso-signal intensity of the DIE lesion in contact with the rectal wall. An ovarian endometrioma is observed at the left side. (C) Fat saturation T1-weighted gradient-echo sequence showing an endometriotic gland with recent bleeding within the endometriotic lesion and an ovarian endometrioma at the left side.
Figure 4.
MRI of the pelvis (sagittal T2-weighted TSE sequence) without (A) and with ultrasound gel (B) injected vaginally and rectally in the same patient. One may clearly identify an important anatomic definition of the pelvis (uterus, rectum, vagina, and bladder) when the ultrasound gel is used, allowing for the proper identification of the DIE lesion.
Types of Surgery for the Treatment of Intestinal DIE
Although the purpose of this article is not to demonstrate surgical techniques for intestinal endometriosis, it is important to have a general idea about the differences between the procedures in order to understand why the preoperative workup can help in the surgical planning of the disease.
Laparoscopic surgical treatment of intestinal DIE involving the rectum and/or sigmoid can be separated into two different surgical concepts: conservative and radical. Conservative surgery can also be called nodulectomy, and it consists of removing only the endometriosis nodule using one of the following techniques13,14:
- Rectal shaving25—consists of removing the DIE nodule from the muscle layer of intestinal wall without, however, completely opening it (ie, not reaching the lumen of the bowel). The defect in the intestinal wall can be sutured or not, depending on the depth of resection. It can be performed by means of two different techniques:
- Traditional technique: the nodule is first released from the anterior rectal wall and then the retrocervical area/posterior vaginal fornix will be treated.
- Reverse technique: the nodule is first released from the retrocervical area and the posterior vaginal fornix [with or without the need of colpectomy (resection of the posterior vaginal fornix), depending on the case] and then it is shaved off the anterior rectal wall.
Mucosal skinning—consists of removing the DIE nodule from the bowel deep in the layers of the intestinal wall, keeping just the mucosa intact. The defect in the rectal wall is sutured at the end of the procedure.
- Full-thickness anterior rectal wall excision (disk resection)—consists of removing the DIE nodule from the bowel including all the layers of the anterior rectal wall using one of the following techniques:
- Scissors or harmonic scalpel and suture.
- Laparoscopic linear stapler.
The “radical” approach is the resection of the bowel segment affected by the disease (segmental bowel resection with primary colorectal anastomosis with or without protective ileostomy, depending on each case).13,14
What Should the Imaging Examinations Show in Order to Help in the Surgical Planning?
The preoperative imaging examinations of intestinal DIE lesions should preferably contain the following information, which are of fundamental importance for the surgeon to plan the procedure:
Size of the lesion (longitudinal and transverse measurements);
Depth of infiltration in the intestinal wall;
Percentage of the intestinal circumference affected by the disease;
Distance between the intestinal DIE lesion and anal verge;
Presence of multifocal/multicentric intestinal DIE lesion.
Size of the lesion
The size of the nodule and the percentage of bowel circumference involved by the DIE lesion can be similarly evaluated by MRI and TVUS. They are both important because they determine the risk of intestinal lumen’s stenosis, which can lead to symptoms of intestinal subocclusion.
In general, only patients with intestinal DIE lesions measuring up to 25–30 mm may be candidates for conservative surgery, either shaving rectal25/mucosal skinning or disk resection.26 Usually we prefer to address such lesions using the technique of full-thickness anterior rectal wall disk resection using the circular stapler. The limit of the resection is the distance between the envil and the stapler because the DIE lesion must be “pushed” into the space between them. Some patients with specific intestinal DIE lesions with up to 40–60 mm in size affecting less than one-third of the bowel circumference may be candidates for discoid resection by the double stapling technique.14,27,28
Depth of infiltration in the intestinal wall
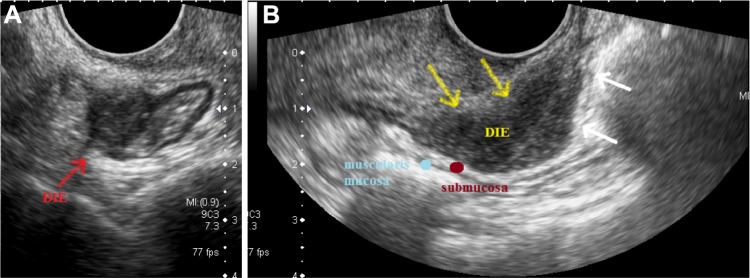
TVUS may visualize and differentiate the layers of the intestinal wall. The muscle layers of the intestinal wall (muscularis propria and muscularis mucosa) are hypoechoic, whereas the serosa, the submucosa, and the mucosa have increased echogenicity on ultrasound (Fig. 5).
Figure 5.
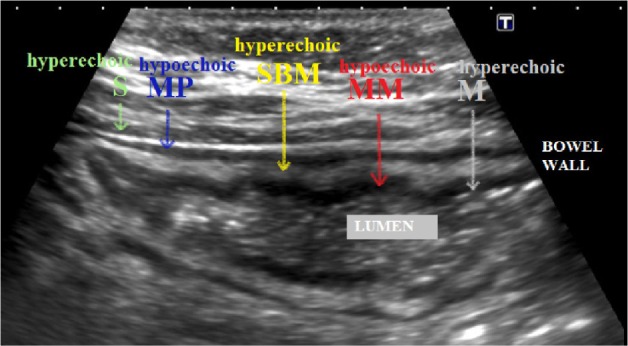
Differentiation of the layers of the bowel wall on TVUS: from outside to inside, the serosa (S) is hyperechoic, the muscularis propria (MP) is hypoechoic, the submucosa (SBM) is hyperechoic, the muscularis mucosa (MM) is hypoechoic, and the mucosa (M) is hyperechoic.
When endometriosis glands and stroma encounter the smooth muscle of the intestinal wall, they determine proliferation of smooth muscle and fibrous reaction, which causes thickening of the muscular layer of the bowel and the specific pattern of image that is hypoechoic. Most of the intestinal DIE lesions do not go deeper than the muscularis propria (Fig. 6A), but large lesions may affect deeper layers of the intestinal wall (Fig. 6B).
Figure 6.
TVUS showing (A) a DIE lesion affecting the anterior rectosigmoid wall up to the muscularis propria, (B) a retrocervical DIE implant affecting the anterior rectal wall about 8 cm from the anal verge, with some areas of infiltration of the submucosal layer. One may see some areas of interruption of the submucosal layer of the intestinal wall (white arrows).
On pelvic/abdominal MRI, when the ultrasound gel is not administered transrectally, the evaluation of the intestinal wall is made as follows:
Infiltration of the muscle layer—thickening of this layer of the bowel wall, hypointense on T2-weighted images.
Infiltration of the submucosa—thickening of this layer of the bowel wall, demonstrated by slightly higher signal shown in the luminal aspect of the intestinal wall.
Although MRI may be useful to detect the involvement of the muscle layer of the bowel by endometriosis, it fails to determine whether there is infiltration or not of the submucosa. The thickening of the submucosa may be due to infiltration of this layer by endometriosis (real involvement of the submucosal layer) or distortion and retraction of the submucosal layer due to inflammatory reaction of the lesion affecting the muscle layer of the bowel (absence of involvement of the submucosa).
In the authors’ experience, the layers of the bowel wall are better identified on TVUS compared to pelvic/abdominal MRI of the pelvis. Our current protocol includes the injection of ultrasound gel into the vagina and the rectum before the MRI of the pelvis in order to maximize the identification of DIE lesions, and whenever bowel endometriosis is identified, TVUS is performed in order to better evaluate the depth of infiltration in the bowel wall.
DIE lesions affecting the serosa
DIE lesions infiltrating only the serosa are not effectively considered as intestinal DIE lesions. In this case, the nodule can be easily excised from the intestinal wall using the shaving technique, usually not requiring to suture the intestinal wall and with a minimal risk of postoperative bowel complications (fistula).
DIE lesions affecting the muscle layer
Intestinal DIE lesions that infiltrate the muscle layer may be treated using conservative or radical surgical approaches, depending on the size of the lesion. Surgeons with extensive experience with conservative surgery are able to treat most of these lesions without the need for segmental bowel resection.25,29,30 Lesions that compromise the outer muscle layer may be resected by rectal shaving technique, with or without suture of the intestinal wall. When there is involvement of the inner muscle layer, the surgical approach may be defined, in theory, by the size of the lesion:
In cases of single lesions with up to 25–30 mm, a deep shaving in the rectal wall may be performed (in fact, a rectal shaving with mucosal skinning) as well as a full-thickness anterior disk resection (discoid resection).
In cases of bulky single lesions, a double discoid resection with circular stapler14,27,28 or a segmental bowel resection may be performed.
Although the choice of the procedure to be performed seems to be theoretically easy, in practice we encounter some difficulties. Our current routine to address patients with intestinal DIE lesions can be summarized as follows:
For single lesions up to 25–30 mm affecting up to the muscle layer of the bowel, an anterior disk resection with circular stapler is usually performed, since rectal shaving seems to increase the risk of residual disease in our experience, because of the difficulty to identify the exact limits among the healthy tissue, the fibrotic reaction without disease, the fibrotic reaction with endometriosis glands/stroma, and the diseased tissue affecting the bowel wall.
For single lesions larger than 25–30 mm, a segmental bowel resection is usually performed; some specific lesions may be treated using the double discoid resection with circular stapler.
For multiple intestinal lesions, regardless of the size, a segmental bowel resection is usually performed.
Certainly, there are several exceptions to these rules, but this is a discussion that distances from the main purpose of the article.
DIE intestinal lesions affecting the submucosa and mucosa
DIE intestinal lesions that infiltrate the submucosa and the mucosa are usually resected using full-thickness anterior intestinal wall disk resection or segmental bowel resection. The difficulty of performing a disk resection in such cases is due to the frequent presence of involvement of a large percentage of the intestinal circumference by the endometriosis implant, what may reduce the caliber of the intestinal lumen and block the progression of the circular stapler. This was demonstrated by Abrão et al,31 who observed that intestinal DIE lesions that infiltrate deeper than the internal muscle layer usually have more than 40% of the circumference of the rectosigmoid affected. Their conclusion was that these lesions usually require a segmental bowel resection because classical indications for disk resection are not applicable.
Percentage of the circumference affected by the disease
The assessment of the percentage of the circumference of the intestinal wall affected by the disease may be conducted by both TVUS (Fig. 7) and MRI (Fig. 8) by means of the acquisition of images in the transverse axis of the bowel loop at the point of greatest involvement by the lesion.
Figure 7.
TVUS demonstrating a DIE lesion affecting the sigmoid. The lesion infiltrates less than 1/4 of the bowel circumference (approximately 20%–25%).
Figure 8.
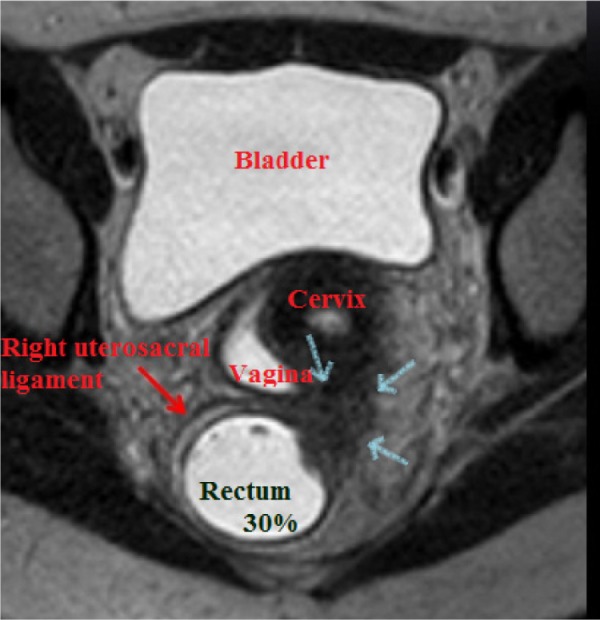
MRI of the pelvis demonstrating a DIE lesion at the retrocervical area affecting the posterior uterine cervix, posterior vaginal fornix, right uterosacral ligament, and anterior and lateral wall of the rectum (about 30% of the intestinal circumference).
As previously discussed, lesions with severe impairment of the intestinal circumference may cause stenosis of the intestinal lumen, what may block the passage of the intraluminal circular stapler, making the discoid resection impossible to be performed.
Distance of the intestinal DIE lesion from the anal verge
The distance of the DIE lesion from the anal verge is important for surgical planning and preoperative patient counseling regarding the possible need for protective stoma.
The most serious postoperative complication after DIE surgery is anastomotic dehiscence/leak that seems to occur in about 3%–7% of intestinal segmental resection, reaching elevated rates (up to 20%) in cases of low rectal anastomosis.32,33 An independent risk factor for the occurrence of anastomotic leaks after intestinal segmental resection is the colorectal anastomosis being less than 10 cm away from the anal verge;34,35 therefore, it is advisable to consider a temporary protective ileostomy in these cases.
This distance can be determined by TVUS using the peritoneal reflection as the main point of reference, which is located about 7–8 cm from the anal verge. The measurement of this distance on pelvic/abdominal MRI is more accurate because of better anatomic resolution in the sagittal T2 sequence. It is recommended to respect the rectal and sigmoid curvatures when measuring this distance (Fig. 9).
Figure 9.
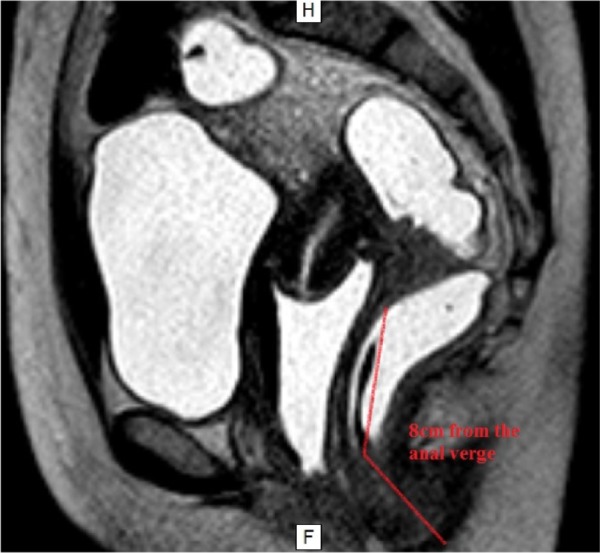
MRI of the pelvis showing a DIE lesion at the retrocervical region affecting the rectum, 8 cm distant from the anal verge.
Presence of multifocal or multicentric lesions
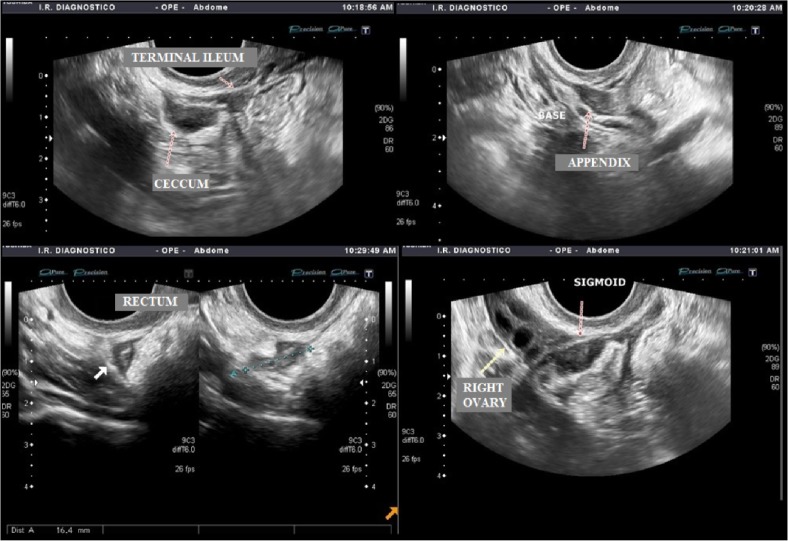
In the presence of multifocal or multicentric lesions (Fig. 10), the option of surgery is usually restricted to intestinal resection in order to obtain the complete treatment of the disease.
Figure 10.
Transvaginal and abdominal US image demonstrating the multifocal pattern of distribution of DIE: cecum, terminal ileum at the level of the ileocecal junction, base of the appendix (at the right iliac fossa), rectum (9 cm distant from the anal verge), and sigmoid (13 cm distant from the anal verge).
Conclusion
DIE is a complex disease that usually requires a complex surgical treatment. The appropriate preoperative workup using imaging examinations may allow for an accurate preoperative diagnosis of the location and extent of the disease, which is the key point for the adequate surgical planning in these patients.
Abbreviations
- DIE
deep infiltrating endometriosis
- TVUS
transvaginal ultrasound
- MRI
magnetic resonance imaging
Footnotes
ACADEMIC EDITOR: Zeev Blumenfeld, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 726 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: CHT, MTZ, WK. Analyzed the data: CHT, MTZ, WK. Wrote the first draft of the manuscript: CHT, MTZ, WK. Contributed to the writing of the manuscript: CHT, MTZ, CRTT, RLSM, RR, WK. Agree with manuscript results and conclusions: CHT, MTZ, CRTT, RLSM, RR, WK. Jointly developed the structure and arguments for the paper: CHT, MTZ, WK. Made critical revisions and approved final version: CHT, MTZ, CRTT, RLSM, RR, WK. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Senapati S, Barnhart K. Managing endometriosis-associated infertility. Clin Obstet Gynecol. 2011;54:720–726. doi: 10.1097/GRF.0b013e3182353e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 4.Donnez J, Nisolle M, Grandjean P, Gillerot S, Clerckx F. The place of GnRH agonists in the treatment of endometriosis and fibroids by advanced endoscopic techniques. Br J Obstet Gynaecol. 1992;99(suppl 7):31–33. doi: 10.1111/j.1471-0528.1992.tb13537.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapron C, Dubuisson JB, Fritel X, et al. Operative management of deep endometriosis infiltrating the uterosacral ligaments. J Am Assoc Gynecol Laparosc. 1999;6:31–37. doi: 10.1016/s1074-3804(99)80037-1. [DOI] [PubMed] [Google Scholar]
- 6.Koninckx PR, Martin DC. Deep endometriosis: a consequence of infiltration or retraction or possibly adenomyosis externa? Fertil Steril. 1992;58:924–928. doi: 10.1016/s0015-0282(16)55436-3. [DOI] [PubMed] [Google Scholar]
- 7.Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53:978–983. doi: 10.1016/s0015-0282(16)53570-5. [DOI] [PubMed] [Google Scholar]
- 8.Kondo W, Ribeiro R, Trippia CH, Zomer MT. Association between ovarian endometrioma and deep infiltrating endometriosis. Rev Bras Ginecol Obstet. 2012;34:420–424. doi: 10.1590/s0100-72032012000900006. [DOI] [PubMed] [Google Scholar]
- 9.Kondo W, Ribeiro R, Trippia C, Zomer MT. Deep infiltrating endometriosis: anatomical distribution and surgical treatment. Rev Bras Ginecol Obstet. 2012;34:278–284. [PubMed] [Google Scholar]
- 10.Kondo W, Zomer MT, Pinto EP, Del Frate G, Bazzocchi M, Zuiani C. Deep infiltrating endometriosis: imaging features and laparoscopic correlation. J Endometr. 2011;3:197–212. [Google Scholar]
- 11.Chapron C, Dubuisson JB, Pansini V, et al. Routine clinical examination is not sufficient for diagnosing and locating deeply infiltrating endometriosis. J Am Assoc Gynecol Laparosc. 2002;9:115–119. doi: 10.1016/s1074-3804(05)60117-x. [DOI] [PubMed] [Google Scholar]
- 12.Piketty M, Chopin N, Dousset B, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. 2009;24:602–607. doi: 10.1093/humrep/den405. [DOI] [PubMed] [Google Scholar]
- 13.Kondo W, Ribeiro R, Trippia C, Zomer MT. Laparoscopic treatment of deep infiltrating endometriosis affecting the rectosigmoid colon: nodulectomy or segmental resection? Gynecol Obstet. 2013;S3:001. doi: 10.1155/2013/837903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo W, Zomer MT, Ribeiro R, Trippia C, Oliveira MA, Crispi CP. Laparoscopic treatment of deep infiltrating endometriosis of the intestine—technical aspects. Braz J Video Surg. 2012;5:23–39. [Google Scholar]
- 15.Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics. 2011;31:E77–E100. doi: 10.1148/rg.314105193. [DOI] [PubMed] [Google Scholar]
- 16.Chamié LP, Pereira RM, Zanatta A, Serafini PC. Transvaginal US after bowel preparation for deeply infiltrating endometriosis: protocol, imaging appearances, and laparoscopic correlation. Radiographics. 2010;30:1235–1249. doi: 10.1148/rg.305095221. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves MO, Podgaec S, Dias JA, Jr, Gonzalez M, Abrao MS. Transvaginal ultrasonography with bowel preparation is able to predict the number of lesions and rectosigmoid layers affected in cases of deep endometriosis, defining surgical strategy. Hum Reprod. 2010;25:665–671. doi: 10.1093/humrep/dep433. [DOI] [PubMed] [Google Scholar]
- 18.Bazot M, Lafont C, Rouzier R, Roseau G, Thomassin-Naggara I, Daraï E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92:1825–1833. doi: 10.1016/j.fertnstert.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Abrao MS, Gonçalves MO, Dias JA, Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22:3092–3097. doi: 10.1093/humrep/dem187. [DOI] [PubMed] [Google Scholar]
- 20.Dousset B, Leconte M, Borghese B, et al. Complete surgery for low rectal endometriosis: long-term results of a 100-case prospective study. Ann Surg. 2010;251:887–895. doi: 10.1097/SLA.0b013e3181d9722d. [DOI] [PubMed] [Google Scholar]
- 21.Kavallaris A, Köhler C, Kühne-Heid R, Schneider A. Histopathological extent of rectal invasion by rectovaginal endometriosis. Hum Reprod. 2003;18:1323–1327. doi: 10.1093/humrep/deg251. [DOI] [PubMed] [Google Scholar]
- 22.Dunselman GA, Vermeulen N, Becker C, et al. European Society of Human Reproduction and Embryology ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 23.Practice Committee of the American Society for Reproductive Medicine Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101:927–935. doi: 10.1016/j.fertnstert.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi H, Kuwatsuru R, Kitade M, et al. A novel technique using magnetic resonance imaging jelly for evaluation of rectovaginal endometriosis. Fertil Steril. 2005;83:442–447. doi: 10.1016/j.fertnstert.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Kondo W, Bourdel N, Jardon K, et al. Comparison between standard and reverse laparoscopic techniques for rectovaginal endometriosis. Surg Endosc. 2011;25:2711–2717. doi: 10.1007/s00464-011-1635-z. [DOI] [PubMed] [Google Scholar]
- 26.Woods RJ, Heriot AG, Chen FC. Anterior rectal wall excision for endometriosis using the circular stapler. ANZ J Surg. 2003;73:647–648. doi: 10.1046/j.1445-2197.2003.02706.x. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira MA, Crispi CP, Oliveira FM, Junior PS, Raymundo TS, Pereira TD. Double circular stapler technique for bowel resection in rectosigmoid endometriosis. J Minim Invasive Gynecol. 2014;21:136–141. doi: 10.1016/j.jmig.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Kondo W, Ribeiro R, Zomer MT, Hayashi R. Laparoscopic double discoid resection with a circular stapler for bowel endometriosis. J Minim Invasive Gynecol. 2015;22:929–931. doi: 10.1016/j.jmig.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98:564–571. doi: 10.1016/j.fertnstert.2012.07.1061. [DOI] [PubMed] [Google Scholar]
- 30.Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949–1958. doi: 10.1093/humrep/deq135. [DOI] [PubMed] [Google Scholar]
- 31.Abrão MS, Podgaec S, Dias JA, Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15:280–285. doi: 10.1016/j.jmig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Fingerhut A, Elhadad A, Hay JM, Lacaine F, Flamant Y. Infraperitoneal colorectal anastomosis: hand-sewn versus circular staples. A controlled clinical trial. French Associations for Surgical Research. Surgery. 1994;116:484–490. [PubMed] [Google Scholar]
- 33.Fingerhut A, Hay JM, Elhadad A, Lacaine F, Flamant Y. Supraperitoneal colorectal anastomosis: hand-sewn versus circular staples—a controlled clinical trial. French Associations for Surgical Research. Surgery. 1995;118:479–485. doi: 10.1016/s0039-6060(05)80362-9. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Choi GS, Kim SH, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665–671. doi: 10.1097/SLA.0b013e31827b8ed9. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. 2009;209:694–701. doi: 10.1016/j.jamcollsurg.2009.09.021. [DOI] [PubMed] [Google Scholar]