Abstract
Tumor-associated inflammation can create an immunosuppressive microenvironment allowing tumor cells to escape immunosurveillance. Inhibiting immunosuppression remains one of the major challenges in cancer immunotherapy via checkpoint inhibitors. Recent preclinical data from Reis e Sousa's group may provide a strong rationale for developing new therapeutics to subvert tumor-induced immunosuppression via prostaglandin inhibition.
Clinical and epidemiologic evidence indicates that chronic inflammation is a risk factor for several gastrointestinal malignancies, including esophageal, gastric, hepatic, pancreatic and colorectal cancer. The tumor tissue microenvironment has been referred to as “wounds that do not heal”, where inflammation prevails. The common pathological features of chronic inflammatory diseases and solid cancers include the elevation of pro-inflammatory mediators such as cytokines, chemokines, and prostaglandins, massive infiltration of deregulated immune cells, and recruitment of endothelial cells and fibroblasts. The best evidence for the contribution of inflammation and inflammatory mediators to cancer development has come from clinical and epidemiologic evidence documenting that daily use of nonsteroidal anti-inflammatory drugs (NSAIDs) has beneficial effects on reducing the incidence, metastasis, and mortality of various solid tumors (1, 2). Although the molecular mechanisms of NSAIDs, especially aspirin, in protecting against cancer are not well understood, NSAIDs are thought to primarily reduce the production of prostaglandins (PGs) by inhibiting the activity of cyclooxygenase enzymes (COX-1 and/or COX-2).
COX-1 is constitutively expressed in most tissues and was thought to be a housekeeping enzyme that maintains certain aspects of tissue homeostasis. By contrast, COX-2 is an immediate-early response gene that is normally absent from most cells but is highly inducible at sites of inflammation, trauma and cancers (2, 3). COX enzymes are responsible for the production of five distinct prostanoids, including PGs such as PGE2, PGD2, PGF2α, PGI2 and thromboxane A2 (TxA2), by specific PG synthases (Figure 1). PGE2 is the only prostanoid demonstrated to play a predominant role in promoting tumor formation, progression, and metastasis by acting directly on tumor cells and also on tumor stromal cells (3, 4). However, the mechanisms underlying the effects of PGE2 on cancer development are elusive. In a recent study, Zelenay et al. provided the first direct evidence that PGE2 could promote tumor growth by evading immune attack in murine tumor transplantation models of melanoma (BrafV600E mouse melanoma tumors in immunocompetent hosts) (5).
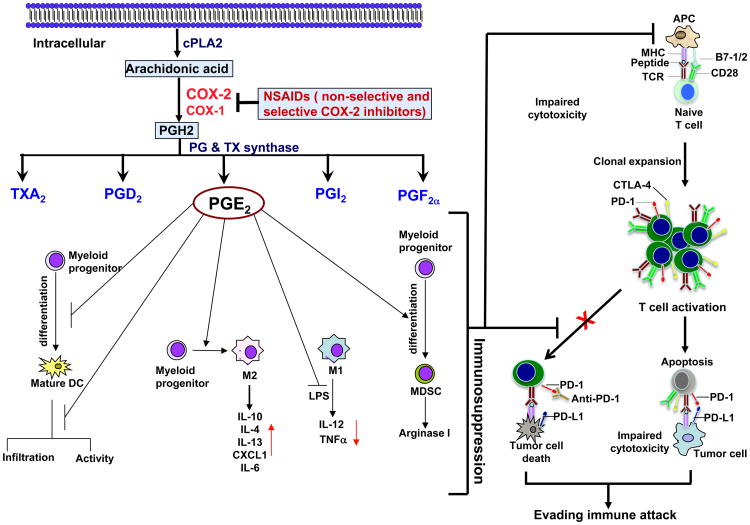
Fig. 1.
Models illustrating a role of pro-inflammatory PGE2 in inducing a shift from anti-tumor to immunosuppressive responses within tumor microenvironments.
Arachidonic acid (AA) constitutes the phospholipid domain of most cell membranes, liberated by cytoplasmic phospholipase A2 (cPLA2). COX enzymes convert free AA to an intermediate PGH2 that is sequentially metabolized to prostanoids, including prostaglandins (PGs) and thromboxanes (TXs) via specific PG and TX synthases. PGE2 produced by tumor epithelial cells and/or their surrounding stromal cells might induce immunosuppression through 1) inhibition of DC differentiation, infiltration, and activation; 2) induction of monocytes into an M2 macrophage phenotype and inhibiting LPS-induced TNFα and IL-12 in M1 macrophages; and 3) inducing MDSC differentiation and production of arginase I. T cell activation requires antigen-specific TCR stimulation and activation of an antigen-independent costimulatory receptor (CD28). PD-1 and CTLA-4 are induced in effector T cells and PD-L1 expression is elevated in various human cancer cells. Interaction of PD-1 with PD-L1 inhibits effector T cell activation and induces effector T cell apoptosis. Malignant cells can escape immunosurveillance by impairing CD8+ T cell cytotoxicity through co-inhibitory receptors such as PD-1 and CTLA-4. Tumor-associated immunosuppression can diminish the anti-tumor effects of PD-1, PD-L1, and CTLA-4 antibodies.
Cancer immune evasion involves a shift from Th1 to Th2 immune responses, defective antigen-presenting cell infiltration and function, impaired cytotoxic activity of CD8+ T cells and natural killer cells (NKs), and enhancement of immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs). PGE2 secretion from colorectal carcinoma cells (CRC) has been reported to induce macrophage production of pro-inflammatory cytokines/chemokines, such as CXCL1 and IL-6 (6). Macrophages are highly plastic and can be activated via a classical pathway to M1 macrophages, with elevated expression of IL-12, TNFα, and MHCII (Th1 response), or alternatively, to M2 macrophages, expressing high levels of IL-10, IL-4, and IL-13 (Th2 responses). In most cancers, tumor-associated macrophages resemble an M2-like phenotype and are a major component of the leukocytic tumor infiltrate. Accordingly, Zelenay et al. found that PGE2 secreted from mouse melanoma tumor cells stimulated myeloid cells to produce CXCL1, IL-6, G-CSF and inhibited the expression of TNFα and IL-12 in LPS-treated myeloid cells (5). These findings suggest that PGE2, secreted from tumor cells, might shift macrophages from an M1 to an M2 phenotype (Figure 1).
Genetic deletion of COX or PGE2 in mouse melanoma, breast or CRC facilitated a shift toward the classic anti-cancer immune pathway. The data presented indicated that PGE2 could promote tumor growth by inhibiting the accumulation of conventional dendritic cells (DC) into tumors, and suppressing tumor-infiltrating DC activation (5). Indeed, PGE2 has been shown to induce immune tolerance via DCs by altering their differentiation, maturation, and their ability to secrete cytokines (7). In addition, PGE2 has been reported to promote tumor progression by inducing the differentiation of MDSCs from bone marrow myeloid progenitor cells and enhancing MDSC-mediated immune suppression (7). Collectively, these findings indicate that PGE2 can shift from anti-tumor to immunosuppressive responses within the tumor microenvironment (Figure 1).
Malignant cells can also escape immunosurveillance by directly impairing the cytotoxic activity and proliferation of CD8+ T cells through PD-1 and CTLA-4 receptors. Interaction of PD-1 with its ligands, PD-L1 and PD-L2, inhibits effector T cell activation and proliferation. PD-L1 expression is elevated in various human cancers, including lung, colon, head and neck, and ovarian cancers as well as melanomas and its expression is associated with poor prognosis among patients with esophageal, colon, ovarian or renal-cell cancers (RCC) (8). However, less information concerning PD-L2 expression in human cancer is available. Although checkpoint antibodies against PD-1, PD-L1, and CTLA-4 offer great promise for treatment of many malignancies, the objective response rate of these is less than 30% in patients with melanoma, RCC, and NSCLC and few responses have been observed in patients with colorectal, pancreatic, gastric, or breast cancer (9). One potential reason for this lack of response is that an immunosuppressive tumor microenvironment might be preventing the effects of these inhibitors. For example, MDSCs have been shown to contribute to cancer immune evasion by suppressing effector T cell activation, proliferation, trafficking, viability, and by inhibiting natural killer cells (NK) and promoting the activation and expansion of Tregs. It thus seems reasonable to assume that tumor-infiltrating MDSCs might remain able to inhibit T cell cytotoxicity against tumor cells, even with blockade of PD-1 or CTLA-4 signaling. The observation that inhibition of tumor-infiltrating MDSCs enhanced anti-PD1 efficacy in rhabdomyosarcoma in vivo (10) supports this notion. Given that PGE2 promotes immunosuppression, it is conceivable that non-selective COX inhibitors such as aspirin or selective COX-2 inhibitors such as celecoxib could inhibit tumor formation and growth by subverting PGE2-inducing immunosuppression in the appropriate context. Zelenay et al. have now reported for the first time that aspirin or celecoxib can facilitate anti-PD-1-mediated anti-tumor responses in mouse transplantation models of melanoma and CRC (5). These findings may provide a rationale for developing new therapeutic approaches which include the reactivation of tumor-inhibited effector T cells through checkpoint blockade, while impairing tumor-induced immunosuppression through COX or PGE2 inhibitors (Figure 1).
In summary, a growing body of evidence supports the hypothesis that effective cancer therapies should include elimination of tumor cells, inhibition of tumor-associated angiogenesis, and subversion of tumor-induced immunosuppression by enhancing infiltration and activation of conventional DCs, targeting immunosuppressive cells, and reactivating tumor-inhibited effector T cells, partly through checkpoint inhibitors. NSAIDs, including aspirin and celecoxib, are able to eliminate tumor epithelial cells and reduce tumor-associated angiogenesis. The exciting observation that aspirin or celecoxib boost the efficacy of immune checkpoint inhibitors by inhibiting PGE2-induced immunosuppression in certain tumors may pave the way for future combination therapies using both checkpoint blockade and NSAIDs in cancer treatment. Although further studies are warranted to evaluate the desired synergistic effects of such combined treatments in patients whose tumors express COX-2 at higher levels, the findings from Reis e Sousa's laboratory provide a significant advance in the field of immunooncology by bringing forth a potentially promising therapeutic approach against cancer.
Acknowledgments
Research conducted in the DuBois laboratory is supported, in part, by the NIH R01 DK47297, NCI R01 CA184820, and NCI P01 CA77839. We thank the National Colorectal Cancer Research Alliance (NCCRA) for its generous support (R.N.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris RE. Cyclooxygenase-2 (Cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 2.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nature medicine. 2012;18:224–226. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Fu L, Ning W, Guo L, Sun X, Dey SK, Chaturvedi R, Wilson KT, DuBois RN. Peroxisome proliferator-activated receptor delta promotes colonic inflammation and tumor growth. Proc Natl Acad Sci U S A. 2014;111:7084–7089. doi: 10.1073/pnas.1324233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, DuBois RN. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013;19:502–510. doi: 10.1097/PPO.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis. 2015 doi: 10.1093/carcin/bgv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368–376. doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra267. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]