Abstract
Circadian disruption is the bane of modern existence and its deleterious effects on health, especially diabetes and metabolic syndrome have been well recognized in shift workers. Recent human studies strongly implicate a ‘dose-dependent’ relationship between circadian disruption and diabetes. Genetic and environmental disruption of the circadian clock in rodents leads to diabetes secondary to β-cell failure. Deletion of Bmal1, a non-redundant core clock gene, leads to defects in β-cell stimulus-secretion coupling, decreased glucose-stimulated ATP production, uncoupling of OXPHOS and impaired glucose-stimulated insulin secretion. Both genetic and environmental circadian disruptions are sufficient to induce oxidative stress and this is mediated by a disruption of the direct transcriptional control of the core molecular clock and Bmal1 on Nrf2, the master anti-oxidant transcription factor in the β-cell. In addition, circadian disruption also leads to a dysregulation of the unfolded protein response and leads to endoplasmic reticulum stress in β-cells. Both the oxidative and ER stress contribute to an impairment of mitochondrial function and β-cell failure. Understanding the basis of the circadian control of these adaptive stress responses offers hope to target them for pharmacological modulation to prevent and mitigate the deleterious metabolic consequences of circadian disruption.
Keywords: β-cell, islet, circadian, clock, Bmal1, Rev-erb, oxidative stress, ER stress, UPR, mitochondria, OXPHOS, shift work, insulin, diabetes
Introduction
The circadian clock is evolutionarily conserved and serves to allow the organism to anticipate environmental changes and adapt its metabolism and behavior to attain a survival advantage [1]. In mammals, the circadian clock is composed of a web of cell-autonomous and self-sustained oscillators residing not only in the hypothalamic suprachiasmatic nucleus (central clock), but also in every cell (peripheral clock) [2]. The importance of the circadian clock in metabolism has been convincingly demonstrated in various gene deletion models which display significant impairments in glucose and lipid metabolism. In addition, human studies have also associated perturbations of the circadian rhythm with metabolic abnormalities, especially diabetes and metabolic syndrome [3–5]. With modern lifestyle with significant light induced circadian disruption, “social jet lag” is widely prevalent and its long term metabolic effects are yet to be studied. Furthermore, mutations in some circadian related genes have been identified to be strongly associated with beta cell dysfunction and diabetes [6–9]. While there has been more insight into the mechanistic underpinnings of circadian regulation of β-cell function, there remains much to be still determined. Here we review the recent understanding on the circadian regulation of β-cell function with an emphasis on stress pathways that are circadian regulated.
Molecular basis of circadian oscillations – the molecular clock
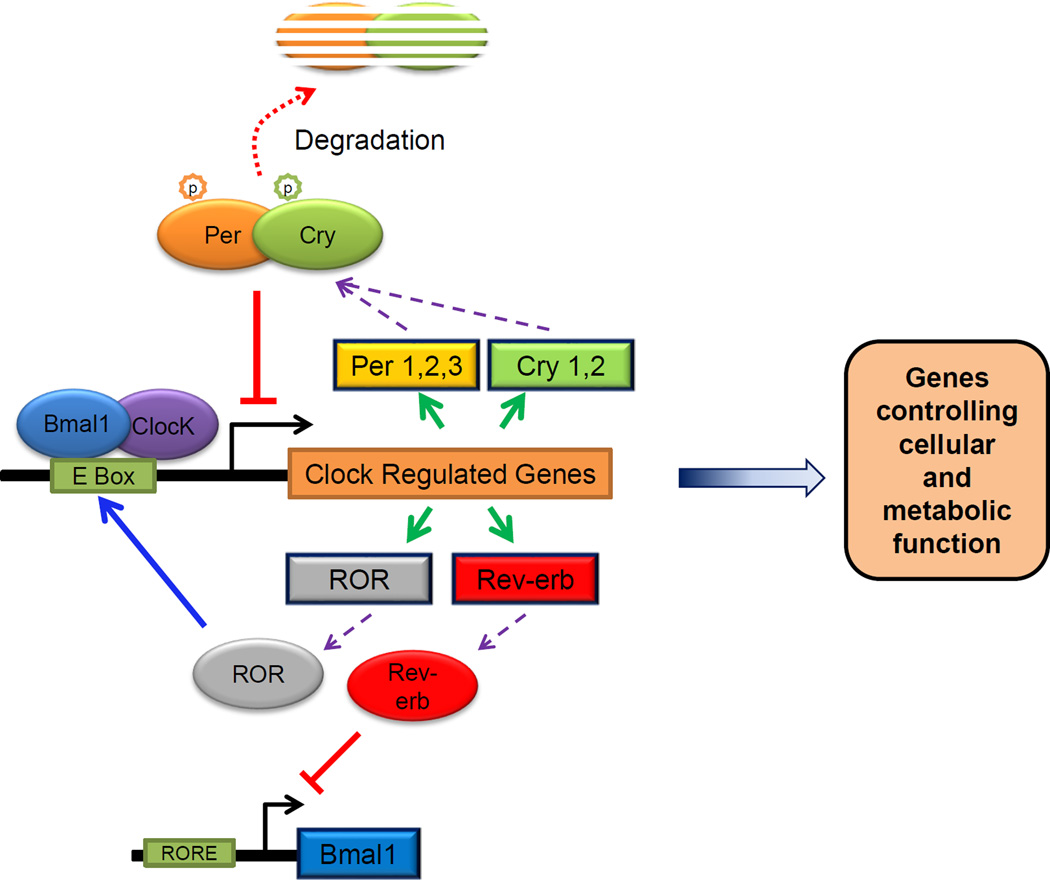
Circadian rhythms are driven by cell-autonomous oscillating circadian molecular clocks built upon molecular feedback loops [10, 11]. The molecular clock (Fig. 1) comprises a core loop that entails the transcription factor Bmal1 (Brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein-1 and also called as Mop3 or Arntl - Aryl hydrocarbon receptor nuclear translocator-like) and its partner Clock (or its orthologue Npas2) transactivating promoter E-box elements of Period (Per1& 2) and Cryptochrome (Cry 1&2), which in turn inhibit the transactivation by Bmal1/Clock. This feedback loop generates an oscillation of ~24 hr rhythmicity [12–18], with other loops (ROR/Rev-erbα/β) adding robustness. Bmla1/Clock transactivate many other genes, by binding to cis-acting promoter E-box elements, to regulate metabolism and many homeostatic processes including cell cycle control, DNA damage response genes, nuclear hormone receptors such as PPARα [19–22] directly or indirectly by regulating other transcription factors such as DBP, TEF and E4BP4 [23]. On the other hand, the clock proteins are themselves regulated by certain metabolic sensors, such as the NAD-dependent histone deacetylase, Sirt1 [24–26] and Sirt3 [27] and PGC-1α [28, 29]. On the other hand, the clock proteins are themselves regulated by certain metabolic sensors, such as the NAD-dependent histone deacetylase, Sirt1 [24–26] and Sirt3 [27] and PGC-1α [28, 29].
Fig. 1.
Organization of the body clocks
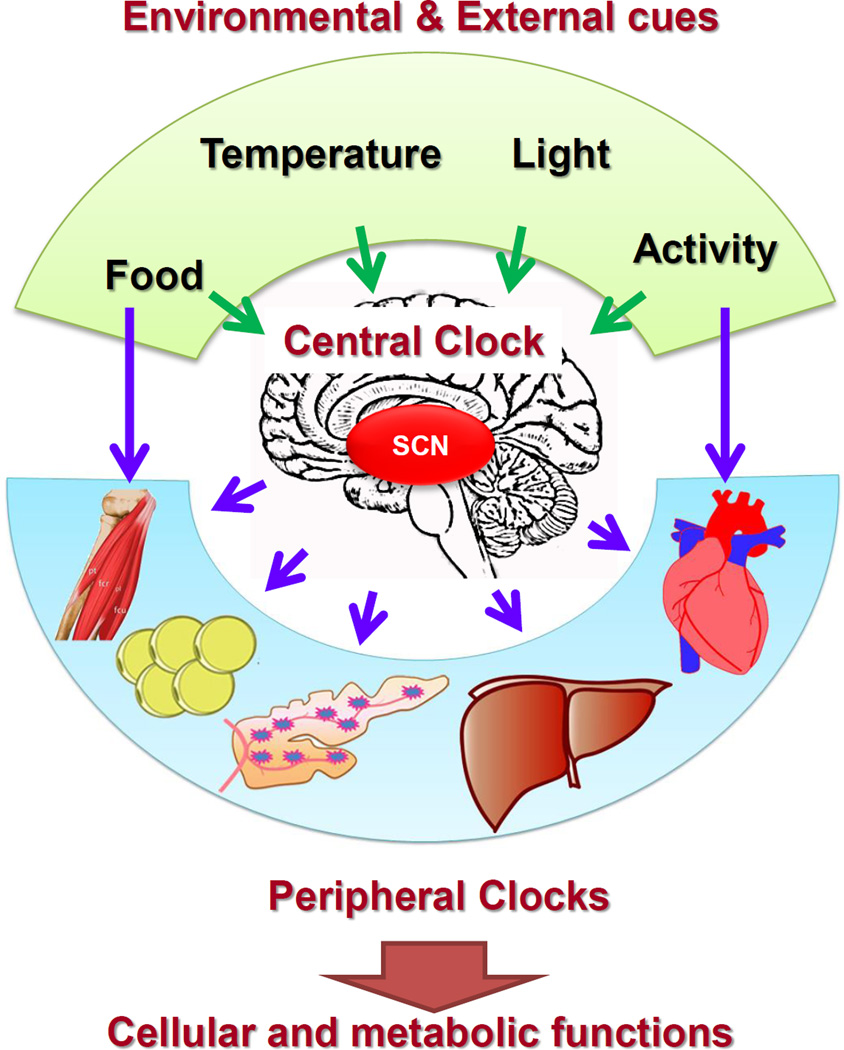
The molecular clock is present in all cell types, including pancreatic islets [30–34] and is maintained ex vivo [33] and in cultured cells [35]. Light is the primary entrainment signal to the central clock in the hypothalamic suprachiasmatic nucleus (SCN), which functions as the pacemaker [36] by synchronizing all peripheral clocks via neuro-humoral pathways [37, 38] (Fig. 2). In addition, other entrainment signals include activity, temperature and food, though food impacts peripheral clocks in metabolically active tissues to a much larger degree as an entraining signal [39–41]. The roles of central and peripheral clocks have been demonstrated by genetic (tissue-specific deletions of clock genes) and environmental (constant light, jet lag and restricted feeding protocols) interventions [42–45].
Fig. 2.
Circadian control of physiology and metabolism
~10% of all transcripts have a circadian rhythm [46, 47] that is tissue-specific, while a third of all nuclear receptors that play critical roles in metabolic homeostasis [22, 48], display circadian rhythm. Furthermore, circadian control of various metabolic pathways appears to be most apparent on rate-limiting steps [46], compelling evidence that it is required for normal homeostasis. Interestingly, metabolic sensors, such as Sirt1 [24–26], AMPK [49] and PGC-1α [28, 29] feed back to the core clock. Finally, targeted disruptions of clock genes result in striking metabolic disturbances (Table 1).
Table 1.
Genetic core clock gene disruptions with impairment of glucose homeostasis
| Gene Disrupted | Metabolic Phenotype | Ref |
|---|---|---|
| Bmal1 (Global) | Impaired gluconeogenesis, adipocyte differentiation, hyperlipidemia, glucose intolerance | [34,42,60,117] |
| Bmal1 (Liver specific) | Fasting hypoglycemia, Impaired gluconeogenesis | [42] |
| Bmal1 (Pancreas using Pdx-1 Cre) | Hyperglycemia, hypoinsulinemia, glucose intolerance | [32–34] |
| Bmal1 (β-cell specific using rip-Cre) | Hyperglycemia, hypoinsulinemia, glucose intolerance | [64] |
| Clock | Hypertriglyceridemia, hypercholesterolemia, hyperglycemia, hyperleptinemia | [33,118] |
| Per1&2 | Glucose intolerance, altered lipid metabolism | [42,119,120] |
| Cry1&2 | Glucose intolerance | [121] |
Circadian disruption and diabetes
Disruption of circadian rhythm, the daily oscillations in the physiology and behavior of organisms has been associated with metabolic disorders [4, 50, 51]. Bmal1, a core clock gene, has been associated with type 2 diabetes (T2D) and hypertension [52], while genome-wide association studies have implicated MTNR1b, a circadian rhythm related gene, in T2D and impaired β-cell function [6–8]. Circadian misalignment, as occurs in over 8.6 million Americans shift workers [53], is associated with obesity, metabolic syndrome and increased mortality [4, 5, 50, 54, 55] and recent data reveal that there is an excess risk of up to 60% of T2D with rotating shift work [56, 57] and this risk is independent of associated obesity and appears cumulative with increasing duration of shift work. There are lot of epidemiological studies from shift workers that indicates that circadian misalignment profoundly affects glucose and metabolic homeostasis, with a higher prevalence of metabolic syndrome, obesity and type 2 diabetes. In addition, there appears to be an increased risk for diabetic patients to worsening of their diabetes control with increasing duration of shift work. Recent studies using healthy young and aged volunteers subjected to various combinations of circadian misalignment and sleep deprivation revealed that sleep deprivation combined with 3 weeks of circadian misalignment led to significant glucose intolerance with a relatively lesser rise in insulin secretion [55, 58]. (Callout #1)
Bmal1−/− mice, a unique model of circadian disruption
The non-redundant role of Bmal1 in molecular clock function has been demonstrated by arrhythmicity of the free-running body clock, without light entrainment, in mice with a global deletion of Bmal1 [13].These mice have metabolic disruptions [32, 33, 42, 59–61] and a premature aging phenotype [62, 63], making it difficult to tease out the tissue specific role of Bmal1. These mice display significant impairments in glucose homeostasis and significant hypoglycemia on fasting secondary to impairments in gluconeogenesis in the liver. However in the fed state they display glucose intolerance despite no significant insulin resistance. This is due to impairment in glucose stimulated insulin secretion in vivo. Isolated islet studies from these mice display impairment in ex vivo glucose stimulated insulin secretion. Since many of these mice become very sick and die by 7–8 months of age, it was necessary to specifically address whether this impairment in β-cell function in the global Bmal1 knockout mice was secondary to the critical function of the molecular clock and Bmal1 in β-cells.
Cell-autonomous Bmal1 function is required for normal β-cell function
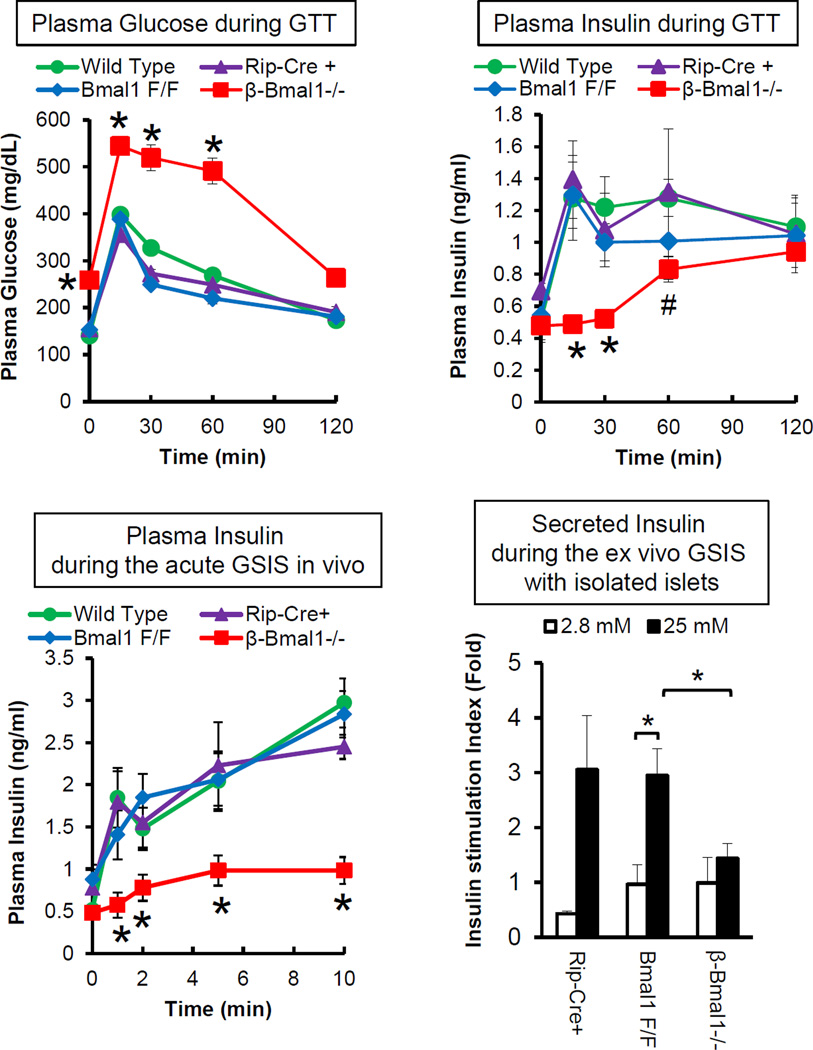
We and others have shown that deletion of Bmal1 leads to progressive glucose intolerance, hypoinsulinemia and diabetes [32, 33, 42, 60]. We demonstrated that this is a consequence of impaired β-cell function with impaired GSIS in vivo and in isolated islets, but not with depolarizing secretagogues such as KCl, suggesting that Bmal1 is required for glucose-induced stimulus-secretion coupling [32]. This was not secondary to insulin resistance[32]. We demonstrated this in knockdown experiments [32], suggesting a cell-autonomous function of Bmal1 in β-cells. In vivo confirmation of the cell-autonomous role of Bmal1 in β-cell function came from three independent labs reporting the phenotype of mice in which Bmal1 was deleted in a tissue specific manner, using Bmal1 floxed mice which had the DNA binding domain flanked by LoxP sites, in the whole pancreas (Bmal1 floxed mice crossed with Pdx1-Cre transgenic mice) [33, 34] or only in the β-cells of the islet (Bmal1 floxed mice crossed with Rip-Cre transgenic mice)[64]. Though both of these transgenes under Pdx1 and Rip promoters are expressed widely in the brain, they were shown not to disrupt Bmal1 in the SCN excluding confounding results from central clock disruption. Both these models displayed diabetes, significant impairment in glucose stimulated insulin secretion (GSIS) due to β-cell dysfunction (Fig. 3). Further in vivo experiments in these mice with a disrupted clock, in β-cells and in vitro using genetic knockdown in insulinoma cells revealed that deletion of Bmal1 was sufficient to impair GSIS in β-cells [32, 64].
Fig. 3.
Circadian regulation of mitochondrial OXPHOS in β-cells
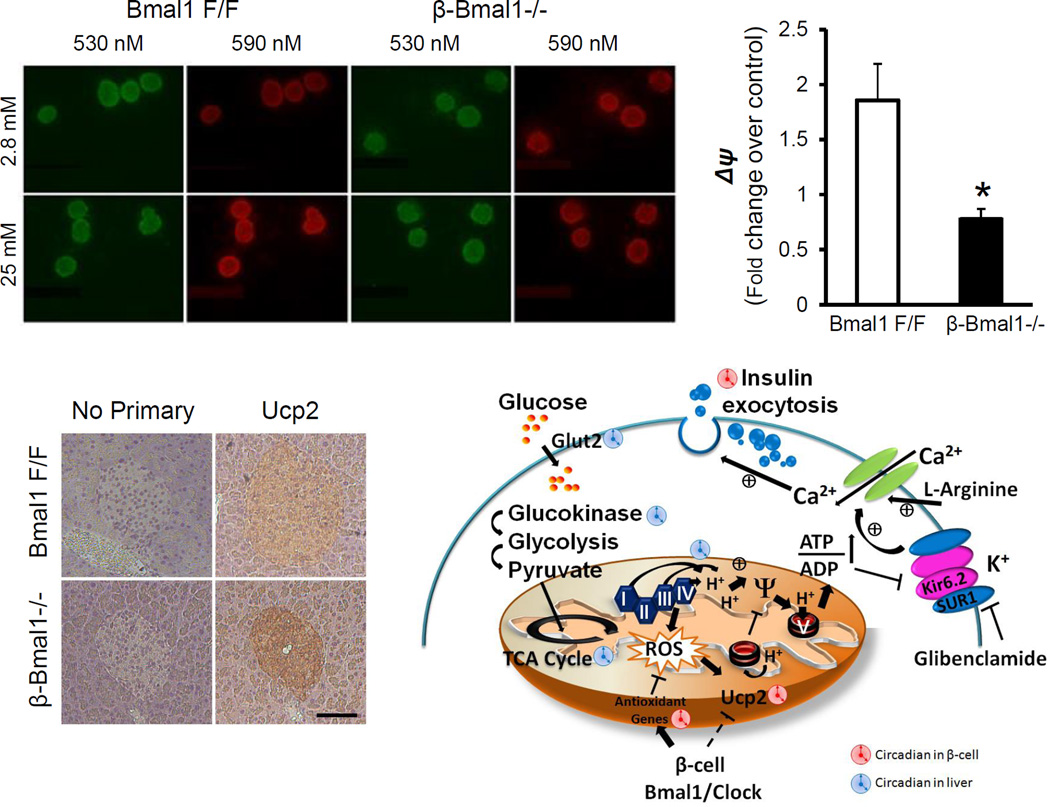
Mitochondrial metabolism is critical for GSIS in β-cells [65, 66]. Circadian control of β-cell mitochondrial metabolism has not been studied though there is evidence that the circadian clock regulates mitochondrial metabolism in heart [67] and skeletal muscle [68]. Our previous studies [32, 64] suggest that Bmal1 is required for GSIS and that this was shown to be a result of impairment in mitochondrial OXPHOS, as shown by a decrease in glucose induced hyperpolarization of the inner mitochondrial membrane (assessed by the JC-1 assay). In this assay, addition of the dye JC-1 to β-cells leads to a green cytoplasmic fluorescence from monomers of the dye in the cytosol. On glucose stimulation, there is increased metabolism of glucose through the TCA cycle and subsequent increase in potential gradient across the inner mitochondrial membrane. This hyperpolarization leads to the import of the JC-1 dye into the mitochondria, wherein it polymerizes and emits red fluorescence. This ratio of red/green fluorescence is a measure of the glucose-induced changes in potential gradient across the inner mitochondrial membrane. As shown in Fig. 4, disruption of the β-cell clock in β-cell specific Bmal1 knockout mouse islets leads to impairment in hyperpolarization of the mitochondria on glucose stimulation. This results in a reduction in the glucose-induced ATP/ADP ratio, the critical signal to the ATP responsive KATP channels and subsequent insulin granule exocytosis. Other experiments have also implicated changes in vesicular trafficking and exocytosis related genes in beta cells in clock disrupted islets [33] that may be contributory factors. The molecular clock regulation of NAD+ bioavailability has been shown in hepatocytes to regulate mitochondrial metabolism [27] via circadian regulation of intracellular Nampt-mediated NAD+ generation that activates Sirt3 and activating mitochondrial metabolism by deacetylation of key proteins. Whether a similar mechanism of circadian regulation of mitochondrial metabolism exists in β–cells needs to be determined, given the reportedly low levels of intracellular Nampt in β-cells [69]. However, non-cell autonomous regulation by systemic nicotinamide mononucleotide (NMN) a precursor of NAD+, may be still involved in β-cell mitochondrial metabolism. (Callout #2)
Fig. 4.
Circadian disruption and Uncoupling in β-cells
Ucp2 has been implicated in β-cell dysfunction [70] that is associated with glucolipotoxicity [71] and its overexpression leads to impaired GSIS [72, 73], while its knockdown or deletion leads to improvement in GSIS [74, 75]. In addition, polymorphisms in Ucp2 that increase its expression have been strongly associated with T2DM especially in patients with BMI>30 [76]. Despite such convincing data, there are still uncertainties as many factors that still remain unknown with respect to the physiological function of Ucp2 [70] that under normal circumstances appears to be expressed at a very low level and is increased only in pathological states associated with impaired GSIS. We have shown that Ucp2 is upregulated in Bmal1−/− islets and contributes to impaired GSIS [32, 64]. Indeed, the use of Genepin, a specific Ucp2 inhibitor, rescues the impaired GSIS in Bmal1-deficinet islets. This rise in Ucp2 appears to be secondary to the ROS increase seen in Bmal1-deficient islets, though other mechanisms may exist for this upregulation in circadian clock disrupted islets, since many known regulators of Ucp2 in β-cells, such as NAD+-dependent Sirt1[77–79], Srebp1c [80] and PGC-1α [81] are also regulated by the circadian clock in other tissues.
Aging, Diet-induced obesity (DIO) and β-cell function
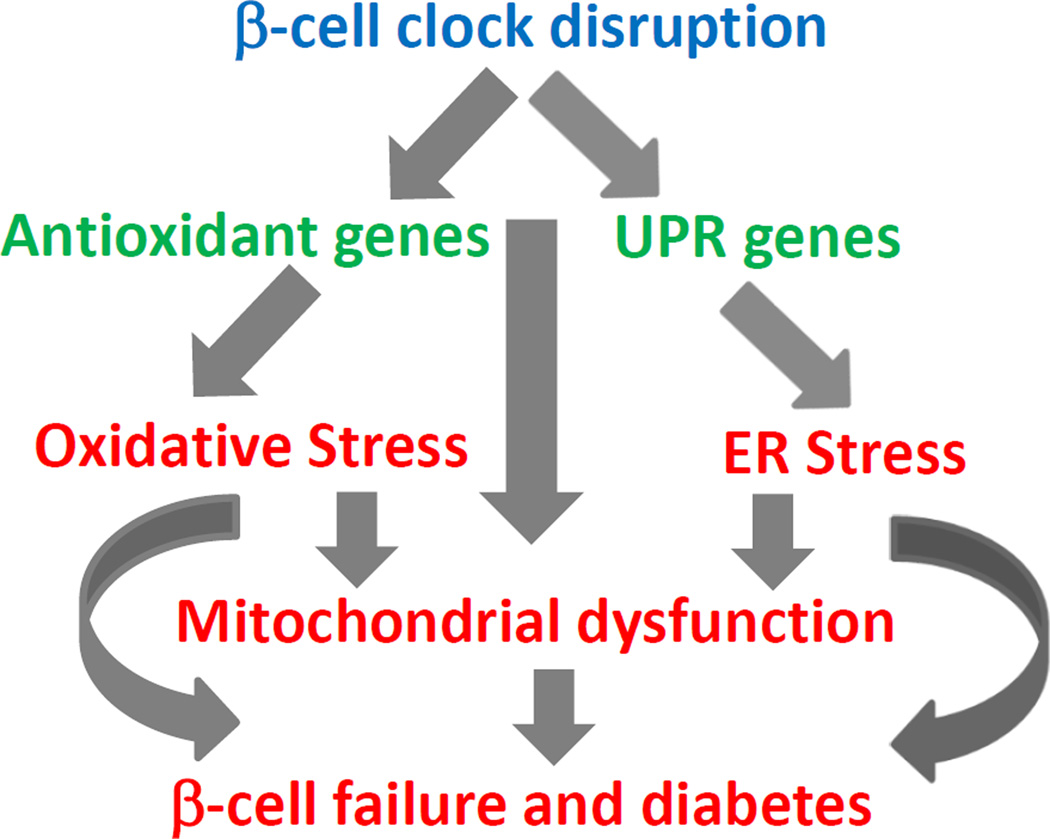
Metabolic demand placed by increasing need for insulin secretion, with insulin resistance associated with aging and DIO, two common risk factors for T2D in humans, leads to compensatory islet hypertrophy and enhanced insulin secretion. When this stress, including glucolipotoxicity, oxidative and ER stress, is very severe or chronic, it leads to activation of stress pathways including oxidative and ER stress, eventually resulting in β-cell dysfunction, apoptosis and failure [82–85]. While circadian disruption also leads to an increased risk of oxidative and ER stress, it appears that there might be an additive effect when these risk factors are put together suggesting that this may be one of the underlying causes of the increased susceptibility of oxidative and ER stress induced β-cell failure in aged and obese individuals with circadian misalignment.
Oxidative stress, Islets and circadian clock
Oxidative metabolism is one of the major contributors of reactive oxygen species (ROS) and mechanisms to mitigate this have evolved concurrently. There are many mechanisms that have evolved to protect the cell from ROS, including the antioxidant enzymes (glutathione peroxidase, catalase, superoxide dismutase) and free radical non-glutathione systems such as the thioredoxin and peroxyredoxin systems among others. ROS fluctuates with the circadian regulated food intake and oxidative metabolism. Hence, it follows that having circadian-regulated ROS scavenging systems would provide survival advantage. β-cells have very low threshold for oxidative stress due to a low expression of antioxidant genes. As compared to other metabolically active tissues, such as the liver, the islets have only 15–38% of the ROS scavenging ability [84–87]. This puts them at risk, to the damage caused by excessive ROS, which in addition is a strong inducer of Ucp2. Indeed this has been hypothesized to be one of the important underlying causes of β-cell failure in many forms of T2D [71]. Regulation of oxidative stress by the circadian clock and Bmal1 has been proposed in the context of the premature aging phenotype in global Bmal1−/− [62, 88, 89].
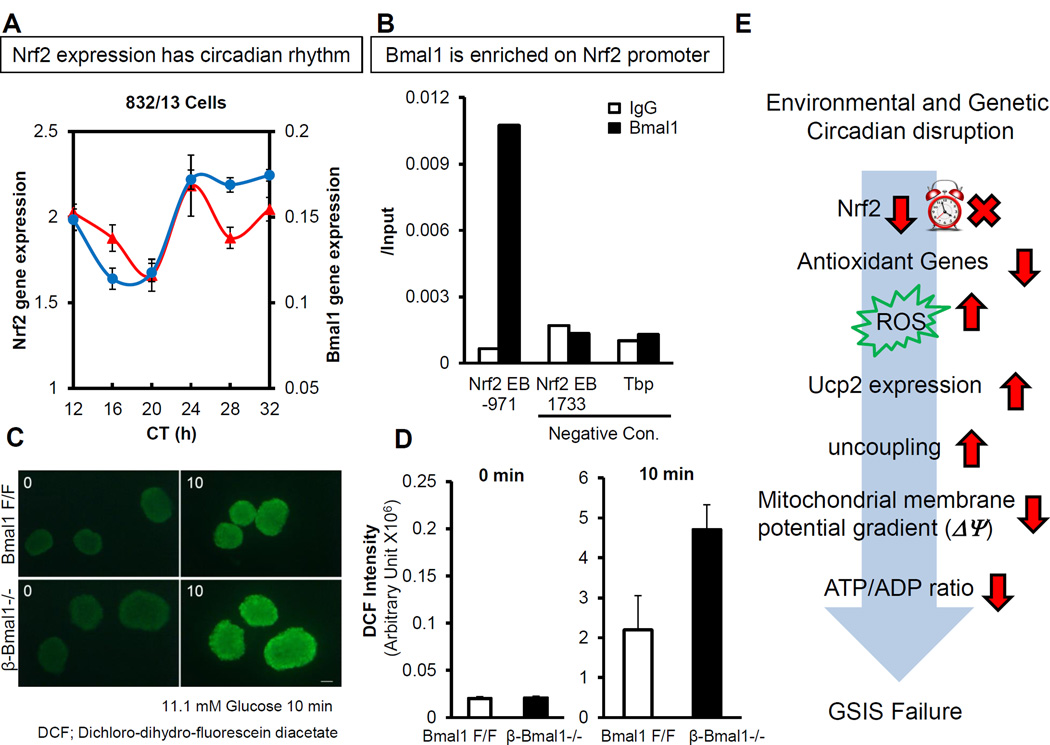
Many of the key ROS scavenging enzyme systems and the rate limiting steps are transcriptionally regulated by Nfe2l2, a leucine zipper transcription factor also commonly referred to as Nrf2, by binding to the Antioxidant Response Elements (ARE) in their promoter regions. Nrf2 is normally inactive and bound to Keap1 in the cytoplasm and on activation by oxidative stress is released to translocate to the nucleus to bind to the ARE to activate the transcription of key antioxidant enzymes. Interestingly, Nrf2 exhibits a peak in the late light/early dark phase in β-cells [64]. Bmal1 also has been shown to bind to the E-box element in the Nrf2 promoter suggesting a direct regulation by the molecular clock. Indeed, the circadian expression of Nrf2 is lost in Bmal1−/− β-cells [64] (Fig. 5). Similar circadian regulation of Nrf2 and the antioxidant system has been demonstrated in the neuronal system [90]. In addition, Bmal1 also directly regulates peroxyredoxin regeneration-related genes, sestrin2 and Prdx3 in β-cells. Interestingly, a recent study demonstrated that Prdx3 is important in preventing oxidative stress-induced apoptosis in β-cells [91]. Furthermore, we demonstrate that sestrin2 is a direct target of Bmal1 in regulating antioxidant defenses in the β-cell, while a recent report identified a direct regulation of sestrin2 by Nrf2 via ARE [92]. Bmal1 and circadian clock have been postulated to regulate oxidative stress in other tissues, including kidney, heart and spleen [62, 88] and recent studies have shown that there is significant increase in ROS in Bmal1−/− mice that can, in part, be rescued by antioxidant n-acetyl cystine (NAC) [88]. In keeping with this, NAC also rescues the impaired GSIS in Bmal1−/− islets confirming the primacy of dysregulated antioxidant system in circadian disrupted β-cells [64].
Fig. 5.
UPR (Unfolded Protein Response) is necessary for the β-cell to adapt to ER stress
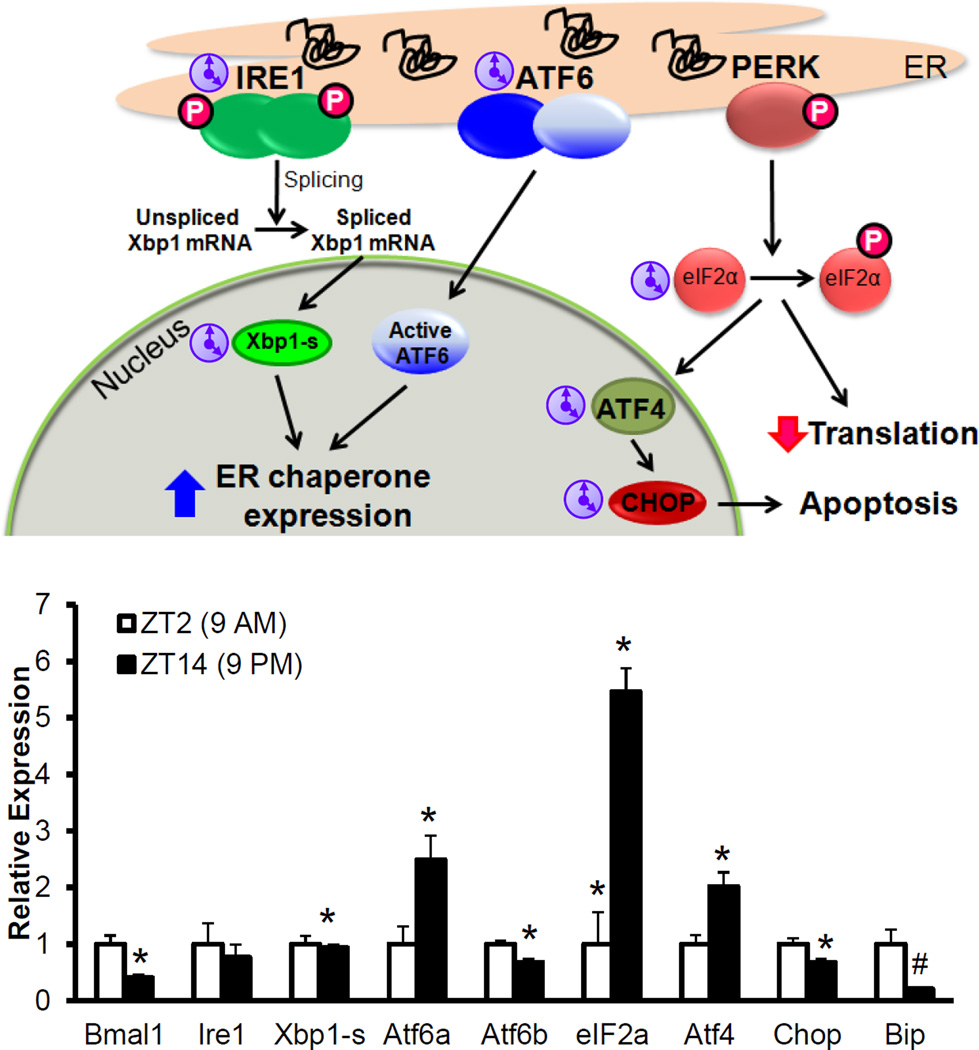
β-cells up regulate proinsulin expression, on exposure to high glucose and post-absorptive state, putting enormous load, up to a million proinsulin molecules per minute, on the ER [82]. Any increase in un-folded proteins triggers the UPR pathway to resolve ER stress by (a) increasing ER folding capacity via chaperone expression (b) reducing ER load by decreasing protein translation and increasing ER associated degradation (ERAD) (c) inducing apoptosis if the ER stress is unresolved. The UPR consists primarily of three trans-membrane sensors, PERK, IRE1-α and ATF6 that are activated by unfolded proteins in the ER (Fig. 6) [83]. PERK, phosphorylates eIF2α, to inhibit translation. Activated IRE1α splices and activates XBP1, a transcription factor that acts in conjunction with ATF6 to increase expression of ER chaperones and CHOP, which by inhibiting the expression of the anti-apoptotic gene Bcl2, induces Bax-induced apoptosis. IRE1α also activates JNK phosphorylation to induce apoptosis. All of these play a determinant role in β-cells with PERK deficiency leading to diabetes in mice [93, 94] and humans (Wolcott-Rallison syndrome [95, 96]) and β-cell specific XBP1 deletion resulting in diabetes [97]. In contrast, CHOP deletion protects β-cells from dysfunction [98]. Deficiency of ER chaperones (p58 [99], Wfs1-the gene associated with Wolfram syndrome [100–102]) increases ER stress, resulting in β-cell death and diabetes. The Akita mouse (mutation in Ins2 with proinsulin mis-folding) has ER stress associated β-cell death and diabetes [103].
Fig. 6.
Circadian clock and ER stress
UPR is circadian regulated, in the liver, with a 12 hour cycle of activation of IRE1α, which if disrupted leads to alterations in hepatic lipid handling [104]. Pinealectomy-induced absence of melatonin also leads to nocturnal hepatic ER stress and insulin resistance [105]. IRE1α with its endo-ribonucleosidase activity has been shown to cause Per1 mRNA decay, disruption of the molecular clock in the context of carcinogenesis [106]. Another study showed both acute and chronic sleep deprivation in mice led to ER stress, more in aged mice, in whole pancreas. However, whether this regulation is operative specifically in β-cells is unknown. Interestingly, a circadian disruption, by phase advancement, accelerated β-cell loss [107] in a diabetes-prone human islet amyloid polypeptide transgenic (HIP) rats that has been shown to have ER stress. This also supports the notion that circadian disruption may worsen unresolved ER stress leading to β-cell apoptosis. Our own observations suggest that the UPR is regulated by the circadian clock with circadian daily rhythms seen in many of the UPR related genes (Fig. 6). In addition, in both Bmal1-deficient and Rev-erbα-deficient mouse islets there is increased markers of ER stress, suggesting that a functional intrinsic molecular clock is required for normal UPR and beta cell homeostasis. (Callout #3)
β-cell stress response pathway signaling
Most metabolic stress in β-cells is accompanied by significant oxidative and ER stress [71, 82, 84, 85, 108–112].This is schematically represented in Fig. 7. Some of the downstream effectors of these pathways that result in β-cell dysfunction and apoptosis are the SAPK/Jnk and p38 MAPK pathways that activate other transcription factors such as AP-1 family, ATF-2, Foxo1 etc. [111, 112]. Though these MAPKs phosphorylation has been studied in the context of β-cell stress, whether they are directly regulated by the circadian clock is unknown.
Fig. 7.
Translational implications
There is mounting evidence that circadian regulation of cellular processes in β-cells is critical for glucose homeostasis and a disruption of this will contribute to β-cell failure and diabetes. Circadian oscillations have been shown in human islets [113, 114] and prospective and cross-sectional studies demonstrate a strong correlation with disturbed glucose homeostasis with circadian misalignment. A better understanding of the time course of the metabolic disruptions with circadian disruption is needed to see if workplace interventions such as those to avoid rapid shifts in light/dark cycles may be of some benefit along with possible benefit of antioxidants to prevent and mitigate circadian disruption induced oxidative stress. It is encouraging to see that that in healthy human volunteers subjected to short-term circadian misalignment and sleep deprivation induced glucose intolerance, there was a full recovery to metabolic normalcy after 9 days of recovery [58]. Whether this is reproducible in shift-workers at large in prospective studies would be an important question to answer.
There have been some recent studies that raise the possibility that with a better understanding of the molecular pathways of circadian regulation, it may be possible pharmacologically to prevent the deleterious consequences of circadian disruption. A recent study has shown that slat inducible kinase 1 (Sik1) and CREB-regulated transcription coactivator 1(CRTC1) have a key role in clock resetting. Sik1 phosphorylates CRTC1 and limits its binding to CREB in the nucleus and thus delays resetting to a new light phase. Blocking this action of Sik1 led to a rapid re-entrainment after experimental jet lag [115]. In another study, deletion of vasopressin receptors V1a and V1b [116] in mice made them resistant to jet lag, thus raising the possibility that modulation of these receptors may allow pharmacological treatment to mitigate circadian disruption and its consequent deleterious metabolic effects. Further studies of the metabolic benefits of these approaches need to be conducted both in animal models and humans to better address the urgent need to prevent and treat the consequences of circadian disruption.
Circadian disruption is an unavoidable consequence of modern day life style and is an occupational consequence of shift work. As detailed above, there is strong emerging data that these disruptions are sufficient to impair β-cell function raising the possibility that circadian disruption could be contributing to the increasing incidence of diabetes. However, avoiding circadian disruption is often not practical. Hence there is an urgent need for further studies to come up with measures that prevent or mitigate the consequences of circadian disruption.
Conclusion
Many pressing questions still remain to be explored. A more comprehensive understanding of the pathways that regulate the metabolic, stress adaptive and survival functions of the β-cells and other cell types in the islet need to be understood. In addition, measures to prevent, treat and reverse the adverse metabolic consequences need to be tested and instituted in high-risk populations, based on sound understanding of underlying the molecular regulatory pathways. Though, understanding circadian control of β-cell function is still in its infancy, the interesting and complementary work being done in many labs offers optimism to deal with this problem and to prevent or mitigate the consequences of circadian disruption.
Callouts.
Circadian disruption, whether environmental or genetic, leads to impaired mitochondrial function in β-cells and blunted glucose stimulated insulin secretion. This β-cell dysfunction leads to diabetes.
Cell autonomous function of Bmal1 and β-cell clock are required for normal β-cell function. Disruption of Bmal1 selectively in β-cells is sufficient to cause diabetes.
Bmal1 and β-cell clock are required for adaptation to β-cell stress. They are required for normal anti-oxidant response and unfolded protein response (UPR). Disruption of this circadian control leads to oxidative stress, ER stress and impaired β-cell function.
Acknowledgements
The work was supported by grants to VKY from NIH: R01DK097160; R56 DK089061-01; P&F grant (DRC-P30DK079638); American Diabetes Association: 7-12-BS-210. The work was also supported by grants to KM from American Diabetes Association (1-13-BS-118).
We also thank the Mouse Metabolism Core of the Diabetes Research Center at Baylor college of Medicine (DRC-P30DK079638).
Footnotes
There are no potential conflicts of interest.
Reference List
- 1.Bell-Pedersen D, Cassone VM, Earnest DJ, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schibler U. The 2008 Pittendrigh/Aschoff lecture: peripheral phase coordination in the mammalian circadian timing system. J Biol Rhythms. 2009;24:3–15. doi: 10.1177/0748730408329383. [DOI] [PubMed] [Google Scholar]
- 3.De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–854. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 5.Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165:175–183. doi: 10.1093/aje/kwj355. [DOI] [PubMed] [Google Scholar]
- 6.Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prokopenko I, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronn T, Wen J, Yang Z, et al. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia. 2009;52:830–833. doi: 10.1007/s00125-009-1297-8. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 11.Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Malgrange B. Cell "circadian" cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8:832–837. doi: 10.4161/cc.8.6.7869. [DOI] [PubMed] [Google Scholar]
- 12.King DP, Zhao Y, Sangoram AM, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitaterna MH, Selby CP, Todo T, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Horst GT, Muijtjens M, Kobayashi K, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 16.Okamura H, Miyake S, Sumi Y, et al. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science. 1999;286:2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 17.Vitaterna MH, King DP, Chang AM, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canaple L, Rambaud J, Dkhissi-Benyahya O, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20:1715–1727. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- 20.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J. 2005;386:575–581. doi: 10.1042/BJ20041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue I, Shinoda Y, Ikeda M, et al. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J Atheroscler Thromb. 2005;12:169–174. doi: 10.5551/jat.12.169. [DOI] [PubMed] [Google Scholar]
- 22.Teboul M, Guillaumond F, Grechez-Cassiau A, Delaunay F. The nuclear hormone receptor family round the clock. Mol Endocrinol. 2008;22:2573–2582. doi: 10.1210/me.2007-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowell IG. E4BP4/NFIL3, a PAR-related bZIP factor with many roles. Bioessays. 2002;24:1023–1029. doi: 10.1002/bies.10176. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama J, Sahar S, Grimaldi B, et al. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 25.Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Peek CB, Affinati AH, Ramsey KM, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 29.Grimaldi B, Sassone-Corsi P. Circadian rhythms: metabolic clockwork. Nature. 2007;447:386–387. doi: 10.1038/447386a. [DOI] [PubMed] [Google Scholar]
- 30.laman-Pillet N, Roduit R, Oberson A, et al. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226:59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Muhlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–96. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Kim MS, Li R, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets. 2011;3:381–388. doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadacca LA, Lamia KA, Delemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 36.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120:2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Minh N, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preitner N, Brown S, Ripperger J, Le-Minh N, Damiola F, Schibler U. Orphan nuclear receptors, molecular clockwork, and the entrainment of peripheral oscillators. Novartis Found Symp. 2003;253:89–99. [PubMed] [Google Scholar]
- 42.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDearmon EL, Patel KN, Ko CH, et al. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science. 2006;314:1304–1308. doi: 10.1126/science.1132430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. doi: 10.2337/db12-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 47.Storch KF, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 48.Yang X, Downes M, Yu RT, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 49.Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller JE, Ludmer PL, Willich SN, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 52.Woon PY, Kaisaki PJ, Braganca J, et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci U S A. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bureau of Labor Statistics. Workers on Flexible and Shift Schedules in 2004 Summary. Washington DC: United States Department of Labor; 2005. [Google Scholar]
- 54.Hermansson J, Gillander GK, Karlsson B, Lindahl B, Stegmayr B, Knutsson A. Ischemic stroke and shift work. Scand J Work Environ Health. 2007;33:435–439. doi: 10.5271/sjweh.1165. [DOI] [PubMed] [Google Scholar]
- 55.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kivimaki M, Batty GD, Hublin C. Shift work as a risk factor for future type 2 diabetes: evidence, mechanisms, implications, and future research directions. PLoS Med. 2011;8:e1001138. doi: 10.1371/journal.pmed.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez JD, Hansen A, Ord T, et al. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudic RD, McNamara P, Curtis M, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimba S, Ogawa T, Hitosugi S, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6:e25231. doi: 10.1371/journal.pone.0025231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, Yang Z, Niu Z, et al. The mortality of MOP3 deficient mice with a systemic functional failure. J Biomed Sci. 2006;13:845–851. doi: 10.1007/s11373-006-9108-4. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Moulik M, Fang Z, et al. Bmal1 and beta-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Mol Cell Biol. 2013;33:2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva JP, Kohler M, Graff C, et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- 66.Maassen JA, 't Hart LM, Van Essen E, et al. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53(Suppl 1):S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 67.Bray MS, Shaw CA, Moore MW, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 68.Andrews JL, Zhang X, McCarthy JJ, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A. 2010;107:19090–19095. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Revollo JR, Korner A, Mills KF, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan CB, Kashemsant N. Regulation of insulin secretion by uncoupling protein. Biochem Soc Trans. 2006;34:802–805. doi: 10.1042/BST0340802. [DOI] [PubMed] [Google Scholar]
- 71.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–1486. doi: 10.2337/diabetes.48.7.1482. [DOI] [PubMed] [Google Scholar]
- 73.Chan CB, De Leo D, Joseph JW, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–1310. doi: 10.2337/diabetes.50.6.1302. [DOI] [PubMed] [Google Scholar]
- 74.Joseph JW, Koshkin V, Zhang CY, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–3219. doi: 10.2337/diabetes.51.11.3211. [DOI] [PubMed] [Google Scholar]
- 75.Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 76.Gable DR, Stephens JW, Cooper JA, Miller GJ, Humphries SE. Variation in the UCP2-UCP3 gene cluster predicts the development of type 2 diabetes in healthy middle-aged men. Diabetes. 2006;55:1504–1511. doi: 10.2337/db05-1645. [DOI] [PubMed] [Google Scholar]
- 77.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moynihan KA, Grimm AA, Plueger MM, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Medvedev AV, Robidoux J, Bai X, et al. Regulation of the uncoupling protein-2 gene in INS-1 beta-cells by oleic acid. J Biol Chem. 2002;277:42639–42644. doi: 10.1074/jbc.M208645200. [DOI] [PubMed] [Google Scholar]
- 81.De Souza CT, Gasparetti AL, Pereira-da-Silva M, et al. Peroxisome proliferator-activated receptor gamma coactivator-1-dependent uncoupling protein-2 expression in pancreatic islets of rats: a novel pathway for neural control of insulin secretion. Diabetologia. 2003;46:1522–1531. doi: 10.1007/s00125-003-1222-5. [DOI] [PubMed] [Google Scholar]
- 82.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 84.Robertson RP. Oxidative stress and impaired insulin secretion in type 2 diabetes. Curr Opin Pharmacol. 2006;6:615–619. doi: 10.1016/j.coph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Kaneto H, Kawamori D, Matsuoka TA, Kajimoto Y, Yamasaki Y. Oxidative stress and pancreatic beta-cell dysfunction. Am J Ther. 2005;12:529–533. doi: 10.1097/01.mjt.0000178773.31525.c2. [DOI] [PubMed] [Google Scholar]
- 86.Acharya JD, Ghaskadbi SS. Islets and their antioxidant defense. Islets. 2010;2:225–235. doi: 10.4161/isl.2.4.12219. [DOI] [PubMed] [Google Scholar]
- 87.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 88.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany NY) 2009;1:979–987. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kondratov RV, Antoch MP. The clock proteins, aging, and tumorigenesis. Cold Spring Harb Symp Quant Biol. 2007;72:477–482. doi: 10.1101/sqb.2007.72.050. [DOI] [PubMed] [Google Scholar]
- 90.Musiek ES, Lim MM, Yang G, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolf G, Aumann N, Michalska M, et al. Peroxiredoxin III protects pancreatic ss cells from apoptosis. J Endocrinol. 2010;207:163–175. doi: 10.1677/JOE-09-0455. [DOI] [PubMed] [Google Scholar]
- 92.Shin BY, Jin SH, Cho IJ, Ki SH. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic Biol Med. 2012;53:834–841. doi: 10.1016/j.freeradbiomed.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 93.Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 94.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 96.Senee V, Vattem KM, Delepine M, et al. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes. 2004;53:1876–1883. doi: 10.2337/diabetes.53.7.1876. [DOI] [PubMed] [Google Scholar]
- 97.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108:8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ladiges WC, Knoblaugh SE, Morton JF, et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- 100.Ishihara H, Takeda S, Tamura A, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet. 2004;13:1159–1170. doi: 10.1093/hmg/ddh125. [DOI] [PubMed] [Google Scholar]
- 101.Yamada T, Ishihara H, Tamura A, et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum Mol Genet. 2006;15:1600–1609. doi: 10.1093/hmg/ddl081. [DOI] [PubMed] [Google Scholar]
- 102.Riggs AC, Bernal-Mizrachi E, Ohsugi M, et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48:2313–2321. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- 103.Wang J, Takeuchi T, Tanaka S, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cretenet G, Le Clech M, Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 105.Nogueira TC, Lellis-Santos C, Jesus DS, et al. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology. 2011;152:1253–1263. doi: 10.1210/en.2010-1088. [DOI] [PubMed] [Google Scholar]
- 106.Pluquet O, Dejeans N, Bouchecareilh M, et al. Posttranscriptional regulation of PER1 underlies the oncogenic function of IREalpha. Cancer Res. 2013;73:4732–4743. doi: 10.1158/0008-5472.CAN-12-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaneto H, Katakami N, Kawamori D, et al. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal. 2007;9:355–366. doi: 10.1089/ars.2006.1465. [DOI] [PubMed] [Google Scholar]
- 109.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177–184. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 110.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 111.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 112.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 113.Pulimeno P, Mannic T, Sage D, et al. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56:497–507. doi: 10.1007/s00125-012-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stamenkovic JA, Olsson AH, Nagorny CL, et al. Regulation of core clock genes in human islets. Metabolism. 2012;61:978–985. doi: 10.1016/j.metabol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 115.Jagannath A, Butler R, Godinho SI, et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell. 2013;154:1100–1111. doi: 10.1016/j.cell.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamaguchi Y, Suzuki T, Mizoro Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi: 10.1126/science.1238599. [DOI] [PubMed] [Google Scholar]
- 117.Shimba S, Ishii N, Ohta Y, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng B, Albrecht U, Kaasik K, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 120.Grimaldi B, Bellet MM, Katada S, et al. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ikeda H, Yong Q, Kurose T, et al. Clock gene defect disrupts light-dependency of autonomic nerve activity. Biochem Biophys Res Commun. 2007;364:457–463. doi: 10.1016/j.bbrc.2007.10.058. [DOI] [PubMed] [Google Scholar]