Abstract
The nature of the process leading to the acellular nonperfused capillaries of diabetic microangiopathy remains unknown. Because these capillaries manifest thickened basement membranes, we asked whether the process causing deposition of excess extracellular matrix in diabetes modifies cell-matrix interactions in a direction that would compromise cell renewal. In 44 individual isolates of human umbilical vein endothelial cells we observed that high glucose concentrations (30 mM) induce coordinate increases in the levels of mRNAs encoding fibronectin and the fibronectin-specific integrin receptor alpha 5 beta 1 as well as in the cognate proteins. Expression of the integrin subunit alpha 3, component of the alpha 3 beta 1 polyspecific receptor for fibronectin, laminin, and collagen, was also up-regulated by high glucose. Overexpression of integrins correlated with increased cell attachment to exogenous fibronectin and laminin as well as to complex matrix. Moreover, cells exhibited firmer steady-state adhesion to their own matrix. To correlate these in vitro observations with events in human diabetic retinopathy we measured integrin levels in retinal trypsin digests prepared from 10 patients with 8.2 +/- 1.6 (mean +/- SE) years of diabetes and 10 age- and sex-matched nondiabetic controls. Microvessels of diabetic patients showed increased immunostaining for beta 1 integrin (P = 0.025) when compared with control microvessels. These data show that high glucose and diabetes increase integrin expression and thus alter the interaction of vascular endothelial cells with their basement membranes in the direction of firmer cell-matrix adhesion. This could compromise the migration and replication critical to the reendothelialization process and contribute to microvascular occlusion.
Full text
PDF

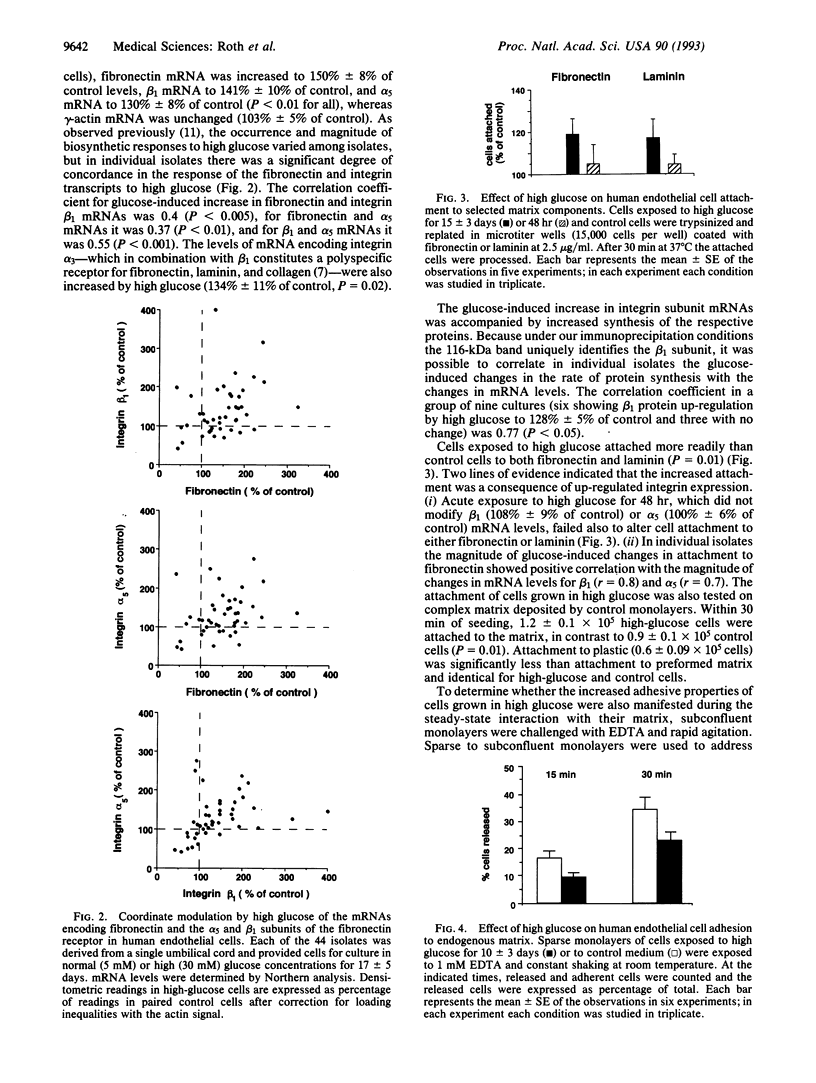

Images in this article
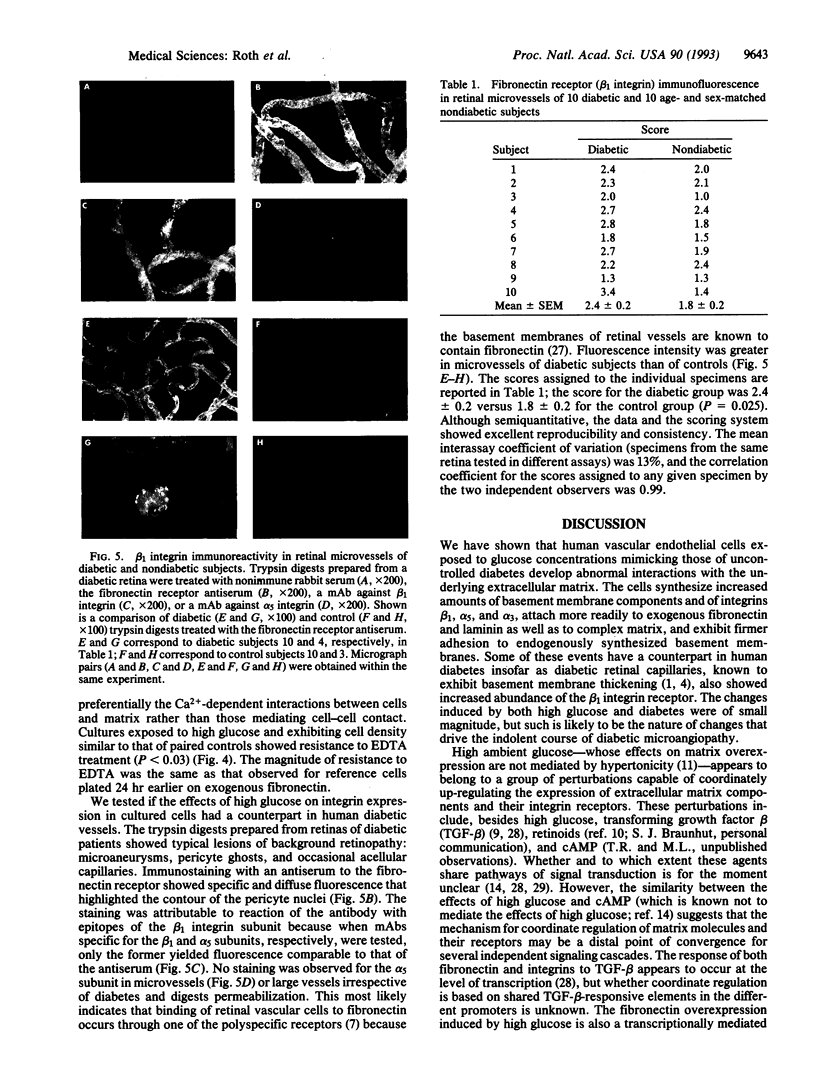
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayo S. H., Radnik R. A., Glass W. F., 2nd, Garoni J. A., Rampt E. R., Appling D. R., Kreisberg J. I. Increased extracellular matrix synthesis and mRNA in mesangial cells grown in high-glucose medium. Am J Physiol. 1991 Feb;260(2 Pt 2):F185–F191. doi: 10.1152/ajprenal.1991.260.2.F185. [DOI] [PubMed] [Google Scholar]
- Basson C. T., Knowles W. J., Bell L., Albelda S. M., Castronovo V., Liotta L. A., Madri J. A. Spatiotemporal segregation of endothelial cell integrin and nonintegrin extracellular matrix-binding proteins during adhesion events. J Cell Biol. 1990 Mar;110(3):789–801. doi: 10.1083/jcb.110.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliero E., Maiello M., Boeri D., Roy S., Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988 Aug;82(2):735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliero E., Roth T., Roy S., Lorenzi M. Characteristics and mechanisms of high-glucose-induced overexpression of basement membrane components in cultured human endothelial cells. Diabetes. 1991 Jan;40(1):102–110. doi: 10.2337/diab.40.1.102. [DOI] [PubMed] [Google Scholar]
- Cagliero E., Roth T., Roy S., Maiello M., Lorenzi M. Expression of genes related to the extracellular matrix in human endothelial cells. Differential modulation by elevated glucose concentrations, phorbol esters, and cAMP. J Biol Chem. 1991 Aug 5;266(22):14244–14250. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Argraves W. S., Suzuki S., Ruoslahti E., Pierschbacher M. D. Human osteosarcoma cells resistant to detachment by an Arg-Gly-Asp-containing peptide overproduce the fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1175–1182. doi: 10.1083/jcb.105.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerman R. L., Kern T. S. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987 Jul;36(7):808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Engerman R. L. Pathogenesis of diabetic retinopathy. Diabetes. 1989 Oct;38(10):1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Pfaffenbach D., Davis M. D. Cell turnover of capillaries. Lab Invest. 1967 Dec;17(6):738–743. [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Madri J. A., Yurchenco P. D. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. J Cell Biol. 1992 Nov;119(4):945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
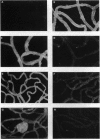
- Jerdan J. A., Glaser B. M. Retinal microvessel extracellular matrix: an immunofluorescent study. Invest Ophthalmol Vis Sci. 1986 Feb;27(2):194–203. [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Shapiro S. S., Poon J. P. Characterization of the action of retinoids on mouse fibroblast cell lines. Exp Cell Res. 1979 Mar 15;119(2):289–299. doi: 10.1016/0014-4827(79)90356-2. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960 Dec;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Li W., Shen S., Khatami M., Rockey J. H. Stimulation of retinal capillary pericyte protein and collagen synthesis in culture by high-glucose concentration. Diabetes. 1984 Aug;33(8):785–789. doi: 10.2337/diab.33.8.785. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Cagliero E. Pathobiology of endothelial and other vascular cells in diabetes mellitus. Call for data. Diabetes. 1991 Jun;40(6):653–659. doi: 10.2337/diab.40.6.653. [DOI] [PubMed] [Google Scholar]
- Lorenzi M., Cagliero E., Toledo S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes. 1985 Jul;34(7):621–627. doi: 10.2337/diab.34.7.621. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- Mascardo R. N. The effects of hyperglycemia on the directed migration of wounded endothelial cell monolayers. Metabolism. 1988 Apr;37(4):378–385. doi: 10.1016/0026-0495(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Newton L. K., Yung W. K., Pettigrew L. C., Steck P. A. Growth regulatory activities of endothelial extracellular matrix: mediation by transforming growth factor-beta. Exp Cell Res. 1990 Sep;190(1):127–132. doi: 10.1016/0014-4827(90)90153-2. [DOI] [PubMed] [Google Scholar]
- Nugent M. A., Newman M. J. Inhibition of normal rat kidney cell growth by transforming growth factor-beta is mediated by collagen. J Biol Chem. 1989 Oct 25;264(30):18060–18067. [PubMed] [Google Scholar]
- Oldberg A., Linney E., Ruoslahti E. Molecular cloning and nucleotide sequence of a cDNA clone coding for the cell attachment domain in human fibronectin. J Biol Chem. 1983 Sep 10;258(17):10193–10196. [PubMed] [Google Scholar]
- Osterby R., Gundersen H. J., Nyberg G., Aurell M. Advanced diabetic glomerulopathy. Quantitative structural characterization of nonoccluded glomeruli. Diabetes. 1987 May;36(5):612–619. doi: 10.2337/diab.36.5.612. [DOI] [PubMed] [Google Scholar]
- Paige K., Palomares M., D'Amore P. A., Braunhut S. J. Retinol-induced modification of the extracellular matrix of endothelial cells: its role in growth control. In Vitro Cell Dev Biol. 1991 Feb;27A(2):151–157. doi: 10.1007/BF02631002. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C., Ingber D. E. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Interactions of retinoids and transforming growth factor-beta in regulation of cell differentiation and proliferation. Mol Endocrinol. 1991 Jan;5(1):3–7. doi: 10.1210/mend-5-1-3. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Gipson I. K. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1829–1844. [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Symington B. E. Fibronectin receptor modulates cyclin-dependent kinase activity. J Biol Chem. 1992 Dec 25;267(36):25744–25747. [PubMed] [Google Scholar]
- Takada Y., Murphy E., Pil P., Chen C., Ginsberg M. H., Hemler M. E. Molecular cloning and expression of the cDNA for alpha 3 subunit of human alpha 3 beta 1 (VLA-3), an integrin receptor for fibronectin, laminin, and collagen. J Cell Biol. 1991 Oct;115(1):257–266. doi: 10.1083/jcb.115.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. G., Faller A. M., Burkhardt J. K., Hoffmann P. L., Kilo C., Williamson J. R. Pericyte degeneration and acellular capillaries are increased in the feet of human diabetic patients. Diabetologia. 1985 Dec;28(12):895–900. doi: 10.1007/BF00703132. [DOI] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]