Abstract
The female genital tract includes several anatomical regions whose luminal fluids successively interact with gametes and embryos and are involved in the fertilisation and development processes. The luminal fluids from the inner cervix, the uterus and the oviduct were collected along the oestrous cycle at oestrus (Day 0 of the cycle) and during the luteal phase (Day 10) from adult cyclic ewes. The proteomes were assessed by GeLC-MS/MS and quantified by spectral counting. A set of 940 proteins were identified including 291 proteins differentially present along the cycle in one or several regions. The global analysis of the fluid proteomes revealed a general pattern of endocrine regulation of the tract, with the cervix and the oviduct showing an increased differential proteins abundance mainly at oestrus while the uterus showed an increased abundance mainly during the luteal phase. The proteins more abundant at oestrus included several families such as the heat shock proteins (HSP), the mucins, the complement cascade proteins and several redox enzymes. Other proteins known for their interaction with gametes such as oviductin (OVGP), osteopontin, HSPA8, and the spermadhesin AWN were also overexpressed at oestrus. The proteins more abundant during the luteal phase were associated with the immune system such as ceruloplasmin, lactoferrin, DMBT1, or PIGR, and also with tissue remodeling such as galectin 3 binding protein, alkaline phosphatase, CD9, or fibulin. Several proteins differentially abundant between estrus and the luteal phase, such as myosin 9 and fibronectin, were also validated by immunohistochemistry. The potential roles in sperm transit and uterine receptivity of the proteins differentially regulated along the cycle in the female genital tract are discussed.
The success of fertilisation in mammals is linked to the correct migration of spermatozoa in the different compartments of the female genital tract and the adequate timing of their interaction with the female gamete. In many mammalian species including human, the deposition of semen in the vagina is followed by the sperm migration through the cervix, the uterus and then the oviduct before reaching the site of fertilisation. This sperm transit within the female tract includes mechanical and biochemical interactions with the luminal fluids leading to selection of spermatozoa able to fertilize the oocyte.
The first physiological barrier the spermatozoa will have to go through is the cervix whose vaginal side is covered by a highly viscous mucus, the cervical vaginal fluid (CVF)1. The CVF proteome was analyzed in humans in various physiological conditions (1, 2), mainly during pregnancy (3–7) or in several pathologies such as human papilloma virus (8) or candida albicans (9) infections. But few studies have investigated the CVF proteome during the cycle (10, 11). The amount of CVF increases at the time of ovulation concomitantly with a higher state of hydration and a reduced viscosity, to facilitate sperm migration (12). The mechanical properties of the mucus are essential to select spermatozoa with the highest fertilizing ability, i.e. with normal morphology and efficient mobility. The main structural components of the cervical mucus are mucins, highly glycosylated, high-molecular-weight proteins assembled into a filamentous and viscous mesh (13). The transcription of the mucin genes is increased at oestrus in the bovine cervical epithelium (14). The amount of mucins and their level of glycosylation are expected to contribute to the viscosity of the mucus (11). Therefore, the quantification of proteins such as mucins in the cervical mucus along the cycle is required to better understand the relation between the proteome and the mechanical properties of the mucus.
Attached to the cervix, the uterus is layered by an endometrial tissue which is capable of physiological remodeling in response to the oestrous cycle and the presence of the embryo (15). Therefore many studies focused on the activation of the uterus genome during pregnancy (16–19). The preimplantation phase, the window of time during which the embryo is present in the uterus but not attached to the endometrium, is critical for the success of the pregnancy. During this phase, the uterus and the embryo have a biochemical dialog leading to modifications of the uterus transcriptome aiming to support embryo development. But few studies investigated the uterus activity along the cycle, including at oestrous when the uterus is putatively interacting with spermatozoa. The expression of the transcriptome of the endometrium along the oestrous cycle was investigated in bovine (20), equine (21) and mouse (22). In human, the proteome of the uterus along the menstrual cycle was studied either on endometrium epithelium (23–26) or on uterine fluid (27–29). However, the variation of the proteome of the uterine fluid during the oestrous cycle is not known.
The oviduct is connected to the uterus through the utero-tubal junction. After having migrated through the uterus, the spermatozoa transit through this utero-tubal junction to fix in the caudal isthmus of the oviduct, the site of the sperm reservoir (30). The quantification of expression of oviduct proteins in the presence of spermatozoa has revealed the dialog between the gametes and the oviduct (31, 32). The regulation of expression of genes in the oviduct along the oestrous cycle was shown using oviduct cells from females in oestrus or luteal phase in porcine (33) and bovine species (34). Again, an extensive description of the proteome of the oviduct fluid along the oestrous cycle is lacking.
Therefore, the aims of this study are 1) to provide for the first time an integrated analysis of the luminal proteomes of the female genital tract by performing a proteomic study of inner cervical mucus, uterine fluid and oviduct fluid in the same biological model, and 2) to quantify the abundance of these luminal proteins throughout the oestrous cycle. The animal model chosen for this study was the sheep because, in this species, the migration of the spermatozoa through the female genital tract, especially in the cervical lumen and the utero-tubal junction, is subjected to a high rate of selection. Therefore, the protein components identified in the lumen of these regions of the tract are candidates for an interaction with spermatozoa during their transit. The proteomes were also assessed after an exogenous hormonal induction of estrus to investigate the endocrine control of the proteomes of each luminal fluid.
EXPERIMENTAL PROCEDURES
Chemicals
Otherwise indicated, chemicals were purchased by Sigma-Aldrich (Saint Quentin Fallavier, France).
Experimental Design
The proteome from the secretions of three segments of the female genital tract (cervix, uterus, and oviduct) was analyzed according to the stage of the oestrous cycle (oestrus versus luteal phase) and the type of cycle (spontaneous versus synchronized).
Females used in this study were adult fertile Ile-de-France ewes housed at the INRA Experimental Farm. The experiment took place during natural season of reproduction in France (December). A group of 8 ewes in spontaneous oestrus was identified within the INRA flock by detection of oestrus with a teaser ram. Among these animals, 4 ewes in estrus were slaughtered on the first day of detection of oestrus whereas the other 4 animals were slaughtered 10 days later, during the luteal phase.
Another group of 8 females were synchronized using a hormonal treatment. Females received a vaginal sponge impregnated with fluorogestone acetate (20 mg) for 14 days followed by an injection of 400 IU PMSG (Pregnant Mare Serum Gonadotropin) at the time of sponge removal. Oestrus occurred 48 h after sponge removal. Four ewes in synchronized oestrus were slaughtered on the day of estrus whereas the 4 remaining ewes were slaughtered 10 days after oestrus during the luteal phase. As such, four groups of ewes were available: spontaneous estrus, spontaneous luteal phase, synchronized estrus and synchronized luteal phase.
Collection of Genital Tract Secretions
Immediately after slaughter of the females, the genital tracts were collected and dissected. The cervix was longitudinally opened with surgical scissors and the inner cervical mucus was aspirated using a positive displacement pipette suited for viscous media (Gilson MicroMan). Each uterine horn was separated from the cervix and the oviduct, and was flushed from the utero-tubal junction to the bottom of the horn with 1 ml of PBS (phosphate buffer saline). Each oviduct was flushed from the isthmus to the ampulla with 200 μl of PBS. The fluids from each uterine horn and each oviduct were kept separate and provided biological variability within each animal. The uterine and oviduct fluids were centrifuged at 10,000 × g to remove the cellular debris and stored at −20 °C until use.
The stage of each ewe (estrus or luteal phase) was confirmed first by the presence/absence of corpora lutea or preovulatory follicles on the ovaries during fluids collection then by blood progesterone assay. The fluids from each region of the genital tract (cervix, uterus, and oviduct) were then pooled by group of ewes according to their physiological state (spontaneous estrus, synchronized estrus, spontaneous luteal phase, and synchronized luteal phase).
Sample Preparation for MS Analyses
Protein concentration was determined in pool samples using Uptima BC Assay kit (Interchim, Montluc̦on, France.) according to manufacturer's instructions and using bovine serum albumin as a standard. Each sample was migrated separately in triplicate (20 μg per lane) on a 10% SDS-PAGE (50V, 30 min). Gels were stained with Coomassie (G-250) and each lane was cut horizontally in 3 bands for quantitative proteomic analysis.
In-gel and Solution Digestion
After SDS-PAGE and cutting of the bands, each band was in-gel digested with bovine trypsin (Roche Diagnostics GmbH, Mannheim, Germany) as previously described (35). Each band was washed in water/acetonitrile (1:1) for 5 min followed by a second wash in acetonitrile for 10 min. Cysteine reduction and alkylation were performed by successive incubations in solutions of 10 mm dithiothreitol in 50 mm NH4HCO3 for 30 min at 56 °C and 55 mm iodoacetamide in 50 mm NH4HCO3 for 20 min at room temperature in the dark, respectively. Gel slices were washed by an incubation in 50 mm NH4HCO3: acetonitrile (1:1) for 10 min followed by an incubation in acetonitrile for 15 min. Proteins were digested overnight in 25 mm NH4HCO3 with 12.5 ng/μl trypsin (Sequencing Grade, Roche, Paris, France). The resulting peptides were extracted from the gel using an incubation in 0.1% formic acid, acetonitrile (1:1) for 10 min followed by an incubation for 5 min, in acetonitrile. The two collected extractions were pooled with the initial digestion supernatant, dried in a SpeedVac, reconstituted with 30 μl of 0.1% formic acid, 2% acetonitrile, and sonicated for 10 min.
To complete the data provided by the in-gel digestion, the in-solution digestion of the cervical mucus, uterus fluid, and oviduct fluid was performed. Each sample (7 μg of total proteins) was diluted in 1% Rapigest (Waters, Milford, MA) in TEAB 100 mm. Cysteine reduction and alkylation were performed by successive incubations in solutions of 10 mm TCEP for 1 h at 37 °C and 50 mm iodoacetamide for 30 min at room temperature in the dark. Proteins were digested overnight with 0.1 μg/μl trypsin (Sequencing Grade, Roche, Paris, France). The resulting peptides were incubated in 1% formic acid to precipitate the Rapigest and centrifuged during 5 min at 10,000 × g. The supernatant was collected and dried in a SpeedVac. TFA (trifluoroacetic acid) was then added at the final concentration of 1% and the peptides were desalted using Zip Tips U-C18 (Millipore, Billerica, MA). Peptides were eluted in a small volume of a solution containing organic solvent (50:50 acetonitrile: 0.1% TFA in water), dried in a SpeedVac, reconstituted with 30 μl of 0.1% formic acid, 2% acetonitrile, and sonicated for 10 min.
NanoLC-MS/MS
All experiments were performed on a dual linear ion trap Fourier transform mass spectrometer (FT-MS) LTQ Orbitrap Velos (Thermo Fisher Scientific, Bremen, Germany) coupled to an Ultimate® 3000 RSLC Ultra High Pressure Liquid Chromatographer (Dionex, Amsterdam, The Netherlands). Five microliters of each sample was loaded on trap column for desalting and separated using nano-column as previously described (35). The gradient consisted of 4–55% B for 90 min at 300 nl/min flow rate. The eluate was ionized using a Thermo Finnigan Nanospray Ion Source 1 with a SilicaTip emitter of 15 μm inner diameter (New Objective, Woburn, MA). Standard mass spectrometric conditions for all experiments were spray voltage 1.2 kV, no sheath and auxiliary gas flow; heated capillary temperature, 275 °C; predictive automatic gain control enabled, and an S-lens RF level of 60%.
Data were acquired using Xcalibur software (version 2.1; Thermo Fisher Scientific, San Jose, CA). The instrument was operated in positive data-dependent mode. Resolution in the Orbitrap was set to r = 60,000. In the scan range of m/z 300–1800, the 20 most intense peptide ions with charge states ≥2 were sequentially isolated (isolation width, 2 m/z; 1 microscan) and fragmented using collision induced dissociation. The ion selection threshold was 500 counts for MS/MS, and the maximum allowed ion accumulation times were 200 ms for full scans and 50 ms for collision induced dissociation-MS/MS in the LTQ. Target ion quantity for FT full MS was 1e6 and for MS/MS it was 1e4. The resulting fragment ions were scanned at the “normal scan rate” with q = 0.25 activation and activation time of 10 ms. Dynamic exclusion was active during 30 s with a repeat count of 1. The lock mass was enabled for accurate mass measurements. Polydimethylcyclosiloxane (m/z, 445.1200025, (Si(CH3)2O)6) ions were used for internal recalibration of the mass spectra.
Protein Identification and Data Validation
Raw data files were converted to MGF using Proteome Discoverer software (version 1.2; Thermo Fischer Scientific, San Jose, USA). Precursor mass range of 350–5000 Da and signal to noise ratio of 1.5 were the criteria used for generation of peak lists. In order to identify the proteins, the peptide and fragment masses obtained were matched automatically against the Swissprot_2013.01 database (66153 entries). MS/MS ion searches were performed using MASCOT Daemon and search engine (version 2.3; Matrix Science, London, UK). The parameters used for database searches include trypsin as a protease with allowed two missed cleavage, carbamidomethylcysteine (+57 Da), oxidation of methionine (+16) and N-terminal protein acetylation (+42) as variable modifications. The tolerance of the ions was set to 5 ppm for parent and 0.8 Da for fragment ion matches. Mascot results from the target and decoy databases were incorporated to Scaffold 3 software (version 3.6, Proteome Software, Portland, OR). Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm (36). Peptides were considered distinct if they differed in sequence. Protein identifications were accepted if they could be established at greater than 95.0% probability as specified by the Protein Prophet algorithm and contained at least two identified peptides (FDR < 1%).
Label-free Protein Quantification
Scaffold 3 Q+ software was employed (version 3.6, Proteome Software, Portland, USA) using spectral count quantitative module. All proteins with greater than two peptides identified in SwissProt database with high confidence were considered for protein quantification. To eliminate quantitative ambiguity into protein groups, we ignored all the spectra matching any peptide which is shared between proteins. Thereby, quantification from normalized spectral counts was carried out on distinct proteins. Student's t test was done to characterize changes between two samples. Statistically significant differences were considered for p < 0.05.
Gene Ontology
The Uniprot accession numbers were submitted to the UniProt Knowledgebase (UniProtKB) and the Gene Ontology data were retrieved for all the 940 identified proteins. The subcellular location data were used to generate a pie graph. A panel of 16 main biological functions was used to categorize the proteins and generate a pie graph.
Data Repository
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://www.proteomexchange.org) via the PRIDE partner repository (37) with the data set identifier PXD000299.
Western Blotting
Primary antibodies directed against the following proteins were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA): zinc α glycoprotein (dilution 1/500, sc-11358), lactoferrin (1/500, sc-52694), α-enolase (1/500, sc-15343), CD109 (1/500, sc-98793), myosin 9 (1/500, sc-98978), ceruloplasmin (1/500, sc-21240), Hsp105 (1/500, sc-1805). Primary antibodies directed against the following proteins were purchased from Abcam (Cambridge, England): gelsolin (1/1000, ab11081), valosin containing protein/VCP (1/2000, ab11433), heat shock protein (HSP) 90 β (1/1000, ab82522), heat shock protein 70 (HSPA2, 1/200, ab1428), and HSPA8 (1/2500, ab1427). Primary antibodies against angiotensin converting enzyme and acrosin were produced from our laboratory after immunization of rabbits with purified angiotensin converting enzyme and acrosin. The antibody directed against bovine oviductin (OVGP or Oviduct specific glycoprotein) was a generous gift from Dr O'Day-Bowman (Laboratory of Dr. Harold Verhage, University of Illinois) The second antibody was goat anti rabbit HRP (1/5000, A6154) for rabbit primary antibodies and goat anti mouse HRP (1/5000, A4416) for mouse primary antibodies. The chemiluminescent HRP substrate was SuperSignal West Pico and West Femto Chemiluminescent Substrate (Thermo Scientific, Waltham, MA).
Each sample was migrated separately in triplicate (20 μg per lane) on a 8–16% gradient SDS-PAGE (180V, 60 min). Liquid transfer of proteins was performed over 75 min at 100V at 4 °C. The western blots were blocked with TBS-Tween 20 (0.5%, w/v), supplemented with lyophilized low-fat milk (5% w/v). Membranes were incubated with primary antibodies under mild agitation overnight at 4 °C or for 1.5h at 37 °C and with secondary antibodies overnight at 4 °C or for 1h at 37 °C. The peroxidase was revealed with chemoluminescent substrates and the images recorded on film or digitized with a cooled CCD camera (ImageMaster VDS-CL, Amersham Biosciences, GE HealthCare Lifesciences, Pittsburgh, PA). The intensity of the signal was quantified using the TotalLab Quant software (TotalLab, Newcastle upon Tyne, UK).
Immunohistochemistry
Tissue-Tek-embedded, 10 μm sections of cervix were prepared on Superfrost Plus microscope slides (Thermo Fisher Scientific) with Cryostar NX70 (Thermo Fisher Scientific), and fixed with acetone at −20 °C for 20 min. After rinsing in PBS, endogenous peroxidase activity was blocked by incubating the sections for 10 min in 1% H2O2. After rinsing again in PBS, slides were pre-incubated in 2.5% normal horse serum blocking solution from the ImmPRESSTM kit (Vector Laboratories, Burlingame, CA) for 30 min at room temperature, and then incubated overnight at 4 °C with the following antibodies : anti-myosin 9 antibody (sc-98978; Santa Cruz Biotechnology; 1/50), anti-fibronectin antibody (produced from our laboratory; 1/100), anti-tubulin α antibody (T9026; 1/100), diluted in 2.5% normal horse serum blocking solution.
After rinsing in PBS, sections were incubated with the anti-mouse/rabbit IgG Reagent from the ImmPRESS™ kit for 30 min at room temperature. For peroxidase detection, all sections were incubated with the NovaREDTM substrate (Vector Laboratories) for 10 min. Slides were then mounted in DePeX mounting medium (GURR). To assess the specificity of the immunolabeling, sections were incubated only with secondary anti-mouse/rabbit IgG Reagent, and all other steps remained the same. Samples were analyzed using bright field microscopy (Olympus BH2, Olympus) at a magnification of 200×.
RESULTS
The Proteome of the Female Genital Tract Fluids
This study aimed to compare the proteome from the luminal fluids at two biologically significant stages of the cycle (estrus and luteal phase) from the three adjacent regions of the female genital tract; namely, the cervix, uterus and oviduct. We collected the cervical mucus originating from the inner part of the cervix only, to characterize as much as possible the secretions from the cervical epithelium. The uterine and the oviduct fluids were collected from gentle flushing with a very limited volume of buffer in order to analyze a fluid being as close as possible to the native fluids. The combination of in-solution and in-gel digestion MS data from the fluids originating from the female tract yielded in the total identification of 940 proteins. The association of the in-solution digestion data to the in-gel digestion data increased the total number of proteins identified when compared with the in-gel digestion data alone. This was particularly evident for cervical mucus as its elevated viscosity reduced the efficiency of protein separation on SDS-PAGE. In the cervix, 527 proteins were identified were identified using the in-gel digestion method whereas 642 proteins were identified with the in-solution digestion protocol. Combined, these methods led to the total identification of 749 proteins, which shows the complementarity of the methods. The combination of data from the two digestion methods also slightly improved the number of proteins identified in uterine and oviductal fluids over in-gel digestion alone with, respectively, 827 uterine proteins identified compared with 789 and 624 oviductal proteins identified compared with 562.
The complete list of the identified proteins is provided in supplemental Table S1. A total of 749, 827, and 624 proteins were identified in the cervix, the uterus and the oviduct fluids, respectively (Fig. 1).
Fig. 1.
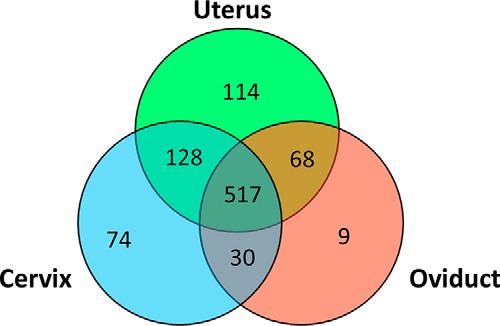
Venn diagram of proteins identified in the female genital tract fluids. The proteins were identified by LC MS/MS in the inner cervical mucus, the uterine fluid and the oviduct fluid.
The comparison of proteomes showed similarity between the different compartments as 517 proteins (out of 940) were found in all three regions (Fig. 1). The comparison of fluid proteomes from the three regions showed that regions anatomically linked to each other shared a higher number of proteins. The cervix proteome shared 645 proteins with the uterus proteome, the uterine proteome shared 585 proteins with the oviduct proteome whereas the cervix and the oviduct only shared 547 proteins. A limited number of proteins were found only in one region as 74, 114, and 9 proteins were found, respectively, only in the cervical mucus, the uterine fluid and the oviduct fluid. However, these proteins may have a marked impact on the function of that particular fluid. Indeed, the cervical mucus is highly concentrated in mucins (mucin 5B and mucin 5C), but this protein is not found in uterine or oviduct fluid. The abundance of mucins of high molecular weight such as the MUC5B and MUC5AC in the cervical mucus explains its higher viscosity compared with those of uterine and oviduct fluids.
Among the proteins found only in uterine fluid, the quantitatively main proteins include Protein FAM115 and Ephrin, more abundant in luteal phase, and β-crystallin S and Niban, more abundant at estrus. In the oviduct fluid, we identified AWN, a member of the spermadhesin family.
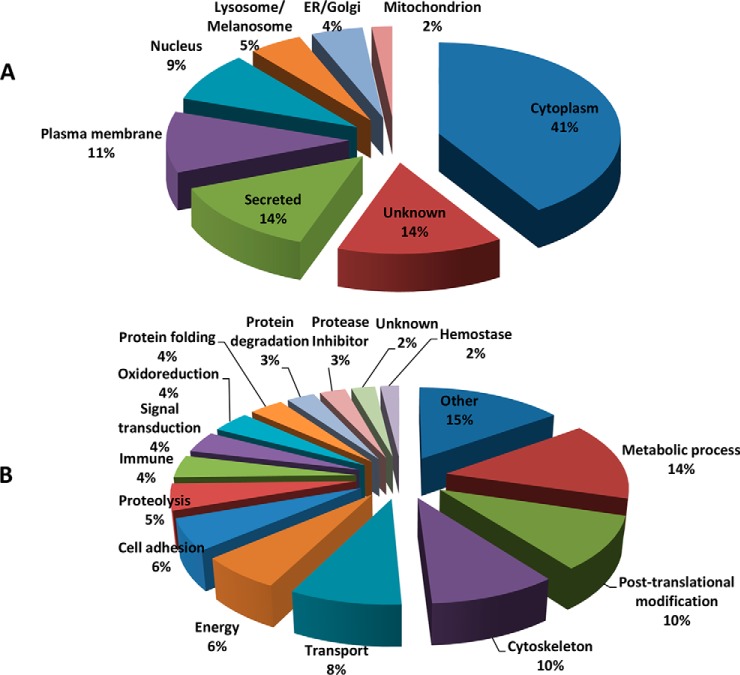
Gene Ontology of the Proteins
According to the Gene Ontology classification, the cellular distribution of proteins found in the total fluids of the female tract in estrus and luteal phase were categorized as cytoplasm proteins (41%), secreted proteins (14%), and plasma membrane (11%) (Fig. 2A). The functional classification of these proteins showed a wide distribution of activities (Fig. 2B).
Fig. 2.
Cellular locations (A) and molecular functions (B) according to the Gene Ontology definition of proteins identified in the female genital tract fluids.
Changes of the Luminal Proteomes Along the Estrous Cycle
Proteomic quantification was performed to assess the effect of the cycle stage (estrus versus luteal phase) and the type of cycle (spontaneous vs. synchronized) on the abundance of proteins in the various fluids of the female tract (cervix, uterus, and oviduct). A total of 694 proteins were identified by GeLC-MS/MS and the data are summarized in the supplemental Table S2. Proteins were considered significantly differentially abundant between estrus and luteal phase if the p value of the Student's t test p < 0.05 and the estrus/luteal phase of normalized spectral counts > 1.5. The results of identification and quantification in the different regions of the tract are provided in supplemental Table S3 (cervix, spontaneous and synchronized estrus), supplemental Table S4 (uterus, spontaneous and synchronized estrus), and supplemental Table S5 (oviduct, spontaneous and synchronized estrus).
In ewes showing spontaneous estrus, a total of 217, 517, and 280 proteins were identified in, the cervix, uterus and oviduct, respectively. During estrus, 61, 97, and 64 proteins were found in higher amounts in respectively, the cervix, the uterus and the oviduct, which corresponded to 28%, 12 and 23% of the total of proteins. In the luteal phase, 26, 102, and 17 proteins were found more abundant in respectively, the cervix, the uterus and the oviduct corresponding to 12%, 17 and 6% of the total of proteins.
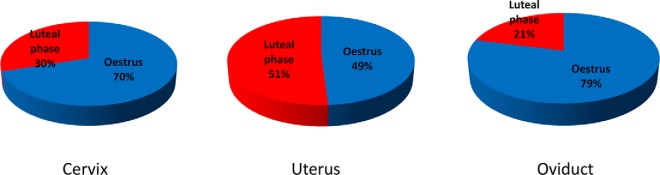
The results show that the regulation of protein abundance along the cycle varies between the different segments of the female tract. Indeed, the cervix and the oviduct exhibit a marked expression of differential proteins during estrus (70 and 79% respectively of differential proteins) whereas the uterus shows a more balanced regulation during estrus and luteal phase (51% of differential proteins in luteal phase) (Fig. 3). Therefore, estrus can be generally characterized by a concomitant increase in the differential abundance of proteins in both the cervix and the oviduct whereas the luteal phase is mainly characterized by an increase in the uterus. This was shown for both types of cycles (spontaneous or synchronized).
Fig. 3.
Pie charts of the differential proteins along the oestrous cycle. The red and blue parts of each pie indicate the proportion of proteins found more abundant in the fluid of each region of the female genital tract (cervix, uterus, and oviduct) during oestrus and luteal phase, respectively.
Proteome of the Cervical Mucus Along the Cycle
In the cervical mucus obtained from ewes in a spontaneous cycle, 87 proteins out of the 217 quantified proteins (40.1%) were found to be differentially abundant between estrus and the luteal phase. The five main proteins more abundant in estrus were transferrin, Heat Shock protein HSP 90-α, complement C3, α-2-HS glycoprotein, and α-2 macroglobulin (Table I). The HSP 90-α showed a marked regulation of its abundance along the cycle with one of the highest estrus/luteal phase ratio (more than 17 times) and a main abundance in cervical mucus (ranked seventh among the 749 identified proteins based on spectral counts). Other members of the Heat Shock Proteins family were also more abundant at estrus such as the HSP 105 kDa protein (HSPH1), the HSP 90-α1 protein (HSPAA1), and the HSP 90-β protein (HSPB1 and HSP90AB1). Beside the complement C3, other components of the complement cascade were found more abundant at estrus i.e. the C4 and C5.
Table I. Proteins differentially abundant between estrus and luteal phase in the cervical mucus. Proteins more abundant in oestrus and luteal phase: list of the 20 quantitatively main proteins based on spectral counting quantitative method at oestrus and luteal phase. Estrus: NSC (normalized spectral counts) at oestrus. Luteal phase: NSC during luteal phase. O/LP ratio: oestrus/luteal phase ratio of NSC.
| Proteins name | Gene symbol | Estrus | Luteal phase | O/LP Ratio |
|---|---|---|---|---|
| More abundant in oestrus | ||||
| Serotransferrin | TF | 217.1 | 107.39 | 2.02 |
| Heat shock protein HSP 90-α | HSP90AA1 | 165.9 | 9.37 | 17.71 |
| Complement C3 | C3 | 109.2 | 65.56 | 1.67 |
| α-2-HS-glycoprotein | AHSG | 52.4 | 17.04 | 3.08 |
| α-2-macroglobulin | A2M | 46.1 | 3.65 | 12.64 |
| Glutathione S-transferase P | GSTP1 | 38.3 | 17.25 | 2.22 |
| Mucin-5B | MUC5B | 37.6 | 8.41 | 4.47 |
| Glutathione S-transferase Mu 1 | GSTM1 | 31.4 | 4.86 | 6.45 |
| Apolipoprotein A-I | APOA1 | 31.1 | 6.31 | 4.92 |
| Triosephosphate isomerase | TPI1 | 24.0 | 6.32 | 3.80 |
| Isocitrate dehydrogenase [NADP] cytoplasmic | IDH1 | 23.5 | 6.72 | 3.50 |
| Fibronectin | FN1 | 21.9 | 5.77 | 3.80 |
| Elongation factor 1-α1 | EEF1A1 | 21.5 | 8.42 | 2.55 |
| Elongation factor 2 | EEF2 | 19.8 | 1.09 | 18.19 |
| 14–3-3 protein epsilon | YWHAE | 19.0 | 5.33 | 3.57 |
| 14–3-3 protein zeta/delta | YWHAZ | 18.0 | 6.96 | 2.58 |
| α-1B-glycoprotein | A1BG | 16.5 | 3.65 | 4.52 |
| α-enolase | ENO1 | 15.8 | 3.59 | 4.40 |
| Heat shock protein β-1 | HSPB1 | 15.4 | 6.02 | 2.56 |
| Chloride intracellular channel protein 1 | CLIC1 | 14.5 | 5.04 | 2.87 |
| More abundant in luteal phase | ||||
| Lactotransferrin | LTF | 273.8 | 937.0 | 0.29 |
| Ceruloplasmin | CP | 129.6 | 278.6 | 0.47 |
| Alkaline phosphatase | ALPL | 15.8 | 80.1 | 0.20 |
| Deleted in malignant brain tumors 1 protein | DMBT1 | 14.8 | 65.3 | 0.23 |
| EGF-containing fibulin-like extracellular matrix protein 1 | EFEMP1 | 0.0 | 63.5 | 0.00 |
| Polymeric immunoglobulin receptor | PIGR | 4.6 | 22.3 | 0.21 |
| Galectin-3-binding protein | LGALS3BP | 0.0 | 22.2 | 0.00 |
| CD9 antigen | CD9 | 0.0 | 17.3 | 0.00 |
| Histone H4 | HIST1H4H | 1.8 | 13.0 | 0.14 |
| Inhibitor of carbonic anhydrase | ICA | 5.0 | 11.4 | 0.44 |
| Aminopeptidase N | ANPEP | 0.6 | 9.6 | 0.06 |
| N-acetylglucosamine-6-sulfatase | GNS | 0.0 | 7.2 | 0.00 |
| Zinc-alpha-2-glycoprotein | AZGP1 | 0.0 | 6.3 | 0.00 |
| Tissue alpha-l-fucosidase | FUCA1 | 0.0 | 5.4 | 0.00 |
| Angiotensin-converting enzyme | ACE | 0.0 | 5.2 | 0.00 |
| β-hexosaminidase subunit alpha | HEXA | 0.0 | 5.0 | 0.00 |
| Complement factor I | CFI | 0.3 | 3.7 | 0.09 |
| Pancreatic secretory granule membrane major glycoprotein GP2 | GP2 | 0.0 | 3.6 | 0.00 |
| Factor XIIa inhibitor | SERPING1 | 0.3 | 3.2 | 0.09 |
| Aldehyde dehydrogenase family 3 member B1 | ALDH3B1 | 0.0 | 2.5 | 0.00 |
The main proteins more abundant in the luteal phase were lactoferrin, ceruloplasmin, alkaline phosphatase, DMBT1, EFEMP1, and PIGR (Table I). Lactoferrin, ceruloplasmin, DMBT1, and PIGR are involved in the immune system. Several other overabundant proteins were involved in tissue remodeling. These include alkaline phosphatase, EFEMP1, CD9, and galectin-3 binding protein. The EFEMP1 (EGF-containing fibulin-like extracellular matrix protein 1 or fibulin 3) and the galectin-3 binding protein were not detected at estrus but found in high amounts both in cervical mucus and uterine fluid during luteal phase.
Proteome of the Uterine Fluid Along the Cycle
In the uterine fluid of ewes in a spontaneous cycle, 199 proteins out of the 517 quantified proteins (38.5%) were found differential between estrus and the luteal phase. In the uterine fluid, the main proteins overabundant in estrus were transferrin, HSP 90-α, myosin-9, α2-macroglobulin, and elongation factor 1-α1 (EEFA1) (Table II). The transferrin, HSP 90-α, and α2-macroglobulin were also among the main overabundant proteins in the cervical mucus.
Table II. Proteins differentially abundant between estrus and luteal phase in the uterine fluid. Proteins more abundant in estrus and luteal phase: list of the 20 quantitatively main proteins based on spectral counting quantitative method at estrus and luteal phase. Estrus: NSC (normalized spectral counts) at estrus. Luteal phase: NSC during luteal phase. O/LP ratio: oestrus/luteal phase ratio of NSC.
| Proteins names | Gene Symbol | Estrus | Luteal Phase | O/LP Ratio |
|---|---|---|---|---|
| More abundant in oestrus | ||||
| Serotransferrin | TF | 202.2 | 133.6 | 1.51 |
| Heat shock protein HSP 90-α | HSP90AA1 | 63.4 | 33.6 | 1.89 |
| Myosin-9 | MYH9 | 56.5 | 5.8 | 9.74 |
| α-2-macroglobulin | A2M | 53.8 | 19.6 | 2.74 |
| Elongation factor 1-α1 | EEF1A1 | 46.5 | 29.0 | 1.60 |
| Clathrin heavy chain 1 | CLTC | 36.7 | 12.2 | 3.00 |
| Transitional endoplasmic reticulum ATPase | VCP | 33.8 | 17.2 | 1.96 |
| Elongation factor 2 | EEF2 | 33.0 | 12.3 | 2.67 |
| α-actinin-4 | ACTN4 | 23.5 | 5.8 | 4.07 |
| High mobility group protein B1 | HMGB1 | 20.2 | 11.3 | 1.79 |
| Myosin-11 | MYH11 | 17.7 | 0.2 | 101.22 |
| Ras GTPase-activating-like protein IQGAP1 | IQGAP1 | 13.1 | 2.7 | 4.91 |
| Complement factor B | CFB | 12.1 | 4.6 | 2.64 |
| Coatomer subunit alpha | COPA | 11.6 | 4.9 | 2.38 |
| 60S ribosomal protein L7 | RPL7 | 11.3 | 0.2 | 65.55 |
| Clusterin | CLU | 9.8 | 2.8 | 3.47 |
| 40S ribosomal protein S3 | RPS3 | 8.4 | 0.9 | 9.66 |
| Complement C4 | C4 | 7.4 | 2.2 | 3.34 |
| 40S ribosomal protein S16 | Rps16 | 6.3 | 0.6 | 9.82 |
| 40S ribosomal protein S8 | RPS8 | 6.2 | 2.2 | 2.85 |
| More abundant in luteal phase | ||||
| Ceruloplasmin | CP | 117.6 | 301.7 | 0.39 |
| Isocitrate dehydrogenase | IDH1 | 66.6 | 180.2 | 0.37 |
| α-enolase | ENO1 | 38.1 | 64.2 | 0.59 |
| Alkaline phosphatase, tissue-nonspecific isozyme | ALPL | 19.0 | 53.6 | 0.35 |
| Ezrin | EZR | 23.3 | 47.6 | 0.49 |
| Fructose-bisphosphate aldolase A | ALDOA | 17.8 | 46.2 | 0.39 |
| Phosphoglycerate kinase 1 | PGK1 | 16.1 | 41.8 | 0.39 |
| Adenosylhomocysteinase | AHCY | 22.8 | 35.2 | 0.65 |
| Peroxiredoxin-1 | PRDX1 | 17.5 | 30.3 | 0.58 |
| Triosephosphate isomerase | TPI1 | 20.3 | 29.9 | 0.68 |
| Annexin A2 | ANXA2 | 16.8 | 27.8 | 0.60 |
| EGF-containing fibulin-like extracellular matrix protein 1 | EFEMP1 | 0.0 | 25.1 | 0.00 |
| D-3-phosphoglycerate dehydrogenase | PHGDH | 12.1 | 24.0 | 0.51 |
| Phosphoserine aminotransferase | PSAT1 | 1.5 | 23.6 | 0.06 |
| α-1B-glycoprotein | A1BG | 13.9 | 23.4 | 0.60 |
| Sialidase-1 | NEU1 | 0.3 | 22.1 | 0.02 |
| Transgelin-2 | TAGLN2 | 8.5 | 19.3 | 0.44 |
| Glutathione S-transferase Mu 5 | Gstm5 | 8.2 | 19.1 | 0.43 |
| N-acetylglucosamine-6-sulfatase | GNS | 0.0 | 18.4 | 0.00 |
| Inhibitor of carbonic anhydrase | ICA | 11.7 | 17.7 | 0.66 |
The main proteins more abundant in the luteal phase were ceruloplasmin, isocitrate dehydrogenase, α-enolase, alkaline phosphatase, and ezrin. Eight components from the component cascade were found overabundant in the uterine fluid: C1s, C3, C4, C5, C6, C7, factor B, and factor H. In accordance with the remodeling of the endometrium occurring during the luteal phase, many of the more abundant proteins in the luteal phase were involved in tissue modeling.
Proteome of the Oviduct Fluid Along the Cycle
In the oviduct fluid from ewes in a spontaneous cycle, 81 proteins out of the 280 quantified proteins (28.9%) were found to be differential between estrus and the luteal phase. In the oviduct fluid, the main proteins overabundant during estrus were oviductin, isocitrate dehydrogenase, elongation factor 1-α1, HSPA8, 14–3-3 protein ε, and annexin A8 (Table III).
Table III. Proteins differentially abundant between estrus and luteal phase in the oviduct fluid. Proteins over abundant in estrus and luteal phase: list of the 20 quantitatively main proteins based on spectral counting quantitative method at estrus and luteal phase. Oestrus: NSC (normalized spectral counts) at estrus. Luteal phase: NSC during luteal phase. O/LP ratio: oestrus/luteal phase ratio of NSC.
| Proteins names | Gene symbol | Oestrus | Luteal phase | O/LP ratio |
|---|---|---|---|---|
| More abundant in oestrus | ||||
| Oviduct-specific glycoprotein | OVGP1 | 258.4 | 152.2 | 1.70 |
| Isocitrate dehydrogenase | IDH1 | 67.5 | 30.5 | 2.21 |
| Heat shock cognate 71 kDa protein | HSPA8 | 60.8 | 21.6 | 2.81 |
| Elongation factor 1-α1 | EEF1A1 | 55.4 | 33.2 | 1.67 |
| 14–3-3 protein epsilon | YWHAE | 31.4 | 20.7 | 1.52 |
| Retinal dehydrogenase 1 | ALDH1A1 | 25.5 | 9.5 | 2.68 |
| Tubulin β-4B chain | TUBB4B | 16.6 | 10.4 | 1.60 |
| Fatty acid-binding protein, epidermal | FABP5 | 14.4 | 6.1 | 2.35 |
| Malate dehydrogenase | MDH1 | 12.6 | 8.0 | 1.57 |
| Transaldolase | TALDO1 | 11.9 | 7.4 | 1.61 |
| Peptidyl-prolyl cis-trans isomerase FKBP4 | FKBP4 | 10.7 | 3.1 | 3.42 |
| Coatomer subunit alpha | COPA | 8.4 | 2.5 | 3.31 |
| Transketolase | TKT | 8.3 | 3.8 | 2.18 |
| Poly(rC)-binding protein 1 | PCBP1 | 8.0 | 3.8 | 2.13 |
| Proteasome subunit alpha type-6 | PSMA6 | 7.5 | 4.1 | 1.82 |
| Adenosylhomocysteinase | AHCY | 6.7 | 1.8 | 3.80 |
| 40S ribosomal protein S16 | Rps16 | 5.8 | 0.6 | 9.17 |
| Annexin A8 | ANXA8 | 5.5 | 2.7 | 2.03 |
| Osteopontin | SPP1 | 4.9 | 2.4 | 2.10 |
| 40S ribosomal protein S4, X isoform | RPS4X | 4.1 | 1.6 | 2.62 |
| More abundant in luteal phase | ||||
| α-2-macroglobulin | A2M | 34.4 | 61.0 | 0.56 |
| Ceruloplasmin | CP | 23.3 | 56.8 | 0.41 |
| Gelsolin | GSN | 24.8 | 40.4 | 0.61 |
| Transthyretin | TTR | 12.1 | 19.5 | 0.62 |
| Complement factor B | CFB | 11.4 | 19.1 | 0.60 |
| Lactotransferrin | LTF | 4.0 | 16.7 | 0.24 |
| Membrane primary amine oxidase | AOC3 | 4.3 | 13.7 | 0.32 |
| Complement C4 | C4 | 5.9 | 13.1 | 0.45 |
| Ig lambda chain V-I region HA | - | 4.2 | 10.2 | 0.41 |
| Hemopexin | HPX | 3.1 | 8.8 | 0.36 |
| Plasminogen (Fragment) | PLG | 2.7 | 8.3 | 0.33 |
| Keratin-associated protein 6–1 | KRTAP6–1 | 0.0 | 4.6 | 0.00 |
| Annexin A3 | ANXA3 | 0.1 | 2.8 | 0.05 |
| Factor XIIa inhibitor | SERPING1 | 0.2 | 2.6 | 0.06 |
| Vinculin | VCL | 0.6 | 2.0 | 0.27 |
| Programmed cell death 6-interacting protein | PDCD6IP | 0.2 | 1.5 | 0.11 |
The proteins which were more abundant in the luteal phase were α-2-macroglobulin, ceruloplasmin, gelsolin, transthyretin, and complement factor B.
Effect of Treatment of Synchronization
The proteomes of the cervix, uterus, and oviduct were also analyzed along the cycle when the estrus was synchronized with a hormonal treatment. A total of 110, 203, and 81 proteins were found differentially abundant between estrus and the luteal phase in the cervix, uterus, and oviduct, respectively. When these proteomes were compared with those obtained from animals in spontaneous cycle, a total of 48, 92, and 34 proteins were both found in spontaneous and synchronized cycles differentially abundant along the cycle in the cervix, uterus, and oviduct, respectively. The ratios estrus/luteal phases of these proteins were similar between spontaneous and synchronized cycles (supplemental Table S6).
Comparison of Fluid Proteomes Along the Cycle in the Female Tract
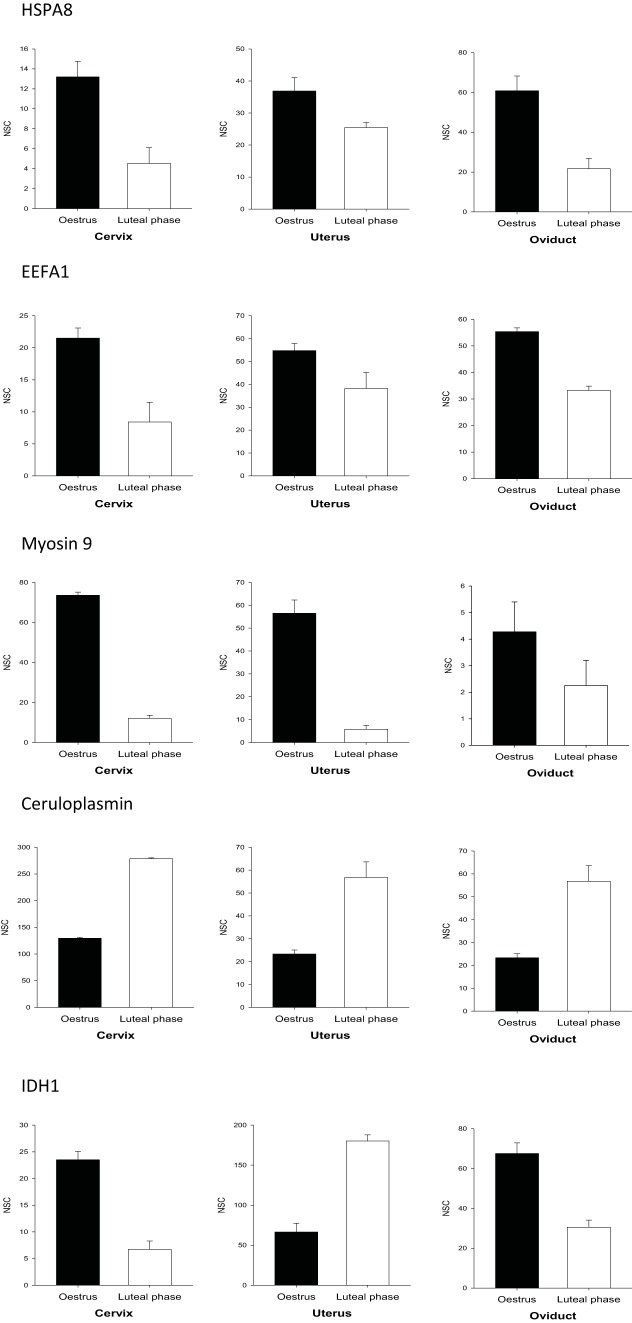
The lists of differentially abundant proteins between the estrus and the luteal phases of each region of the genital tract of spontaneous cycling ewes were compared with each other (supplemental Table S7). Very few proteins were found to be differentially abundant in all the regions and the abundance of proteins in the female tract showed various patterns (Fig. 4). The proteasome subunit α type-7, oviduct-specific glycoprotein, and elongation factor 1-α1 (EEFA1) were all found increased at estrus in the different regions of the female genital tract. Beside elongation factor 1 α, four members of the elongation factor family were also found in all fluids: β, γ, and Δ, and elongation factor 2. The ceruloplasmin was clearly a main marker of the luteal phase in the genital tract as it is the only protein we found in high amounts with an increased abundance in the cervix, the uterus, and the oviduct (Tables I–III, supplemental Table S7, Fig. 4).
Fig. 4.
Patterns of proteins abundance in the fluids from the female genital tract. Quantitative data from several examples of differentially abundant proteins along the cycle (estrus versus luteal phase) in the fluids from different compartments of the female genital tract (cervix, uterus, oviduct). Several proteins are more abundant in all regions of the tract at estrus such as HSPA8, EEFA1 and myosin 9 or in luteal phase such as ceruloplasmin. IDH1 showed a different abundance along the cycle depending on the site of collection. Whereas IDH1 was more abundant in cervix and oviduct at estrus, it was a strong marker of luteal phase in the uterus. Data are means ± S.E. of normalized spectral counts (NSC).
When the proteomes were compared between two regions, the differential proteomes of the cervical mucus and the uterine fluid showed the highest similarity. Indeed, they showed a common variation along the cycle for 25 proteins including several main quantitative proteins such as serotransferrin, Heat shock protein HSP 90-α, elongation factors 1/2, and α-2-macroglobulin which are more abundant at estrus, as well as ceruloplasmin, alkaline phosphatase, EFEMP1, and galectin-3-binding protein which are more abundant during the luteal phase.
The different members of the HSP family (HSPH1, HSPAA1, HSP90AB1, HSPB1, HSPA4, and HSPA8) were all abundant in all the fluids and were all more abundant at estrus in at least one region. IDH1 showed a different regulation along the cycle depending on the site of expression. Whereas IDH1 was more abundant in the cervix and oviduct at estrus, it was a strong marker of luteal phase in the uterus (Fig. 4). This was observed for both spontaneous and synchronized cycles.
Comparison with data reported in previous studies focused on the stage of the cycle
Variation throughout the oestrous cycle in the abundance of proteins in the fluids assessed in this study was manually compared with data reported in previous studies on the regulation of the genes in the female genital tract during the cycle. We produced from our study a list of 367 proteins including 61, 97, and 64 proteins found in higher amounts at estrus and 26, 102, and 17 proteins found more abundant during the luteal phase in the cervix, uterus and oviduct, respectively. We searched for these genes in seven studies of the transcriptome or proteome of the female tract during the cycle in other species. These include the transcriptome of the bovine cervix (14), the transcriptomes of the horse (21) and bovine (20) endometrium, the proteome of human endometrium (23, 25), the transcriptome of bovine oviduct epithelial cells (34), and the proteome of porcine oviduct epithelial cells (33).
A total of 59 proteins were found to be regulated similarly, i.e. up or down regulated at estrus or the luteal phase, in the female tracts of other species (Table IV). Among these proteins, mucin 5B, and protein disulfide isomerase A3 (PDIA3) were found both in ovine and bovine cervices to be more expressed at estrus.
Table IV. Comparison of abundance of sheep proteins during the oestrus cycle with other species. The proteins identified in the female genital tract of the sheep were classified into two groups: more abundant either (1) at estrus or (2) during luteal phase. Based on previous studies, the genes (mRNAs or proteins) showing the same regulation along the cycle in other species are indicated as black boxes in the table. These data are taken from the following references.

aPluta et al., 2012, Transcriptome of the bovine cervix.
bGebhardt et al., 2012, Transcriptome of the horse endometrium.
cMitko et al., 2008, Transcriptome of the bovine endometrium.
dChen et al., 2015, Proteome of human endometrium.
eLi et al., 2011, Proteome of human endometrium.
fBauersachs et al., 2004, Transcriptome of bovine oviduct epithelial cells.
gSeytanoglu et al., 2008, Proteome of porcine oviduct epithelial cells.
In ovine uterine fluid, cyclic variation in the abundance of 16 proteins such as heat shock protein 90 β, elongation factor 2, and ezrin were found to match the proteomic changes that occurred in the human endometrium along the menstrual cycle. Moreover, the abundance of 41 ovine uterine fluid proteins in the present study corresponded with transcription of their matching genes at the same points in the cycle in equine and bovine uterine tissue. Among these proteins, ezrin and superoxide dismutase were found to show similar regulation, being more abundant in the luteal phase, in several models, i.e. in the ovine uterine fluid, in the human endometrium proteome and the bovine uterus transcriptome.
Endoplasmin and oviductin proteins were more abundant during estrus in ovine oviduct fluid, a result supported by data in cattle which showed a similar modification in the amounts of mRNA that code for each of these proteins in bovine oviduct epithelial cells. Gelsolin protein, that was more abundant in the ovine oviduct fluid during the luteal phase, was also described as more abundant in porcine oviduct epithelial cells during the luteal phase.
Immunodetection of Differential Proteins
Among the proteins found to be differentially abundant between estrus and the luteal phase in spontaneous estrus, 14 proteins were quantified by western blotting (Fig. 5). The relative abundance in estrus or the luteal phase quantified by western blotting was in accordance with that observed by MS. Gelsolin, hsp90b, ezrin, α-enolase, and VCP were confirmed to be more abundant in the cervical mucus during estrus. Lactoferrin, CD109, ceruloplasmin, and ZAG were confirmed to be more abundant in the cervical mucus during the luteal phase. Several HSPs (HSPA8, hsp105, hsp70) and oviductin were confirmed to be more abundant in the oviduct fluid during estrus. Myosin 9 was found more abundant both in oviduct fluid and cervical mucus during estrus.
Fig. 5.
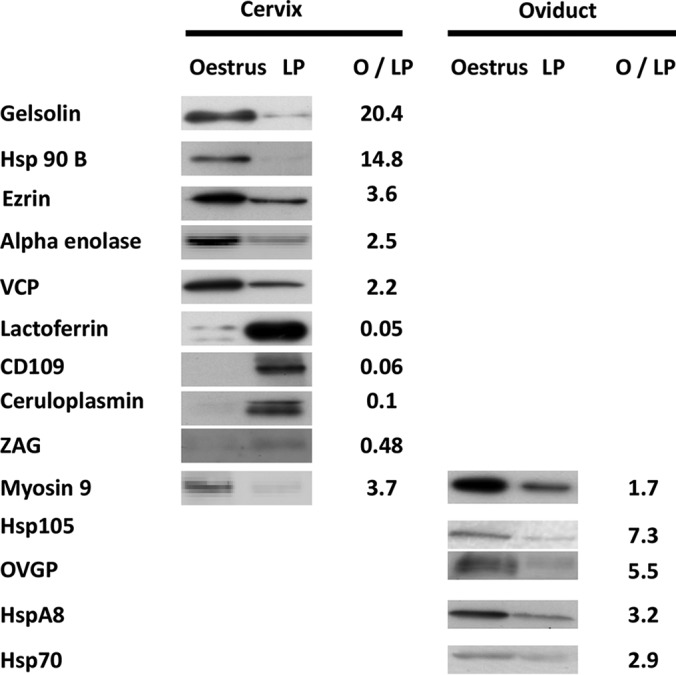
Immunodetection of differential proteins along the cycle. The cervical mucus and the oviduct fluid were analyzed by western blot using primary antibodies directed against several differential proteins between estrus and luteal phase. O/LP: oestrus/luteal phase ratio of amounts of proteins quantified after western blotting.
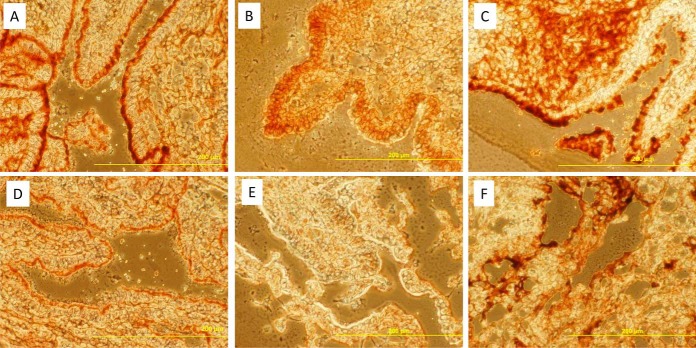
Immunohistochemistry was performed to confirm the origin of secretion and the increased expression of several differential proteins along the cycle. Fibronectin and myosin 9 were found more abundant in cervical mucus at estrus. The abundance of fibronectin and myosin 9 at estrus in the cervical mucus was associated with their increased expression in the luminal epithelium of the cervical tissue (Fig. 6).
Fig. 6.
Immunodetection in the cervix of differentially expressed proteins along the oestrous cycle. The myosin 9 was detected by immunohistochemistry in the luminal epithelium of the cervix at estrus (A) and in luteal phase (D). The fibronectin was clearly detected at estrus (B) but the signal was very weak during luteal phase (E). The tubulin was detected in the whole tissue with similar intensity at estrus (C) and luteal phase (F).
DISCUSSION
This study is the first to investigate in the same biological model the luminal proteomes from different regions of the female genital tract along the oestrous cycle.
The main result from this work is the differential regulation of protein abundance in the fluid from the different regions, depending on the stage of the oestrous cycle. Whereas the cervix and the oviduct show an increased number of differential proteins mainly at estrus, the uterus shows a more balanced pattern during both estrus and the luteal phase.
The patterns of protein abundance in the fluids along the cycle could result from the regulation of secretion and also from other origins such as immune system cells (38). The comparison of previously published transcriptomes (14, 20, 21, 33, 34) and tissue proteomes (23, 25) in other models with the sheep luminal proteomes from this study provided some information about the possible secretory origin of many proteins, suggesting that the regulation of the protein abundance along the cycle may be associated to a significant extent with endocrine control of the expression of several genes.
The comparison of proteomes from the different regions showed similarity as 55% of the proteins were found in all three regions. The similarity could be explained by the high proportion of these proteins being common to biological fluids but also by transfer between contiguous compartments. Despite anatomical barriers such as the utero-tubal junction between the oviduct and the uterus, the secretions flow from the oviduct to the cervix due to peristaltic contractions of the tract (39). Indeed, the quantitatively main protein identified in the oviduct fluid, the oviductin, was also found, albeit as traces, in the uterine fluid and the cervical mucus. The secretions of the uterus also enter the cervix, located anatomically at the basis of the uterine body, and can mix with cervical secretions to constitute the cervical mucus.
As we provide data of luminal proteomes along the reproductive cycle, it is of interest to relate the modifications of these proteomes with the specific functions of reproduction of the different regions of the tract. In species where the semen is deposited in the vagina, the cervix (mainly through the properties of the cervical mucus) plays a role in selection of spermatozoa (40, 41). Only a small percentage of the motile and morphologically normal spermatozoa will be able to cross the cervical mucus and fill the lumen of the cervix. The rheological properties of this mucus are known to change along the cycle, with a reduced viscosity at estrus to allow spermatozoa to reach the uterus and an increased viscosity during luteal phase to protect the uterus from external contamination (12). The viscosity of the cervical mucus is mainly due its high content of mucins, such as the mucins 5B and 5AC (13). During the menstrual cycle in humans, the cervical production of mucins peaks midcycle (42, 43) and the transcription of several mucin genes in the cervix is also increased at estrus during the bovine oestrus cycle (14). Our results in the sheep show that the amount of mucins present in the inner cervical mucus also peaks at estrus.
Like the cervix, our results indicated that the oviduct also had a higher number of proteins with increased abundance at estrus when compared with the luteal phase. Given the spermatozoa fertilize the oocyte in the oviduct at estrus, the higher abundance of several proteins in the lumen of the oviduct at the time of ovulation may suggest a putative role in fertilisation. Indeed, several of the oviduct proteins we found to be more abundant at estrus such as oviductin, osteopontin, and HSPA8 were described in previous studies as potentially interacting with gametes (44–46).
Oviductin was shown to be preferentially secreted in the oviduct at estrus (47) where it binds to the zona pellucida of the oocyte (48) and the spermatozoa (49), suggesting a role in the fertilisation process (45). Our data are in accordance with previous studies (34) and show that oviductin is the quantitatively main protein in the lumen of the sheep oviduct and increases in abundance at estrus.
Myosin 9 was also increased in the oviduct fluid at estrus which could be consistent with an increase of oviductin, as it was suggested that myosin 9 would bind to oviductin and be involved in the binding of oviductin to the spermatozoa (50). Interestingly, we observed that myosin 9 was also more abundant in other compartments of the tract at estrus, with an increased synthesis by the luminal epithelium of the cervix. Therefore, the putative interaction of myosin 9 with spermatozoa may also occur when they enter the cervical canal.
Another family of proteins overabundant at estrus are the Heat Shock Proteins as we identified 8 members with an increased presence in different parts of the tract. One member of this family, HSPA8, secreted by the oviduct, was shown to support the viability of spermatozoa in the boar, the ram, and the bull (46, 51). The increase of abundance of HSPA8 we found in the oviduct fluid at estrus may be linked to a role in sperm survival around ovulation.
Osteopontin is another secreted oviduct protein suggested to modulate sperm function, gametes interaction and migration in the oviduct (44). The transcription of the osteopontin gene was not found to vary along the oestrous cycle in the cow oviduct (52) but was found to be up-regulated in the mouse oviduct at estrus (53). Our data showed an increase of osteopontin protein in the oviduct fluid at estrus in sheep.
The AWN protein is a member of the spermadhesins family, named after their sperm binding properties (54) and was proposed to be involved in sperm egg primary binding (55). A previous study showed that AWN was synthesized by the female tract as it was identified by immunohistochemistry in the sow oviduct (56). We have identified AWN in the oviduct fluid, suggesting its secretion. Given its known properties of sperm binding, the role of AWN in the oviduct fluid might be linked to sperm oocyte interactions.
The complement C3 was found in high amounts in fluids from the cervix, the uterus and the oviduct in our study with an increased abundance in the cervical mucus and the uterine fluid at estrus. Apart from C3, we observed an increased abundance of the complement cascade at estrus with eight components overabundant in the uterine fluid: C1s, C3, C4, C5, C6, C7, factor B, and factor H. The synthesis of complement C3 and complement factor B are increased at estrus in the mouse endometrium and luminal fluid (57, 58) and complement C5 was found in rabbit uterine flushings with a higher prevalence at estrus (59). This increase of abundance at estrus suggests a positive regulation by estrogens. Indeed, the secretion of C3 is under estrogen regulation in the mouse and human oviducts (60) and the 5′-flanking region of the C5 gene contains sequences homologous with an estrogen responsive element (61). In human, the complement C3 plays an embryo trophic role after conversion into iC3b by the oviduct cells (60). Complement C3 has also been proposed as a bridging ligand between sperm and oocyte receptors (62, 63).
Beside proteins directly involved in the reproduction process, other groups of proteins were more abundant at estrus such as the antioxidant enzymes. The peroxiredoxins 1, 5, and 6, the glutathione reductase, the glutathione s-transferase Mu1, Mu5, and P, and the thioredoxin were all found in increased amounts in the cervical mucus at estrus. The thioredoxin pathway was shown to be positively regulated by estradiol in the mouse uterus (64) and peroxidase and cyclooxygenase were more elevated at estrus in rat uterus tissue (65, 66) or luminal fluid (67). Glutathione peroxidase 1 mRNA was more abundant at estrus in the cow oviduct (68).
The luteal phase was characterized by a higher abundance of proteins in the uterine fluid associated with the immune system. The cervical mucus was also modified with higher amounts of proteins associated with the immune system such as the ceruloplasmin, the lactoferrin, DMBT1 and PIGR emphasizing the role of the cervical mucus as an immunological barrier in the luteal phase.
This is consistent with the need to provide an environment suited for the development of a putative embryo. Other proteins more abundant in ovine uterine fluid during luteal phase are associated with tissue remodeling like galectin 3 binding protein, alkaline phosphatase, CD9, CD109, or fibulin. The expression of alkaline phosphase in the hamster was shown to be higher in diestrus in the luminal epithelium of the uterus and under the control of progesterone suggesting a role in uterine receptivity (69). EFEMP1, also named fibulin 3, belongs to the group of fibulins, a family of secreted glycoproteins associated with basement membranes, elastic fibers, and other matrices (70). In human endometrial tissues, the fibulin-1 transcript levels were higher during the secretory phase than during the proliferative phase (71) and progesterone was shown to up-regulate the fibulin 1 and fibulin 2 genes in human endometrial stromal cell in culture (72). In our study, the presence of EFEMP1/fibulin 3 in the uterine fluid and the cervical mucus was observed only during the luteal phase suggesting a control of its expression by progesterone in vivo.
The increased abundance of several proteins in the uterine fluid during luteal phase also suggests that the activity of secretion is focused toward the preparation of the implantation of a putative embryo. The regulation of the uterine transcriptome during the preimplantation phase has been intensively studied in several species (sheep (73), bovine (74, 75), horse (21, 76), and pig (77–79)) and the proteome of uterine fluid during the early stages of pregnancy was analyzed in cattle and sheep (80–82). Several proteins we found more abundant in the uterine luminal fluid during the luteal phase were also described as uterine markers of early pregnancy with an increased production in the presence of an embryo. The transgelin protein was found in higher amounts in uterine luminal fluid from early pregnant ewes compared with cyclic ewes (82). The mRNA of galectin-3 binding protein (LGALS3BP) was found in higher amounts in the luminal endometrium of the cow (74), the mRNA of β2 microglobulin was found in higher amounts in the endometrium of the ewe (73) and the Annexin A2 gene was found up-regulated in the myometrium of pregnant pigs compared with cyclic pigs (83). VEGF, which was found more abundant during luteal phase, supports the in vitro development of the mouse embryo (84).
Taken together, the data shown in this article suggest a general pattern of regulation of protein presence in the fluid of the female genital tract. More than 70% of differential proteins in the cervix and the oviduct were found in higher abundance at estrus. During the cycle, the predominant functions of the cervix and the oviduct in the process of reproduction involve an interaction with gametes, occurring only at estrus. Therefore, it could be hypothesized that the regulation of protein abundance in the cervical and oviductal fluid at estrus is orientated toward an interaction with gametes. On the other hand, the number of differential proteins in the uterus was higher during the luteal phase. Given the function of the uterus during the cycle involves preparation for embryo implantation and development, the regulation of protein abundance in the uterine fluid could be orientated toward an interaction with the embryo.
This study of the proteome of the fluids from the different regions of the female genital tract along the oestrous cycle provides a basis for a future global understanding of the regulation of the secretory activity of the female tract. Further, it forms the basis for the identification of components that potentially interact with the gametes in the fertilisation process and the embryo during the early phases of its development.
Supplementary Material
Acknowledgments
We thank Damien Capo and the INRA Experimental Farm (UEPAO). We thank Olivier Sandra and Marie Saint-Dizier for their comments on the manuscript.
Footnotes
Author contributions: C.S., V.L., S.P.d., N.G., and X.D. designed research; C.S., C.R., G.T., V.L., G.H., P.K., K.R., and X.D. performed research; V.L., G.H., and P.K. contributed new reagents or analytic tools; C.S., V.L., G.H., and X.D. analyzed data; C.S., K.R., S.P.d., N.G., and X.D. wrote the paper.
* This work was supported by various grants. P. Kohnke was supported by a post-doctoral fund provided by the French Agence Nationale de la Recherche. SP de Graaf is supported by Australian Wool Innovation and the NSW Stud Merino Breeders Association Trust. The high resolution mass spectrometer was financed (SMHART project) by the European Regional Development Fund (ERDF), the Conseil Régional du Centre, the French National Institute for Agricultural Research (INRA) and the French National Institute of Health and Medical Research (Inserm).
 This article contains supplemental Tables S1 to S7.
This article contains supplemental Tables S1 to S7.
1 The abbreviations used are:
- CVF
- cervical vaginal fluid
- HSP
- heat shock protein.
REFERENCES
- 1.Zegels G., Van Raemdonck G. A., Tjalma W. A., and Van Ostade X. W. (2010) Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci 8, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw J. L. V., Smith C. R., and Diamandis E. P. (2007) Proteomic Analysis of Human Cervico-Vaginal Fluid. J. Proteome Res. 6, 2859–2865 [DOI] [PubMed] [Google Scholar]
- 3.Klein L. L., Jonscher K. R., Heerwagen M. J., Gibbs R. S., and McManaman J. L. (2008) Shotgun proteomic analysis of vaginal fluid from women in late pregnancy. Reprod. Sci. 15, 263–273 [DOI] [PubMed] [Google Scholar]
- 4.Dasari S., Pereira L., Reddy A. P., Michaels J. E., Lu X., Jacob T., Thomas A., Rodland M., Roberts C. T. Jr., Gravett M. G., and Nagalla S. R. (2007) Comprehensive proteomic analysis of human cervical-vaginal fluid. J. Proteome Res. 6, 1258–1268 [DOI] [PubMed] [Google Scholar]
- 5.Di Quinzio M. K. W., Oliva K., Holdsworth S. J., Ayhan M., Walker S. P., Rice G. E., Georgiou H. M., and Permezel M. (2007) Proteomic analysis and characterisation of human cervico-vaginal fluid proteins. Austral. N Zeal. J. Obstet. Gynaecol. 47, 9–15 [DOI] [PubMed] [Google Scholar]
- 6.Pereira L., Reddy A. P., Jacob T., Thomas A., Schneider K. A., Dasari S., Lapidus J. A., Lu X., Rodland M., Roberts C. T. Jr., Gravett M. G., and Nagalla S. R. (2007) Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J. Proteome Res. 6, 1269–1276 [DOI] [PubMed] [Google Scholar]
- 7.Lo J. O., Reddy A. P., Wilmarth P. A., Roberts V. H., Kinhnarath A., Snyder J., Rincon M. P., Gravett M. G., Nagalla S. R., and Pereira L. M. (2014) Proteomic analysis of cervical vaginal fluid proteins among women in recurrent preterm labor. J. Matern. Fetal Neonatal Med. 27, 1183–1188 [DOI] [PubMed] [Google Scholar]
- 8.Zegels G., Van Raemdonck G. A., Coen E. P., Tjalma W. A., and Van Ostade X. W. (2009) Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome Sci. 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang L.-J., De Seta F., Odreman F., Venge P., Piva C., Guaschino S., and Garcia R. C. (2007) Proteomic analysis of human cervical-vaginal fluids. J. Proteome Res. 6, 2874–2883 [DOI] [PubMed] [Google Scholar]
- 10.Muthukumar S., Rajkumar R., Karthikeyan K., Liao C. C., Singh D., Akbarsha M. A., and Archunan G. (2014) Buffalo cervico-vaginal fluid proteomics with special reference to estrous cycle: heat shock protein (HSP)-70 appears to be an estrus indicator. Biol. Reprod. 90, 1–8 [DOI] [PubMed] [Google Scholar]
- 11.Andersch-Björkman Y., Thomsson K. A., Holmén Larsson J. M., Ekerhovd E., and Hansson G. C. (2007) Large Scale Identification of Proteins, Mucins, and Their O-Glycosylation in the Endocervical Mucus during the Menstrual Cycle. Mol. Cell. Proteomics 6, 708–716 [DOI] [PubMed] [Google Scholar]
- 12.Katz D. F., Slade D. A., and Nakajima S. T. (1997) Analysis of pre-ovulatory changes in cervical mucus hydration and sperm penetrability. Adv. Contracept. 13, 143–151 [DOI] [PubMed] [Google Scholar]
- 13.Gipson I. K. (2001) Mucins of the human endocervix. Front. Biosci. 6, 1245–1255 [DOI] [PubMed] [Google Scholar]
- 14.Pluta K., McGettigan P. A., Reid C. J., Browne J. A., Irwin J. A., Tharmalingam T., Corfield A., Baird A., Loftus B. J., Evans A. C. O., and Carrington S. D. (2012) Molecular aspects of mucin biosynthesis and mucus formation in the bovine cervix during the periestrous period. Physiol. Genomics 44, 1165–1178 [DOI] [PubMed] [Google Scholar]
- 15.Chae J. I., Kim J., Lee S. G., Jeon Y. J., Kim D. W., Soh Y., Seo K. S., Lee H. K., Choi N. J., Ryu J., Kang S., Cho S. K., Lee D. S., Chung H. M., and Koo A. D. (2011) Proteomic analysis of pregnancy-related proteins from pig uterus endometrium during pregnancy. Proteome Sci. 9, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forde N., and Lonergan P. (2012) Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J. Reprod. Dev. 58, 189–195 [DOI] [PubMed] [Google Scholar]
- 17.Salilew-Wondim D., Schellander K., Hoelker M., and Tesfaye D. (2012) Oviductal, endometrial and embryonic gene expression patterns as molecular clues for pregnancy establishment. Anim. Reprod. Sci. 134, 9–18 [DOI] [PubMed] [Google Scholar]
- 18.Bauersachs S., and Wolf E. (2012) Transcriptome analyses of bovine, porcine and equine endometrium during the pre-implantation phase. Anim. Reprod. Sci. 134, 84–94 [DOI] [PubMed] [Google Scholar]
- 19.Garrido-Gómez T., Ruiz-Alonso M., Blesa D., Diaz-Gimeno P., Vilella F., and Simón C.. Profiling the gene signature of endometrial receptivity: clinical results. Fertility Sterility 99, 1078–1085 [DOI] [PubMed] [Google Scholar]
- 20.Mitko K., Ulbrich S. E., Wenigerkind H., Sinowatz F., Blum H., Wolf E., and Bauersachs S. (2008) Dynamic changes in messenger RNA profiles of bovine endometrium during the oestrous cycle. Reproduction 135, 225–240 [DOI] [PubMed] [Google Scholar]
- 21.Gebhardt S., Merkl M., Herbach N., Wanke R., Handler J., and Bauersachs S. (2012) Exploration of Global Gene Expression Changes During the Estrous Cycle in Equine Endometrium. Biol. Reprod. 87, 1–13 [DOI] [PubMed] [Google Scholar]
- 22.Yip K. S., Suvorov A., Connerney J., Lodato N. J., and Waxman D. J. (2013) Changes in mouse uterine transcriptome in estrus and prestrous. Biol. Reprod. 89, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Tan Z., Li M., Xia T., Liu P., and Yu W. (2011) Proteomic analysis of endometrium in fertile women during the prereceptive and receptive phases after luteinizing hormone surge. Fertility Sterility 95, 1161–1163 [DOI] [PubMed] [Google Scholar]
- 24.Rai P., Kota V., Sundaram C. S., Deendayal M., and Shivaji S. (2010) Proteome of human endometrium: Identification of differentially expressed proteins in proliferative and secretory phase endometrium. Proteomics Clin. Appl. 4, 48–59 [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Zhang A., Yu F., Gao J., Liu Y., Yu C., Zhou H., and Xu C. (2015) Label-free proteomics uncovers energy metabolism and focal adhesion regulations responsive for endometrium receptivity. J. Proteome Res. 14, 1831–1842 [DOI] [PubMed] [Google Scholar]
- 26.Dominguez F., Garrido-Gomez T., Lopez J. A., Camafeita E., Quinonero A., Pellicer A., and Simon C. (2009) Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum. Reprod. 24, 2607–2617 [DOI] [PubMed] [Google Scholar]
- 27.Salamonsen L. A., Edgell T., Rombauts L. J. F., Stephens A. N., Robertson D. M., Rainczuk A., Nie G., and Hannan N. J. (2013) Proteomics of the human endometrium and uterine fluid: a pathway to biomarker discovery. Fertility Sterility 99, 1086–1092 [DOI] [PubMed] [Google Scholar]
- 28.Scotchie J. G., Fritz M. A., Mocanu M., Lessey B. A., and Young S. L. (2009) Proteomic analysis of the luteal endometrial secretome. Reprod. Sci. 16, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casado-Vela J., Rodriguez-Suarez E., Iloro I., Ametzazurra A., Alkorta N., García-Velasco J. A., Matorras R., Prieto B., González S., Nagore D., Simón L., and Elortza F. (2009) Comprehensive proteomic analysis of human endometrial fluid aspirate. J. Proteome Res. 8, 4622–4632 [DOI] [PubMed] [Google Scholar]
- 30.Coy P., García-Vázquez F. A., Visconti P. E., and Avilés M. (2012) Roles of the oviduct in mammalian fertilization. Reproduction 144, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgiou A. S., Sostaric E., Wong C. H., Snijders A. P., Wright P. C., Moore H. D., and Fazeli A. (2005) Gametes alter the oviductal secretory proteome. Mol. Cell. Proteomics 4, 1785–1796 [DOI] [PubMed] [Google Scholar]
- 32.Georgiou A. S., Snijders A. P., Sostaric E., Aflatoonian R., Vazquez J. L., Vazquez J. M., Roca J., Martinez E. A., Wright P. C., and Fazeli A. (2007) Modulation of the oviductal environment by gametes. J. Proteome Res. 6, 4656–4666 [DOI] [PubMed] [Google Scholar]
- 33.Seytanoglu A., Georgiou A. S., Sostaric E., Watson P. F., Holt W. V., and Fazeli A. (2008) Oviductal cell proteome alterations during the reproductive cycle in pigs. J. Proteome Res. 7, 2825–2833 [DOI] [PubMed] [Google Scholar]
- 34.Bauersachs S., Rehfeld S., Ulbrich S., Mallok S., Prelle K., Wenigerkind H., Einspanier R., Blum H., and Wolf E. (2004) Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J. Mol. Endocrinol. 32, 449–466 [DOI] [PubMed] [Google Scholar]
- 35.Labas V., Grasseau I., Cahier K., Gargaros A., Harichaux G., Teixeira-Gomes A. P., Alves S., Bourin M., Gerard N., and Blesbois E. (2015) Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteomics 112, 313–335 [DOI] [PubMed] [Google Scholar]
- 36.Keller A., Nesvizhskii A. I., Kolker E., and Aebersold R. (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 [DOI] [PubMed] [Google Scholar]
- 37.Vizcaino J. A., Cote R. G., Csordas A., Dianes J. A., Fabregat A., Foster J. M., Griss J., Alpi E., Birim M., Contell J., O'Kelly G., Schoenegger A., Ovelleiro D., Perez-Riverol Y., Reisinger F., Rios D., Wang R., and Hermjakob H. (2013) The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 41, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S. K., Kim C. J., Kim D. J., and Kang J. H. (2015) Immune cells in the female reproductive tract. Immune Netw. 15, 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zervomanolakis I., Ott H. W., Hadziomerovic D., Mattle V., Seeber B. E., Virgolini I., Heute D., Kissler S., Leyendecker G., and Wildt L. (2007) Physiology of upward transport in the human female genital tract. Ann. N. Y. Acad. Sci. 1101, 1–20 [DOI] [PubMed] [Google Scholar]
- 40.Katz D. F., Morales P., Samuels S. J., and Overstreet J. W. (1990) Mechanisms of filtration of morphologically abnormal human sperm by cervical mucus. Fertil. Steril. 54, 513–516 [PubMed] [Google Scholar]
- 41.Mattner P. E., and Braden A. W. (1969) Comparison of the distribution of motile and immotile spermatozoa in the ovine cervix. Aust. J. Biol. Sci. 22, 1069–1070 [DOI] [PubMed] [Google Scholar]
- 42.Gipson I. K., Moccia R., Spurr-Michaud S., Argueso P., Gargiulo A. R., Hill J. A. 3rd, Offner G. D., and Keutmann H. T. (2001) The Amount of MUC5B mucin in cervical mucus peaks at midcycle. J. Clin. Endocrinol. Metab. 86, 594–600 [DOI] [PubMed] [Google Scholar]
- 43.Gipson I. K., Spurr-Michaud S., Moccia R., Zhan Q., Toribara N., Ho S. B., Gargiulo A. R., and Hill J. A. 3rd (1999) MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol. Reprod. 60, 58–64 [DOI] [PubMed] [Google Scholar]
- 44.Killian G. (2011) Physiology and endocrinology symposium: evidence that oviduct secretions influence sperm function: a retrospective view for livestock. J. Animal Sci. 89, 1315–1322 [DOI] [PubMed] [Google Scholar]
- 45.Avilés M., Gutiérrez-Adán A., and Coy P. (2010) Oviductal secretions: will they be key factors for the future ARTs? Mol. Hum. Reprod. 16, 896–906 [DOI] [PubMed] [Google Scholar]
- 46.Lloyd R. E., Elliott R. M. A., Fazeli A., Watson P. F., and Holt W. V. (2009) Effects of oviductal proteins, including heat shock 70 kDa protein 8, on survival of ram spermatozoa over 48 h in vitro. Reproduction, Fertility Develop. 21, 408–418 [DOI] [PubMed] [Google Scholar]
- 47.DeSouza M. M., and Murray M. K. (1995) An estrogen-dependent secretory protein, which shares identity with chitinases, is expressed in a temporally and regionally specific manner in the sheep oviduct at the time of fertilization and embryo development. Endocrinology 136, 2485–2496 [DOI] [PubMed] [Google Scholar]
- 48.Gonçalves R. F., Staros A. L., and Killian G. J. (2008) Oviductal fluid proteins associated with the bovine zona pellucida and the effect on in vitro sperm–egg binding, fertilization and embryo development. Reproduction Domestic Animals 43, 720–729 [DOI] [PubMed] [Google Scholar]
- 49.King R. S., and Killian G. J. (1994) Purification of bovine estrus-associated protein and localization of binding on sperm. Biol. Reprod. 51, 34–42 [DOI] [PubMed] [Google Scholar]
- 50.Kadam K. M., D'Souza S. J., Bandivdekar A. H., and Natraj U. (2006) Identification and characterization of oviductal glycoprotein-binding protein partner on gametes: epitopic similarity to non-muscle myosin IIA, MYH 9. Mol. Hum. Reprod. 12, 275–282 [DOI] [PubMed] [Google Scholar]
- 51.Elliott R. M., Lloyd R. E., Fazeli A., Sostaric E., Georgiou A. S., Satake N., Watson P. F., and Holt W. V. (2009) Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 137, 191–203 [DOI] [PubMed] [Google Scholar]
- 52.Gabler C., Chapman D., and Killian G. (2003) Expression and presence of osteopontin and integrins in the bovine oviduct during the oestrous cycle. Reproduction 126, 721–729 [PubMed] [Google Scholar]
- 53.Liu Q., Xie Q. Z., Zhou Y., and Yang J. (2014) Osteopontin is expressed in the oviduct and promotes fertilization and preimplantation embryo development of mouse. Zygote 29, 1–9 [DOI] [PubMed] [Google Scholar]
- 54.Topfer-Petersen E., Romero A., Varela P. F., Ekhlasi-Hundrieser M., Dostalova Z., Sanz L., and Calvete J. J. (1998) Spermadhesins: a new protein family. Facts, hypotheses and perspectives. Andrologia 30, 217–224 [DOI] [PubMed] [Google Scholar]
- 55.Topfer-Petersen E., and Calvete J. J. (1996) Sperm-associated protein candidates for primary zona pellucida-binding molecules: structure-function correlations of boar spermadhesins. J. Reprod. Fertil. Suppl. 50, 55–61 [PubMed] [Google Scholar]
- 56.Ekhlasi-Hundrieser M., Sinowatz F., Greiser De Wilke I., Waberski D., and Topfer-Petersen E. (2002) Expression of spermadhesin genes in porcine male and female reproductive tracts. Mol. Reprod. Dev. 61, 32–41 [DOI] [PubMed] [Google Scholar]
- 57.Li S. H., Huang H. L., and Chen Y. H. (2002) Ovarian steroid-regulated synthesis and secretion of complement C3 and factor B in mouse endometrium during the natural estrous cycle and pregnancy period. Biol. Reprod. 66, 322–332 [DOI] [PubMed] [Google Scholar]
- 58.Brown E. O., Sundstrom S. A., Komm B. S., Yi Z., Teuscher C., and Lyttle C. R. (1990) Progesterone regulation of estradiol-induced rat uterine secretory protein, complement C3. Biol. Reprod. 42, 713–719 [DOI] [PubMed] [Google Scholar]
- 59.Jones M. A., Kolb W. P., and Harper M. J. (1988) The presence of the fifth component of complement (C5) in rabbit uterine flushings in relation to reproductive state. Immunol. Invest. 17, 63–75 [DOI] [PubMed] [Google Scholar]
- 60.Lee Y.-L., Cheong A. W. Y., Chow W.-N., Lee K.-F., and Yeung W. S. B. (2009) Regulation of complement-3 protein expression in human and mouse oviducts. Molecular Reproduction Development 76, 301–308 [DOI] [PubMed] [Google Scholar]
- 61.Carney D. F., Haviland D. L., Noack D., Wetsel R. A., Vik D. P., and Tack B. F. (1991) Structural aspects of the human C5 gene. Intron/exon organization, 5′-flanking region features, and characterization of two truncated cDNA clones. J. Biol. Chem. 266, 18786–18791 [PubMed] [Google Scholar]
- 62.Fabryova K., and Simon M. (2009) Function of the cell surface molecules (CD molecules) in the reproduction processes. Gen. Physiol. Biophys. 28, 1–7 [PubMed] [Google Scholar]
- 63.Anderson D. J., Abbott A. F., and Jack R. M. (1993) The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc. Natl. Acad. Sci. U.S.A. 90, 10051–10055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deroo B. J., Hewitt S. C., Peddada S. D., and Korach K. S. (2004) Estradiol regulates the thioredoxin antioxidant system in the mouse uterus. Endocrinology 145, 5485–5492 [DOI] [PubMed] [Google Scholar]
- 65.Baiza-Gutman L. A., Flores-Sanchez M. M., Diaz-Flores M., and Hicks J. J. (2000) Presence of uterine peroxidase activity in the rat early pregnancy. Int. J. Biochem. Cell Biol. 32, 255–262 [DOI] [PubMed] [Google Scholar]
- 66.Fang L., Chatterjee S., Dong Y. L., Gangula P. R., and Yallampalli C. (1998) Immunohistochemical localization of constitutive and inducible cyclo-oxygenases in rat uterus during the oestrous cycle and pregnancy. Histochem. J. 30, 383–391 [DOI] [PubMed] [Google Scholar]
- 67.Hosoya T., and Saito T. (1981) Comparative studies on estrogen-dependent peroxidases contained in uterine microsomes and fluid of rats and pigs. J. Biochem. 89, 203–215 [DOI] [PubMed] [Google Scholar]
- 68.Lapointe J., and Bilodeau J. F. (2003) Antioxidant defenses are modulated in the cow oviduct during the estrous cycle. Biol. Reprod. 68, 1157–1164 [DOI] [PubMed] [Google Scholar]
- 69.Lei W., Nguyen H., Brown N., Ni H., Kiffer-Moreira T., Reese J., Millán J. L., and Paria B. C. (2013) Alkaline phosphatases contribute to uterine receptivity, implantation, decidualization, and defense against bacterial endotoxin in hamsters. Reproduction 146, 419–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Vega S., Iwamoto T., and Yamada Y. (2009) Fibulins: multiple roles in matrix structures and tissue functions. Cell. Mol. Life Sci. 66, 1890–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haendler B., Yamanouchi H., Lessey B. A., Chwalisz K., and Hess-Stumpp H. (2004) Cycle-dependent endometrial expression and hormonal regulation of the fibulin-1 gene. Mol. Reprod. Dev. 68, 279–287 [DOI] [PubMed] [Google Scholar]
- 72.Okada H., Nakajima T., Yoshimura T., Yasuda K., and Kanzaki H. (2003) Microarray analysis of genes controlled by progesterone in human endometrial stromal cells in vitro. Gynecol. Endocrinol. 17, 271–280 [DOI] [PubMed] [Google Scholar]
- 73.Gray C. A., Abbey C. A., Beremand P. D., Choi Y., Farmer J. L., Adelson D. L., Thomas T. L., Bazer F. W., and Spencer T. E. (2006) Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol. Reprod. 74, 383–394 [DOI] [PubMed] [Google Scholar]
- 74.Bauersachs S., Ulbrich S. E., Gross K., Schmidt S. E. M., Meyer H. H. D., Wenigerkind H., Vermehren M., Sinowatz F., Blum H., and Wolf E. (2006) Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction 132, 319–331 [DOI] [PubMed] [Google Scholar]
- 75.Walker C. G., Meier S., Littlejohn M. D., Lehnert K., Roche J. R., and Mitchell M. D. (2010) Modulation of the maternal immune system by the pre-implantation embryo. BMC Genomics 11, 1471–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merkl M., Ulbrich S. E., Otzdorff C., Herbach N., Wanke R., Wolf E., Handler J., and Bauersachs S. (2010) Microarray Analysis of Equine Endometrium at Days 8 and 12 of Pregnancy. Biol. Reprod. 83, 874–886 [DOI] [PubMed] [Google Scholar]
- 77.Samborski A., Graf A., Krebs S., Kessler B., Reichenbach M., Reichenbach H.-D., Ulbrich S. E., and Bauersachs S. (2013) Transcriptome changes in the porcine endometrium during the preattachment phase. Biol. Reprod. 89, 1–16 [DOI] [PubMed] [Google Scholar]
- 78.Samborski A., Graf A., Krebs S., Kessler B., and Bauersachs S. (2013) Deep Sequencing of the Porcine Endometrial Transcriptome on Day 14 of Pregnancy. Biol. Reprod. 88, 1–13 [DOI] [PubMed] [Google Scholar]
- 79.Chen X., Li A., Chen W., Wei J., Fu J., and Wang A. (2015) Differential gene expression in uterine endometrium during implantation in pigs. Biol. Reprod. 92, 1–17 [DOI] [PubMed] [Google Scholar]
- 80.Forde N., McGettigan P. A., Mehta J. P., O'Hara L., Mamo S., Bazer F. W., Spencer T. E., and Lonergan P. (2014) Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 147, 575–587 [DOI] [PubMed] [Google Scholar]
- 81.Ledgard A. M., Lee R. S. F., and Peterson A. J. (2009) Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus. Mol. Reproduction Development 76, 65–74 [DOI] [PubMed] [Google Scholar]
- 82.Koch J. M., Ramadoss J., and Magness R. R. (2010) Proteomic Profile of Uterine Luminal Fluid from Early Pregnant Ewes. J. Proteome Res. 9, 3878–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Franczak A., Wojciechowicz B., Kolakowska J., Zglejc K., and Kotwica G. (2014) Transcriptomic analysis of the myometrium during peri-implantation period and luteolysis–the study on the pig model. Funct. Integr. Genomics 14, 673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Binder N. K., Evans J., Gardner D. K., Salamonsen L. A., and Hannan N. J. (2014) Endometrial signals improve embryo outcome: functional role of vascular endothelial growth factor isoforms on embryo development and implantation in mice. Hum. Reprod. 29, 2278–2286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.