Synopsis
Osteonecrosis of the jaw, at one time considered to be infrequent has now become a major public health concern not only in the United States, but throughout the world. The wide-spread use of radiotherapy for head and neck cancer as well as bone antiresorptives and antiangiogenic agents have increased the incidence of osteonecrosis. While the exact pathophysiological process of osteonecrosis is yet to be clearly defined, there has been a much higher incidence of medication-related osteonecrosis of the jaw relative to the other types of osteonecrosis. The traditional osteoradionecrosis still occurs despite better treatment planning and shielding to minimize collateral damage to bone. There are other related necrotic lesions secondary to usage of recreation drugs and the use of steroids. This chapter will give comprehensive information about these different types of bone necrosis; provide the readers with radiographic diagnostic criteria and updates on current theories on pathophysiology of osteonecrosis.
Keywords: Osteoradionecrosis, Medication-related Osteonecrosis, chemical, radiation, recreational drug, Damage, Diseases, Necrosis
1. Introduction
Bone is a unique connective tissue because it is functionally dynamic, consisting of different cells that continuously interact together. Unlike other connective tissues within the body, bone is physiologically mineralized. There is also an abundance of osteoprogenitor cells that reside within the bone microenvironment that can be activated to form different cell types 1. The ability of bone to constantly remodel plays a vital role in the maintenance of mineral homeostasis as old bone is removed by the activities of osteoclasts and new bone matrix is deposited by osteoblasts. Essentially, external and internal insults from radiation, drugs or other chemical insults can induce a pathological process that disrupts the bone microenvironment, turnover and homeostasis. The outcome is dysregulation of the bone healing process that can potentially lead to loss of bone tissue as in osteonecrosis.
Osteonecrosis is characterized by tissue dehiscence, chronic bone devitalization, hypocellularity and osteolysis. The term osteonecrosis is often used interchangeably with ischemic necrosis, avascular necrosis or aseptic necrosis but there are different types of osteonecrosis. Depending on the etiological agent, osteonecrosis can occur in any bone including the orofacial, appendicular and axial bones. Osteonecrosis may or may not be associated with exposed bone with delayed healing. Specifically, the femoral head and mandible are highly susceptible to osteonecrosis. In the orofacial region, jaw osteonecrosis can lead to significant loss of bone tissue, tooth loss and facial disfigurement. The unfortunate outcomes are significant morbidity, debility and diminished quality of life 2. The high susceptibility of the femoral bone to osteonecrosis is associated with a variety of factors that include alcohol abuse and steroid therapy. However, jaw osteonecrosis is much more associated with complications of radiation therapy, and long term therapy with bone antiresorptives used to control skeletal events of cancer metastasis and osteoporosis 3, 4. In a randomized controlled study that assessed 792 cases of osteonecrosis in general, 76% of the cases occurred in the hip while 4.4% occurred in the jaw mainly as a result of bisphosphonate therapies. The remaining cases were associated with either the wrist, knee, foot or ankle 5. Several pathophysiologic theories have been proposed for osteonecrosis based on correlations of clinical signs with histologic and radiologic analyses. Although many of these theories have not been conclusively established, radiographic imaging has played a major role in the diagnosis, management and follow up assessment of osteonecrosis. Even more importantly, is the increasing use of the combination of functional imaging with planar images to fully understand the metabolic changes that lead to osteonecrosis 6.
2. Types of osteonecrosis
Osteoradionecrosis
Osteoradionecrosis (ORN) of the jaw is defined as non-healing bony exposure and necrosis that starts with a breach in the oral mucosa, and persists for at least 3 months in a patient that has undergone previous radiation therapy. The necrosis however, must be evidently different from a recurrent, vestigial or metastatic tumor 7, 8. This definition however does not include cases of ORN in which the oral mucosa is intact, but osteonecrotic changes can be observed by diagnostic imaging 9. ORN is a chronic condition that can last for months or even years after the initial radiation therapy. The incidence of ORN can range from 2.6 - 22% 10 and it develops when the radiation dose exceeds 50 Gy. Specifically, radiation doses between 50-70Gy have been implicated in the etiology of ORN 11. Within the orofacial complex, the mandible is commonly affected because the mandible is usually in the line of radiation delivery and it is believed that the mandible is less vascularized than the maxilla 12, 13. Radiation also affects teeth secondarily due to pronounced xerostomia noted in patients receiving radiation therapy (fig 1). The extensive carious lesions can be readily noted on radiographs as demonstrated in these bitewings (figs 2, 3)
Fig 1.

Intraoral photograph of a patient who received radiation therapy for oral cancer. Note the several complications of radiation therapy that include extensive caries involving multiple surfaces including incisal edges (arrow heads). The chalky white appearance of teeth is characteristic of radiation caries. Also note the angular cheilitis due to radiation induced xerostomia (arrow)
Fig 2.

Right premolar bitewing radiograph of a patient who received radiation therapy for head and neck cancer. Note the extensive carious lesions and failing restorations.
Fig 3.

Left premolar bitewing of the same patient as in fig 2 with evidence of extensive dental caries due to a combination of decreased salivation and increased biofilm.
Pathogenesis
Osteoradionecrosis was first described in 1926 14. It was not until 1970 that a triad of radiation, trauma and infection was proposed as the mechanistic process in ORN. However, this theory was later replaced in 1983 by another proposal that radiation causes development of hypoxic-hypocellular-hypovascular tissue (3H theory) 15 when it was reported that microorganisms do not play any causative but rather a contaminant role in ORN. It was also reported that trauma mainly creates a portal of entry for microorganisms to invade the radiation-suppressed bone. The 3H theory of ORN takes into account that several tissues from exterior to interior are damaged by radiation ranging from the skin or mucosa to periosteum, bone and finally endothelium within the bone marrow compartment 16. So the combination of tissue fibrosis, vascular and cellular damage induces a hypoxic environment within the radiated tissue 16. The 3H theory was quickly followed by development of hyperbaric oxygen (HBO) therapy protocols to prevent and treat ORN, but this had only modest effectiveness and HBO therapy is limited because it is contraindicated in patients with metastatic cancer 3, 4, 17.
The radiation induced fibroatrophic process is another mechanism associated with radiation damage more commonly to the superficial structures. This is associated with the activity of reactive oxygen species that cause damage to fibroblasts, endothelium and bone cells eventually causing tissue and bone necrosis (fig 4). However, the direct application of this theory to deep seated radiation damage within the bone is yet to be conclusively clarified 16. Another proposed pathophysiologic hypothesis is that ORN can be precipitated by a combination of dysregulated turnover, osteoclast depletion, local tissue injury and infection 11. As all these theories do not conclusively define the pathophysiological process of ORN, more work is still needed to further our understanding of ORN pathogenesis.
Fig 4.

Osteoradionecrosis of the mandible. This patient received radiation therapy for head and neck cancer. The panoramic radiographs before radiation therapy (A) show intact and well corticated outline of the mandible. However, the patient developed left mandibular osteoradionecrosis (B, red arrow) after undergoing post-irradiation extraction of the left mandibular premolars.
Risk factors and classification
Several risk factors predispose to ORN; these include poor oral health, smoking, alcohol abuse and most importantly type and dose of radiation. Brachytherapy and radiation doses greater than 50 Gy have been associated with higher incidence of ORN. Additionally, any surgical manipulations of the irradiated area including dental extractions pose a significant risk. Several different classification of ORN have been proposed, but one recently proposed theory combines clinical description, presence or absence of symptoms and the treatment option for each of the different stages of ORN (Table 1) 9.
Table 1.
Classification of ORN.
Adapted from Lyons A, Osher J, Warner E, Kumar R, Brennan PA. Osteoradionecrosis A review of current concepts in defining the extent of the disease and a new classification proposal. British Journal of Oral and Maxillofacial Surgery 2014 5;52(5):392; with permission.
| Stage | Length of affected bone/associated structures (damaged/exposed) | Presence/absence of symptoms | Treatment |
|---|---|---|---|
| 1 | <2.5cm | Asymptomatic | Medication treatment only |
| 2 | >2.5cm | Asymptomatic. Includes pathological fracture and/or involvement of inferior dental nerve. |
Medication treatment only; except for presence of dental sepsis and loose/necrotic bone |
| 3 | >2.5cm | Symptomatic. No other features of bone necrosis. However, symptoms persist despite medication treatment. |
Debridement of loose/necrotic bone. Local pedicle flap |
| 4 | >2.5cm | Symptomatic. Pathological fracture. Involvement of Inferior alveolar nerve and/or orocutaneous fistula |
Reconstruction with free flap, if patient's overall health allows |
Clinical presentation
ORN patients usually present with pain, typically neuropathic in nature, swelling and may be accompanied by fever depending on the extent of the inflammatory process. Follow up clinical evaluation may reveal tissue breakdown and bone necrosis that may be accompanied by paresthesia/anesthesia. If untreated, ORN especially in the mandible may result in pathological fracture. Definitive diagnosis of ORN is a combination of clinical, radiologic and histologic evaluations.
Medication-Related Osteonecrosis of the Jaws
Medication related osteonecrosis of the jaw (MRONJ) is a more recent class of jaw osteonecrosis first described in 2003 in patients taking nitrogen-containing bisphosphonates 18. It is defined as exposed bone in the intraoral cavity persisting for 8 weeks or more, in patients that have previously undergone, or are currently undergoing treatment with antiresorptives and/or antiangiogenic agents and with no prior history of radiation therapy to the jaw. This excludes primary or metastatic cancer within the jaw region (Ruggiero et al. 2014). However, this definition does not take into account the non-exposed bone variant of the disease process, which makes up about a third of all cases of MRONJ cases 19.
Nitrogen containing bisphosphonates (nBP) especially the intravenous nBP such as zoledronic acid were the first group of drugs initially associated with MRONJ. The high efficacy of intravenous nBP such as zoledronic acid and pamidronate to control altered bone remodeling make them highly favored for the treatment of skeletal events of cancer metastasis, Paget's disease, osteogenesis imperfecta and hyperparathyroid jaw tumors, so it is understandable that nBP were the first to be associated with osteonecrosis exclusive to the jaws. Other medication have also been implicated in MRONJ; these include another antiresorptive drug, denosumab that acts as an inhibitor of receptor activator for NFκB ligand (RANKL) and anti-angiogenic drugs like bevacizumab, an inhibitor of VEGF and sunitinib, a tyrosine kinase inhibitor.
Due to the vast array of medications associated with osteonecrosis of the jaw (ONJ), the nomenclature for this disorder has evolved over the years from ONJ, BON, BRONJ and ARONJ to the more recent MRONJ 20. The incidence of MRONJ in patients taking intravenous nBP ranges from 0 - 27.5% 19, 20, denosumab 1.7% 19.The relative risk of MRONJ occurring in patients taking intravenous nBP, denosumab or bevacizumab are 0.7- 6.7%, 0.7 – 1.9% and 0.2% respectively 20. MRONJ affects the mandible and maxilla at a ratio of 2: 1 21 because the mandible is partly associated with a single vascular supply from the inferior alveolar artery compared to the superior, inferior and middle arteries in the maxilla. Severe cases of MRONJ in the mandible can lead to pathological fracture while in the maxilla it can result in oro-antral fistulation.
Pathogenesis
The pathophysiology of MRONJ is still unclear but different investigators have proposed several theories 22, 23. These include a decrease in bone turnover; presence of infection (especially Actinomyces species); inhibition of angiogenesis; and a dysregulation or dysfunction of innate and acquired immunity 24. The infection theory is based on the premise that a “complex biofilm” is present on the surface of exposed necrotic bone 25. Definitive elucidation of MRONJ pathogenesis is hampered by the fact that the offending drugs have different mechanisms of action (see Table 2) and non-oral bones are spared by MRONJ. The role of bone mesenchymal stem (MSCs) can also not be overlooked considering that jaw MSCs are phenotypically and functionally different from those of axial and appendicular bones 1 and are disproportionately more sensitive to both zoledronic acid and pamidronate both of which are strongly associated with MRONJ 26.
Table 2. Implicated drugs in MRONJ and their mechanisms of action.
| Zoledronic acid | Denosumab | Bevacizumab | |
|---|---|---|---|
| Half life | Binds to bone-longer half life | Does not bind to bone – short half- life (approx. 28 days) | Does not bind to bone – short half-life (approx. 20 days) |
| Mechanism | Apoptosis of osteoclasts | Prevents formation of osteoclasts | Inhibits angiogenesis |
| Target pathways/transcription proteins | Inhibits Farnesyl Pyrophosphate (FPP) through mevalonate pathway | Inhibits Receptor activator of nuclear factor kappa-B ligand (RANKL) | Inhibits tyrosine kinase by binding to :Vascular Endothelial Growth Factor (VEGF) |
| Effects on immune system | No effect on immune system | Tendency to cause immunosuppression by action on B and T cells | Has effects on the immune system |
| Clearance | Clearance through kidneys – nephrotoxic (up to 12 years) | Clearance through immunoglobulin pathway in reticuloendothelial system (within 6 months) | Clearance by pinocytosis with binding to neonatal Fc receptor 27 |
Risk factors and classification
The local risk factors that may predispose to MRONJ include trauma, overall poor dental health, presence of tori or bony exostosis, and invasive dental procedures such as dental extractions and periodontal treatment. In addition, the systemic risk factors include diabetes, smoking, alcohol and an ongoing immunosuppressive therapy 28.
Clinical presentation
Patients with MRONJ have variable clinical presentation depending on the clinical course of the disease. This can vary from non-exposed bone to extensive bone loss and pathological fracture (fig 5). Therefore, a staging algorithm has been proposed to aid not only in the diagnosis but also management of MRONJ (see table 3). A detailed history, clinical examination and carefully selected radiographic imaging will aid in the diagnosis of MRONJ. (figs 6-7)
Fig 5.

Exposed bone in the right mandibular posterior region in a patient with medication related osteonecrosis of the jaw.
Courtesy of Drs. Arthur Kuperstein DDS and Mel Mupparapu, DMD, University of Pennsylvania School of Dental Medicine.
Table 3. Clinical staging of MRONJ.
Adapted from Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update. Journal of Oral and Maxillofacial Surgery 2014 9 March 2015;72(10):1938; with permission.
| Clinical staging | Presentation |
|---|---|
| Stage 0 | Non- specific odontogenic symptoms in patients with a history of antiresorptive treatments |
| Stage 1 | Asymptomatic bony exposure |
| Stage 2 | Bony exposure, pain and infection, well contained in the dento-alveolar area |
| Stage 3 | Stage 2 disease symptoms extending beyond the alveolar area. Includes pathological fractures, oroanthral communication, maxillary sinusitis, sinuses and fistula. |
Fig 6.

Panoramic reconstruction of the mandible and maxilla in a patient with medication related osteonecrosis of the jaw (arrow points to the area of osteonecrosis).
Courtesy of Drs. Arthur Kuperstein DDS and Mel Mupparapu, DMD, University of Pennsylvania School of Dental Medicine.
Fig 7.
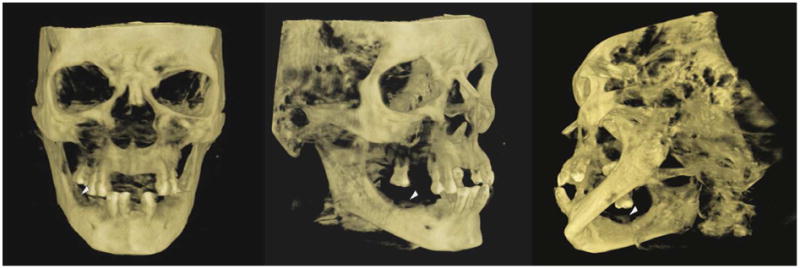
Same patient as in figures 5 and 6. CBCT three dimensional reconstruction of the facial skeleton showing the separated osteonecrotic alveolar portion of the mandible (arrows heads).
Courtesy of Drs. Arthur Kuperstein DDS and Mel Mupparapu, DMD, University of Pennsylvania School of Dental Medicine.
Recreational drug induced osteonecrosis
Chronic use of recreational or illicit drugs such as cocaine, amphetamine and methamphetamine are established independent risk factors for osteonecrosis, termed Recreational drug induced osteonecrosis (RDIO) 29-32. It is more common in the maxilla 29. The incidence of recreational drug induced osteonecrosis is unknown because many addicts do not seek medical care. Additionally, most illicit drug addicts are also heavy smokers and may be abusing alcohol or other prescription drugs, which also heightens their predisposition to osteonecrosis 31. The pathogenesis of RDIO is multifactorial. Although several recreational drugs have been implicated, cocaine in particular induces vascular constriction that results in local ischemia of the adjacent soft and hard tissues 33. It has been proposed that the combined effect of chemical irritation from additives to the recreational drug, trauma and superimposed microbial infection accentuate the necrotic process that leads to bone destruction 32. Therefore, excessive bone destruction caused by nasal inhalation or snorting of a recreational drug like cocaine initially starts as ulceration of mucosal tissue that progressively leads to osteocartilaginous necrosis. If uncontrolled, the nasal septum and palate become perforated consequently leading to oro-nasal and oro-antral fistulations 30, 34. Specifically, cocaine-induced midline destructive lesion (CIMDL) has been used to describe extensive destruction caused by cocaine addiction to the oro-nasal structures including the hard palate 32. This extensive osteonecrosis of the bony structures is one of the hallmarks used to identify CIMDL in the assessment of human skeletal remains by medical examiners and forensic scientists. If the addicted individual seeks treatment early, which often does not happen, RDIO can be controlled before extensive bone destruction occurs by discontinuation of the offending recreational drug
Steroid Induced Osteonecrosis
Steroid induced osteonecrosis also referred to as aseptic, ischemic, or avascular necrosis develops as a result of long-term administration of corticosteroids. It is more common in the appendicular bones such as the femur or humerus rather than the jaw35, 36 and it is not induced by trauma or external insults, so it is a form of atraumatic osteonecrosis. Corticosteroids affects multiple organs and systems, it has immunosuppressive effects and can disrupt bone homeostasis 36. Long-term use of corticosteroids for more than 20 mg per day can result in osteopenia and eventually osteoporosis. To further complicate the situation, the patient may need to be placed on bisphosphonate therapy to control dysregulated bone remodeling, consequently resulting in the development of medication-induced osteonecrosis of the jaw. Smoking, alcohol abuse and comorbid conditions like osteoporosis and systemic lupus erythematosus predispose patients to steroid induced osteonecrosis. 37
3. Histological features of osteonecrosis
Definitive diagnosis of osteonecrosis is based on clinical, histological and radiological findings. Histologically, vascular damage with hyperemia, inflammatory cells and osteoclastic activity are often seen at the early stages of osteonecrosis. Thereafter, osteonecrosis displays regions of acellular marrow with loss of hematopoietic cells, adipocytic infiltration suggestive of fatty marrow and some regions of patchy calcifications within the marrow components due to osteoclastic activity. The bone is also hypocellular with regions of empty lacunae devoid of osteocytic nuclei. There may be attempts at healing demonstrated by reparative granulation tissue and some degree of new osteoid deposition. Histological features of osteonecrosis are similar in the oral and non-oral bone and are independent of the bone type.
4. Radiological features of osteonecrosis
Radiological features of osteonecrosis must be correlated with histological findings. A good starting point for diagnosis of osteonecrosis is the use of plain film and panoramic radiography (figure 2), cone beam computerized tomography (CBCT) (figure 3) conventional CT (figure 4). Plain film radiographs will display mixed radiolucent and radiopaque trabecular pattern. Depending on the cause of the osteonecrosis, there could be regions of osteolysis and sclerosis. Similarly, CT will display altered trabeculation, cortical thinning and sclerosis. Unfortunately, there needs to be about 30-50% loss of bone mineral content for a bone lesion to be better defined in a panoramic radiograph or CT 6.
Other limits of panoramic radiograph and CT are that some of the radiographic features are not specific to osteonecrosis; also these imaging modalities cannot differentiate necrotic bone from metastatic lesion especially in oncology patients where this may be a concern 38. Bone scintigraphy or bone scan provides better information about the metabolic activities and pathophysiological changes in osteonecrosis much earlier than panoramic radiograph as osteonecrosis will display high uptake of 99mTc-methylene diphosphonate in a bone scan. But a major diagnostic challenge of using bone scintigraphy to diagnose osteonecrosis is its high signal sensitivity and low specificity as many dental disorders can present as osteonecrosis in a bone scan.
The additional use of the combinations of fluorodeoxyglucose/positron emission tomography (FDG/PET) and single photon emission computed tomography (SPECT)/CT combination have also been shown to improve diagnostic accuracy because they provide functional and anatomic co-registration of the extent of the osteonecrosis 32, 39-41. Magnetic resonance imaging (MRI) can display osteonecrosis as decreased marrow signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. Interestingly, studies using osteonecrosis of the hip samples have also shown that magnetic resonance imaging (MRI) findings correlate well with histological features of osteonecrosis 42. As MRI displays excellent visualization of soft tissues, it may be useful in the diagnosis of osteonecrosis in the mandibular condyle and around the TMJ complex where it may be clinically impracticable to obtain biopsy samples
5. Conclusion
The role of radiographic imaging in diagnosis of osteonecrotic lesions cannot be overemphasized. However, there are limitations in the use of radiologic imaging only as the standard of diagnosis without correlating the findings with other aspects of diagnosis - clinical presentation and histologic findings. This is because most types of jaw osteonecrosis have similar radiographic presentations and cannot be differentiated from other diseases affecting the jaw bone e.g. osteomyelitis and periapical lesions.
Treatment options for jaw osteonecrosis includes the use of local and systemic antibiotics; pain medications; debriding; sequestrectomy; hyperbaric oxygen treatment; use of antioxidants-tocopherol and Pentoxifylline 43. Surgical resection is usually a last resort, when all other forms of therapy fail.
The effects on the quality of life in patients with jaw osteonecrosis, makes it an important area of research especially to researchers interested in bone and tissue engineering. An interesting area of ongoing research right now is the possible role of mesenchymal stem cells in possible reconstruction of the defect caused by jaw osteonecrosis and the use of pharmacologic compounds e.g. anti-sclerostin antibody in the prevention of the osteonecrotic process especially in patients with MRONJ 44-47.
Key Points.
The role of radiographic imaging in diagnosis of osteonecrotic lesions cannot be overemphasized.
Treatment options for jaw osteonecrosis includes the use of local and systemic antibiotics; pain medications; debriding; sequestrectomy; hyperbaric oxygen treatment; use of antioxidants-tocopherol and pentoxifylline. Surgical resection is usually a last resort, when all other forms of therapy fail.
The effects on the quality of life in patients with jaw osteonecrosis, make it an important area of research especially to researchers interested in bone and tissue engineering.
Acknowledgments
This work was supported in part by the grants K22CA169089 and R21DE022826 (awarded to SOA) by United States Department of Health and Human Services/National Institutes of Health, Bethesda MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo. copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006 Jun;38(6):758–68. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010 Feb 1;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aapro M, Saad F, Costa L. Optimizing clinical benefits of bisphosphonates in cancer patients with bone metastases. Oncologist. 2010;15(11):1147–58. doi: 10.1634/theoncologist.2007-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauri D, Valachis A, Polyzos NP, Tsali L, Mavroudis D, Georgoulias V, et al. Does adjuvant bisphosphonate in early breast cancer modify the natural course of the disease? A meta-analysis of randomized controlled trials. J Natl Compr Canc Netw. 2010 Mar;8(3):279–86. doi: 10.6004/jnccn.2010.0020. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int. 2009 Jun 23; doi: 10.1007/s00198-009-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedogni A, Fedele S, Bedogni G, Scoletta M, Favia G, Colella G, et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br J Oral Maxillofac Surg. 2014 Sep;52(7):603–8. doi: 10.1016/j.bjoms.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Madrid C, Abarca M, Bouferrache K. Osteoradionecrosis: An update. Oral Oncol. 2010 Mar 4;46(6):471. doi: 10.1016/j.oraloncology.2010.03.017. 2015. [DOI] [PubMed] [Google Scholar]
- 8.Pitak-Arnnop P, Sader R, Dhanuthai K, Masaratana P, Bertolus C, Chaine A, et al. Management of osteoradionecrosis of the jaws: An analysis of evidence. European Journal of Surgical Oncology. 2008 Apr 8;34(10):1123. doi: 10.1016/j.ejso.2008.03.014. 2015. [DOI] [PubMed] [Google Scholar]
- 9.Lyons A, Osher J, Warner E, Kumar R, Brennan PA. OsteoradionecrosisóA review of current concepts in defining the extent of the disease and a new classification proposal. British Journal of Oral and Maxillofacial Surgery. 2014;52(5):392. doi: 10.1016/j.bjoms.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza J, Lowe D, Rogers SN. Changing trends and the role of medical management on the outcome of patients treated for osteoradionecrosis of the mandible: experience from a regional head and neck unit. British Journal of Oral and Maxillofacial Surgery. 2014;52(4):356. doi: 10.1016/j.bjoms.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 11.McCaul JA. Pharmacologic modalities in the treatment of osteoradionecrosis of the jaw. Oral Maxillofac Surg Clin North Am. 2014 May;26(2):247–52. doi: 10.1016/j.coms.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Parliament M, Alidrisi M, Munroe M, Wolfaardt J, Scrimger R, Thompson H, et al. Implications of radiation dosimetry of the mandible in patients with carcinomas of the oral cavity and nasopharynx treated with intensity modulated radiation therapy. Int J Oral Maxillofac Surg. 2005 Mar;34(2):114–21. doi: 10.1016/j.ijom.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Salama JK, Vokes EE, Chmura SJ, Milano MT, Kao J, Stenson KM, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006 Feb 1;64(2):382–91. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Phemister DB. Radium necrosis of bone. Am J Roentgenol. 1926;16:340. [Google Scholar]
- 15.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 1983 May;41(5):283–8. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 16.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004 Nov;73(2):119–31. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Marx RE. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg. 1983;41(6):351–7. doi: 10.1016/s0278-2391(83)80005-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004 May;62(5):527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Campisi G, Fedele S, Fusco V, Pizzo G, Di Fede O, Bedogni A. Epidemiology, clinical manifestations, risk reduction and treatment strategies of jaw osteonecrosis in cancer patients exposed to antiresorptive agents. Future Oncology. 2014 Apr 2;10(2):257. doi: 10.2217/fon.13.211. 2015. [DOI] [PubMed] [Google Scholar]
- 20.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update. Journal of Oral and Maxillofacial Surgery. 2014 Mar 9;72(10):1938. doi: 10.1016/j.joms.2014.04.031. 2015. [DOI] [PubMed] [Google Scholar]
- 21.Franco-Pretto E, Pacheco M, Moreno A, Messa O, Gnecco J. Bisphosphonate-induced osteonecrosis of the jaws: clinical, imaging, and histopathology findings. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology. 2014;118(4):408. doi: 10.1016/j.oooo.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Landesberg R, Woo V, Cremers S, Cozin M, Marolt D, Vunjak-Novakovic G, et al. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann N Y Acad Sci. 2011 Feb;1218:62–79. doi: 10.1111/j.1749-6632.2010.05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarin J, DeRossi SS, Akintoye SO. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Dis. 2008 Apr;14(3):277–85. doi: 10.1111/j.1601-0825.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Gromov K, Proulx ST, Xie C, Li J, Crane DP, et al. Effects of antiresorptive agents on osteomyelitis: novel insights into the pathogenesis of osteonecrosis of the jaw. Ann N Y Acad Sci. 2010 Mar;1192:84–94. doi: 10.1111/j.1749-6632.2009.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsarelis H, Shah NP, Dhariwal DK, Pazianas M. Infection and medication-related osteonecrosis of the jaw. J Dent Res. 2015 Apr;94(4):534–9. doi: 10.1177/0022034515572021. [DOI] [PubMed] [Google Scholar]
- 26.Stefanik D, Sarin J, Lam T, Levin L, Leboy PS, Akintoye SO. Disparate osteogenic response of mandible and iliac crest bone marrow stromal cells to pamidronate. Oral Diseases. 2008 Jul;14(5):465–71. doi: 10.1111/j.1601-0825.2007.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist. 2010;15(8):819–25. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M. Management of bisphosphonate-associated osteonecrosis: Pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2010 Mar 4;110(5):593. doi: 10.1016/j.tripleo.2010.05.067. 2015. [DOI] [PubMed] [Google Scholar]
- 29.Rustemeyer J, Melenberg A, Junker K, Sari-Rieger A. Osteonecrosis of the maxilla related to long-standing methamphetamine abuse: a possible new aspect in the etiology of osteonecrosis of the jaw. Oral Maxillofac Surg. 2014 Jun;18(2):237–41. doi: 10.1007/s10006-014-0449-2. [DOI] [PubMed] [Google Scholar]
- 30.Seyer BA, Grist W, Muller S. Aggressive destructive midfacial lesion from cocaine abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002 Oct;94(4):465–70. doi: 10.1067/moe.2002.126020. [DOI] [PubMed] [Google Scholar]
- 31.Ziraldo L, O'Connor MB, Blake SP, Phelan MJ. Osteonecrosis following alcohol, cocaine, and steroid use. Subst Abus. 2011 Jul;32(3):170–3. doi: 10.1080/08897077.2011.562751. [DOI] [PubMed] [Google Scholar]
- 32.Rubin K. The manifestation of cocaine-induced midline destructive lesion in bone tissue and its identification in human skeletal remains. Forensic Sci Int. 2013 Sep 10;231(1-3):408 e1–11. doi: 10.1016/j.forsciint.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Nastro Siniscalchi E, Gabriele G, Cascone P. Palatal fistula resulting from cocaine abuse: a case report. Eur Rev Med Pharmacol Sci. 2012 Feb;16(2):280–2. [PubMed] [Google Scholar]
- 34.Goodger NM, Wang J, Pogrel MA. Palatal and nasal necrosis resulting from cocaine misuse. Br Dent J. 2005 Mar 26;198(6):333–4. doi: 10.1038/sj.bdj.4812171. [DOI] [PubMed] [Google Scholar]
- 35.Chiu CT, Chiang WF, Chuang CY, Chang SW. Resolution of oral bisphosphonate and steroid-related osteonecrosis of the jaw--a serial case analysis. J Oral Maxillofac Surg. 2010 May;68(5):1055–63. doi: 10.1016/j.joms.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Powell C, Chang C, Gershwin ME. Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin Rev Allergy Immunol. 2011 Aug;41(1):102–13. doi: 10.1007/s12016-010-8217-z. [DOI] [PubMed] [Google Scholar]
- 37.Nowak DA, Yeung J. Steroid-Induced Osteonecrosis in Dermatology: A Review. J Cutan Med Surg. 2015 Mar 30; doi: 10.1177/1203475415579759. [DOI] [PubMed] [Google Scholar]
- 38.Gander T, Obwegeser JA, Zemann W, Gratz KW, Jacobsen C. Malignancy mimicking bisphosphonate-associated osteonecrosis of the jaw: a case series and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014 Jan;117(1):32–6. doi: 10.1016/j.oooo.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Fleisher KE, Raad RA, Rakheja R, Gupta V, Chan KC, Friedman KP, et al. Fluorodeoxyglucose positron emission tomography with computed tomography detects greater metabolic changes that are not represented by plain radiography for patients with osteonecrosis of the jaw. J Oral Maxillofac Surg. 2014 Oct;72(10):1957–65. doi: 10.1016/j.joms.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Lapa C, Linz C, Bluemel C, Mottok A, Mueller-Richter U, Kuebler A, et al. Three-phase bone scintigraphy for imaging osteoradionecrosis of the jaw. Clin Nucl Med. 2014 Jan;39(1):21–5. doi: 10.1097/RLU.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 41.Dore F, Filippi L, Biasotto M, Chiandussi S, Cavalli F, Di Lenarda R. Bone scintigraphy and SPECT/CT of bisphosphonate-induced osteonecrosis of the jaw. J Nucl Med. 2009 Jan;50(1):30–5. doi: 10.2967/jnumed.107.048785. [DOI] [PubMed] [Google Scholar]
- 42.Larheim TA, Westesson PL, Hicks DG, Eriksson L, Brown DA. Osteonecrosis of the temporomandibular joint: correlation of magnetic resonance imaging and histology. J Oral Maxillofac Surg. 1999 Aug;57(8):888–98. doi: 10.1016/s0278-2391(99)90001-0. discussion 99. [DOI] [PubMed] [Google Scholar]
- 43.Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 Nov;110(5):593–6. doi: 10.1016/j.tripleo.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 44.Xu J, Zheng Z, Fang D, Gao R, Liu Y, Fan Z, et al. Mesenchymal stromal cell-based treatment of jaw osteoradionecrosis in Swine. Cell Transplant. 2012;21(8):1679–86. doi: 10.3727/096368911X637434. [DOI] [PubMed] [Google Scholar]
- 45.Damek-Poprawa M, Stefanik D, Levin LM, Akintoye SO. Human bone marrow stromal cells display variable anatomic site-dependent response and recovery from irradiation. ArchOral Biol. 2010 Mar 4;55(5):358. doi: 10.1016/j.archoralbio.2010.03.010. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandra A, Lin T, Tribble MB, Zhu J, Altman AR, Tseng WJ, et al. PTH1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone. 2014 Oct;67:33–40. doi: 10.1016/j.bone.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fessel J. There are many potential medical therapies for atraumatic osteonecrosis. Rheumatology (Oxford) 2013 Feb;52(2):235–41. doi: 10.1093/rheumatology/kes241. [DOI] [PubMed] [Google Scholar]


