Summary
Background
sonoelastography helps in the detection of abnormalities not yet evident on B-mode exam.
Methods
in this observational study, we report a collection of cases of symptomatic patients without alterations at ultrasound imaging but with evidence of pathological findings at sonoelastography. Patients, with clinical history suggestive for tendinopathies or surgically treated, and negative at the ultrasound exam, were submitted to sonoelastography. Out of 846, 632 patients with positive ultrasound exam were excluded. Sonoelastography was therefore performed in the remaining 214.
Results
the examination was positive in 168 cases: 78 patients were affected with shoulder diseases, while elbow pathology was observed in 31 subjects; patellar, Achilles and plantar fascia disorders were reported in 19, 27, and 13 patients, respectively.
Conclusion
sonoelastography can reveal tendon abnormalities of clinical relevance in a high percentage of cases, where the ultrasound exam was negative, making the method a complementary tool to ultrasound evaluation.
Keywords: imaging, sonoelastography, tendon, tendinopathy, ultrasound
Introduction
Ultrasound elastography (EUS) is a new technique recently introduced in the clinical practice that allows qualitative evaluations and quantitative measurements of the mechanical properties of tissues1, 2. It is based on the principle that tissue compression produces changes within it and that the displacement (strain), depending on the elastic properties, is less pronounced in hard than in soft tissues. Strain EUS, the most commonly method used, allows the direct visualization, on B-mode image, of strain distribution map or “elastogram”, where blue, red, and yellow/green colours indicate hard, soft, and intermediate tissue stiffness, respectively2.
EUS has been employed for musculo-skeletal diseases showing, in general, a good correlation with the clinical and ultrasound (US) examination, in agreement with the histopathological features of the lesion3–5.
It is well known that, in some cases, it is difficult or even impossible to distinguish pathological tissues using conventional US because these tissues show the same echogenicity of the surrounding healthy structures6. In these cases, EUS could detect or differentiate abnormalities, which are thought to correspond to sub-clinical changes not yet evident on B-mode evaluation, providing supplementary information useful for diagnostic, therapeutic (ultrasound guided procedures) and follow-up purposes.
However, only few systematic studies have compared EUS with traditional US3–5, 7, 8; in these studies little information has been reported about the characteristics of lesions, which more frequently remain undetected by US, but are made evident by EUS. For instance, EUS could detect areas of cleavage inside tendons after partial tears, or scar tissue not strong enough to withstand load, or could provide early information on the functional recovery after surgery.
Aim of the present paper was to report a collection of illustrative cases of symptomatic patients without evident alterations at US imaging but with evidence of pathological findings at EUS.
Materials and methods
The study was perfomed according to the Declaration of Helsinki and to the ethical standard of the Muscles, Ligaments and Tendons Journal9, and informed written consent was obtained from all the patients.
Subjects referred to our Ultrasound Services (Rizzoli Orthopaedic Institute, Bologna; Madre Fortunata Toniolo Clinic, Bologna) with clinical history suggestive for tendinopathies (pain, tenderness and/or functional limitation) or surgically treated for tendon tears and in the late phases of rehabilitation, and negative for acute pathology, were enrolled.
We excluded patients with a positive history of systemic inflammatory arthritis (rheumatoid, psoriatic and reactive arthritis, arthritis associated with inflammatory bowel diseases, and spondiloarthritis), suffering from severe osteoarthritis of the scanned district, malignancy, endocrinopathies and severe chronic diseases (renal, hepatic, cardiac, etc.).
Clinical examination was aimed at evaluating the presence of pain during the previous week, local tenderness, functional limitation of the involved district and of the surrounding tissues (i.e. joints, muscles, ligaments and subcutaneous tissue), and, where possible (palpable tendons), tendon thickening. Pain, at rest and during common activities of daily living, was assessed using 10 cm visual analogue scale (VAS), with 0 representing no pain and 10 representing the worst pain.
Afterwards, participants underwent an US and Colour Doppler (CD) evaluation of the affected region, using a high-resolution, multi-frequency (6–15 MHz) linear array transducer (Hitachi Preyrus). The US criteria adopted for the diagnosis of tendinopathies were the following.
The presence of dishomogeneous hypo- or hyperechoic thickening, diffuse or focal, of the tendon, associated with loss of the normal fibrillar pattern and/or irregularity of the tendon margins, was interpreted as sign of degeneration10.
Fluid within and patchy thickening of the paratenon, associated or not with irregularities of tendon margins, were classified as peritendinitis11, while an enthesopathy was reported when, at US scans, focal tendon thickening, abnormal tendon echotexture, calcifications, bone erosions, and bursal fluid distension at the site of tenderness were observed12.
Involvement of bursae was diagnosed when accumulation of anechoic fluid, with or without hypoechoic swelling of the synovia, appeared within it13.
Presence of neovascularization was estimated, by means of CD and graded as (0), (1+), (2++), (3+++), (4++++), according to the appearance of vessels inside the tendon14. To avoid artifacts, sensitivity was optimised for low flow, and colour gain was set just below the noise level.
When the US scan was not suggestive for tendon diseases, a real-time EUS examination (Hitachi Preyrus, Philips IU22) was carried out.
EUS was performed with the tendon not in extension by applying light repetitive compression, both in the longitudinal and transverse plane, with the hand-held transducer over the region of interest; the size of the EUS window was selected in relationship to the size of the tendon to be examined. Force applied was set according to the quality factor of the equipment and was displayed on the screen: the visual indicator facilitated the acquisition by showing the average strain applied. During the examination, B-mode and EUS images were displayed side by side on the monitor, with EUS superimposed on B-mode image as a colour-coded, real-time picture. Colour scale, which represented the relative stiffness of the tissues, ranged from red (soft tissue), yellow/green (intermediate stiffness) to blue (hard tissue). The most representative EUS image (defined as the adequate depiction of tissue structure and constant reproduction of the scanned images) of at least three concordant was chosen and recorded on communication system for a further analysis.
Care was taken to hold the probe perpendicular to the target tissue to avoid anisotropy and tissue shifting when performing US and EUS respectively.
Both the US and EUS evaluations were performed by the same radiologist (GS) with ultra-decennial experience in musculo-skeletal imaging.
The demographic and clinical characteristics of the EUS positive and negative subjects were compared in general and for each region (shoulder, elbow, patellar, Achilles, and plantar fascia). Data are reported as mean ± SD for continuous variables, whereas categorical and dichotomous variables are reported as frequencies and percentage. The two-sample Student’s t-test was used to compare continuous variables, when the distribution of data was normal; the Wilcoxon’s rank sum test was used otherwise. The χ2 test was used to evaluate associations between categorical data. The significance level was determined at p < 0.05.
Results
846 subjects (50.1 ± 20.4, range: 13–83, M:F 501:345) with referred symptoms suggestive of tendinopathy were evaluated clinically and by means of sonography. In 74.7% (632/846) the US scan was positive for tendon abnormalities (data not reported), and they were excluded from the trial.
EUS was then performed in the remaining 214 (25.2%) subjects (Tendinopathy= 203; surgically treated= 11), whose B-mode and color doppler US scans were negative. The US negative patients (mean age: 49.6 ± 13.8, range: 13–81, M:F 116:98), were complaining of mild symptoms (VAS at rest: 1.8 ± 1.1; VAS during activities: 2.6 ± 0.8), with a mean symptoms duration of 2.4 ± 1.1 months (Tab. 1).
Table 1.
Demographic and clinical characteristics of included patients.
| Patients | EUS positive | EUS negative | p | |
|---|---|---|---|---|
| Number | 214 | 168 | 46 | |
| M:F | 116:98 | 93:75 | 23:23 | |
| Age (range) | 49.6 ± 13.8 | 49.7 ± 13.9 | 49.2 ± 13.4 | ns |
| Symptoms duration | 2.4 ± 1.1 | 2.4 ± 1 | 2.6 ± 1.1 | ns |
| VAS at rest | 1.8 ± 1.1 | 1.8 ± 1.1 | 1.7 ± 1.1 | ns |
| VAS during activities | 2.6 ± 0.8 | 2.7 ± 0.9 | 2.6 ± 0.8 | ns |
Regarding the upper limbs, 97 (45.3%) shoulders and 38 (17.7%) elbows were evaluated, while at the lower limbs, 26 (12.1%) patellar tendons, 35 (16.3%) Achilles tendons, and 18 (8.4%) plantar fasciae were studied. Concerning the 11 surgically treated tendons, 7 Achilles (6 complete and 1 incomplete tears), 3 Patellar (jumpers’ knee), and 1 elbow extensors tendons (chronic tendinopathies) were noted (Tabs. 2, 3). The tendon structure showed a normal or non diagnostic EUS pattern in 46 (21.4%) patients.
Table 2.
Demopraghic and clinical characteristics of included patients, and EUS diagnosis of the upper limbs district.
| UPPER LIMBS | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SHOULDER | ELBOW | ||||||
|
|
|
|
|||||
| EUS pos | EUS neg | p | EUS pos | EUS neg | p | ||
| Number | 78/97 (80.4%) | 19/97 (19.5%) | 31/38 (81.5%) | 7/38 (18.4%) | |||
| Age (range) | 55.4 ± 12.8 | 56.6 ± 13.3 | 0.3 | 43.1 ± 11.6 | 43.7 ± 9.7 | 0.4 | |
| Symptoms duration | 2.5 ± 1.2 | 2.5 ± 1.1 | 0.4 | 2.4 ± 1 | 3 ± 1.2 | 0.07 | |
| VAS at rest | 1.7 ± 1 | 1.6 ± 1.1 | 0.3 | 1.9 ± 1.1 | 1.4 ± 1.1 | 0.1 | |
| VAS during activities | 2.6 ± 0.7 | 2.5 ± 0.8 | 0.3 | 2.9 ± 1.1 | 2.7 ± 1.1 | 0.3 | |
| EUS diagnoses | |||||||
| - Post-trauma | 69/78 (88.4%) | ||||||
| - Biceps tendon | 9/78 (11.5%) | ||||||
| - Peritendinopathy | 26/31 (83.8%) | ||||||
| - Enthesis | 4/31 (12.9%) | ||||||
| - Post-surgery | 1/31 (3.2%) | ||||||
Table 3.
Demographic and clinical characteristics of included patients, and EUS diagnosis of the lower limbs district.
| LOWER LIMBS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| PATELLAR | ACHILLES | PLANTAR FASCIA | |||||||
|
|
|
|
|||||||
| EUS pos | EUS neg | p | EUS pos | EUS neg | p | EUS pos | EUS neg | p | |
| Number | 19/26 (73%) | 7/26 (26.9%) | 0.002 | 27/35 (77.1%) | 8/35 (22.8%) | 0.0000 | 13/18 (72.2%) | 5/18 (27.7%) | 0.01 |
| Age (range) | 37.9 ± 16.7 | 38.1 ± 13.9 | 0.4 | 46.9 ± 12.5 | 48.5 ± 10.6 | 0.3 | 52.8 ± 6.3 | 50.2 ± 4.4 | 0.2 |
| Symptoms duration | 2.4 ± 1.1 | 2.7 ± 1.2 | 0.2 | 2.5 ± 1 | 3.1 ± 1.5 | 0.08 | 1.7 ± 0.5 | 1.8 ± 0.4 | 0.3 |
| VAS at rest | 1.9 ± 1.1 | 1.8 ± 1.1 | 0.3 | 2.1 ± 1.4 | 2.4 ± 1.1 | 0.2 | 1.8 ± 0.8 | 1.2 ± 0.8 | 0.1 |
| VAS during activities | 2.9 ± 0.9 | 2.7 ± 0.7 | 0.2 | 2.9 ± 1.1 | 3.1 ± 0.6 | 0.2 | 2.6 ± 0.8 | 2.2 ± 0.8 | 0.1 |
| EUS diagnoses | |||||||||
| - Peritendinopathy | 11/19 (57.8%) | 11/27 (40.7%) | |||||||
| - Post-surgery | 3/19 (15.7%) | 7/27 (25.9%) | |||||||
| - Proximal tendinopathy | 2/19 (10.5%) | 9/13 (69.2%) | |||||||
| - Distal tendinopathy | 2/19 (10.5%) | 3/27 (11.1%) | |||||||
| - Pre-patellar thickening | 1/19 (5.2%) | ||||||||
| - Midportion tendinopathy | 6/27 (22.2%) | 4/13 (30.7%) | |||||||
The results of EUS evaluation were the following.
Shoulder: out of 97 tendons, 19 subject (19.5%) did not show any EUS abnormalities (symptoms related to gleno-humeral instability), while EUS was positive in the remaining 78 patients (80.4%).
In the EUS positive group, 88.4% (69/78) patients were affected from post-trauma diseases: indeed, rotator cuff tendons appeared stiff (blue), and inflammation/oedema (red areas) of the sub-acromial bursa and surrounding tissues were associated (Fig. 1). Biceps tendon disorders (red circle surrounding the tendon) were reported only in 9 subjects (11.5%).
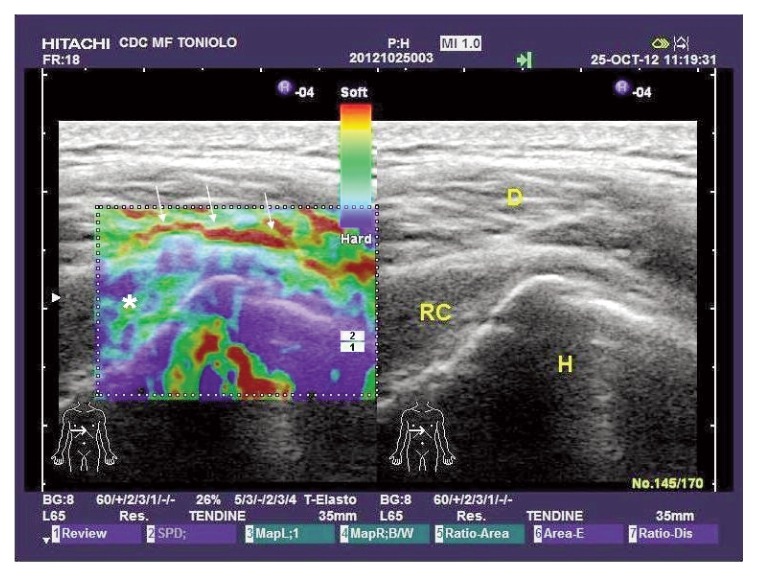
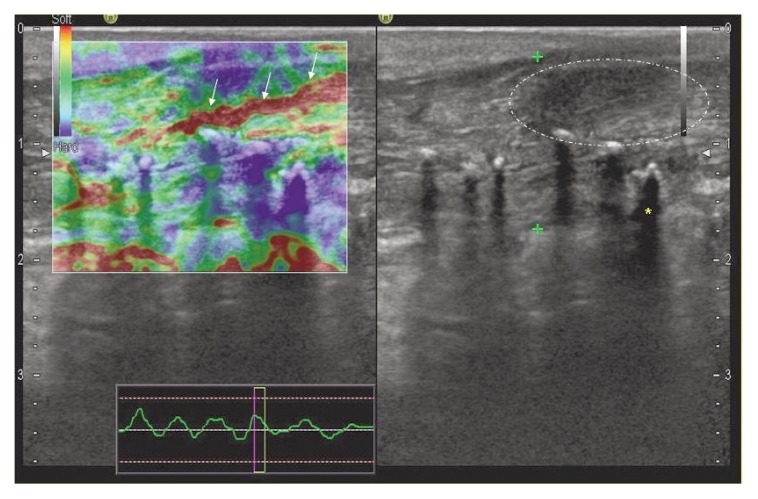
Figure 1.
Post-trauma shoulder: in the left panel, EUS shows a stiff (blue, green colours) rotator cuff (*), expression of initial fibro-adesive process; oedema and inflammation (arrows) (red areas) are present at the site of sub-acromial bursa (synovial contusion without effusion). RC= Rotator Cuff; D= Deltoid muscle; H= Humeral head.
Elbow: of 38 tendons, EUS showed abnormalities in 31 subjects (81.5%). Signs of peritendinopathy (oedema and inflammation along the peritenon) were observed in 26 patients (83.8%); in the remaining 5 cases, enthesitis (blue areas at the attachment site of the tendon, oedema in the surrounding bursae and tissue) and post-surgery features (mixture of irregular red and blue areas) were observed in 4 and 1 patients, respectively.
Patellar: EUS alterations were observed in 19/26 patients (73%). Eleven patients (57.8%) complained peritendinopathy, while proximal and distal tendinopathies were observed in 2 cases respectively; 3 post-surgery alterations and 1 pre-patellar thickening (red areas) were observed in the remaining patients (Fig. 2).
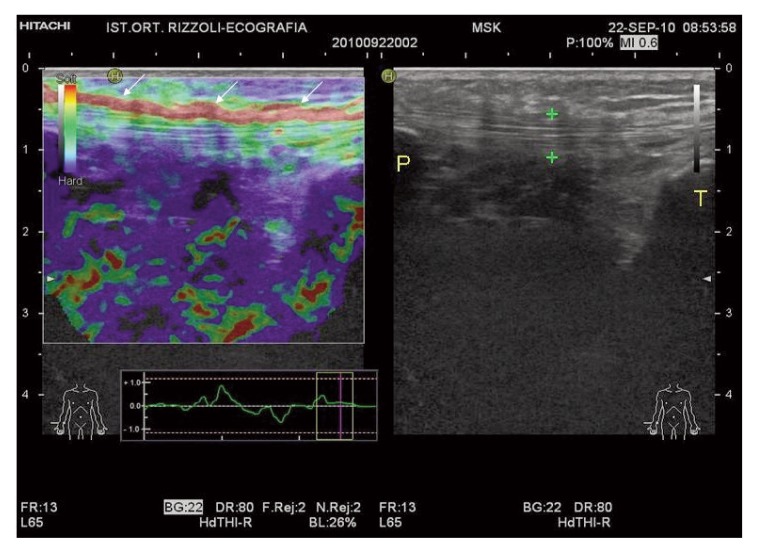
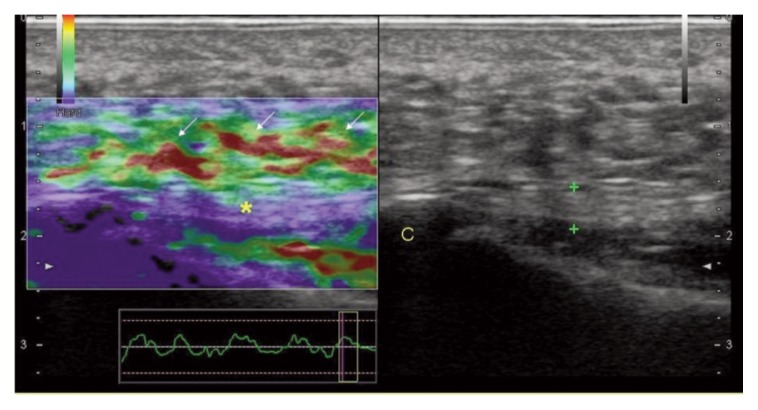
Figure 2.
Patellar peritendinopathy: patellar tendon (calipers) appears normal on B-mode exam (right panel). At EUS evaluation, red areas (arrows) along the full length of the ventral portion of the tendon are expression of peritenon inflammation. P= patella; T= Tibia.
Achilles: 27/35 (77.1%) patients showed EUS positive features: peritendinopathy (Fig. 3) was observed in 11 (40.7%) subjects, while EUS revealed signs of focal mid-portion and of distal tendinopathy (Fig. 4) in 6 (22.2%) and 3 cases (11.1%), respectively. Finally, post-surgery features (Fig. 5) were observed in 7/27 (25.9%) patients.
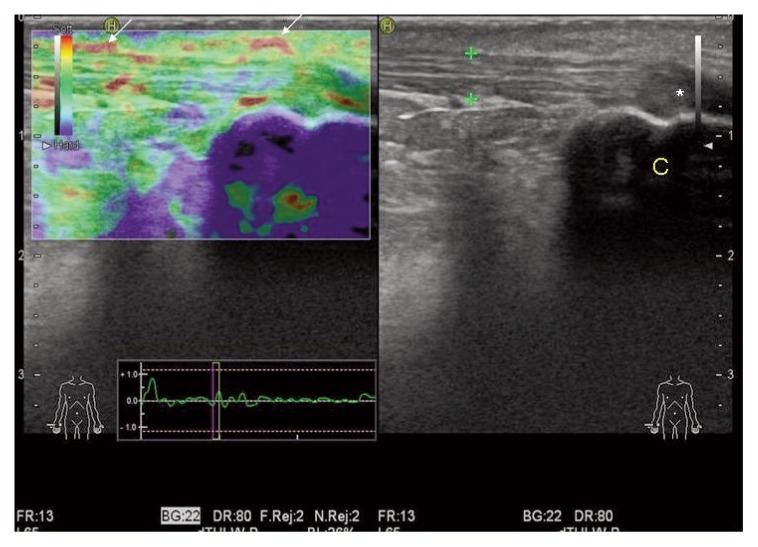
Figure 3.
Achilles peritendinopathy: EUS reveals areas of inflammation and oedema (red spots, arrows) in the ventral side of the tendon. Achilles tendon (calipers) is normal at US evaluation (right panel). C= calcaneal bone; *= anisotropy effect.
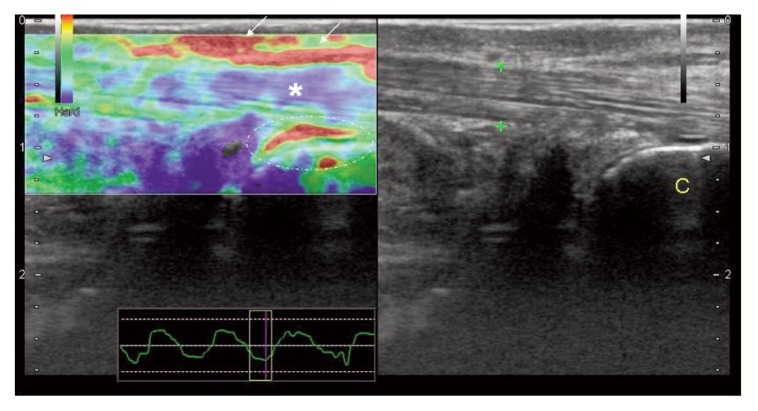
Figure 4.
Distal Achilles tendinopathy: at the EUS exam, peritenon is severely inflamed (red areas, arrows); synovial thickening (red spot) in the retrocalcaneal bursa (dot circle) is present, while the distal portion of the tendon (*) is stiff (blue and green spots). Achilles tendon (calipers) is normal at US evaluation. C= calcaneal bone.
Figure 5.
Achilles tendon tenorraphy: at US evaluation, Achilles tendon (calipers) appears hypoechoic, patchy (dot circle) and thick; surgical suture is present (*).
Worthnoting, the EUS exam (left panel) shows an oedematous area (arrows) at the tear site, which indicate that the scar tissue is not strong enough to withstand load. Patchy Achilles tendon appears as a mixture of blue, green, red and yellow areas.
Plantar fascia: 13/18 (72.2%) patients were EUS positive for plantar fascitiis. Plantar fascia appeared homogeneously blue (hard structures), probably related to anelastic tissue alterations. Red areas (tissue edema) was present in the surrounding tissue under the fascia (9 cases proximal and 4 cases mid-portion tendinopathy) (Fig. 6). In 5 cases (27.7%) the results of EUS evaluation were negative or inconclusive.
Figure 6.
Plantar fascitiis: at B-mode evaluation, plantar fascia (calipers) appears normal, while at EUS exam it is stiff (*, blue areas). In the surrounding tissues (arrows), oedema and inflammation (red areas) can be observed. C= calcaneal bone.
Discussion
In several symptomatic subjects US examination fails to reveal tendon abnormalities of clinical relevance. In this observational study we illustrate a case series of patients with negative or inconclusive US exam where EUS allowed to show tendon pathologies.
Indeed, EUS abnormal features explaining the mild symptomatology of our patients were found in several tendons, such rotator cuff, elbow, patellar, Achilles and plantar fascia. In these patients we observed tissue softening (red-yellow) within the tendon, paratenon and surrounding tissues (fascia, bursae), that might be explained by very early changes in tissue elasticity, probably due to histopatological alterations (oedema and inflammation)15, 16.
We also observed, in quite all cases of suspected plantar fasciitis, that plantar fascia, which showed a normal echogenic pattern at US evaluation, behaved as an hard tissue (blue), probably related to the increased content of type III collagen.
Our observations are in agreement with previous studies where EUS detected intra and peritendinous alterations17–19. Indeed, in a comparison study between healthy volunteers and patients suffering from lateral epicondylitis, De Zordo et al.4 found that normal tendons appeared blue (hard) in 96% of volunteers, while tissue edema (red areas) was observed in 67% of patients (p< 0.001). In the same study, the authors observed that EUS was more sensitive than US in discovering early oedema and inflammation (red-yellow areas) in the collateral ligament (26 vs 21%) and paratenon (29 vs 13%).
Similar patterns were also described for Achilles tendon20, 21 where normal tendons3 were found to be homogeneously hard in 93% of cases, while marked (red) and mild softening (yellow) was observed in 57 and 32% of patients, respectively3.
On the basis of these observations, EUS appears as a complementary method to US evaluation, because it can discover small changes in the elastic and mechanical properties of tissue1, 2, expression of pathology, which are not evident on B-mode evaluation due to the same echogenicity of the surrounding healthy tissues6. Only in few cases (46/214, 21.4%) the tendon’s structure showed a normal or non diagnostic EUS pattern. We hypothesize that this can be due to a misleading clinical history (low pain threshold of the patient?) or to artifacts which masked subtle pathological findings. The potential advantages of EUS can be summarized as follows: first, it could be used to differentiate identical grey-scale images, better detecting at an early stage alterations which could progress to higher stages of tendinopathy. Second, it may be used as a tool allowing the subjects to modify exercise regimen to prevent further tendon damage. Third, it may be useful for therapeutic purposes and to monitor treatment effectiveness. EUS could be also applied in the athletic population, but this requires further validation studies, because the tendinous structures of athletes are partly different from those of the non athletic subjects.
Despite these advantages, the method suffer the limitation of being in some way operator dependent in terms of application of pressure to the probe and the differentiation of artifacts from diagnostic image information in real time. At this regard, a visual indicator on the screen may give an optimal dynamic range of pressure, helping to decrease inter-observer variability and facilitate image acquisition. However, because in the present study all the evaluations were performed by the same radiologist, these features must be confirmed by investigations aiming the intra- and inter-observer variability of the method.
Some limitations of the present study must be acknowledged. First, this is not a randomized control study and a control group, made of asymptomatic subjects matched for age and sex, is lacking; second, we did not evaluate the controlateral side (probably normal) and not investigate differences between the sexes, and the dominant and non-dominant side (important in the athletic population); third, we did not follow up the evolution of the EUS alterations; finally, no histopatological exam was available to confirm our results.
In addition, it must be added that the EUS systematic evaluation of all the patients (i.e. including those positive to the traditional US) would have allowed to detect a higher number of tendon abnormalities.
In conclusion, EUS may be considered a powerful diagnostic adjunct to US and CD evaluation in the diagnostic approach to tendinopathies.
References
- 1.Drakonaki EE, Allen GM, Wilson DJ. Ultrasound elastography for musculoskeletal applications. Br J Radiol. 2012;85(1019):1435–1445. doi: 10.1259/bjr/93042867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13(2):111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 3.De Zordo T, Chhem R, Smekal V, et al. Real-time sonoelastography: findings in patients with symptomatic achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010;31(4):394–400. doi: 10.1055/s-0028-1109809. [DOI] [PubMed] [Google Scholar]
- 4.De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. 2009;193(1):180–185. doi: 10.2214/AJR.08.2020. [DOI] [PubMed] [Google Scholar]
- 5.Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28(3):373–378. [PubMed] [Google Scholar]
- 6.Frey H. Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity. Radiologe. 2003;43(10):850–855. doi: 10.1007/s00117-003-0943-2. [DOI] [PubMed] [Google Scholar]
- 7.Wu CH, Chang KV, Mio S, Chen WS, Wang TG. Sonoelastography of the plantar fascia. Radiology. 2011;259(2):502–507. doi: 10.1148/radiol.11101665. [DOI] [PubMed] [Google Scholar]
- 8.Park GY, Kwon DR. Application of real-time sonoelastography in musculoskeletal diseases related to physical medicine and rehabilitation. Am J Phys Med Rehabil. 2011;90(11):875–886. doi: 10.1097/PHM.0b013e31821a6f8d. [DOI] [PubMed] [Google Scholar]
- 9.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research. MLTJ. 2013;4:250–252. [PMC free article] [PubMed] [Google Scholar]
- 10.Grassi W, Filippucci E, Farina A, Cervini C. Sonographic imaging of tendons. Arthritis Rheum. 2000;43:969–976. doi: 10.1002/1529-0131(200005)43:5<969::AID-ANR2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Gibbon WW, Cooper JR, Radcliffe GS. Distribution of sonographically detected tendon abnormalities in patients with a clinical diagnosis of chronic achilles tendinosis. J Clin Ultrasound. 2000;28:61–66. doi: 10.1002/(sici)1097-0096(200002)28:2<61::aid-jcu1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Kamel M, Eid H, Mansour R. Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. J Rheumatol. 2003;30:774–778. [PubMed] [Google Scholar]
- 13.Gibbon WW, Cooper JR, Radcliffe GS. Sonographic incidence of tendon microtears in athletes with chronic Achilles tendinosis. Br J Sports Med. 1999;33:129–130. doi: 10.1136/bjsm.33.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 15.Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 16.Järvinen TA, Kannus P, Paavola M, Järvinen TL, Józsa L, Järvinen M. Achilles tendon injuries. Curr Opin Rheumatol. 2001;13(2):150–155. doi: 10.1097/00002281-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Klauser AS, Faschingbauer R, Jaschke WR. Is sonoelastography of value in assessing tendons? Semin Musculoskelet Radiol. 2010;14(3):323–333. doi: 10.1055/s-0030-1254521. [DOI] [PubMed] [Google Scholar]
- 18.Lalitha P, Reddy MCh, Reddy KJ. Musculoskeletal applications of elastography: a pictorial essay of our initial experience. Korean J Radiol. 2011;12(3):365–375. doi: 10.3348/kjr.2011.12.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botar Jid C, Vasilescu D, Damian L, Dumitriu D, Ciurea A, Dudea SM. Musculoskeletal sonoelastography. Pictorial essay. Med Ultrason. 2012;14(3):239–245. [PubMed] [Google Scholar]
- 20.De Zordo T, Fink C, Feuchtner GM, Smekal V, Reindl M, Klauser AS. Real-time sonoelastography findings in healthy Achilles tendons. AJR Am J Roentgenol. 2009;193(2):W134–138. doi: 10.2214/AJR.08.1843. [DOI] [PubMed] [Google Scholar]
- 21.Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol. 2009;64(12):1196–1202. doi: 10.1016/j.crad.2009.08.006. [DOI] [PubMed] [Google Scholar]