Abstract
Group B Streptococci (GBS) are ß-hemolytic, gram-positive bacteria that are typically associated with infections in human newborns or immunocompromised adults. However, mutation in the two-component regulator CovR/S relieves repression of hemolysin, potentially increasing virulence of GBS.
We report the isolation of hyperhemolytic/hyperpigmented GBS strain from an adolescent patient who presented to the University of Washington clinic with symptoms of sore throat. While the patient also tested positive for mononucleosis, a GBS strain with increased hemolysis was isolated from the throat swab obtained from the patient. As hyperhemolytic/hyperpigmented GBS strains are typically associated with mutations in the regulator CovR/CovS, we sequenced the covR/S loci in the clinical isolate. An adenine to cytosine mutation resulting in a change in amino acid coding sequence from glutamine at position 120 to proline in CovR (Q120P) was identified. Introduction of the Q120P amino acid substitution in a CovR complementation plasmid abolished complementation of a ΔcovR mutant derived from the wild type GBS serotype Ia strain A909; these results confirm that the hyperhemolysis observed in the clinical isolate is due to the Q120P substitution in CovR. Antibiotic was prescribed and the patient's symptoms resolved without reported complications. This study represents the first report of the isolation of a hyperhemolytic/hyperpigmented GBS strain due to a covR/S mutation from an adolescent patient with persistent sore throat who was also diagnosed with mononucleosis. The isolation of GBS CovR/S mutants indicates their presence in settings of co-infections and includes adolescents.
Keywords: Streptococcus agalactiae, CovR, Hyperpigmentation, Hyperhemolysis, Co-infections, Adolescent
INTRODUCTION
Group B Streptococci (GBS) are beta-hemolytic, grampositive bacteria that cause invasive infections in human newborns and elderly adults. Although these bacteria typically exist as commensal organisms in the rectovaginal tract in about 30% of healthy adult women, increasing incidence of invasive GBS infections have been reported in both newborns and elderly adults [1-3]. Common symptoms of GBS infections in non-pregnant adults include bacteremia, skin/soft tissue infections and pneumonia [3].
GBS strains with increased expression of hemolysis and pigmentation have been described [4-8]. Molecular studies revealed the existence of a two component regulatory system known as CovR/CovS (CovR/S or CsrR/S) which, down-regulates the expression of many virulence factors including the pluripotent hemolysin [4-8]. Recently, we showed that hemolysis is due to the ornithine rhamnolipid pigment produced by GBS [9]. GBS isolates with increased expression of the hemolytic pigment due to mutations in CovR/S have been associated with increased virulence [9-12]. Sendi et al., [10] also reported a case of necrotizing fasciitis due to a GBS covR mutant in a 50 year old male. We have also observed that CovR/S mutants accelerated GBS virulence in animal models of adult infections and preterm birth, in a hemolytic pigment dependent manner [11,12] Although GBS infections in non-pregnant adolescents are relatively uncommon, here, we describe the isolation of a hyperhemolytic/hyperpigmented GBS CovR mutant from an adolescent patient with symptoms of sore throat.
CASE PRESENTATION
A 16-year-old female presented to the University of Washington outpatient clinic complaining of a 3-week history of sore throat. She was a non-smoker and denied any recent antibiotic use. The patient was a febrile, without complaints of fatigue or weight loss, and was noted to have mild pharyngeal erythema and Grade 2+ tonsillar hypertrophy without neck lymphadenopathy on exam. Throat swabs were obtained and sent to the microbiology laboratory for culture to rule out ß-hemolytic streptococci, which includes testing for groups A, C, and G). Tryptic soy agar (TSA) and TSA supplemented with 5% sheep blood (blood agar plate; BAP) was inoculated and incubated an aerobically at 37°C. Strong ß-hemolytic, orange pigmented colonies were observed after 24 h (strain hereafter called RM003, see (Figure1)A, B& C). The isolate tested negative for group A, C and G streptococci (by latex agglutination, PathoDX, Remel), which are commonly associated with sore throat infections. Although not routine laboratory practice, the orange pigment production by the organism initiated further investigation. Ultimately, the strain was identified as Streptococcus agalactiae (or Group B Streptococcus, GBS) based on the positive latex agglutination test for the group B antigen. These observations prompted further tests for Group B Streptococcus and we confirmed that orange to red pigmented bacteria were also observed after overnight growth on Granada Media and TSA (see RM003 in Figure 1B& C).Using methods described [13], we then determined that RM003 belongs to GBS capsular serotype II (Figure 1E).
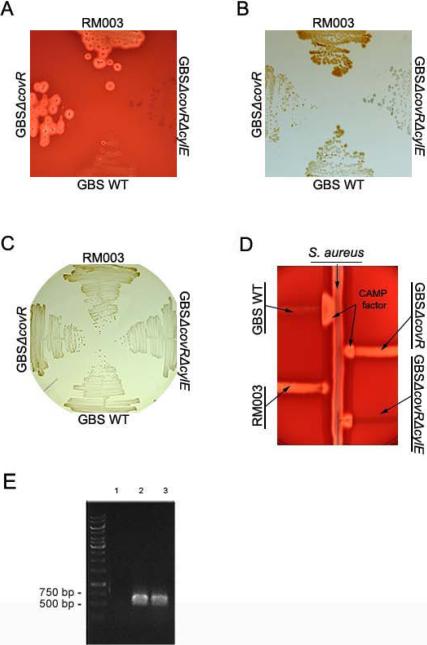
Figure 1. The GBS strain isolated from the patient with sore throat exhibits increased hemolytic activity, pigment production and decreased CAMP factor expression and belongs to capsula serotype II.
(A) The zone of lysis around the colonies on sheep blood agar represents hemolytic activity. RM003 is the GBS clinical isolate obtained from the patient with sore throat. Controls included are wild type (WT) GBS of serotype III, strain COH1; GBSΔcovR represents a covR mutant derived from COH1 that is hyperhemolytic and GBSΔ covRΔ cylE represents the GBSΔ covR mutant lacking the cylE gene important for GBS hemolysis.
(B,C) RM003, GBS WT (COH1), GBSΔcovR and GBSΔcovRΔcylE on Granada Media and TSA (Tryptic Soy Agar), respectively. Note that RM003 and GBSΔcovR exhibit increased pigmentation
(D) CAMP factor activity is denoted by the triangular zone of lysis at the junction between GBS and a β-lysin producing strain of S. aureus. Note that WT GBS COH1 exhibits increased CAMP factor activity but RM003, GBSΔcovR and GBSΔcovRΔcylE exhibit decreased CAMP factor activity.
(E)RM003 belongs to GBS capsular serotype II. The capsular serotype of RM003 was determined as described [20]. Primers specific for capsular serotype II amplified capsule regions in DK23, a known serotype II isolate (lane 2) and RM003 (lane 3) but not in DK14 (lane 1), which belongs to GBS serotype Ib.
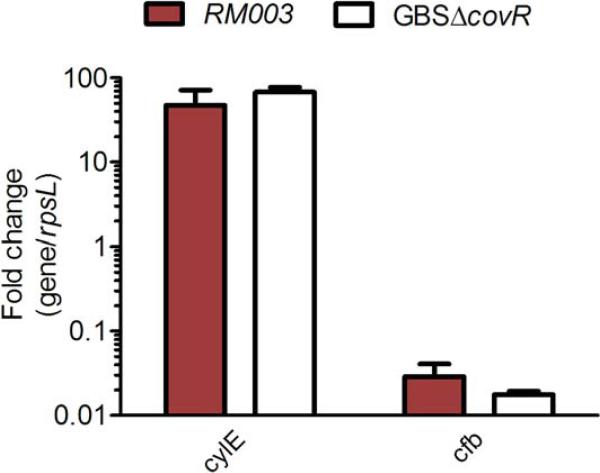
In the GBS strain obtained from the patient with sore throat, we also noted decreased CAMP factor expression apart from increased hemolysis (see RM003 in Figure 1D). Previous studies have indicated that GBS strains with mutations in the two-component system covR/S exhibit increased hemolysis/pigmentation and decreased CAMP factor expression [4,5,8]. We therefore sequenced the covR/S locus in RM003 as described [9] and compared the sequence to the GBS genome [14]. We identified an adenine to cytosine substitution (A359C) in the CovR sequence of RM003 that resulted in a predicted amino acid change from glutamine at position 120 to proline in CovR (Q120P; CAA-CCA). No mutations were noted in the coding sequence of CovS. Amino acid 120 is located between the receiver and effector domains of CovR; see homology model in [7]. To confirm that the phenotypes demonstrating increased hemolysis and decreased CAMP factor expression can be linked to decreased CovR function, qRT-PCR analysis was performed to assess transcription of key CovR regulated genes such as cylE and cfb as described ([9], also see below). These data revealed that RM003 showed > 50- fold increase in transcription of the gene cylE important for hemolytic pigment biosynthesis [9] and > 15- fold decrease in expression of the CovR activated gene cfb encoding CAMP factor [15] (Figure 2).
Figure 2. The GBS clinical isolate RM003 exhibits increased transcription of the cylE gene associated with hemolytic pigment production and decreased transcription of the cfb gene associated with CAMP factor.
qRT-PCR was performed on RNA isolated from exponential phase cultures (O.D600nm =0.3) of GBS strains RM003, WT GBS (COH1) and GBSΔcovR. Data are normalized to relative expression of the house keeping gene rpsL for each strain. Fold difference in gene expression (gene/rpsL) shown relative to gene expression (gene/rpsL) observed with WT GBS (COH1). Data shown are the average and SD from five independent biological replicates performed in triplicate (n=5).
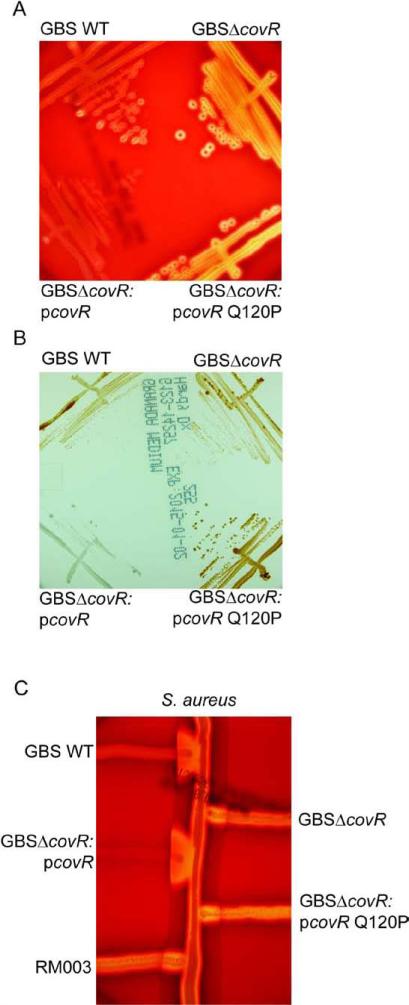
To further confirm that the Q120P substitution abolished CovR function in GBS, we also constructed a site directed substitution in a CovR complementation plasmid (pCovR) that was previously shown to complement the ΔcovR strain derived from WT GBS serotype Ia strain A909 [9]. The results shown in (Figures 3A-C) confirmed that while pCovR complemented GBSA909 ΔcovR for repression of hemolytic activity and pigment and also restored activation of CAMP factor, the pCovRQ120P plasmid failed to do so. We also performed western blots using the GBS CovR antibody as described [11] and confirmed that GBS strains RM003 and A909/pCovRQ120P expressed CovR protein (Figure 4). Collectively, these data indicate that a Q120P substitution alleviates CovR function in GBS.
Figure 3. The Q120P amino acid substitution in CovR abolished complementation of a GBSΔcovR mutant.
(A) The zone of lysis around the colonies on sheep blood agar represents hemolytic activity. Note that hemolytic activity is increased in GBSΔcovR and introduction of the plasmid encoding WT CovR (pCovR) into GBSΔcovR decreased hemolytic activity similar to WT GBS whereas at the pCovR plasmid with the Q120P (pCovRQ120P) substitution did not.
(B) WT, mutant and complemented strains on Granada Media. Note that complementation of GBSΔcovR with WT pCovR plasmid substantially decreased pigment expression whereas the pCovR plasmid with the Q120P (pCovRQ120P) substitution did not.
(C) CAMP factor activity is denoted by the triangular zone of lysis at the junction between GBS and a β-lysin producing strain of S. aureus. Note that complementation of GBSΔcovR with WT pCovR plasmid restored CAMP factor expression to levels observed in WT GBS whereas the pCovR plasmid with the Q120P (pCovRQ120P) substitution did not.
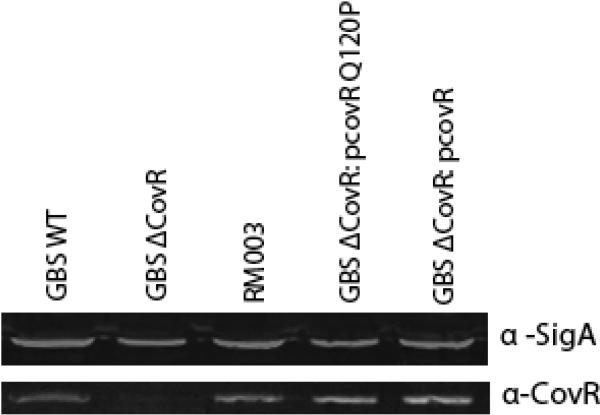
Figure 4. CovR protein is present in GBS strains expressing Q120P CovR.
Quantitative western analysis was performed on equal protein concentrations of GBS WT (A909), ΔcovR, ΔcovR/pCovR, ΔcovR/pCovRQ120P and RM003 using CovR antibody (lower panel). As a control, quantitative westerns were also hybridized to anti-Sigma A antibody (top panel). Note that CovR protein is expressed in all strains except the control GBSΔcovR strain indicating that the lack of CovR function in RM003 and ΔcovR/pCovRQ120P is not due to the absence of CovR protein.
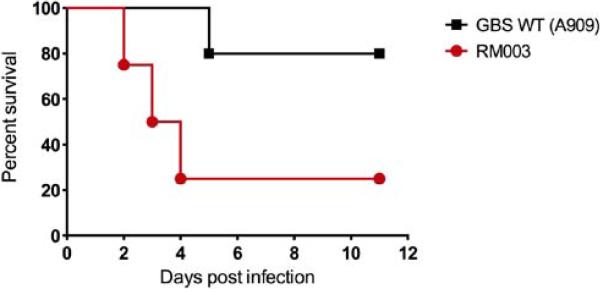
To determine if RM003 is more virulent than WT GBS such as A909, we used the murine sepsis model of infection to compare virulence potential these GBS strains as described as described [11]. The results shown in (Figure 5) indicate that 80% of mice infected with RM003 succumbed to the infection whereas only 20% of mice infected with GBS WT A909 succumbed to the infection.
Figure 5. RM003 is more virulent than WT GBS such as A909.
Six - eight week old C57BL6/J mice were intravenously injected with 5 X 107 CFU of WT GBS A909 or RM003 (n = 5/group). Kalpan Meier survival curve shows percent survival of mice after the infection. Note that only 20% of mice infected with RM003 survived the infection whereas only 80% of mice infected with A909 succumbed to the infection (p = 0.057, Log-rank test; p = 0.0477, Gehan –Breslow-Wilcoxon test). Performance of the animal experiment was approved by the Seattle Children's ResearchI nstitutional Animal Care and Use Committee (protocol #13311) and performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (Eighth Edition).
While it is not possible to conclude that the sore throat infection observed in the patient was solely due to the GBS CovRQ120P strain as mononucleosis was also diagnosed based on a qualitative whole blood Mono Test (OSOM Mono Test, Sekisui diagnostics, San Diego CA.), exacerbation of symptoms observed in the patient may be related to the GBS strain with the covR mutation. As the patient was allergic to penicillin and macrolides, cephalexin was prescribed empirically (bid for 10 days) to treat strep throat and her symptoms resolved without reported complications.
DISCUSSION
Previous studies have shown that hyperhemolytic/hyperpigmented GBS strains with mutations in CovR/S exhibit increased virulence. Both, Sendi et al., and we have observed that GBS CovR/S mutants accelerate mortality in animal models of GBS sepsis and meningitis [10,11]. We further demonstrated that the hyperhemolytic GBS strains are proficient for penetration of human placenta ex vivo, and can be isolated from the amniotic fluid and placental membranes from women in preterm labor [9]. Furthermore, we observed that these strains accelerated fetal injury and preterm birth, in a hemolytic pigment dependent manner, in a pregnant mouse model, [12]. Collectively, these observations emphasize the enhanced pathogenesis of hyperhemolytic/hyperpigmented GBS strains.
GBS clinical isolates with mutations in CovR/S have also been isolated from atypical GBS infections that include a 50 year old adult male with necrotizing fasciitis [10], and elderly patients presenting with either a prosthetic joint infection, conjunctivitis, or urinary tract infection [16]. However, hyperhemolytic/hyperpigmented GBS have not been reported from adolescents presenting with clinical symptoms. Although co-infection with infectious mononucleosis and ß-hemolytic group A streptococci has been reported in children with pharyngitis [17], we are not aware of reports of co-infection with GBS. Taken together, our observations indicate that hyperhemolytic/hyperpigmented GBS clinical isolates with spontaneous mutations in the covR/S locus can be isolated from adolescents presenting with clinical symptoms and co-infections.
Table 1. Hemolytic titers of GBS strains.
Hemolytic tires were quantified as described [18,19]. RM003 is the GBS clinical isolate obtained from the patient with sore throat. Controls included are wild type (WT) GBS of serotype III, strain COH1; GBSΔcovR represents a ΔcovR mutant derived from COH1 that is hyperhemolytic and GBSΔcovRΔcylE represents the GBSΔcovR mutant lacking the cylE gene important for GBS hemolysis.
| Strain | Hemolytic Titer |
|---|---|
| WT GBS (COH1) | 2 |
| COH1ΔCovR | > 32 |
| COH1ΔcovRΔcylE | < 2 (No hemolysis detected) |
| RM003 | > 32 |
ACKNOWLEDGEMENTS
This work was supported by funding from the National Institutes of Health Grants R01AI100989, R01AI112619 and R21AI109222 to L.R.
Whidbey C and Vornhagen J were supported by the NIH training grant (T32 AI07509, PI: Lee Ann Campbell). Whidbey C was also supported by UW GO-MAP Fellowship. McAdams R was supported by the UW Postdoctoral Training program in Medical and Public Health Microbiology. We thank Dr. Brad Cookson for his support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Cite this article
Whidbey C, Burnside K, Martinez RM, Gendrin C, Vornhagen J, et al. (2015) A Hyperhemolytic/Hyperpigmented Group B Streptococcus Strain with a CovR Mutation Isolated from an Adolescent Patient with Sore Throat. Clin Res Infect Dis 2(2): 1018.
REFERENCES
- 1.Porta K, Rizzolo D. Preventing group B streptococcal infections in newborns. JAAPA. 2015;28:24–29. doi: 10.1097/01.JAA.0000460915.52391.70. [DOI] [PubMed] [Google Scholar]
- 2.Bekker V, Bijlsma MW, van de Beek D, Kuijpers TW, van der Ende A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis. 2014;14:1083–1089. doi: 10.1016/S1473-3099(14)70919-3. [DOI] [PubMed] [Google Scholar]
- 3.Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin Infect Dis. 2009;49:85–92. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 4.Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, Pellegrini E, et al. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol. 2004;54:1250–1268. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR. Regulation of virulence by a two-component system in group B streptococcus. J Bacteriol. 2005;187:1105–1113. doi: 10.1128/JB.187.3.1105-1113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang SM, Ishmael N, Dunning Hotopp J, Puliti M, Tissi L, Kumar N, et al. Variation in the group B Streptococcus CsrRS regulon and effects on pathogenicity. J Bacteriol. 2008;190:1956–1965. doi: 10.1128/JB.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin WJ, Walthers D, Connelly JE, Burnside K, Jewell KA, Kenney LJ, et al. Threonine phosphorylation prevents promoter DNA binding of the Group B Streptococcus response regulator CovR. Mol Microbiol. 2009;71:1477–1495. doi: 10.1111/j.1365-2958.2009.06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol. 2006;62:941–957. doi: 10.1111/j.1365-2958.2006.05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, et al. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med. 2013;210:1265–1281. doi: 10.1084/jem.20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sendi P, Johansson L, Dahesh S, Van-Sorge NM, Darenberg J, Norgren M, et al. Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg Infect Dis. 2009;15:223–232. doi: 10.3201/eid1502.080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lembo A, Gurney MA, Burnside K, Banerjee A, de los Reyes M, Connelly JE, et al. Regulation of CovR expression in Group B Streptococcus impacts blood-brain barrier penetration. Mol Microbiol. 2010;77:431–443. doi: 10.1111/j.1365-2958.2010.07215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, et al. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol Med. 2015;7:488–505. doi: 10.15252/emmm.201404883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forquin MP, Tazi A, Rosa-Fraile M, Poyart C, Trieu-Cuot P, Dramsi S. The putative glycosyltransferase-encoding gene cylJ and the group B Streptococcus (GBS)-specific gene cylK modulate hemolysin production and virulence of GBS. Infect Immun. 2007;75:2063–2066. doi: 10.1128/IAI.01565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc Natl Acad Sci U S A. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensler ME, Quach D, Hsieh CJ, Doran KS, Nizet V. CAMP factor is not essential for systemic virulence of Group B Streptococcus. Microb Pathog. 2008;44:84–88. doi: 10.1016/j.micpath.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupo A, Ruppen C, Hemphill A, Spellerberg B, Sendi P. Phenotypic and molecular characterization of hyperpigmented group B Streptococci. Int J Med Microbiol. 2014;304:717–724. doi: 10.1016/j.ijmm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Rush MC, Simon MW. Occurrence of Epstein-Barr virus illness in children diagnosed with group A streptococcal pharyngitis. Clin Pediatr (Phila) 2003;42:417–420. doi: 10.1177/000992280304200505. [DOI] [PubMed] [Google Scholar]
- 18.Nizet V, Gibson RL, Chi EY, Framson PE, Hulse M, Rubens CE. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gendrin C, Vornhagen J, Ngo L, Whidbey C, E. B, Santana-Ufret V, et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv. 2015:E1400225. doi: 10.1126/sciadv.1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyart C, Tazi A, Réglier-Poupet H, Billoët A, Tavares N, Raymond J, Trieu-Cuot P. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J Clin Microbiol. 2007;45:1985–1988. doi: 10.1128/JCM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]