Abstract
Ovarian tissue collected by biopsy procedures allows the performance of many studies with clinical applications in the field of female fertility preservation. The aim of the present study was to investigate the influence of reproductive phase (anestrous vs. diestrous) and ovarian structures (antral follicles and corpus luteum) on the quality, class distribution, number, and density of preantral follicles, and stromal cell density. Ovarian fragments were harvested by biopsy pick-up procedures from mares and submitted to histological analysis. The mean preantral follicle and ovarian stromal cell densities were greater in the diestrous phase and a positive correlation of stromal cell density with the number and density of preantral follicles was observed. The mean area (mm2) of ovarian structures increased in the diestrous phase and had positive correlations with number of preantral follicles, follicle density, and stromal cell density. Biopsy fragments collected from ovaries containing an active corpus luteum had a higher follicle density, stromal cell density, and proportion of normal preantral follicles. In conclusion, our results showed: (1) the diestrous phase influenced positively the preantral follicle quality, class distribution, and follicle and stromal cell densities; (2) the area of ovarian structures was positively correlated with the follicle and stromal cell densities; and (3) the presence of an active corpus luteum had a positive effect on the quality of preantral follicles, and follicle and stromal densities. Therefore, herein we demonstrate that the presence of key ovarian structures favors the harvest of ovarian fragments containing an appropriate number of healthy preantral follicles.
Introduction
The mare has been advocated by many researchers as an appropriate comparative animal model to study antral follicular dynamics in women [1–9] due to some similarities in reproductive events (e.g., follicular waves, hormonal concentration changes, age-related decline in fertility, acyclic conditions, and anovulatory dysfunctions such as cysts or luteinized unruptured follicles/hemorrhagic anovulatory follicles). Women and mares are monovular species and although the seasonality does not occur in women, this characteristic in an animal model allows the evaluation of which internal ovarian factors may be operating when acyclic patterns are occurring [3]. More recently, the mare has also been suggested as a model for studies related to preantral follicles [10–13]. Therefore, the search for an appropriate animal model for comparative studies of preantral follicle population, density, and distribution has been a major focus of ovarian translational studies [14].
The preantral follicle population represents the finite and available reserve of gametes that can be recruited during the reproductive lifespan. In order to explore such potential, it is necessary to understand some anatomical-physiological aspects related to the ovary. The follicular heterogeneity observed in ovarian fragments of different species (mares: [10]; women: [15]; cows: [16]; and ewes: [17]) limits the harvesting and more widespread use of preantral follicles in assisted reproductive technologies (ARTs), such as in vitro follicle culture, and ovarian cryopreservation and transplant. The ovarian parenchyma contains a considerable area of stroma which suffers profound structural changes during the different reproductive phases due to the dynamics of antral follicles and corpus luteum. Furthermore, stromal cell density is an important indicator of tissue integrity, plays a role in follicle development [18–20], and is considered an important parameter for maintaining the functionality of preantral follicles. Previous studies reported that ovarian structures produce hormones and growth factors that can stimulate the development and viability of preantral follicles [21–25]. In addition, cellular mechanochemical processes and changes in the extracellular matrix govern tissue morphogenesis during different reproductive phases [26]. Therefore, studies evaluating the influence of ovarian parenchymal structures such as antral follicles (tertiary and preovulatory follicles) and corpus luteum on the number, density, and quality of preantral follicles are needed.
Ovarian biopsy has been an important tool used to harvest preantral follicles for use in clinical and research settings, allowing female fertility preservation in several species (mares: [11, 12]; women: [15, 27, 28, 29]; and cows: [16, 30]). This safe method ensures the collection of preantral follicles without jeopardizing the individual’s reproductive life [10, 31]. To the best of our knowledge, there are no studies in horses reporting established guidelines to identify the most suitable reproductive phase (e.g., diestrous vs. anestrous) to perform ovarian biopsy procedures for harvesting a suitable number of healthy preantral follicles associated with higher follicular and stromal cell densities.
The aim of this study was to assess the influence of reproductive phases (anestrous and diestrous) and ovarian structures (antral follicles and corpus luteum) on the: (1) quality, class distribution, number, and density of preantral follicles, and (2) stromal cell density in ovarian fragments harvested by biopsy procedure.
Materials and Methods
Animals
All experimental procedures were performed according to the United States Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training. The research protocol (#11–007) was approved by the Institutional Animal Care and Use Committee of Southern Illinois University. The study was carried out during the winter (December to March) and spring seasons (April to June) in the northern hemisphere. Light horse mares (n = 10) that weighed between 400 and 600 Kg and were 5 to 11 years old were used. No hormonal treatments were administered during the experimental period. Mares were kept on pasture and orchard grass/alfalfa mixed hay, supplemented with balanced grain ration and minerals.
Area of ovarian structures
The ovaries and uterus of each mare were scanned every other day for two months during the winter and spring seasons using a transrectal ultrasound scanner (Aloka SSD-900, Aloka Co., LTD., Wallingford, CT, USA) equipped with a 5 to 10 MHz linear array transducer (Aloka UST-5821-7.5). At the moment of the biopsy procedure, the ovarian structures were recorded as small follicles (6 to 26 mm of diameter), preovulatory follicles (≥36 mm), and corpus luteum (≥30 mm, days 4 to 12 of estrous cycle). Therefore, the following classifications were generated: a) small follicles (ovary with only small follicles); b) corpus luteum (ovary with small follicles and a corpus luteum); and c) preovulatory follicle (ovary with small follicles and a preovulatory follicle). The height and width of the ovarian structures were recorded at the maximal length using the caliper of the ultrasound scanner software and the average of these measures was used to determine each structure’s diameter [32]. Thereafter, the area (mm2) of each ovarian structure was calculated by the following formula: Area = π × (D/2)2; where: π = 3.14; D = diameter (mm).
Ovarian tissue collection
Biopsy fragments (n = 3 to 4) were collected from each ovary (left and right) separately via the biopsy pick-up technique [10]. Briefly, before each biopsy procedure, analgesia (flunixin meglumine; Flunixiject, 1.1 mg/kg iv; Butler Schein Animal Health, Dublin, OH, USA), rectal relaxation (hyoscine N-butyl bromide; Buscopan, 0.2 mg/kg iv), and sedation (xylazine; AnaSed, 1 mg/kg iv; Lloyd Laboratories, Shenandoah, IA, USA and buthorphanol tartrate; Dolorex, 0.05 mg/kg iv; Intervet / Shering-Plough Animal Health, Millsboro, DE, USA) were induced. Mares were administered penicillin (Agri-Cillin, 6500 U/kg im; AgriLabs, ST. Joseph, MO, USA) immediately after each procedure and for the next two days. The BPU device used was a 48 cm-long, automated, spring-loaded instrument with an inner trocar point plunger containing a 15 × 1.6 mm specimen notch surrounded by an outer 16 ga cutting needle (US Biopsy, Franklin, IN, USA). This device was introduced through a needle guide mounted on a probe handle with a 5 to 10 MHz transvaginal ultrasound-guided convex array transducer (Aloka UST-987-7.5), which was used for placement of the biopsy needle within the ovary. The ovary was manipulated transrectally and positioned against the vaginal wall so that the projected needle path could be visualized. When the needle was properly positioned in the ovary, the inner stylet was advanced to expose the specimen notch. The spring-loaded device was then fired, which propelled the cutting cannula over the specimen notch, thus collecting any ovarian tissue resting within the notch. The Biopsy Pick-Up (BPU) needle was then removed from the transvaginal ultrasound extension probe and the specimen notch exposed in order to retrieve the biopsy fragment. All procedures were performed by the same operator. The ultrasound-guided BPU is a non-invasive method that allows the repeatedly harvesting of ovarian tissue fragments containing large numbers of preantral follicles from living mares without affecting their normal short-term ovarian function or general reproductive health [10, 11, 12].
The procedures were performed during the winter (anestrous phase; follicles ≤20 mm, absence of corpus luteum, ovulation, and estrus signs) and spring seasons (diestrous phase; days 4 to 12 of estrous cycle, presence of a corpus luteum and compatible uterine echotexture). The same mares were used in both seasons.
Histological processing and follicle density
Ovarian fragments were fixed in Bouin’s solution for 2 hours and then kept in 70% alcohol until histological preparation. The fragments were embedded in paraffin wax and totally cut into serial sections (7 μm). Every section was mounted and stained with acid Schiff (PAS) and hematoxylin. All slides were scanned and the perimeter of digital images from histological sections was delimited with a photo editing program (Adobe Photoshop CS4, San Jose, CA, USA) after a scale calibration, and the area’s measurement (cm2) was recorded. Thereafter, the follicle density was determined by the following formula: follicle density = number of preantral follicles/area of the ovarian section (cm2) as previously reported [13].
Microscopy and end points
Histological sections were analyzed on a light microscope (Nikon E200, Tokyo, Japan) at magnification ×400 and an image capture system (LEICA Imaging Software, Wetzlar, Germany). The following end points were recorded: number of preantral follicles and follicle density per ovarian fragment, follicle class distribution, follicle morphology (normal and abnormal), and ovarian stromal cell density. The preantral follicles were classified according to their developmental stage into primordial, transitional, primary, and secondary, as previously described [10].
Morphology of preantral follicles
Only preantral follicles with visualized oocyte nucleus were counted and morphologically classified as normal (follicle containing an intact oocyte and oocyte nucleus surrounded by granulosa cells well organized in one or more layers) or abnormal (follicles with a retracted cytoplasm or disorganized granulosa cell layers detached from the basement membrane and oocyte with pyknotic nucleus [33]).
Ovarian stromal cell density
Ovarian stromal cell density was evaluated as described by Commin et al. [34], with some modifications. Briefly, a total of 10% of histological sections for each ovarian fragment were analyzed. Four random fields (50 × 50 μm = 2,500 μm2) were selected and the stromal cell nuclei were counted to calculate the mean of stromal cell density per ovarian fragment. All evaluations and measurements were performed by a single operator.
Statistical analyses
All statistical analyses were performed using R statistical software version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Data for end points that were not normally distributed were transformed (base 10 logarithmic). The number of preantral follicles and follicle density per ovarian fragment within the same reproductive phase (anestrous or diestrous) and side ovary (left or right) were compared by Kruskal-Wallis test, and among reproductive phases and side ovary by Wilcoxon-Mann-Whitney test. The follicle class distribution and the percentage of normal preantral follicles among reproductive phases were analyzed by chi-square test and G-test. Wilcoxon-Mann-Whitney test was used to compare the stromal cell density and area of the ovarian structures among the reproductive phases and side ovary. In addition, the former test was used to analyze the number of preantral follicles, follicle density, and stromal cell density in biopsy fragments harvested from ovaries with antral follicles and corpus luteum. Correlations between the number of preantral follicles and follicle density with stromal cell density, and among the number of preantral follicles, follicle density and stromal cell density with the area of ovarian structures were estimated by Spearman correlation analysis. Data are presented as mean ± SEM, unless otherwise stated. A probability of P < 0.05 indicated that a difference was significant, and P > 0.05 and ≤ 0.1 indicated that a difference approached significance.
Results
Ovarian biopsy fragments
A total of 142 ovarian fragments were collected by the biopsy pick-up method (mean, 3.7 fragments per ovary in each reproductive phase) and 13,462 histological sections were evaluated. Overall, 1,493 preantral follicles were recorded with a mean of 10.5 ± 1.7 follicles per ovarian fragment [range, 0 to 165; mean coefficient of variation (CV) = 195%]. No adverse effects were observed in the mares’ cyclicity or general reproductive health after any procedure.
Effect of reproductive phase on preantral follicle density
The mean follicle density was 2.7 follicles per cm2 (range, 0 to 39; CV = 214%) and differed (P < 0.05) among mares (Table 1). This parameter varied within the same mare in different reproductive phases and in the overall mean a higher (P < 0.05) follicle density was observed in the diestrous when compared with the anestrous phase. Data of follicle density related to the ovary side (left and right) in different reproductive phases are shown (Table 2). Regardless of reproductive phase, the overall mean follicle density was greater (P < 0.05) in the right ovary. Furthermore, there was an increase (P < 0.05) in the overall mean follicle density in the diestrous phase for both ovary sides.
Table 1. Mean (± SEM) density of equine preantral follicles in ovarian biopsy fragments collected during anestrous and diestrous phases.
| Follicle density (number of preantral follicles/cm2) | |||
|---|---|---|---|
| Mare | Anestrous (n = 73)† | Diestrous (n = 69) | Total (n = 142) |
| 1 | 0.6 ± 0.1aA | 1.5 ± 0.2bA | 1.0 ± 0.1AC |
| 2 | 0.8 ± 0.1aAB | 1.0 ± 0.1bAB | 0.9 ± 0.1AC |
| 5 | 0.6 ± 0.1aA | 0.7 ± 0.3aB | 0.7 ± 0.1A |
| 8 | 17.3 ± 2.3aD | 16.0 ± 0.9bD | 16.4 ± 1.0B |
| 9 | 1.5 ± 0.2aAB | 1.1 ± 0.2aAB | 1.3 ± 0.1AC |
| 10 | 0.7 ± 0.1AB | 0.7 ± 0.1AC | |
| 11 | 0.7 ± 0.1aAB | 0.5 ± 0.2aB | 0.7 ± 0.1A |
| 13 | 2.6 ± 0.3aCE | 3.0 ± 0.4aC | 2.7 ± 0.2CD |
| 21 | 3.1 ± 0.3aC | 3.8 ± 0.4aC | 3.5 ± 0.2D |
| 32 | 1.3 ± 0.2aBE | 0.9 ± 0.2bAB | 1.1 ± 0.1AC |
| Overall mean | 2.1 ± 0.1a | 3.5 ± 0.1b | 2.7 ± 0.1 |
† Total number of ovarian fragments collected via ovarian biopsy pick-up method.
a,b Within a row, values without a common superscript differed (P < 0.05).
A,B,C,D,E Within a column, values without a common superscript differed (P < 0.05).
Mare #10 did not participate in the diestrous phase of the experiment.
Table 2. Mean (± SEM) density of equine preantral follicles in ovarian biopsy fragments collected from left and right ovaries in anestrous and diestrous phases.
| Follicle density (number of preantral follicles/cm2) | ||||
|---|---|---|---|---|
| Anestrous | Diestrous | |||
| Mare | Left ovary (n = 37)† | Right ovary (n = 36) | Left ovary (n = 36) | Right ovary (n = 33) |
| 1 | 0.5 ± 0.1aAC | 0.7 ± 0.2aA | 1.2 ± 0.2aA | 1.8 ± 0.3bAD |
| 2 | 0.9 ± 0.1aAC | 0.8 ± 0.2aAC | 1.0 ± 0.2aA | 1.1 ± 0.2aAB |
| 5 | 1.0 ± 0.2aAC | 0.2 ± 0.1bA | 1.4 ± 0.5aA | 0.04 ± 0.0bB |
| 8 | 10.9 ± 1.5aBD | 24.6 ± 4.6aD | 16.4 ± 1.7aC | 15.7 ± 1.1aC |
| 9 | 0.3 ± 0.1aA | 3.3 ± 0.5bB | 0.5 ± 0.2aA | 1.7 ± 0.3bAD |
| 10 | 0.9 ± 0.2aAC | 0.5 ± 0.1aAC | ||
| 11 | 0.7 ± 0.2aAC | 0.7 ± 0.2aAC | 0.3 ± 0.2aA | 0.8 ± 0.3aAB |
| 13 | 3.0 ± 0.4aD | 2.3 ± 0.4bBC | 4.2 ± 0.7aB | 1.8 ± 0.4bAD |
| 21 | 4.6 ± 0.5aB | 1.4 ± 0.3bAB | 4.1 ± 0.7aB | 3.7 ± 0.6aD |
| 32 | 1.3 ± 0.2aC | 1.4 ± 0.3aAB | 0.1 ± 0.0aA | 2.3 ± 0.6bA |
| Overall mean | 1.9 ± 0.1W | 2.3 ± 0.2X | 3.0 ± 0.2Y | 3.9 ± 0.2Z |
† Total number of ovarian fragments collected via ovarian biopsy pick-up method.
a,b Within a row and same reproductive phase (anestrous or diestrous), values without a common superscript differed (P < 0.05).
A,B,C,D Within a column, values without a common superscript differed (P < 0.05).
W,X,Y,Z Within a row, regardless of the reproductive phase and ovary side, values without a common superscript differed (P < 0.05).
Mare #10 did not participate in the diestrous phase of the experiment.
The mean number of preantral follicles recorded per ovarian fragment during the anestrous and diestrous phases is shown (Table 3). The overall mean was similar among reproductive phases (P > 0.05), and among mares within the same phase a higher variability was observed (P < 0.05). Regardless of reproductive phase, no difference (P > 0.05) was observed for the number of preantral follicles between the left and right ovaries, but once again there was heterogeneity among animals and ovaries of the same animal (Table 4).
Table 3. Mean (± SEM) number of equine preantral follicles in ovarian biopsy fragments collected during anestrous and diestrous phases.
| Number of preantral follicles per ovarian fragment | |||
|---|---|---|---|
| Mare | Anestrous (n = 73)† | Diestrous (n = 69) | Total (n = 142) |
| 1 | 2.7 ± 1.0aA | 9.1 ± 5.3aACE | 5.7 ± 2.6AE |
| 2 | 4.7 ± 2.9aAC | 4.7 ± 1.2aAE | 4.7 ± 1.5ACE |
| 5 | 2.7 ± 0.9aA | 1.7 ± 0.9aB | 2.2 ± 0.6AE |
| 8 | 31.0 ± 2.4aB | 60.8 ± 19.6aD | 50.9 ± 13.4D |
| 9 | 7.2 ± 3.5aAD | 4.6 ± 1.8aAE | 5.8 ± 1.9E |
| 10 | 4.1 ± 1.2AD | 4.1 ± 1.2AE | |
| 11 | 2.3 ± 0.8aA | 1.2 ± 0.8aB | 1.8 ± 0.5A |
| 13 | 14.2 ± 5.6aBCD | 8.6 ± 3.8aE | 11.4 ± 3.3BCE |
| 21 | 22.3 ± 10.3aBD | 16.0 ± 4.5aCE | 18.7 ± 4.9B |
| 32 | 8.2 ± 3.4aAD | 3.2 ± 1.9bAB | 5.7 ± 2.0AE |
| Overall mean | 8.5 ± 1.4a | 12.6 ± 3.1a | 10.5 ± 1.7 |
† Total number of ovarian fragments collected via ovarian biopsy pick-up method.
a,b Within a row, values without a common superscript differed (P < 0.05).
A,B,C,D,E Within a column, values without a common superscript differed (P < 0.05).
Mare #10 did not participate in the diestrous phase of the experiment.
Table 4. Mean (± SEM) number of equine preantral follicles in ovarian biopsy fragments collected from left and right ovaries in anestrous and diestrous phases.
| Number of preantral follicles per ovarian fragment | ||||
|---|---|---|---|---|
| Anestrous | Diestrous | |||
| Mare | Left ovary (n = 37)† | Right ovary (n = 36) | Left ovary (n = 36) | Right ovary (n = 33) |
| 1 | 2.5 ± 1.8 | 3.0 ± 1.2 | 5.2 ± 2.5 | 14.3 ± 12.8 |
| 2 | 6.7 ± 6.0 | 2.7 ± 0.6 | 5.0 ± 1.8 | 4.5 ± 1.8 |
| 5 | 4.2 ± 1.6 | 1.2 ± 0.6 | 2.7 ± 1.6 | 0.3 ± 0.3 |
| 8 | 29.5 ± 5.5 | 32.5 ± 1.5 | 44.0 ± 26.4 | 77.7 ± 30.1 |
| 9 | 1.7 ± 0.7 | 14.6 ± 6.4 | 1.7 ± 0.2 | 7.5 ± 3.2 |
| 10 | 3.7 ± 1.3 | 4.5 ± 2.2 | ||
| 11 | 2.2 ± 1.2 | 2.5 ± 1.2 | 0.7 ± 0.4 | 2.0 ± 2.0 |
| 13 | 17.5 ± 10.2 | 11.0 ± 5.9 | 11.2 ± 7.6 | 6.0 ± 2.2 |
| 21 | 34.6 ± 18.2 | 10.0 ± 7.5 | 12.0 ± 5.2 | 20.0 ± 7.5 |
| 32 | 9.2 ± 4.9 | 7.2 ± 5.5 | 1.2 ± 1.2 | 5.2 ± 3.6 |
| Overall mean§ | 9.5 ± 2.4 | 7.4 ± 1.6 | 9.3 ± 3.5 | 16.1 ± 5.4 |
† Total number of ovarian fragments collected via ovarian biopsy pick-up method.
§ No difference (P > 0.05) was detected between ovaries within each reproductive phase.
Comparisons between ovaries and among mares within the same ovary and reproductive phase were not done because of the low number of observations (range, 2 to 4 fragments per ovary).
Mare #10 did not participate in the diestrous phase of the experiment.
Effect of reproductive phase on preantral follicle class distribution and quality
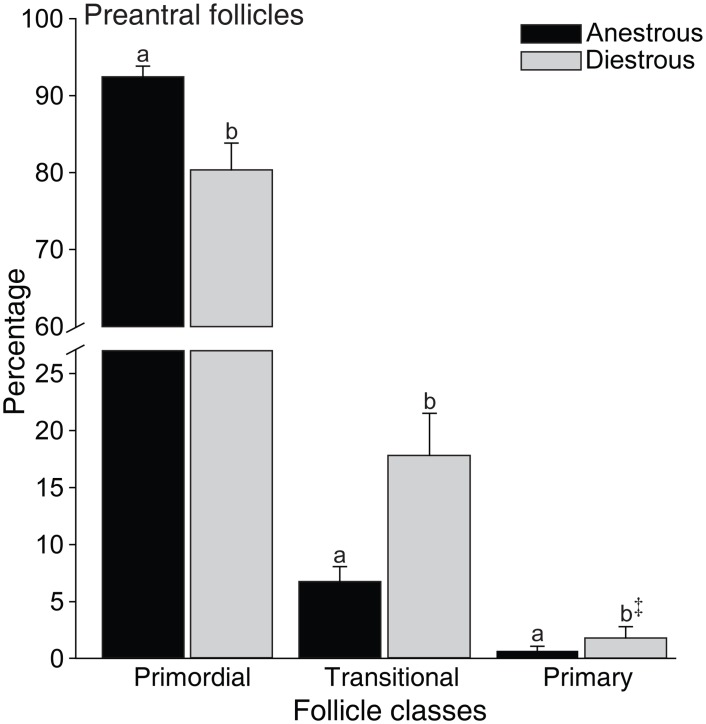
The distribution of preantral follicle population was 85.3% primordial, 13.1% transitional, 1.4% primary, and 0.13% secondary. The percentage of primordial follicles was higher (P < 0.05) in the anestrous compared to the diestrous phase. In contrast, the proportion of growing follicles (transitional, P < 0.05; primary, P < 0.09) was higher in the diestrous phase (Fig 1).
Fig 1. Mean (± SEM) percentage of equine preantral follicles according to class distribution in ovarian biopsy fragments collected during anestrous and diestrous phases.
a,b Within the same follicle class, values without a common letter differed (P < 0.05). ‡ Tended to differ from transitional follicles during the same phase (P < 0.09).
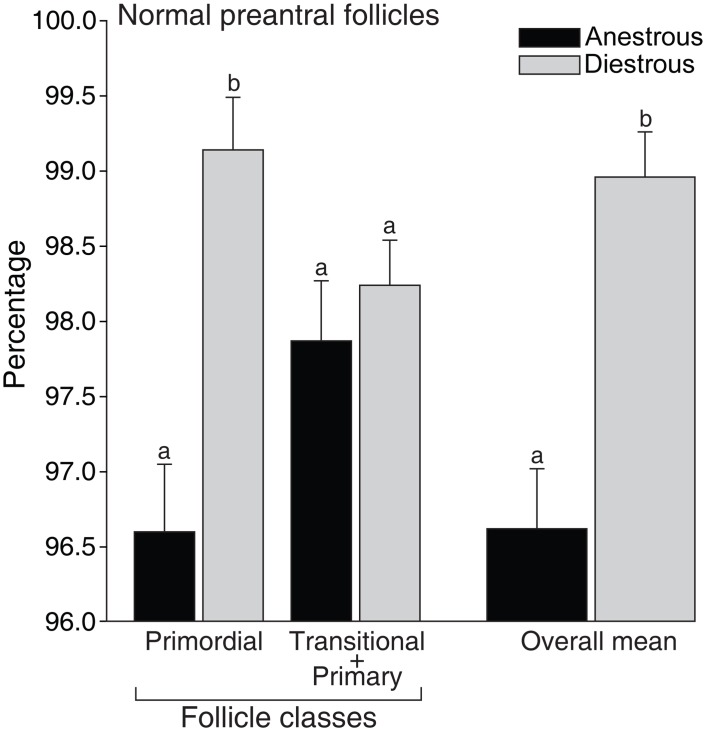
The overall percentage of normal preantral follicles differed (P < 0.05) among anestrous (96%) and diestrous phases (98%) (Fig 2). The percentage of normal primordial follicles was greater (P < 0.05) during the diestrous phase (99%); however, this pattern was not observed (P > 0.05) for growing follicles (transitional and primary follicles combined).
Fig 2. Mean (± SEM) percentage of morphologically normal equine preantral follicles according to class distribution in ovarian biopsy fragments collected during anestrous and diestrous phases.
a,b Within the same follicle class and overall mean, values without a common letter differed (P < 0.05). Because of the low number of primary follicles (n = 5) observed in the anestrous phase, transitional and primary follicles classes were combined.
The percentage of normal preantral follicles in biopsy fragments collected during anestrous and diestrous phases according to the presence of different ovarian structures is shown (Table 5). Within ovaries with the same structures (follicles ≤26 mm), a greater (P < 0.05) percentage of normal preantral follicles was observed during the diestrous phase. Moreover, in the diestrous phase the percentage of normal preantral follicles tended to be higher (P < 0.09) in fragments collected from ovaries with a corpus luteum compared to ovaries with preovulatory follicles (≥36 mm). Overall, a higher (P < 0.05) preantral follicle normality rate was observed in biopsy fragments harvested from ovaries with the presence of a corpus luteum. The combination of preovulatory follicle with a corpus luteum in the same ovary was not considered for assessing the effect of ovarian structures on preantral follicle quality due to the small sample size (7.9%; n = 3/38).
Table 5. Percentage of morphologically normal equine preantral follicles in biopsy fragments collected during anestrous and diestrous phases, and presence of different ovarian structures (antral follicles and corpus luteum).
| Normal preantral follicles (%) | |||
|---|---|---|---|
| Ovarian structures† | Anestrous (n = 582)‡ | Diestrous (n = 796) | Overall (n = 1,378) |
| Follicles ≤26 mm | 96.6a (562/582) | 98.8bAB (352/356) | 97.4A (914/938) |
| Preovulatory follicle | 97.9A (96/98) | 97.9A (96/98) | |
| Corpus luteum | 99.7B§ (341/342) | 99.7B§ (341/342) | |
† Antral follicles ≤26 mm: 6 to 26 mm in diameter; Preovulatory follicle: ≥36 mm; and Corpus luteum: ≥30 mm, days 4 to 12 of estrous cycle.
‡ Total number of preantral follicles evaluated.
a,b Within a row, values without a common superscript differed (P < 0.05).
A,B Within a column, values without a common superscript differed (P < 0.05).
§ Tended to differ from the preovulatory follicle (P < 0.09).
Effect of reproductive phase on stromal cell density
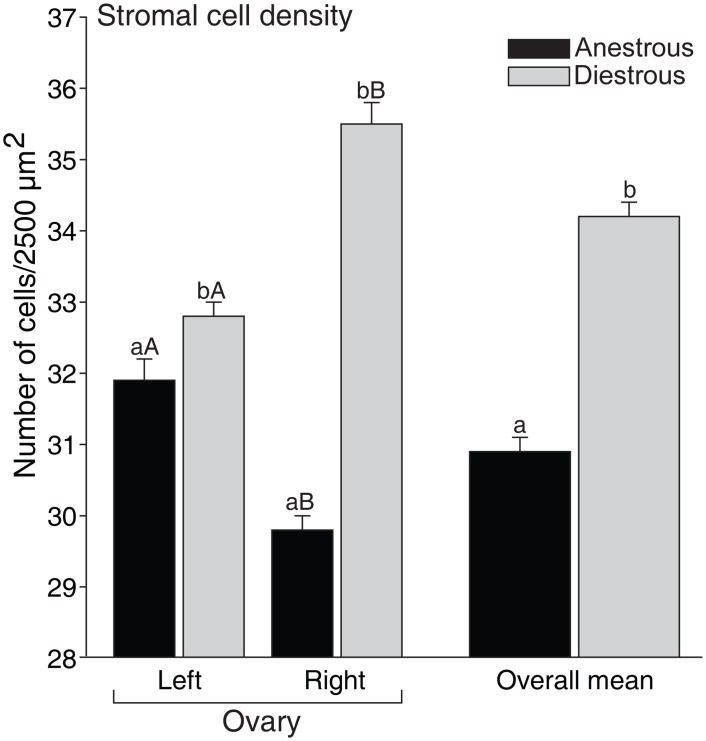
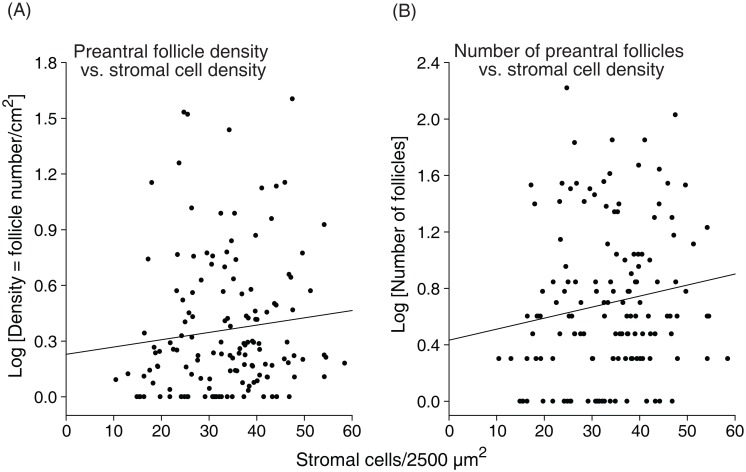
The mean stromal cell density (cells/2,500 μm2) of the ovarian fragments was 32.0 ± 0.1 cells (range, 6 to 64 cells; CV = 37%). The cell density increased (P < 0.05) in the diestrous phase (34.2 ± 0.1) when compared to the anestrous phase (30.9 ± 0.1), regardless of the ovary side (Fig 3). In addition, when comparing the ovary sides within the same reproductive phases, the left ovary in anestrous and right ovary in diestrous phases had higher (P < 0.05) stromal cell densities. Positive correlations of stromal cell density with follicle density (P < 0.07) and number of preantral follicles (P < 0.05) per ovarian fragment were observed (Fig 4A and 4B).
Fig 3. Mean (± SEM) density of equine ovarian stromal cells in biopsy fragments collected from left and right ovaries during anestrous and diestrous phases.
a,b Within the same ovarian side and overall mean, values without a common letter differed (P < 0.05). A,B Within the same reproductive phase, left and right ovary values without a common letter differed (P < 0.05).
Fig 4. Correlation between (A) follicular density and stromal cell density, and (B) number of preantral follicles and stromal cell density.
The association among variables (black line) was evaluated by Spearman correlation coefficient [(A): r = 0.15, P < 0.07; (B): r = 0.16, P < 0.05]. Each point on the chart represents one ovarian fragment evaluated (n = 142).
Effect of ovarian structures on preantral follicle and stromal cell densities
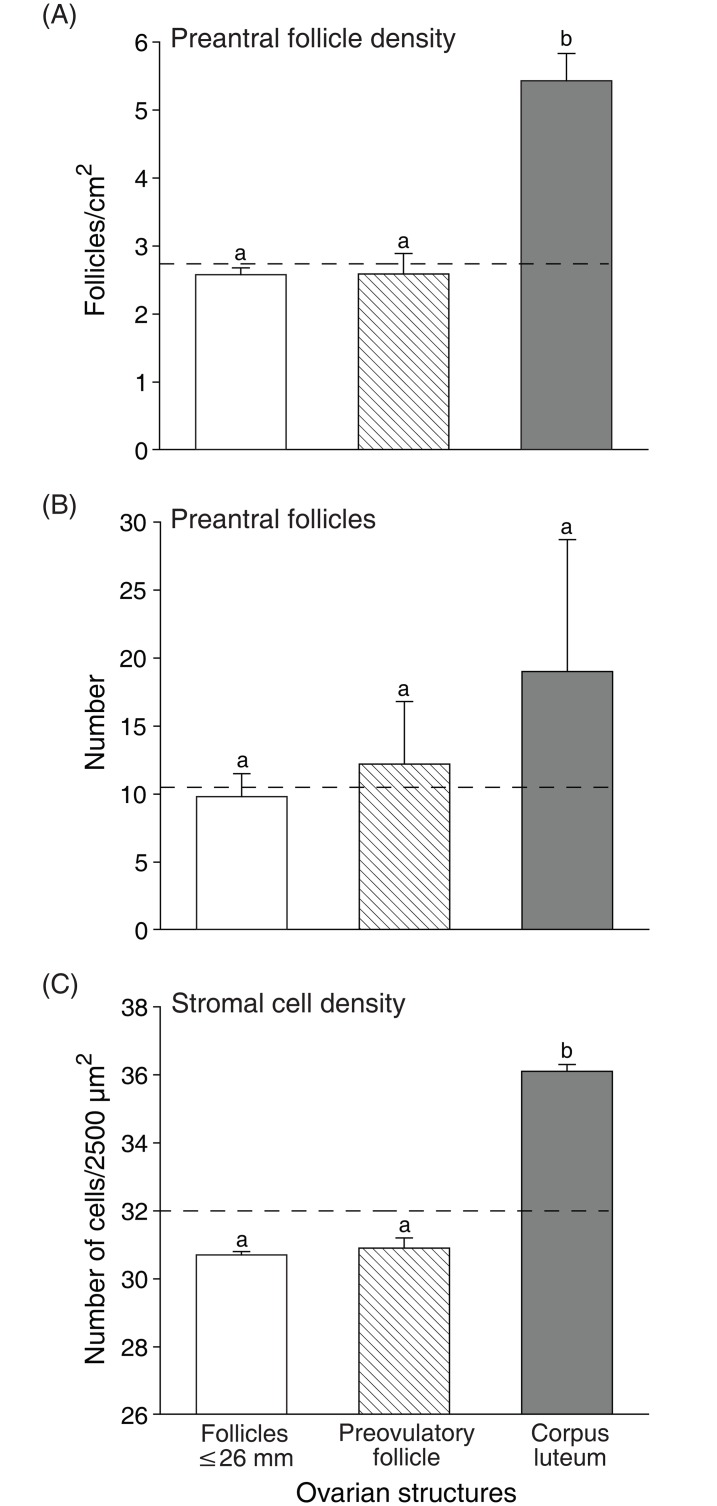
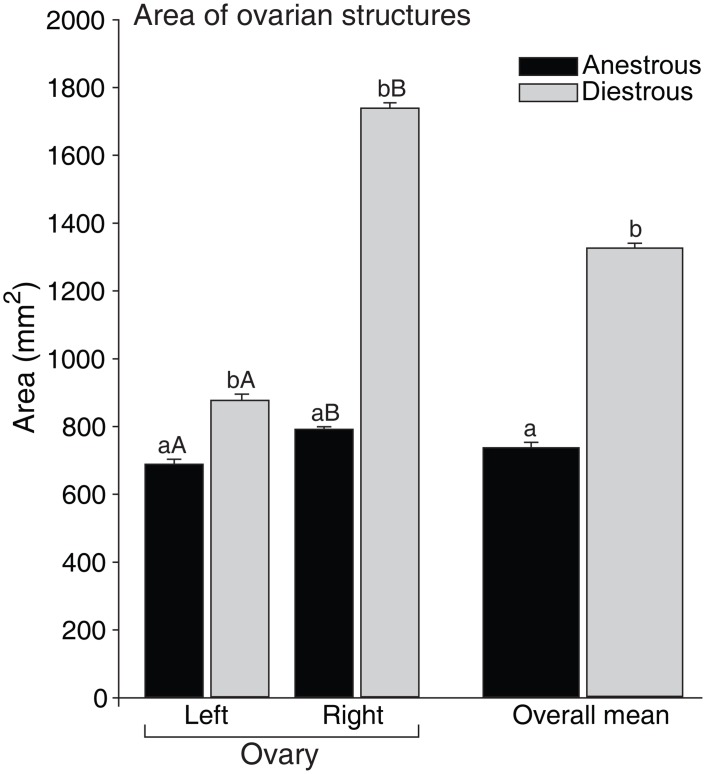
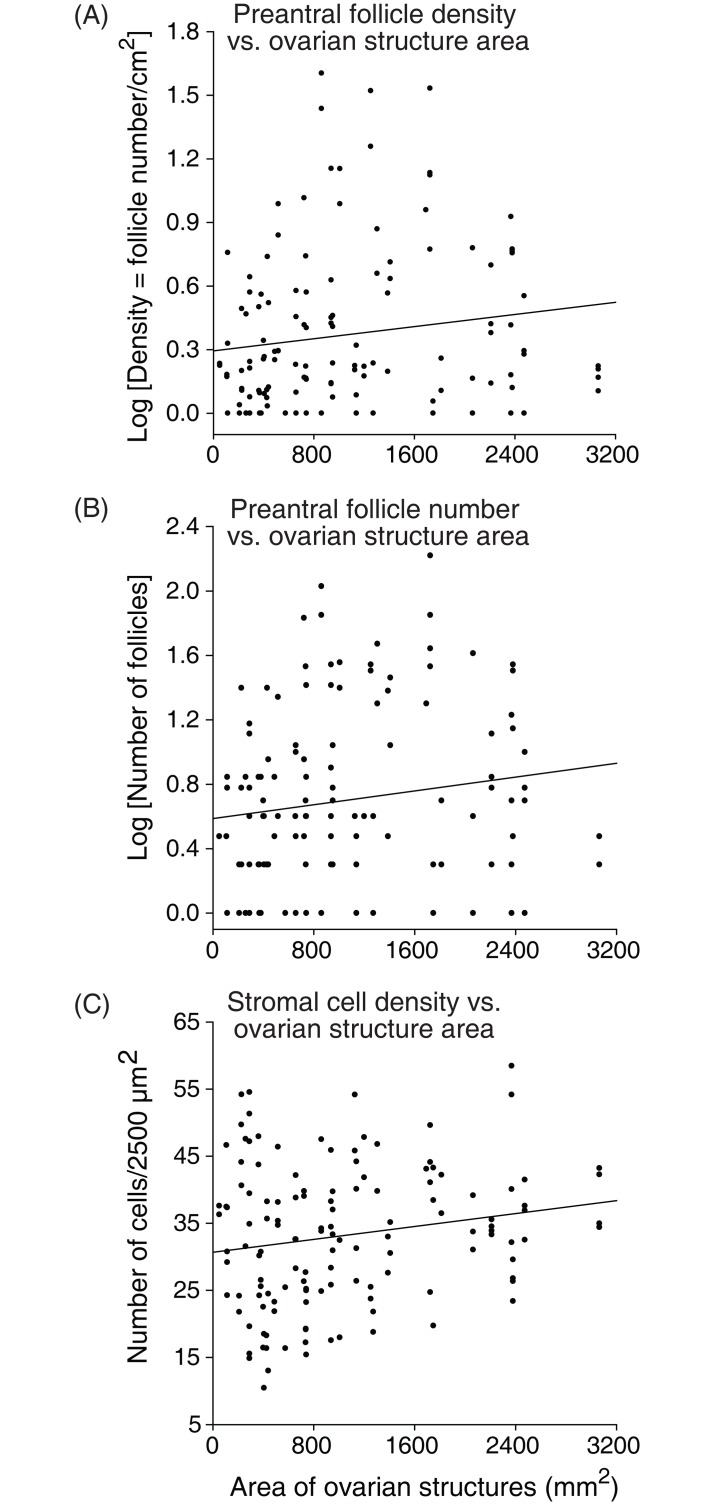
Number of preantral follicles, and follicle and stromal cell densities evaluated according to the type of ovarian structures present in the ovary are shown (Fig 5). Biopsy fragments harvested from ovaries with corpus luteum showed a greater (P < 0.05) mean follicle density (5.4 ± 0.4) when compared with fragments from ovaries with preovulatory follicles (2.5 ± 0.3), and follicles ≤26 mm (2.5 ± 0.1; Fig 5A). Nevertheless, the mean number of preantral follicles recorded was similar (P > 0.05) among fragments from ovaries with all types of structures (follicles ≤26 mm: 9.8 ± 1.7; preovulatory follicle: 12.2 ± 4.6; and corpus luteum: 19.0 ± 9.7; Fig 5B). The mean stromal cell density was similar (P > 0.05) among ovaries with follicles ≤26 mm (30.7 ± 0.1) and preovulatory follicles (30.9 ± 0.3), and differed (P < 0.05) from ovaries with corpus luteum (36.1 ± 0.2; Fig 5C). In addition, the overall mean area (mm2) of ovarian structures was greater (P < 0.05) in the diestrous phase and the right ovary had a greater area (P < 0.05) of ovarian structures when compared to the left ovary regardless of reproductive phase (Fig 6). Positive correlations (P < 0.05) were observed between the area of ovarian structures and follicle density, number of preantral follicles, and stromal cell density per ovarian fragment (Fig 7A–7C).
Fig 5. Mean (± SEM) (A) follicle density, (B) number of preantral follicles, and (C) ovarian stromal cell density in biopsy fragments collected from ovaries with antral follicles and corpus luteum.
Dashed line represents the average for (A) follicle density, (B) number of preantral follicles, and (C) stromal cell density. a,b Within the same parameter evaluated, values without a common letter differed (P < 0.05). Follicles ≤26 mm: 6 to 26 mm in diameter; Preovulatory follicle: ≥36 mm; and Corpus luteum: ≥30 mm, days 4 to 12 of estrous cycle.
Fig 6. Mean (± SEM) area (mm2) of equine ovarian structures (antral follicles and corpus luteum) during the biopsy pick-up procedures performed during anestrous and diestrous phases.
a,b Within the same ovarian side and overall mean, values without a common letter differed (P < 0.05). A,B Within the same reproductive phase, left and right ovary values without a common letter differed (P < 0.05).
Fig 7. Correlation coefficients between (A) follicle density, (B) number of preantral follicles, and (C) ovarian stromal cell density with area of ovarian structures (antral follicles and corpus luteum).
The association among variables (black line) was evaluated by Spearman correlation [(A): r = 0.21, (B): r = 0.21, (C): r = 0.10; P < 0.05]. Each point on the chart represents one ovarian fragment evaluated.
Discussion
Studies with human ovaries face ethical barriers and limited availability of material for research, concerns which are lesser with domestic animals. Considering the similarities between women and mares relative to the dynamics of reproductive cycles [5, 7–9], age-associated changes in fertility [11], and the physiological preantral follicle heterogeneity [13], the mare can be considered an important animal model to advance knowledge regarding follicular population and density in ovarian biopsy fragments. Thus, studies with preantral follicles in mares (as an animal model) may provide relevant information that could be applied in the future on clinical human reproduction.
To our knowledge, this study reports for the first time in mares the influence of reproductive phases (anestrous and diestrous), ovary side (left and right), and ovarian structures (antral follicles and corpus luteum) on number and density of preantral follicles, and on ovarian stromal cell density from ovarian biopsy fragments.
Primordial follicles constitute the sole and critical reserve for all further follicle recruitment [35]. In this study, a higher percentage of primordial follicles was observed in the anestrous phase; however, the percentage of growing follicles (transitional and primary combined) and the overall percentage of normal follicles were greater during the diestrous phase. These results indicated that an increase in primordial follicle activation [36] occurred during the diestrous phase potentially due to higher levels of gonadotropins (FSH and LH) and several growth factors (epidermal growth factor, EGF; transforming growth factor beta, TGF-β; and vascular endothelial growth factor, VEGF) during the breeding season [37]. In addition, studies in vitro have shown the benefits of the aforementioned stimulatory factors on the survival and development of preantral follicles [38, 39]. A higher ovarian stromal cell density in both ovary sides was observed in the diestrous phase compared to the anestrous. Overall, a positive correlation of stromal cell density with number and density of preantral follicles was observed. Stromal cell density (number of cells per μm2) in mares was different when compared to bitches (1.7-fold higher; [34]), or goats and ewes (8.5-fold lower; [40]). Stromal cells are essential for the development and survival of grafted isolated follicles and have an important role in co-culture systems [41, 42]. Furthermore, theca cells are derived from stromal cells and together produce steroids (androgens) and growth factors (TGF-β, EGF, bone morphogenetic protein 7, BMP-7), which play a role in follicle activation and survival [43–46]. Once stromal cell and preantral follicle density have an important role in female fertility, the association of these factors should be considered when selecting the most suitable ovarian biopsy fragments to be used for ART programs.
In the present study, an increase in follicle density during the diestrous phase was observed when compared to the anestrous phase, regardless of the ovary side. In addition, a high disparity in follicle density among and within animals was detected in both reproductive phases. In a recent study [13] in which the reproductive phase of the estrous cycle was unknown, our group also reported a high heterogeneity in follicle density among and within mares. Contrary to the results from follicle density, the overall mean number of equine preantral follicles in biopsy fragments in the present study was similar for both ovaries (left and right) and reproductive phases (anestrous and diestrous). One possible explanation for these two findings is that the biopsies were performed in different areas of the ovarian stroma and recovered fragments with different sizes and heterogeneous follicle population [27]. On the other hand, the follicle density in biopsy fragments uses an established measure unity (area) which equalizes all samples, providing a more accurate parameter to evaluate preantral follicle population for further use in ART programs.
In our study, the biopsies were performed in different reproductive conditions (anestrous and diestrous). In order to explain the influence of ovarian structures on the number of preantral follicles and follicle and stromal cell densities, we analyzed the data in two ways: (1) total area of ovarian structures (small antral follicles, preovulatory follicle, and corpus luteum), and (2) type of ovarian structures present at the moment of the biopsy procedure. Regarding the first aspect, the overall mean area of ovarian structures increased by over 79% in the diestrous phase. In addition, the area of structures had a positive correlation with the number of preantral follicles and follicle and stromal cell densities, and the right ovary showed a greater area than the left ovary in both studied reproductive phases. Moreover, biopsy fragments collected from ovaries with a corpus luteum had greater follicle and stromal cell densities and percentage of normal preantral follicles. The influence of corpus luteum on number of preantral follicles in different species has been reported (bovine: [47]; bubaline: [23]; ovine: [21]). The primary function of the corpus luteum is the production of progesterone [48] and prostaglandin F2α [49], hormones that can improve the quality of preantral follicles [50]. Furthermore, the proliferation index of endothelial cells is intense in the early luteal phase [51], which is characterized by a dense network of capillaries in the ovary. Therefore, a potential physiological mechanism behind our findings might be that the presence of an active and highly vascularized corpus luteum may contribute to better diffusion of growth factors and hormones throughout the ovarian stroma, favoring the quality of preantral follicles. In addition, a positive correlation between the degree of cell proliferation and vascular area has been reported during early preantral follicle growth [52]. Consequently, the greater percentage of transitional and primary follicles and normal primordial follicles observed in our data during the diestrous phase makes it tempting to speculate that preantral follicle recruitment in mares may be influenced by stromal vascular supply and blood flow perfusion. Further studies need to be performed to address this subject.
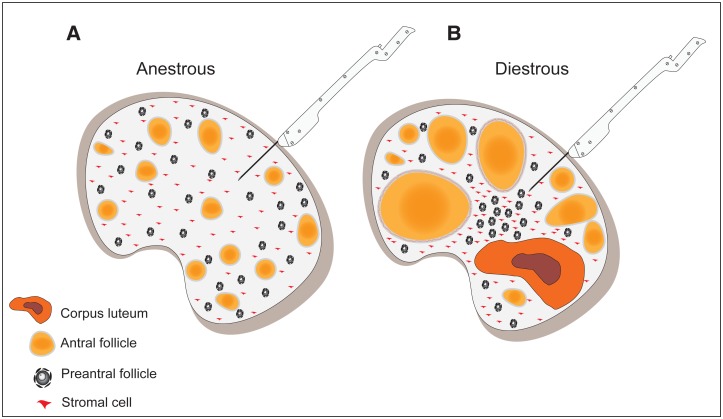
To our knowledge, there are no studies that described the association among the number of preantral follicles, follicle density, and stromal cell density with the area of ovarian structures for any species. This fact allowed us to propose a hypothetical mechanism to support the results observed in our study (Fig 8). During the anestrous phase in mares, there is low follicle activity and small follicles are present in the ovarian stroma. In contrast, in the diestrous phase, multiple structures such as small and medium antral follicles, preovulatory follicles, and corpus luteum can be present in the same ovary. This fact indicates that denser structures, such as the corpus luteum associated or not with larger antral follicles, can exert a greater pressure in the ovarian parenchyma and cause a consequent clustering of preantral follicles and stromal cells per unit area. In the anestrous phase, the absence of large structures allows lower compression in the ovarian tissue and therefore a possible dispersion of the preantral follicle population in the ovary.
Fig 8. Model proposing the difference of follicle density, number of preantral follicles, and stromal cell density among anestrous (A) and diestrous (B) phases.
The increase of the area of ovarian structures during the diestrous phase due to the presence of large follicle(s) and corpus luteum may increase the ovarian compression and consequently the clustering of preantral follicles and stromal cells per unit area in ovarian fragments harvested by biopsy procedure.
In summary, our results indicated that: (1) the diestrous phase influenced positively the quality of preantral follicles, percentage of growing follicles, and follicle and stromal cell densities; (2) the area of ovarian structures affected the stromal cell density, and the number of preantral follicles and follicle density; and (3) the corpus luteum had a positive effect on the quality of preantral follicles, and follicle and stromal cell densities. In addition, we discovered herein a potential ideal scenario for collection of richer ovarian fragments (i.e. higher preantral follicle and stromal cell densities) using ovarian biopsy procedure. These findings reinforce the concept of the use of the mare as a model to provide comparative insights about preantral follicle density and ovarian plasticity.
Supporting Information
(XLS)
Acknowledgments
Research was supported by Southern Illinois University (SIU), USA, and Foundation for Research Support of the State of Goiás (FAPEG; 201210267001032), Brazil. Alves K.A. was the recipient of a PhD sandwich scholarship from The National Council for Scientific and Technological Development (CNPq; 203563/2013-1), Brazil.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research was supported by Southern Illinois University (SIU), USA, and Foundation for Research Support of the State of Goiás (FAPEG; 201210267001032), Brazil. KAA was the recipient of a PhD sandwich scholarship from The National Council for Scientific and Technological Development (CNPq; 203563/2013-1), Brazil.
References
- 1.Ginther OJ, Gastal EL, Gastal MO, Bergfelt DR, Baerwald AR, Pierson RA. Comparative study of the dynamics of follicular waves in mares and women. Biol Reprod 2004; 71:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginther OJ, Beg MA, Gastal EL, Gastal MO, Baerwald AR, Pierson RA. Systemic concentrations of hormones during the development of follicular waves in mares and women: a comparative study. Reproduction 2005; 130:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnevale EM. The mare model for follicular maturation and reproductive aging in the woman. Theriogenology 2008; 69:23–30. [DOI] [PubMed] [Google Scholar]
- 4.Mihm M, Evans ACO. Mechanisms for dominant follicle selection in monovulatory species: a comparison of morphological, endocrine and intraovarian events in cows, mares and women. Reprod Dom Anim 2008; 43:48–56. [DOI] [PubMed] [Google Scholar]
- 5.Baerwald AR. Human antral folliculogenesis: what we have learned from the bovine and equine models. Anim Reprod 2009; 6:20–29. [Google Scholar]
- 6.Baerwald AR, Walker RA, Pierson RA. Growth rates of ovarian follicles during natural menstrual cycles, oral contraception cycles, and ovarian stimulation cycles. Fertil Steril 2009; 91:440–449. 10.1016/j.fertnstert.2007.11.054 [DOI] [PubMed] [Google Scholar]
- 7.Gastal EL. Recent advances and new concepts on follicle and endocrine dynamics during the equine periovulatory period. Anim Reprod 2009; 6:144–158. [Google Scholar]
- 8.Gastal EL, Gastal MO, Wischral A, Davis J. The equine model to study the influence of obesity and insulin resistance in human ovarian function. Acta Sci Vet 2011; 39:57–70. [Google Scholar]
- 9.Ginther OJ. The mare: a 1000-pound guinea pig for study of the ovulatory follicular wave in women. Theriogenology 2012; 77:818–828. 10.1016/j.theriogenology.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 10.Haag KT, Magalhaes-Padilha DM, Fonseca GR, Wischral A, Gastal MO, King SS, et al. Equine preantral follicles obtained via the Biopsy Pick-Up method: histological evaluation and validation of a mechanical isolation technique. Theriogenology 2013; 79:735–743. 10.1016/j.theriogenology.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 11.Haag KT, Magalhaes-Padilha DM, Fonseca GR, Wischral A, Gastal MO, King SS, et al. Quantification, morphology, and viability of equine preantral follicles obtained via the Biopsy Pick-Up method. Theriogenology 2013; 79:599–609. 10.1016/j.theriogenology.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 12.Haag KT, Magalhaes-Padilha DM, Fonseca GR, Wischral A, Gastal MO, King SS, et al. In vitro culture of equine preantral follicles obtained via the Biopsy Pick-Up method. Theriogenology 2013; 79:911–917. 10.1016/j.theriogenology.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 13.Alves KA, Alves BG, Rocha CD, Visonna M, Mohallem RF, Gastal MO, et al. Number and density of equine preantral follicles in different ovarian histological section thicknesses. Theriogenology 2015; 83:1048–1055. 10.1016/j.theriogenology.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 14.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013; 99:1523–1533. 10.1016/j.fertnstert.2013.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt KL, Byskov AG, Andersen NA, Muller J, Andersen YC. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod 2003; 18:1158–1164. [DOI] [PubMed] [Google Scholar]
- 16.Aerts JM, Oste M, Bols PE. Development and practical applications of a method for repeated transvaginal, ultrasound-guided biopsy collection of the bovine ovary. Theriogenology 2005; 64:947–957. [DOI] [PubMed] [Google Scholar]
- 17.Fransolet M, Labied S, Henry L, Masereel MC, Rozet E, Kirschvink N, et al. Strategies for using the sheep ovarian cortex as a model in reproductive medicine. PLoS One 2014; 9:e91073 10.1371/journal.pone.0091073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci 2004; 82:431–446. [DOI] [PubMed] [Google Scholar]
- 19.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132:191–206. [DOI] [PubMed] [Google Scholar]
- 20.Gerritse R, Beerendonk CC, Westphal JR, Bastings L, Braat DD, Peek R. Glucose/lactate metabolism of cryopreserved intact bovine ovaries as a novel quantitative marker to assess tissue cryodamage. Reprod Biomed Online 2011; 23:755–764. 10.1016/j.rbmo.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 21.Al-Gubory KH, Martinet J. Effect of the corpus luteum on ovarian follicular populations and growth in the ewe. Anim Reprod Sci 1987; 13:269–281. [Google Scholar]
- 22.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update 2005; 11:461–471. [DOI] [PubMed] [Google Scholar]
- 23.Gupta PS, Ramesh HS, Nandi S, Ravindra JP. Recovery of large preantral follicles from buffalo ovary: effect of season and corpus luteum. Anim Reprod Sci 2007; 101:145–152. [DOI] [PubMed] [Google Scholar]
- 24.Yang MY, Fortune JE. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol Reprod Dev 2007; 74:1095–1104. [DOI] [PubMed] [Google Scholar]
- 25.Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction 2009; 138:869–881. 10.1530/REP-09-0283 [DOI] [PubMed] [Google Scholar]
- 26.Thorne JT, Segal TR, Chang S, Jorge S, Segars JH, Leppert PC. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol Reprod 2015; 92:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lass A, Silye R, Abrams DC, Krausz T, Hovatta O, Margara R, et al. Follicular density in ovarian biopsy of infertile women: a novel method to assess ovarian reserve. Hum Reprod 1997; 12:1028–1031. [DOI] [PubMed] [Google Scholar]
- 28.Kwok R, Johnson NP. Ovarian biopsy has no role as a routine diagnostic test of ovarian reserve: a systematic review. Reprod Biomed Online 2012; 24:492–495. 10.1016/j.rbmo.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 29.Vanacker J, Luyckx V, Amorim C, Dolmans MM, Van Langendonckt A, Donnez J, et al. Should we isolate human preantral follicles before or after cryopreservation of ovarian tissue? Fertil Steril 2013; 99:1363–1368. 10.1016/j.fertnstert.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 30.Jorssen EP, Langbeen A, Fransen E, Martinez EL, Leroy JL, Bols PE. Monitoring preantral follicle survival and growth in bovine ovarian biopsies by repeated use of neutral red and cultured in vitro under low and high oxygen tension. Theriogenology 2014; 82:387–395. 10.1016/j.theriogenology.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 31.Lass A. Assessment of ovarian reserve—Is there a role for ovarian biopsy? Hum Reprod 2001; 16:1055–1057. [DOI] [PubMed] [Google Scholar]
- 32.Gastal EL, Gastal MO, Bergfelt DR, Ginther OJ. Role of diameter differences among follicles in selection of a future dominant follicle in mares. Biol Reprod 1997; 57:1320–1327. [DOI] [PubMed] [Google Scholar]
- 33.Silva JR, Lucci CM, Carvalho FC, Bao SN, Costa SH, Santos RR, et al. Effect of coconut water and Braun-Collins solutions at different temperatures and incubation times on the morphology of goat preantral follicles preserved in vitro. Theriogenology 2000; 54:809–822. [DOI] [PubMed] [Google Scholar]
- 34.Commin L, Buff S, Rosset E, Galet C, Allard A, Bruyere P, et al. Follicle development in cryopreserved bitch ovarian tissue grafted to immunodeficient mouse. Reprod Fertil Dev 2012; 24:461–471. 10.1071/RD11166 [DOI] [PubMed] [Google Scholar]
- 35.Forabosco A, Sforza C. Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. Fertil Steril 2007; 88:675–683. [DOI] [PubMed] [Google Scholar]
- 36.Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci 2003; 78:135–163. [DOI] [PubMed] [Google Scholar]
- 37.Donadeu FX, Watson ED. Seasonal changes in ovarian activity: lessons learnt from the horse. Anim Reprod Sci 2007; 100:225–242. [DOI] [PubMed] [Google Scholar]
- 38.Thomas FH, Walters KA, Telfer EE. How to make a good oocyte: an update on in-vitro models to study follicle regulation. Hum Reprod Update 2003; 9:541–555. [DOI] [PubMed] [Google Scholar]
- 39.Araújo VR, Gastal MO, Figueiredo JR, Gastal EL. In vitro culture of bovine preantral follicles: a review. Reprod Biol Endocrinol 2014; 12:78 10.1186/1477-7827-12-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faustino LR, Santos RR, Silva CM, Pinto LC, Celestino JJ, Campello CC, et al. Goat and sheep ovarian tissue cryopreservation: effects on the morphology and development of primordial follicles and density of stromal cell. Anim Reprod Sci 2010; 122:90–97. 10.1016/j.anireprosci.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 41.Tingen MC, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 2011; 141:809–820. 10.1530/REP-10-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dath C, Dethy A, Van Langendonckt A, Van Eyck AS, Amorim CA, Luyckx V, et al. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum Reprod 2011; 26:1431–1439. 10.1093/humrep/der073 [DOI] [PubMed] [Google Scholar]
- 43.Palermo R. Differential actions of FSH and LH during folliculogenesis. Theriogenology 2007; 15:326–337. [DOI] [PubMed] [Google Scholar]
- 44.Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res 2009; 2:9 10.1186/1757-2215-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction 2010; 140:489–504. 10.1530/REP-10-0094 [DOI] [PubMed] [Google Scholar]
- 46.Qiu M, Quan F, Han C, Wu B, Liu J, Yang Z, et al. Effects of granulosa cells on steroidogenesis, proliferation and apoptosis of stromal cells and theca cells derived from the goat ovary. J Steroid Biochem Mol Biol 2013; 138:325–333. 10.1016/j.jsbmb.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 47.Gao J, Yandong W, Yaxin F, Xuenqing W, Yong G, Liyun G, et al. Relationship between situation of the bovine ovarian corpus luteum and the numbers of isolated preantral follicles from them. Chin J Vet Sci 2001; 21:181–183. [Google Scholar]
- 48.Auletta FJ, Flint AP. Human ovarian oxytocin and steroid hormones. Am J Obstet Gynecol 1987; 157:1010–1011. [DOI] [PubMed] [Google Scholar]
- 49.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 2000; 80:1–29. [DOI] [PubMed] [Google Scholar]
- 50.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 2003; 144:3329–3337. [DOI] [PubMed] [Google Scholar]
- 51.Al-zi’abi MO, Watson ED, Fraser HM. Angiogenesis and vascular endothelial growth factor expression in the equine corpus luteum. Reproduction 2003; 125:259–270. [PubMed] [Google Scholar]
- 52.Martelli A, Palmerini MG, Russo V, Rinaldi C, Bernaból N, Di Giacinto O, et al. Blood vessel remodeling in pig ovarian follicles during the periovulatory period: an immunohistochemistry and SEM-corrosion casting study. Reprod Biol Endocrinol 2009; 7:72 10.1186/1477-7827-7-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.