Abstract
Border cell migration in the Drosophila ovary has emerged as a genetically tractable model for studying collective cell movement. Over many years border cell migration was exclusively studied in fixed samples due to the inability to culture stage 9 egg chambers in vitro. Although culturing late-stage egg chambers was long feasible, stage 9 egg chambers survived only briefly outside the female body. We identified culture conditions that support stage 9 egg chamber development and sustain complete migration of border cells ex vivo. This protocol enables one to compare the dynamics of egg chamber development in wild-type and mutant egg chambers using time-lapse microscopy and taking advantage of a multiposition microscope with a motorized imaging stage. In addition, this protocol has been successfully used in combination with fluorescence resonance energy transfer biosensors, photo-activatable proteins, and pharmacological agents and can be used with wide-field or confocal microscopes in either an upright or an inverted configuration.
Keywords: Border cell migration, Drosophila stage 9 egg chambers, Organ culture, Collective cell migration, Time-lapse live imaging
1 Introduction
Collective cell migration refers to the concerted movement of groups of cells. Unlike single moving cells such as fibroblasts or fish keratinocytes, collectively migrating cells maintain some level of adhesion among themselves during movement [1, 2]. Though this kind of cellular movement is characteristic of several physiological processes during embryonic development [3], wound healing, and tumor metastasis [1], it has been studied less extensively than the movements of single cells. Recently, a number of model systems have emerged for the study of collective movement using the powerful combination of genetic manipulations and live imaging [4, 5]. One of these, border cell migration in the Drosophila ovary, is the focus of this chapter.
Drosophila females bear a pair of ovaries within the abdomen (Fig. 1). Each ovary consists of 15–20 strings of egg chambers of increasing stages of maturity, called the ovarioles. At the tip of each ovariole resides the germarium, which contains germline and somatic stem cells and their immediate progeny. Egg chambers assemble in the germarium when somatic follicle cells surround a cyst of 16 interconnected germline cells, one of which develops into the oocyte while the other 15 differentiate as support cells called nurse cells [6]. Egg chambers bud off from the germarium and then grow and progress through 14 developmental stages [7]. Whereas germline cells do not divide further, follicle cells continue to undergo mitotic divisions until stage 6 when they switch to endoreplication without cytokinesis [8]. During early oogenesis at each end of each egg chamber a pair of specialized follicle cells differentiates into the polar cells [9]. The polar cells secrete a cytokine, unpaired, which activates JAK-STAT signaling in nearby follicle cells [10]. In late stage 8 and early stage 9, anterior follicle cells (4–6 in number) that perceive the highest level of JAK-STAT signal round up [11, 12]. These cells are the border cells.
Fig. 1.
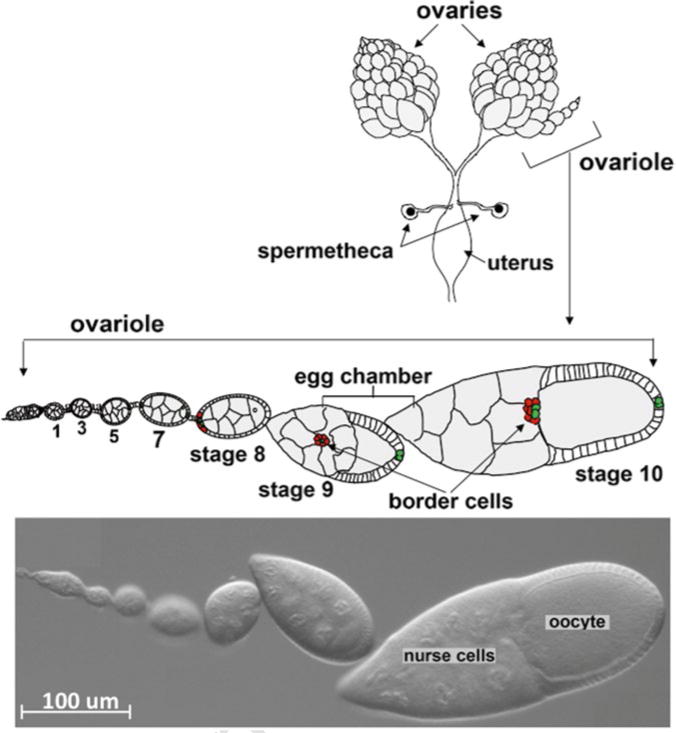
Anatomy of the Drosophila ovary. Top—Schematic drawing of a pair of ovaries dissected from female fruit fly. A schematic drawing of an enlarged single ovariole containing egg chambers of the indicate stages of development. Bottom—DIC image of an ovariole with similar stages of egg chamber development
One or two of the cells extend protrusions in between the nurse cells. Some of these protrusions retract right away but sooner or later a protrusion attaches stably to the nurse cells and the border cell cluster detaches from the other follicle cells and from the basement membrane that surrounds the egg chamber [13, 14]. The border cells migrate directly down the center of the egg chamber toward the oocyte, in response to secreted signals. One such signal is the PDGF- and VEGF-related factor 1 (PVF1), which binds to a receptor tyrosine kinase, PVR, expressed on the border cells [15, 16]. PVR functions redundantly with epidermal growth factor receptor (EGFR) [15]. Three ligands for the EGFR are expressed in the oocyte [16, 17], Spitz, Keren, and Gurken, all of which are TGFα homologs. Spitz and keren mRNAs are distributed throughout the oocyte at stage 9 and these two ligands can redirect border cells when either one is misexpressed [16]. Thus, these ligands promote migration of the border cells to the oocyte. When the border cells get very near to the oocyte, they turn and move toward the dorsal side [17] (Movie 1). Grk mRNA and protein are restricted to the dorsal/anterior corner of the oocyte and promote the dorsal turn [17]. It is unlikely that border cells sense Grk until they get near the oocyte because there is no dorsal bias to the migration before that point [16]. Moreover, when Grk is expressed ectopically it is not sufficient to redirect border cells during the posterior migration [16].
The border cells cover a distance of approximately 150–200 μm in 4–6 h [13]. Their migration speed is variable and is faster in the beginning and slower near the end [14, 18]. In the migrating cluster, individual border cells can change relative position within the group, while the polar cells remain in the center [13, 14]. Until 2007, border cell migration was studied exclusively in fixed tissue due to the lack of suitable culture conditions for stage 9 egg chambers. Recently, we identified the culture conditions and subsequently optimized the imaging conditions for capturing the complete migration while minimizing phototoxicity [13] (Movie 1). This protocol has enabled more detailed phenotypic analysis and use of pharmacological agents, fluorescence resonance energy transfer (FRET) probes, and photo-activatable proteins [12, 19, 20]. In addition, this protocol can be used for studying other aspects of oogenesis including epithelial morphogenesis of follicle cells [21], RNA localization in the oocyte [22], actin dynamics in nurse cells [23, 24], and stem cell division in the germarium [24]. Key features of the protocol are optimization of pH and addition of insulin, which may generally enhance cultures of Drosophila tissues including imaginal discs. Longer term cultures (>6 h) may require perfusion systems to allow medium exchange.
2 Materials
2.1 Culture Reagents and Equipment
Drosophila Schneider’s medium (Drosophila S2 Medium) supplemented with 20 % fetal bovine serum (FBS).
Insulin: A 10 mg/ml stock solution was prepared in acidified water (see Note 1) and used to supplement the culture media to a final concentration of 0.2 mg/ml.
(Optional) FM4-64 lipophilic dye dissolved in dH2O or DMSO (see Note 2) used for labeling plasma membranes of all cells.
A fluorescent reporter that marks the border cells, e.g., slbo-GAL4; UAS-mCD8GFP [25, 26] or slbo-GAL4; UAS- MoesinGFP [13].
(Optional) Streptomycin/penicillin 10,000 U/ml of penicillin G–sodium, 10,000 mg/ml streptomycin sulfate in 0.85 % saline.
Lumox® dish (50 mm) (Sarstedt).
Cover slip glasses: 22 × 22 mm2, thickness: 0.13–0.17 mm.
Steriflip®-GP, 0.22 μm filter (EMD Millipore SCGP00525).
Two pairs of forceps (Dumostar forceps #5) and a dissecting microscope.
Concavity dissection slides.
Fine-Tip Transfer Pipettes.
Halocarbon oil 27.
(Optional) Poly-D-lysine, 10 mg/ml in PBS.
Dry baker’s yeast.
Heat-filter KG1 (Chroma Technologies).
BG38 IR suppression filter (Chroma Technologies).
Neutral-density filters (ND 0.3) (Chroma Technologies).
Wide-field microscope: We use a Zeiss Axio Imager upright epifluorescence microscope and Plan-Apochromat 20×/0.8 N.A. dry objective. Illumination source is X-Cite 120 metal halide lamp. Filter cube sets: BP470/40, FT495, BP525/50 for Alexa 488/GFP and BP550/25, FT570, BP605/70 for Alexa 568/DsRed.
Confocal microscope: We use a Zeiss 510-Meta inverted confocal microscope and Plan-Apochromat 63×/1.4 N.A. oil objective with argon (488 nm) and HeNe (542 nm) lasers. Filter sets are for FITC and TRITC.
2.2 Preparation of Culture Medium
Drosophila S2 medium was supplemented with 20 % FBS (needed) and 0.6× streptomycin/penicillin (optional). Sterilize it by passing through a 0.22 μm filter.
The final pH of the medium was adjusted to 6.85–6.95. Verify the pH of the medium before every use (see Note 3).
Supplement with insulin to a final concentration of 0.2 mg/ml. Henceforth, the medium with all the supplements would be referred to as complete medium.
(Optional) FM4-64 dye can be used at a final concentration of 9 μM to stain plasma membranes of all cells.
3 Methods
3.1 Preparing Poly-D-Lysine-Coated Cover Slips (Optional)
Take 5–10 cover slips (22 × 22 mm2), wash with MilliQ H2O, and dry. Cover the cover slips evenly with approximately 200 μl of 2 mg/ml of poly-D-lysine in PBS and dry them completely in a 37 °C oven. Rinse cover slips in flowing tap H2O and subsequently rinse with MilliQ H2O and then dry at room temperature.
3.2 Preparing Female Flies for Dissection
Transfer 5–10 females (2–4 days old) of the desired genotype along with 3–4 males into a new fly vial with fresh fly food, a small amount of baker’s yeast, and incubate at 25 °C for 14–18 h (see Note 4).
3.3 Dissection of Ovaries to Egg Chambers
Anesthetize female flies on a CO2 pad.
Fill the cavity of the dissection slide with complete medium and place the slide under the dissecting microscope.
Transfer one anesthetized fly to the slide using forceps.
Gently hold the thorax or upper abdomen of the immobile fly using a pair of forceps, submerge it in the medium, while with another pair of forceps grasp and peel off a bit of abdominal cuticle at the middle of the abdomen (Fig. 2a).
This reveals a large pair of ovaries, which are white and opaque after overnight fattening. Grasp the base of the ovaries (the common oviduct) and pull them away from the rest of the fly and into the medium (Fig. 2b). Remove the carcass using the forceps and discard it on a dampened Kimwipe. Keep the ovaries covered in medium at all times.
Repeat this process for all female flies, accumulating the ovaries in the complete medium on the dissection slide. Handle the ovaries very gently (see Note 5).
With one pair of forceps, hold the posterior part of a single ovary. Use another pair of forceps to gently grasp the anterior tip of the ovary (Fig. 2c) (older egg chambers reside at the posterior while the germarium and early-stage egg chambers occupy the anterior tip).
Gently pull the ovarioles out of the muscular sheath around ovary. Strings and sometimes individual egg chambers will pop out of the muscle sheath (Fig. 2c, d). Repeat this action until you get 5–10 stage 9 egg chambers. Never touch the stage 9 egg chambers directly with the forceps! You can pull on early- or very-late-stage egg chambers such that stage 9 chambers get isolated (see Note 5).
Use a plastic fine-tip transfer pipette to transfer egg chambers to a 500 μl Eppendorf tube. After the egg chambers settle down, aspirate old medium and replace with 300 μl fresh complete medium.
Break a 22 × 22 mm2 cover slip into two halves (spacers). Align them 1 cm apart on a Lumox® dish and immobilize them with 5 μl H2O under each of them.
Transfer 55 μl complete media with egg chambers between two cover slip spacers.
Carefully cover the samples with another 22 × 22 mm2 cover slip. Cover slip should land on two spacers. Avoid bubbles (see Note 6).
Remove excess media from around the cover slips with Kimwipe so that egg chambers just barely move when you gently rock the Lumox® dish. Take care not to compress the egg chambers.
Seal every edge of the cover slips with a thin layer of halocarbon oil 27 (~20 μl in total) to minimize evaporation of the media during imaging (see Note 7).
For the upright microscope, the glass spacers and cover slips are mounted on the top of Lumox® dish; for the inverted microscope, the sample is mounted on the bottom of the dish.
Fig. 2.
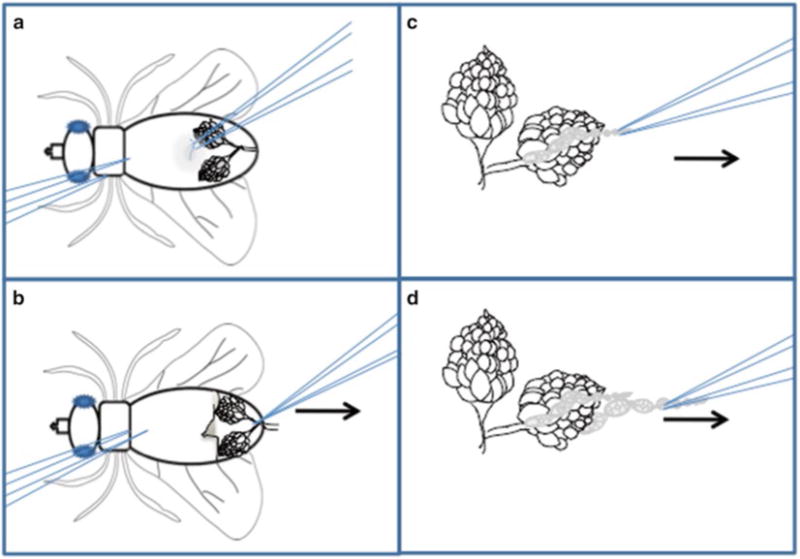
Ovary dissection technique: Schematic representation of egg chamber dissection. (a) The female fly should be immobilized on its back using the left forceps. The right forceps are used to pinch the soft cuticle of the ventral side of the abdomen. (b) Pull the cuticle toward the right (arrow), revealing the ovaries. Grasp the common oviduct with the right forceps and pull to the right (arrow) to free the ovaries from the carcass. (c) Immobilize the ovary pair by pinching the oviduct using the left forceps. Use the right forceps to grasp the tip of an ovariole and slowly pull to the right (arrow). (d) Repeat until multiple ovarioles have been freed from the sheath
3.4 Time-Lapse Imaging of the Egg Chambers
Transfer the Lumox® dish to a microscope stage equipped with a petri dish holder.
Set up the center of the Lumox® dish as a reference point. Move to a location on the dish, identify an egg chamber of the desired stage, and mark its coordinates. Repeat this step for other locations, avoiding egg chambers that have abnormal morphology (i.e., uneven follicle cell layer). In addition, avoid choosing egg chambers that are located near a germarium. The inherent pulsating movement of germarium might move the egg chamber during imaging.
Lower the light intensity using 25 % neutral density filter. Include KG1 and BG38 filters to suppress heating of the sample (see Note 8). When imaging more than one egg chamber at a time, mark the position of the center of each border cell cluster and identify the correct exposure for each channel and each egg chamber. Try not to exceed 150 ms for each channel. With this exposure setting one can collect 16 z-sections, 1.25 μm apart at 20× magnification, for each channel. Egg chambers generally tolerate a time interval of 2.25 min between successive frames when imaging four egg chambers with 16 z-sections for each of the two channels.
Start time-lapse image acquisition. Use modest speed of the mobile stage. We have successfully used an Axiocam MRm camera mounted on a Zeiss Axioimager (upright, wide field) microscope with 20× magnification for 5–6 h. Higher magnification, such as 40×, can be used for shorter total imaging times.
During acquisition, it is necessary to refocus on the border cells as they move. In addition, look for signs of normal development such as egg chamber growth, outer follicle cell rearrangement, dynamic changes in gene expression (e.g., expression of the slow border cell enhancer in centripetal cells), and of course border cell movement.
The Lumox® dish can be reused as long as the film base is intact. After the experiment, carefully remove the cover slips and wash away the halocarbon oil by rinsing several times with Windex cleaner and then thoroughly with H2O.
After imaging, one can process the images in different ways depending on the software available. Movie 1 shows a maximal intensity projection of the 16 z-slices over the entire time interval of acquisition.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences grant GM73164 to D.J.M.
Footnotes
Insulin powder dissolves in slightly acidic condition. For preparing acidic H2O, dilute 1 μl of concentrated HCl in 1 ml of MilliQ H2O.
If using DMSO for dissolving FM4-64, the final dilution of DMSO in complete medium should be 1:1000. A higher concentration of DMSO impedes border cell migration.
The pH of the medium is critical for the experiment. Low pH impedes border cell migration and high pH leads to early degeneration of the egg chamber.
The age of the female fly is very important. The ovaries of newly hatched flies do not fatten very well, while the ovaries of older fly have large number of mature egg chambers.
Gentle handling of the ovaries is critical as even inconspicuous damage to egg chambers inhibits border cell migration.
While lowering the cover slip onto the medium with egg chambers, one needs to be very careful that the ends of the cover slip should land on the spacer to avoid any damage to the egg chambers.
Do not use nail polish.
The addition of a heat-filter KG, infrared suppression filter (BG38), and 25 % neutral density filter suppresses phototoxicity to the sample during long-term imaging.
Contributor Information
Mohit Prasad, Department of Biological Chemistry, Johns Hopkins School of Medicine, Baltimore, MD, 21205, USA; Department of Biological Sciences, IISER, Kolkata, West Bengal, 741252, India.
Xiaobo Wang, Department of Biological Chemistry, Johns Hopkins School of Medicine, Baltimore, MD, 21205, USA; Université P. Sabatier Toulouse III, 31062, Toulouse cedex 9, France.
Li He, Department of Biological Chemistry, Johns Hopkins School of Medicine, Baltimore, MD, 21205, USA; Department of Genetics, Harvard Medical School, Boston, MA, 02115, USA.
Danfeng Cai, Department of Biological Chemistry, Johns Hopkins School of Medicine, Baltimore, MD, 21205, USA; Molecular, Cellular and Developmental Biology Department, University of California, Santa Barbara, CA, 93106-9625, USA.
Denise J. Montell, Email: denise.montell@lifesci.ucsb.edu, Department of Biological Chemistry, Johns Hopkins School of Medicine, Baltimore, MD, 21205, USA; Molecular, Cellular and Developmental Biology Department, University of California, Santa Barbara, CA, 93106-9625, USA.
References
- 1.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 2.Aman A, Piotrowski T. Cell migration during morphogenesis. Dev Biol. 2010;341:20–33. doi: 10.1016/j.ydbio.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Weijer CJ. Collective cell migration in development. J Cell Sci. 2009;122:3215–3223. doi: 10.1242/jcs.036517. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Schier H. Fly fishing for collective cell migration. Curr Opin Genet Dev. 2010;20:428–432. doi: 10.1016/j.gde.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Rorth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 6.Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. pp. 1–70. [Google Scholar]
- 7.King RC. Ovarian development in Drosophila melanogaster. Academic; New York, NY: 1970. [Google Scholar]
- 8.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles-more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 9.Ruohola H, Bremer KA, Baker D, et al. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 10.Silver DL, Montell DJ. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell. 2001;107:831–841. doi: 10.1016/s0092-8674(01)00607-9. [DOI] [PubMed] [Google Scholar]
- 11.Montell DJ, Rorth P, Spradling AC. Slow border cells, a locus required for a developmentally regulated cell-migration during oogenesis, encodes Drosophila C/Ebp. Cell. 1992;71:51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- 12.Starz-Gaiano M, Melani M, Wang XB, et al. Feedback inhibition of JAK/STAT signaling by apontic is required to limit an invasive cell population (vol 14, pg 726, 2008) Dev Cell. 2008;15:330. doi: 10.1016/j.devcel.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Prasad M, Montell DJ. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev Cell. 2007;12:997–1005. doi: 10.1016/j.devcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Bianco A, Poukkula M, Cliffe A, et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448:362–365. doi: 10.1038/nature05965. [DOI] [PubMed] [Google Scholar]
- 15.Duchek P, Somogyi K, Jekely G, et al. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 16.McDonald JA, Pinheiro EM, Kadlec L, et al. Multiple EGFR ligands participate in guiding migrating border cells. Dev Biol. 2006;296:94–103. doi: 10.1016/j.ydbio.2006.04.438. [DOI] [PubMed] [Google Scholar]
- 17.Duchek P, Rorth P. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science. 2001;291:131–133. doi: 10.1126/science.291.5501.131. [DOI] [PubMed] [Google Scholar]
- 18.Prasad M, Jang AC, Starz-Gaiano M, et al. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat Protoc. 2007;2:2467–2473. doi: 10.1038/nprot.2007.363. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, He L, Wu YI, et al. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–597. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai D, Chen S-C, Prasad M, et al. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–574. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- 22.Grunert S, St Johnston D. RNA localization and the development of asymmetry during Drosophila oogenesis. Curr Opin Genet Dev. 1996;6:395–402. doi: 10.1016/s0959-437x(96)80059-1. [DOI] [PubMed] [Google Scholar]
- 23.Hudson AM, Cooley L. Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis. Annu Rev Genet. 2002;36:455–488. doi: 10.1146/annurev.genet.36.052802.114101. [DOI] [PubMed] [Google Scholar]
- 24.Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–2215. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rorth P, Szabo K, Bailey A, et al. Systematic gain-of-function genetics in Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Bo J, Bridges T, et al. Analysis of cell migration using whole-genome expression profiling of migratory cells in the Drosophila ovary. Dev Cell. 2006;10:483–495. doi: 10.1016/j.devcel.2006.02.003. [DOI] [PubMed] [Google Scholar]


