Abstract
Using the extinction-reinstatement model of cocaine relapse, we and others have demonstrated that the antibiotic ceftriaxone attenuates cue- and cocaine-primed reinstatement of cocaine-seeking. Reinstatement is contingent on the release of glutamate in the nucleus accumbens core (NAc) and manipulations that reduce glutamate efflux or block post-synaptic glutamate receptors attenuate reinstatement. We have demonstrated that the mechanism of action by which ceftriaxone attenuates reinstatement involves increased NAc GLT-1 expression and a reduction in NAc glutamate efflux during reinstatement. Here we investigated the effects of ceftriaxone (100 and 200 mg/kg) on context-primed relapse following abstinence without extinction training and examined the effects of ceftriaxone on GluA1, GluA2 and GLT-1 expression. We conducted microdialysis during relapse to determine if an increase in NAc glutamate accompanies relapse after abstinence and whether ceftriaxone blunts glutamate efflux. We found that both doses of ceftriaxone attenuated relapse. While relapse was accompanied by an increase in NAc glutamate, ceftriaxone (200 mg/kg) was unable to significantly reduce NAc glutamate efflux during relapse despite its ability to upregulate GLT-1. GluA1 was reduced in the NAc by both doses of ceftriaxone while GluA2 expression was unchanged, indicating that ceftriaxone altered AMPA subunit composition following cocaine. Finally, GLT-1 was not altered in the PFC by ceftriaxone. These results indicate that it is possible to attenuate context-primed relapse to cocaine-seeking through modification of post-synaptic receptor properties without attenuating glutamate efflux during relapse. Furthermore, increasing NAc GLT-1 protein expression is not sufficient to attenuate glutamate efflux.
Keywords: glutamate, nucleus accumbens, cocaine, relapse, GluA1, GluA2, GLT-1
Introduction
Cocaine addiction is a chronic relapsing disorder characterized by loss of control over drug-seeking and drug-taking, and continued drug use regardless of adverse consequences (APA, 2000). Despite almost 50 years of experimental research, effective treatments for psychostimulant addiction have not been identified. Animal models of relapse have been designed to elucidate the neurobiological processes involved in relapse behavior and to evaluate potential pharmacotherapies that may prevent or reduce the risk of relapse.
The extinction-reinstatement model is one of the most commonly used animal models in addiction research (see Epstein et al. 2006 for review). Following operant drug self-administration, the drug-seeking response is extinguished by no longer delivering drug or cues upon performance of the operant response. Over the course of days to weeks, drug- seeking behavior declines and can be reinstated by exposure to stimuli known to cause relapse in humans, including stress (Erb et al. 1996; Shaham and Stewart, 1995), discrete cues previously associated with drug delivery (Davis and Smith, 1976; Meil and See, 1996), and/or the drug itself (de Wit and Stewart, 1981; Stretch et al. 1971). Thus, “reinstatement’ is considered to be a model of relapse with adequate face and construct validity (Epstein et al. 2006). Typically, extinction training occurs in the drug-associated context (context “A”), but when it occurs in a different “B” context, the placement of animals back into context A is sufficient to “renew” lever pressing without the presentation of drug, discrete cues or stress (Crombag and Shaham, 2002).
While relapse models that utilize extinction training possess construct validity (see Epstein et al. 2006), humans do not typically experience explicit extinction training, even in clinical settings, during drug abstinence. Thus, it has been proposed that the “forced abstinence” model has greater face validity (see Reichel and Bevins, 2009 for review) in that it captures the ability of a drug-associated context to induce relapse after a period of abstinence. In this model, animals do not undergo extinction training and instead remain in the home-cage with only daily handling. “Relapse” is induced by re-exposing animals to the drug taking context (operant chamber) and execution of the previously reinforced response during this test does not result in the delivery of cues or drug (for review, Reichel and Bevins, 2009). Because extinction of the operant response does not occur in this model, the response cannot be “reinstated” and is thus referred to as “context-induced relapse after abstinence” (Knackstedt, et al. 2014). A variation of this procedure is the “incubation of craving” model in which animals only experience abstinence without extinction training for 21–90 days, but the relapse test re-introduces discrete cues or drug administration (for review see Pickens et al. 2011).
The neurocircuitry underlying relapse has been found to differ substantially based on whether either extinction or abstinence procedures are employed. The stimulus used to induce reinstatement (e.g. cue vs. drug) also influences the brain regions responsible for mediating relapse. Both pharmacological and optogenetic inhibition of the nucleus accumbens core (NAc), as well as its afferents from the dorsal medial PFC (dmPFC) attenuate cue- and cocaine-primed reinstatement following extinction training (Fuchs et al. 2004; McFarland et al. 2001; McLaughlin and See, 2003; Stefanik and Kalivas 2013). While the basolateral amygdala (BLA) is not involved in cocaine-primed reinstatement (McFarland and Kalivas 2001), its pharmacological (McLaughlin and See, 2003) and optogenetic (Stefanik and Kalivas 2013) inactivation attenuates cue-primed reinstatement after extinction. When abstinence replaces extinction training, pharmacological inhibition of the BLA and dmPFC has no effect on relapse (Fuchs et al. 2006). Conversely, inactivation of the dorsal lateral caudate putamen (dlCPu) attenuates context-primed relapse following abstinence, but has no effect on reinstatement following extinction (Fuchs et al. 2006; Mclaughlin and See, 2003). The NAc has been reported as unnecessary for abstinent-relapse to occur when animals are trained to self-administer cocaine without discrete cues (Fuchs et al. 2006; See et al. 2007). However, when cocaine self-administration is accompanied by discrete drug-associated cues (light+tone), the NAc is then demonstrated to mediate abstinent-relapse (Knackstedt, et al. 2014). Additionally, the NAc has been found as essential for context-primed (ABA) renewal of drug-seeking when extinction occurs in a different context (Fuchs et al. 2008).
Glutamate dysregulation in the NAc has been identified as a primary driver of cocaine-induced reinstatement of cocaine-seeking behavior after extinction training (for review see Knackstedt and Kalivas, 2009). During cocaine-primed reinstatement following extinction training, glutamate efflux in the NAc mediates reinstatement; its release is action potential-dependent and prevented by pharmacological inactivation of the dmPFC (McFarland et al., 2003). Reduced NAc expression of the major glutamate transporter GLT-1 is present following 2–3 weeks of extinction training and is thought to contribute to the glutamate efflux observed during reinstatement (Trantham-Davidson et al. 2012). The beta-lactam antibiotic ceftriaxone increases GLT-1 expression in the NAc and attenuates cue- and cocaine-induced reinstatement (Knackstedt et al. 2010a). Ceftriaxone also prevents the increase in NAc glutamate that drives cocaine-primed reinstatement after extinction (Trantham-Davidson et al. 2012). The ability of ceftriaxone to attenuate context-primed relapse and influence GLT-1 expression and glutamate levels after abstinence have not been tested and this was the first goal of the present work.
The second, related goal of the present work was to determine the role of glutamate efflux in the NAc and post-synaptic AMPA receptor modifications in mediating context-primed relapse. Evidence for persistent glutamate adaptations following abstinence (without extinction) includes a loss of the ability to induce LTP (Knackstedt et al. 2010b) and adaptations in AMPA-receptor subunit composition (Conrad et al. 2008; Wolf and Tseng, 2012). Cocaine self-administration followed by abstinence results in the formation of GluA2-lacking, calcium-permeable AMPA receptors (CP-AMPAs) in the NAc, which underlie incubated cocaine-seeking withdrawal (Conrad et al. 2008; Mameli et al. 2009). CP-AMPAs are found under conditions of increased total and surface GluA1 expression and unaltered GluA2 expression (Conrad et al. 2008; Wolf and Tseng, 2012). Here we examined the effects of ceftriaxone on NAc expression of GluA1 and GluA2.
Experimental Procedures
Drugs
Cocaine hydrochloride was acquired from the NIDA Controlled Substances Program (Research Triangle Institute, NC, USA), ceftriaxone (β-lactam antibiotic) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Cocaine (4 mg/mL; 0.25 mg/infusion) and ceftriaxone (100 and 200 mg/kg, i.p.) were both dissolved in saline (0.9% sodium chloride).
Animals
Fifty-five adult male Sprague Dawley rats (Charles River Laboratories, Raleigh, NC, USA) weighing 275–300 g were single-housed in a temperature and humidity controlled vivarium on a reversed 12-hour light/dark cycles with water available ad libitum. Animals were food-restricted to 20–25 g of standard rat chow per day, resulting in a reduction to approximately 85% of free-feeding weight. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy Press, 1996).
Catheter and stereotaxic surgery
Animals were anesthetized using a mixture of ketamine (87.5 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.) and surgically implanted with jugular vein catheters. Ketorolac (2 mg/kg, i.p.) was administered pre-operatively for pain. Catheters (SILASTIC silicon tubing, ID 0.51 mm, OD 0.94 mm, Dow Corning, Midland, MI, USA) were implanted in the right jugular vein, secured with sutures, and then passed subcutaneously between the shoulder blades to exit though the skin on the back. The catheter was then attached to a stainless-steel cannula that was held stable within a harness (Instech, Plymouth Meeting, PA, USA). For Experiment 2, stereotaxic surgery to implant intracranial guide cannulas occurred directly after the catheter implantation. Animals were placed in a stereotaxic frame (Stoelting, Wood Dale, IL. USA) and a stainless guide cannula (22 gauge, Synaptech, Marquette, MI, USA) was unilaterally aimed at the NAc according to the following coordinates (AP +1.2 mm, ML +/−1.6 mm, DV −5.5 mm; Paxinos & Watson, 2005). Cannulas were secured to the skull with stainless steel skull screws and dental acrylic (Co-Oral-Ite Dental MFG. Co., Diamond Springs, CA, USA). Post-operatively animals received 0.3 ml of ketorolac (2 mg/kg, i.p.) for 3 days for pain. The antibiotic timentin (200 mg/ml) was administered via catheter for 7 days and followed by 0.1 ml of heparinized saline (100 U/ml). Catheters were flushed with 0.2 ml of heparinized saline (100 U/ml) before and after each self-administration session to ensure continued catheter patency. Animals were allowed to recover for 5 days before self-administration procedures were initiated. Catheter patency was verified periodically via methohexital sodium (10 mg/ml; Eli Lilly, Indianapolis, IN, USA).
Cocaine self-administration, abstinence, and relapse procedures
Animals were trained to self-administer cocaine under an FR1 schedule of reinforcement in standard operant chambers (30 × 24 × 30 cm; Med Associates, St. Albans, VT, USA) equipped with two retractable levers. Pressing the right lever (active) resulted in an intravenous infusion of cocaine (0.25 mg/infusion) and was simultaneously paired with a 5-second presentation of auditory (2900 Hz tone) and visual (stimulus light) conditioned cues. Each infusion of cocaine was followed by a 20- second ‘time-out’ period during which time presses on the active lever produced neither drug nor cue presentation. Pressing on the left lever (inactive) was not reinforced, but presses were recorded. Med PC software version IV (Med Associates) controlled data and programmed all sequences. Animals maintained daily 2 hr. self-administration sessions (during their dark cycle) until a criterion of 10 or more infusions of cocaine/session for 12 days was attained. Upon reaching the criterion, animals entered the abstinence phase of the experiment. During this time, animals were handled daily but not placed into the operant chambers. Instead, they were removed from the housing room, weighed, and remained in their home cages in a holding room in which they had previously been placed prior to each self-administration session. Following 20–21 days of abstinence, animals were placed into operant chambers and tested for context-primed relapse. During the test session, lever pressing did not produce either drug infusion or the presentation of cues. Presses on the previously associated active and inactive levers were recorded for 2 hours.
Experiment 1
Twenty four rats were trained to self-administer cocaine; three were eliminated from the study for either catheter failure (n=1) or failure to acquire self-administration (n=2). After cocaine self-administration animals underwent abstinence procedures for ~21 days. During the last 6 days of abstinence animals received consecutive daily injections of either ceftriaxone [100 mg/kg (n=6) or 200 mg/kg (n=8)] or vehicle (0.9% saline; n=7). The following day, animals were placed in operant chambers for relapse testing and then euthanized by rapid decapitation. Brains were extracted and the PFC and NAc regions were dissected. Western blotting was performed on this tissue for GLT-1 expression in the PFC and NAc, and GluA1 and GluA2 expression in the NAc.
Tissue Preparation and Immunoblotting
The PFC and NAc regions were dissected, homogenized in sucrose buffer containing protease inhibitors, and spun at 1000 g. The supernatant was retrieved and then spun at 10000 g. The resulting pellet was re-suspended in sucrose buffer and protease inhibitors, and spun at 10000 g. The supernatant was then discarded and the pellet containing the membrane fraction was suspended in 1% sodium dodecyl sulfate (SDS) in RIPA. Proteins were separated using 10% SDS-PAGE and transferred to PVDF membrane. The membranes were probed overnight at 4°C with primary antibodies diluted in 5% milk/Tris-buffered saline with 0.1% Tween 20. GLT-1 (1:5000, Chemicon, Billerica, MA) was measured in both the PFC and NAc, and GluA1 and GluA2 (1:1000, Chemicon, Billerica, MA) were measured in the NAc only. After incubation with HRP-conjugated secondary antiserum (Jackson Immuno; 1:10,000), immunoreactive bands on the membranes were detected by ECL Plus. Band density was measured using NIH Image J software.
Experiment 2
Thirty-one rats self-administered cocaine for 12 days followed by ~21 days of abstinence. During the last 6 days of abstinence animals received daily injections of either ceftriaxone (200 mg/kg) or vehicle. After the last daily treatment of ceftriaxone or vehicle, microdialysis probes were implanted into the NAc. Probes were constructed of 2 mm cuprophane membranes (20 kDa cut-off weight, outer diameter 0.36 mm; Synaptech, Marquette, MI). Animals were housed overnight in their home cages that were placed adjacent to operant boxes equipped with liquid swivels mounted onto counterbalanced lever arms (Instech Laboratories, Plymouth Meeting, PA). Probes were perfused overnight with artificial cerebrospinal fluid (aCSF) containing (125 mM NaCl, 2.5 mM KCL, 1 mM MgCl26H2O, 5 mM D-glucose, 1.2 mM CaCl2H2O, 0.75 mL 10 x phosphate buffered saline, pH-7.3–7.5) at a flow rate of 0.2 μl/min. The following morning, the flow rate was increased to 2.0 μl/min and allowed to two hours for adjustment. Baseline samples were collected every 10 minutes while the animals remained in their home cages for a total of 6 dialysate collections. Animals were then removed from their home cage and placed into the operant chambers for the 2 hr context-primed relapse test. Levers were extended during testing, but had no programmed consequences. Presses on the active and inactive levers were recorded, and 6 additional dialysate samples were collected. Three rats were removed from analysis due to active lever pressing that was greater than 2 standard deviations from the mean (CEF = 1; VEH = 2) during the relapse test. One rat was removed from this study for failing to meet self-administration criteria, and two rats were not tested for relapse because their microdialysis probes failed overnight. Data from animals with misplaced cannula are not shown and were excluded from analysis (n=5), yielding a total of 20 rats that were tested for context-primed relapse during microdialysis (CEF: n=8; VEH: n=12).
HPLC
Glutamate was quantified in dialysate samples using isocratic high-performance liquid chromatography with electrochemical detection (HPLC-ED; Thermo Scientific., Waltham, MA, USA). Microdialysis samples were derivatized with o-pthalaldehyde (Sigma-Aldrich, St. Louis, MO) automatically by an autosampler (Thermo Scientific, Inc.) immediately before injection onto a CAPLCELL PAK C18 column (5 μm, 2.0mm I.D. X 50mm; Shiseido Inc., Tokyo, Japan). The mobile phase consisted of 100 mM Na2HPO4, 16% (vol/vol) methanol, and 2.5% (vol/vol) acetonitrile (pH=6), and was pumped at a flow rate of 0.650 mL/min. Glutamate levels in the dialysis samples were quantified by comparing computer-integrated peak areas of samples with those of L-glutamate standards using a 5-point calibration curve (10, 5, 2.5, 1.25, and 0.625 μM).
Histology
After the completed of the dialysis procedures, animals were deeply anesthetized with pentobarbital (100 mg/kg, i.p.), and underwent transcardial perfusion with phosphate-buffered saline (PBS) (pH = 7.4). After perfusion, brains were extracted and preserved in 4% PFA for 24 hrs and then stored in a 20% sucrose PBS solution. Brains were then sectioned into 200 μm slices using a vibratome. Sections were mounted on gelatin-coated slides, stained with cresyl violet and examined for probe placement using the rat brain atlas of Paxinos and Watson (2007). Only animals with probe placements in the NAc were included in the data analysis.
Statistical analysis
SPSS (IBM, Armonk, NY) was used to analyze the behavioral data (self-administration and relapse) and western blot data. For all statistical analyses used, the alpha level was set at p < 0.05. Self-administration and extinction learning data were analyzed with mixed-factorial 2-way analyses of variance (ANOVAs), with time as the repeated measure. Group differences in lever pressing during the relapse test were investigated using one-way ANOVAs (Experiments 1 and 2) or an independent-sample t-test (Experiment 1). Significant main effects and/or interactions were followed by Bonferonni post-hoc analyses to examine group or time differences. Immunoblotting data, represented by integrated density of individual protein bands, were normalized for the density of calnexin immunoreactivity within the same sample and the three treatment groups (Ceftriaxone 100 mg/kg or 200 mg/kg or Vehicle) were compared using one-way ANOVAs.
GraphPad Prism (version 5.00, GraphPad Software, La Jolla, CA) was used to analyze the HPLC data. Microdialysis samples were converted to a percent baseline by averaging the dialysate glutamate values at each time point prior to the relapse test. All dialysate glutamate concentrations were then converted to percentages based on the animal’s individual basal dialysate level of glutamate. A 2-way RM ANOVA was used to determine group and/or time differences. Because animals displayed variability in when the peak glutamate value occurred during the relapse test, glutamate content was converted to area under the curve (AUC) to identify group and time differences (Chefer et al, 2011). The formula used was AUC = [0.5(B+S1)d + 0.5(S1+S2)d +……..0.5(Sn-1+Sn)d] – Bdn, where B is average of baseline samples, Sx are the values of each 10-minute sample during the test, n is the total number of samples collected (6) and d is the duration of sample collection (10 min). We then conducted an independent – sample t-test on this data.
Results
Experiment 1
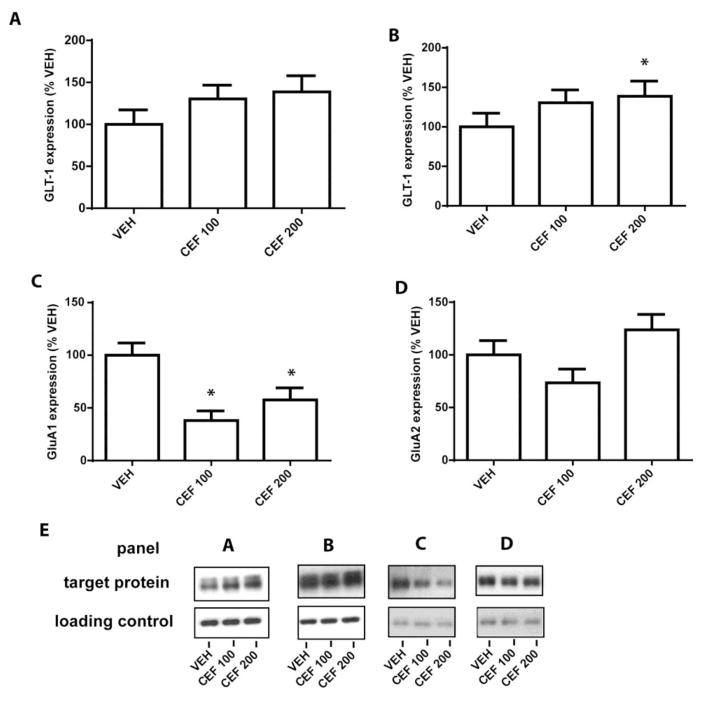
Mean cocaine infusions did not differ between groups later assigned to receive CEF or VEH, as a two-way RM ANOVA found no significant effect of Group (F(2,18) = 0.277, n.s.; Figure 1a). A significant effect of Time was found (F(11,198) = 5.932, p<0.001) as groups increased cocaine intake over the course of the experiment. A significant Group x Time interaction was detected (F(2,18) = 1.625, p<0.05), indicating potential group differences in cocaine intake over time. Thus, a one-way ANOVA was conducted on total number of cocaine infusions during the 12 days and found no group differences (F(2, 18) = 0.2438, n.s.; mean for VEH= 235.6 ± 16.1; CEF100= 233.5 ± 12.8; CEF200 = 223.8 ± 10.5). A one-way ANOVA conducted on the active lever presses during the relapse test revealed a significant effect of Group (F(2,18) = 5.025, p<0.05; Figure 1B) and Bonferonni post-hoc analyses found that rats treated with both doses of CEF displayed significantly fewer presses on the previously active lever relative to VEH-treated rats (p<0.05). No significant Group effect was detected for previously inactive lever presses during the context-primed relapse test (F(2,18) = 1.050, n.s.). Following the abstinent-relapse test, animals were sacrificed and western blotting was performed to determine treatment effects on protein expression of GLT-1, GluA1, and GluA2 in the NAc and GLT-1 in the PFC. A one-way ANOVA conducted found no group differences in GLT-1 expression in the PFC (F(2,20) = 0.798, n.s.; Figure 2A). A one-way ANOVA detected a trend towards a significant Group effect in NAc GLT-1 expression (F(2,19) = 3.082, p=0.06; Figure 2B). However, as CEF (200 mg/kg) has previously been demonstrated to increase GLT-1 expression in the NAc (Knackstedt et al. 2010a) we used a planned comparison to confirm that this dose was sufficient to significantly increase expression of GLT-1 in the NAc (t(1,13) = 1.832, p<0.05; Figure 2C). A one-way ANOVA found a significant effect of Group on NAc GluA1 expression (F(2,14) = 7.064, p<0.01; Figure 2C). Post-hoc analyses revealed that rats treated with both doses of CEF had significantly decreased expression of GluA1 relative to VEH-treated rats. We found no Group differences in the expression of GluA2 in the NAc (F(2,17) = 1.737, n.s.).
Fig. 1.
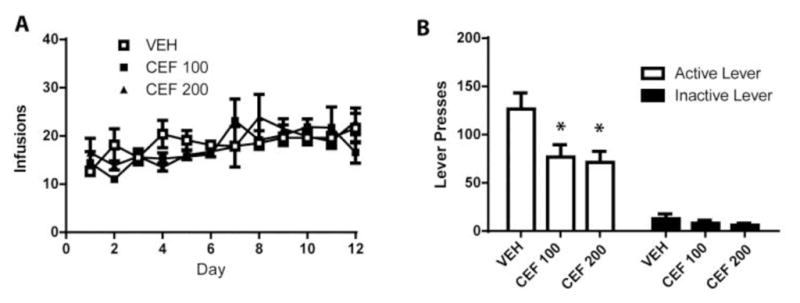
Subchronic administration of ceftriaxone attenuates context-primed relapse after abstinence (A) Mean infusions during the self-administration period did not differ between groups later given VEH or CEF. (B) CEF treatment significantly decreased responding on the previously active lever while having no effect on inactive lever presses during the relapse test. (n=6–8/group). Error bars depict the SEM.
Figure 2.
Subchronic administration of ceftriaxone increases NAc GLT-1 expression while reducing GluA1 expression. (A) CEF did not significantly alter GLT-1 expression in the PFC. (B) CEF (200 mg/kg) significantly increased GLT-1 in the NAc. (C) CEF (100 and 200 mg/kg) decreased NAc GluA1 expression (D) CEF did not alter GluA2 expression in the NAc. (E) Sample western blot bands for each panel. (N=6–8/group). Error bars depict the SEM.
Experiment 2
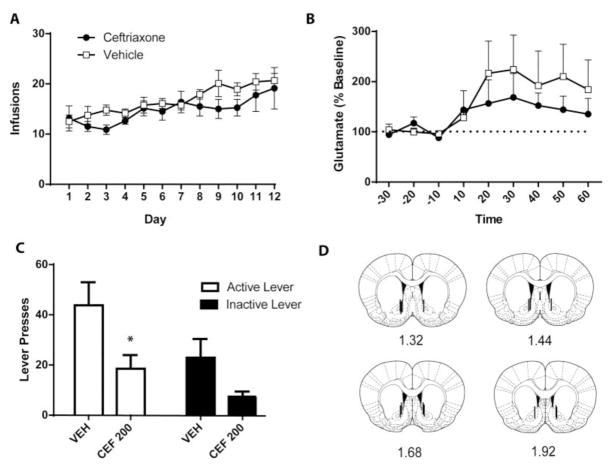
Mean cocaine infusions did not differ between groups later assigned to receive CEF or VEH, as a 2-way RM ANOVA found no significant effect of Group (F(1,18)=1.89, n.s.) and no Group x Time interaction (F(11,198) = 0.65, n.s.; Figure 3A). A significant effect of Time was found (F(11,198) = 5.35, p <0.001) as both groups increased cocaine intake over the course of the experiment. Glutamate concentration in the last three baseline samples were averaged (“B”) for each rat. These baseline samples and each of the 6 samples for the first hour of the abstinent-relapse test were divided by B to yield percent change in baseline during the test (Figure 3b). A two-way RM ANOVA conducted on this data found no significant effects of Group (F(1,18)=0.42, n.s.) and no Group x Time interaction (F(8,104)=0.55, n.s.). A significant effect of Time was found (F(8,104)= 3.24, p<0.01), indicating that both CEF and VEH-treated groups experienced an increase in glutamate upon placement into the self-administration context. We also calculated the area under the curve (AUC) glutamate during the relapse test and no Group difference was detected (t(1,18) = 0.868, n.s.; data not shown). While there was no trend towards a Group effect on glutamate levels, the SEM is large for the data points following placement into the operant chamber for the relapse test, despite an n of 8–10/group. Thus, we also calculated effect size for the Group factor and found 2=0.056, a further indication that Group differences in glutamate levels during relapse are not present here. We chose to examine the first hour after placement into the cocaine context as this is when previous publications have demonstrated the greatest release of glutamate (e.g. Trantham-Davison et al., 2012). When examining the entire 2 hr test session we still did not find a significant Group x Time interaction (F(14,154) =0.8217, n.s.), but a significant effect of Time remained (F(14, 154) = 2.183, p<0.05).
Figure 3.
Subchronic administration of ceftriaxone did not attenuate context-induced elevated glutamate levels in the NAc. (A) Mean cocaine infusions did not differ between groups later treated with VEH or CEF. (B) Glutamate levels represented by percent change from baseline, demonstrating that both CEF and VEH-treated rats displayed increases in glutamate during the context-primed relapse test. (C) Ceftriaxone attenuated context-primed relapse, indicated by CEF-treated rats pressing the previously active lever significantly fewer times than VEH-treated rats. (D) Represents placement of microdialysis probes. (N = 8–10/group). Error bars depict the SEM.
Rats treated with CEF displayed significantly less presses on the previously active lever during this test relative to VEH-treated rats (t(1,18) = 2.355, p=0.05; Figure 3C). Figure 3D displays the placement of the microdialysis probes. Accurate placement was achieved when two-thirds of the active dialysis membrane was within the NAc.
Discussion
The present study is the first to demonstrate an increase in NAc glutamate during context-primed relapse to cocaine-seeking after a period of forced abstinence. In agreement with a role for glutamate efflux in the NAc in mediating abstinent-relapse, we found that CEF attenuated relapse (Figures 1B and 3C). However, while successfully attenuating relapse, CEF did not significantly attenuate glutamate efflux during the relapse test (Figure 3B). This is interesting in light of the observed CEF-induced elevation in NAc GLT-1(Figure 2B), which was previously proposed to reduce glutamate levels during reinstatement (Trantham-Davidson et al. 2012). The expression of GluA1 was significantly reduced in the NAc by both doses of CEF that attenuated relapse while GluA2 expression was unchanged, providing a likely mechanism by which CEF attenuated relapse. Finally, CEF treatment did not significantly alter GLT-1 expression in the PFC (Figure 2A).
Glutamate efflux has been demonstrated to accompany the reinstatement of cocaine-seeking following 2–3 weeks of extinction training (McFarland et al. 2003; Trantham-Davidson et al. 2012) and strategies that reduce glutamate efflux such as inactivation of the dmPFC (McFarland et al. 2003) attenuate cocaine-primed relapse in this model. Here we demonstrate that a similar increase in glutamate also occurs upon placement in the drug-associated context after three weeks of abstinence (Figure 3B). The standard error of mean glutamate concentration is large throughout the relapse test, but not during baseline sample collection (Figure 3B). We believe that this is due to the rise in glutamate being a consequence of placement into the drug-associated context and the cognitive effects of that placement, as opposed to a pharmacological stimulus administered at the same time to every animal. This resulted in a rise of glutamate that began, ended and peaked at different times for each rat.
Pharmacological strategies that decrease NAc synaptic glutamate efflux release have been demonstrated to attenuate relapse in multiple animal models. For example, we previously demonstrated that chronic administration of CEF (200 mg/kg) attenuates the increase in glutamate that occurs during cocaine-induced reinstatement (Trantham-Davidson et al. 2012). Both 100 and 200 mg/kg of chronic CEF (5–7 days) has been demonstrated to reliably attenuate cue-and cocaine-induced reinstatement following extinction training (Knackstedt et al. 2010a; Sari et al. 2009), and the dose of 200 mg/kg significantly decreases cue-primed relapse following abstinence (Fischer et al. 2013). Here we demonstrate that both 100 and 200 mg/kg CEF attenuates relapse induced by placement into the drug self-administration context. N-acetylcysteine is a nutritional supplement with a similar, but not identical, mechanism of action as CEF, increasing GLT-1 and xCT (Knackstedt et al. 2010a) and reducing glutamate levels during reinstatement (Baker et al. 2003). Chronic N-acetylcysteine administered during the abstinence period after cocaine self-administration also attenuates context-primed relapse after abstinence (Reichel et al. 2011). We have demonstrated previously that MTEP (3-((2-Methyl-4-thiazolyl) ethynyl) pyridine), the allosteric antagonist of the metabotropic glutamate receptor 5, infused into the NAc attenuates abstinent-relapse (Knackstedt et al. 2014). Thus, while little work has been done to examine pharmacological strategies to decrease relapse induced solely by drug context after abstinence, the present results are in agreement with the two studies that have done so.
Interestingly, while CEF reduces glutamate efflux during cocaine-primed reinstatement (Trantham-Davidson et al. 2012), it failed to do so here during context-primed relapse. We have previously reported that CEF in combination with extinction training increases GLT-1 expression in the NAc (Knackstedt et al. 2010a; Sondheimer and Knackstedt 2011). Theoretically, the ability of CEF to up-regulate GLT-1 in the NAc could account for the attenuation of glutamate levels during cocaine-primed reinstatement following extinction training (Trantham-Davidson et al. 2012). While the present results demonstrate that CEF increases GLT-1 expression in the NAc following abstinence, similarly to following extinction training, here increases in GLT-1 expression were not sufficient to diminish glutamate efflux. A second important contributor to the increased glutamate efflux that drives cocaine reinstatement after extinction is the loss of metabotropic glutamate receptor 2/3 (mGlu2/3) function (Moussawi et al. 2011). This receptor is a Gi-coupled autoreceptor for glutamate and can dampen presynaptic glutamate release. Thus, CEF in combination with extinction training, but not abstinence, may normalize presynaptic mGluR2/3 to reduce glutamate release during reinstatement. In agreement with the idea that extinction training can influence mGlu2/3 expression, Schwendt et al. (2012) demonstrated that daily extinction sessions reversed the down-regulation of NAc and dorsal striatum mGlu2/3 observed in rats following abstinence from methamphetamine. Thus, we hypothesize that CEF in combination with extinction training, but not abstinence, may normalize presynaptic mGlu2/3 to reduce glutamate release during relapse and future experiments will target this question.
It should be noted that the increase in glutamate upon placement into the self-administration context may be either or both glial and/or neuronal in source. Approximately 60% of basal glutamate in the NAc is derived from glial sources (Baker et al., 2002), but microdialyis techniques are capable of sampling neuronal glutamate release as well (e.g. Gabriele, Pacchioni & See 2013; McFarland et al., 2003). Future experiments are necessary to determine whether the glutamate rise observed during context-primed relapse is action potential-dependent and if so, which projection to the NAc is responsible for this increase. Based on the established role of the dorsal hippocampus in mediating context-primed relapse (Fuchs et al., 2005), and the existence of glutamate projections from this region to the NAc, there is a strong possibility that glutamate release along this pathway initiates context-primed relapse.
CEF treatment reduced the expression of GluA1 in the NAc, indicating that the potential alteration of post-synaptic AMPA receptor subunit composition may underlie the ability of CEF to attenuate relapse. Conrad et al. (2008) reported that following cocaine self-administration and abstinence, surface membrane expression of the AMPA receptor subunit GluA1 increased while GluA2 did not change, resulting in an accumulation of GluA2-lacking, CP-AMPARs in the NAc. Furthermore, naspm, a selective CP-AMPAR antagonist, prevents cue-primed cocaine-seeking following abstinence when infused into the NAc (Conrad et al. 2008), indicating that blockade of these receptors will prevent relapse. Another strategy to prevent CP-AMPAR-induced relapse is by stimulating mGluR1 receptors, which decreases the amount of CP-AMPARs present in the NAc and decreases cue-primed relapse after abstinence (Loweth et al. 2014). We propose that while CEF-treated animals experience an increase in glutamate transmission upon placement into the self-administration context, CEF altered the post-synaptic AMPAR-mediated response to the elevated glutamate levels in such a way to diminish cocaine-seeking. It should be noted that Purgianto et al. (2013) found no evidence of NAc CP-AMPAR formation after a similar short-access cocaine self-administration regimen (11 days, 2 hr/day), however this report used a 40-day abstinence period as opposed to 21 days employed here. Similarly, White et al. (2015) did not observe the characteristic alterations in rectification index characteristic of CP-AMPARs in the NA shell following extinction training after 2 hr/day cocaine self-administration. However, these authors observed that intra-shell naspm attenuated cocaine-primed reinstatement and thus White et al. (2015) proposed that the reinstatement act itself may serve to insert CP-AMPARs into the membrane. As the protein measurements here (Figure 2) were conducted in animals sacrificed after a relapse test and not before (as in Purgianto et al. 2013), we may also observe changes in GluA1 expression that were due to the relapse test. When examining total protein expression in the NAc, GluA1 were found to be elevated after 1 and 90 days withdrawal from cocaine but not 30 days while GluA2 expression were increased only on Day 1 of withdrawal (Lu et al. 2003). Here at 21 days withdrawal, we see that CEF decreased total GluA1 but not GluA2 expression, leading us to hypothesize that this alteration in subunit expression leading away from a tendency toward the formation of GluA2-lacking AMPAs may contribute to the ability of CEF to attenuate relapse. One weakness of the present study is that we did not employ a cocaine-naïve group with which to compare cocaine/CEF-induced changes in protein expression and thus we cannot conclude that cocaine followed by 21 days of abstinence resulted in an increase in GluA1 expression as previously demonstrated after 90 days of abstinence (Lu et al. 2003).
Previous research has reported that administration of CEF during extinction training increases GLT-1 expression in the PFC (Sari et al. 2009). We found no evidence of GLT-1 up-regulation in the PFC when CEF is administered during abstinence. These results highlight neurobiological changes that can be dependent on the type of relapse model chosen, in this case either abstinence or extinction. These findings indicate that CEF treatment, in combination with extinction training, increases PFC and NAc GLT-1 expression that in accordance with other neuroadaptations produced by extinction is sufficient to decrease reinstatement-driven glutamate efflux.
In conclusion, the data presented here indicate that while CEF attenuates context-primed relapse after abstinence, CEF treatment alone is not sufficient to normalize glutamate efflux following abstinence from cocaine. We suggest that the full therapeutic potential of CEF treatment is engaged by behavioral and neuronal changes induced by extinction training. Thus, extinction training in combination with CEF may represent the most effective “treatment” to attenuate cocaine relapse and ameliorate cocaine-induced neuroadaptations. Extinction-based therapies (e.g. cue exposure) have been demonstrated to be ineffective at reducing the risk of relapse in human addicts (for review see Tiffany & Conklin, 2002). However, this may be due to the fact that, unlike in animal studies, human extinction sessions take place outside the drug-taking context (for review see Perry et al., 2014). Accordingly, a movement towards conducting extinction training in combination with virtual reality reconstruction of the drug-taking environment is underway (e.g. Garcia-Rodriguez et al., 2002). We further propose that the efficacy of extinction therapy in humans may be enhanced by a pharmacotherapy such as CEF. In the future, we will examine whether the ability of CEF to reduce glutamate efflux following extinction training involves upregulation of mGluR2/3 function.
Acknowledgments
Funding source
This research was supported by the National Institute of Health (NIH) Grant DA033436, awarded to LK. NIH had no role in experimental design or in the collection, analysis and interpretation of data. They also had no role in the writing of the report or in the decision to submit the paper for publication.
The authors would like to thank Lizhen Wu and Brooke Jackson for their technical contributions to the project.
Footnotes
Contributors
Dr. Knackstedt designed the experiments, analyzed relapse and western blot data, and co-wrote the manuscript. Dr. LaCrosse and Ms. Hill ran subjects through the microdialysis experiment and analyzed samples via HPLC. Dr. Lacrosse conducted statistical analysis of the HPLC data and co-wrote the manuscript with Dr. Knackstedt.
Confilict of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22(20):9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addiction Biology. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–73. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT-1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci. 2013;33:9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2004;176:459–65. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate- putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Pacchioni AM, See RE. Dopamine and glutamate release in the dorsolateral caudate putamen following withdrawal from cocaine self-administration in rats. Pharmacol Biochem Behav. 2012;103(2):373–9. doi: 10.1016/j.pbb.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodríguez O, Pericot-Valverde I, Gutiérrez-Maldonado J, Ferrer-García M, Secades-Villa R. Validation of smoking-related virtual environments for cue exposure therapy. Addict Behav. 2012;37(6):703–8. doi: 10.1016/j.addbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9(1):59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010a;67(1):81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010b;30(23):7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addiction Biology. 2014;19(1):87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85(6):1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1–2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behavioural Pharmacology. 1996;7(8):745–763. [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences. 2011;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic/Elsevier; Amsterdam: 2007. [Google Scholar]
- Perry CJ, Zbukvic I, Kim JH, Lawrence AJ. Role of cues and contexts on drug-seeking behaviour. Br J Pharmacol. 2014;171(20):4636–72. doi: 10.1111/bph.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology. 2013;38(9):1789–1797. doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2(2):184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;37(2):487–93. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29(29):9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, See RE. Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self- administration in rats. PLoS One. 2012;7(3):e34299. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194(3):321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue-and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225(1):252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, et al. Optogenetic inhibition of cocaine seeking in rats. Addiction Biology. 2013;18(1):50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32(36):12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SL, Ortinski PI, Friedman SH, Zhang L, Neve RL, Kalb RG, Schmidt HD, Pierce RC. A Critical Role for the GluA1 Accessory Protein, SAP97. Cocaine Seeking Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.199. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29(12):3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]