Abstract
Progenitor cells in the adult pancreas are potential sources of endocrine beta cells for treating type 1 diabetes. Previously, we identified tri-potent progenitor cells in the adult (2–4 month-old) murine pancreas that were capable of self-renewal and differentiation into duct, acinar, and endocrine cells in vitro. These progenitor cells were named pancreatic colony-forming units (PCFUs). However, because PCFUs are a minor population in the pancreas (~1%) they are difficult to study. To enrich PCFUs, strategies using cell-surface marker analyses and fluorescence-activated cell sorting were developed. We found that CD133highCD71low cells, but not other cell populations, enriched PCFUs by up to 30 fold compared to the unsorted cells. CD133highCD71low cells generated primary, secondary, and subsequent colonies when serially re-plated in Matrigel-containing cultures, suggesting self-renewal abilities. In the presence of a laminin hydrogel, CD133highCD71low cells gave rise to colonies that contained duct, acinar, and Insulin+Glucagon+ double-hormonal endocrine cells. Colonies from the laminin hydrogel culture were implanted into diabetic mice, and five weeks later duct, acinar, and Insulin+Glucagon− cells were detected in the grafts, demonstrating tri-lineage differentiation potential of CD133highCD71low cells. These CD133highCD71low cells will enable future studies of putative adult pancreas stem cells in vivo.
Keywords: 4. Key words: Pancreatic colony-forming units, Fluorescence-activated cell sorting (FACS), Differentiation, Self-renewal, Heterogeneity of ductal cells
INTRODUCTION
The pancreas plays a critical role in regulating metabolism and is composed of three major cell lineages: acinar cells, which secrete digestive enzymes such as amylase; duct cells, which transport digestive enzymes into the gut and secrete mucin to fend off pathogens; and endocrine cells, which secrete hormones such as insulin and glucagon that are important for glucose homeostasis.
Pancreas development follows a sequence of events regulated by transcription factors. Around embryonic day (E) 8.5, murine pancreatic endoderm expresses critical transcription factors, such as pancreas duodenal homeobox (Pdx) 1 and Sox9. Between E8.5 and E12.5, Pdx1+ and Sox9+ ductal cells are multi-potential and capable of giving rise to all three lineages in the adult pancreas [1–3]. After E12.5, the lineage potential of the ductal epithelium becomes restricted [1–3], and the up-regulation of another key transcription factor, neurogenin (Ngn) 3 is necessary for subsequent differentiation into endocrine cells [4, 5].
There has been an intense debate about whether the adult pancreas harbors stem and progenitor cells that can give rise to new insulin-producing beta cells. Because of their roles as progenitors in the early embryo, duct cells in the adult pancreas have long been the assumed source of new beta cells [6]. However, in mouse models using Cre-Lox lineage-tracing techniques, contradictory results either positively [7, 8] or negatively [3, 9–13] implicate ducts as the source of progenitor cells in the adult pancreas. In addition to ducts, cells expressing markers for acinar (Ptf1a [14, 15], carboxypeptidase A [16], or elastase [15]) or endocrine cells (insulin [9] or glucagon [17]) have been shown to give rise to new beta cells in the adult murine pancreas. Together, these results suggest that there are multiple mechanisms by which beta cells can be replenished from different cellular sources in the adult pancreas. However, the aforementioned in vivo lineage-tracing studies did not directly address properties of multi-potentiality and self-renewal—two criteria necessary to define a stem cell.
To investigate progenitor cells in the adult pancreas, we and others have turned to in vitro single cell analysis. We designed unique 3D colony assays that allow quantitative analyses of single progenitor-like cells from adult murine pancreas. Using these assays, we found that the adult murine pancreas contains tripotent progenitor cells that are capable of multi-lineage differentiation and robust self-renewal in vitro [18–20]. These progenitor-like cells were named “pancreatic colony-forming units” (PCFUs). Consistent with our findings, other laboratories confirmed that dissociated single cells from the adult pancreas of mice [21, 22] and humans [23] can be propagated in high concentrations (>33% vol/vol) of Matrigel in vitro where they generate cystic “organoid” colonies similar to what we observed [18–20]. Together, these results demonstrate that single cells with the capacities for self-renewal and multi-lineage differentiation in vitro are present in the adult pancreas. However, these PCFU progenitor cells remain poorly characterized.
One major roadblock to effective characterization of PCFUs is that they constitute a minor population in the adult pancreas (~1% in 2–4 month-old mice) [18, 20]. Thus, purification of these progenitor cells is needed. Previously, a transgenic mouse model was used for enrichment of PCFUs [18]. In this mouse model, expression of enhanced green fluorescence protein (EGFP) reporter was driven by Sox9 regulatory loci (75 kb upstream and 150 kb downstream sequences) in a CD1 out-bred background [24]. However, this strategy prohibits enrichment of PCFUs from other mouse models. Therefore, identification of cell surface markers expressed by PCFUs could lead to an alternative enrichment method. CD133 (prominin 1) is a cell-surface marker commonly used to enrich various stem cells from adult tissues [25], and adult pancreatic ducts of humans and mice express CD133 [26–29]. Our prior work demonstrated that CD133+, but not CD133− pancreatic cells from adult C57Bl/6 (B6) mice, contained PCFUs [18, 20]. However, only one in about twenty pancreatic CD133+ cells was a PCFU [20], consistent with a recent report [22].
The goal of this study was to identify an additional cell-surface marker that, when combined with CD133, could further distinguish and enrich PCFUs. Cell surface markers that were previously known to enrich non-pancreas progenitor cells, including CD71 (transferrin receptor), were screened. CD71 transports iron from the extracellular space into cells, and higher levels of CD71 expression are detected in erythroid progenitor cells [30] as well as in some cancer cells [31]. The adult pancreas expresses CD71 [32]; however, its role in the pancreas has not been characterized. Here, we report that CD71 expression status fractionates pancreatic CD133+ ductal cells in adult mice. Among the subpopulations, CD133highCD71low cells are the most enriched for PCFUs, and approximately one in three CD133highCD71low cells is a PCFU. This enriched population will enable further studies on putative pancreas stem and progenitor cells in vivo.
RESULTS
Expression of CD133 and CD71 in the adult murine pancreas
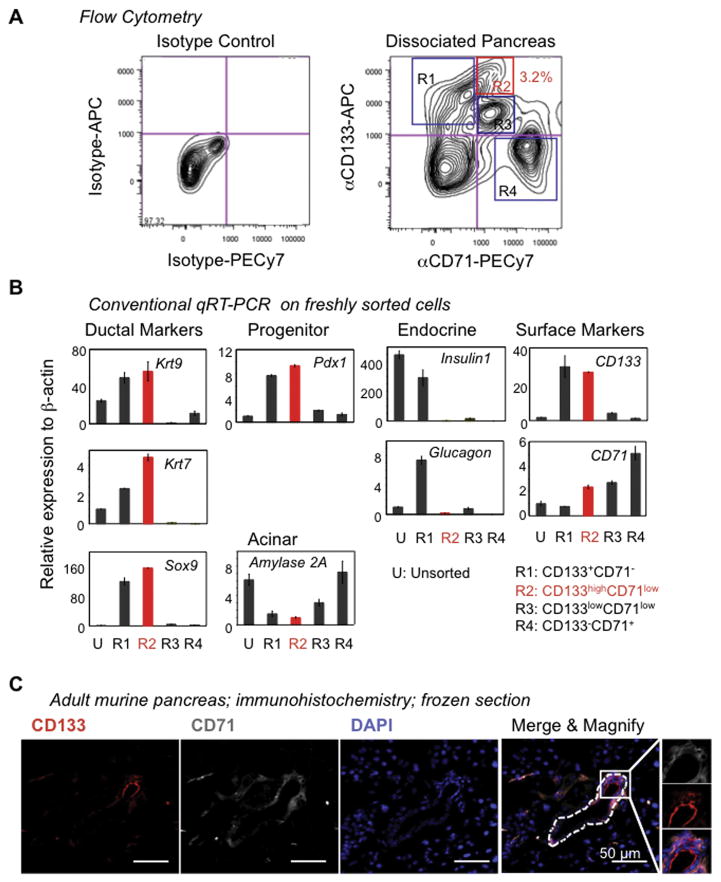
CD133 is expressed by the ductal cells of the adult pancreas [26–29]. We used flow cytometry analysis to screen other known cell-surface markers to determine their ability to fractionate adult CD133+ pancreatic cells. CD71 was found to divide CD133+ ductal cells into three major subpopulations: CD133+CD71−, CD133highCD71low, and CD133lowCD71low cells, as indicated by regions (R) 1 to 3, respectively (Fig. 1A), suggesting heterogeneity of ductal cells.
Figure 1. CD133 and CD71 are expressed in the adult murine pancreas.
(A) Flow cytometry analyses of dissociated pancreatic cells stained with antibodies against CD133 and CD71. CD71 sub-fractionates CD133-expressing ductal cells, suggesting heterogeneity of ductal cells. (B) Regions (R) 1 to 4 indicated in (A) were analyzed for gene expression by conventional qRT-PCR. (C) Double immunohistochemical staining on frozen sections showed that CD133 and CD71 expression was located mostly in the ductal structures (outlined by a dotted line) in the adult murine pancreas. (D) Immunohistochemical staining on adjacent slides prepared from formalin-fixed, paraffin-embedded pancreas. Both primary antibodies for Sox9 and CD71 were rabbit antibodies. Results showed that Sox9+ ductal regions were also positive for CD71 staining. (E) Double immunostaining on frozen sections showed that Pdx1 protein was expressed in the CD133+ ductal cells with various intensities. The yellow arrow indicates a CD133+ duct cell expressing a higher level of Pdx1 than other duct cells. (F) Representative genes identified from RNA-seq analyses which are significantly up-regulated in R2 versus R1 cells.
Quantitative (q) RT-PCR analysis of freshly sorted cells indicated that cells in both R1 and R2 expressed higher levels of ductal genes (Krt19, Krt7 and Sox9) and Pdx1 compared to unsorted cells (Fig. 1B). Compared to R2, R1 cells expressed higher levels of endocrine markers, Insulin1 and Glucagon (Fig. 1B). Because the lower limit of the R1 gate was close to the double negative cells, some endocrine cells may have been contaminated during sorting. In the subsequent RNA-seq experiments (see below), the lower boundary of the sorting gate for R1 was moved upward, and as a result no difference in the expression of Insulin1 and Glucagon between R1 and R2 cells was observed (Supplementary Table 1). R3 cells did not express significant levels of the aforementioned pancreatic lineage markers (Fig. 1B).
To further confirm the expression of CD71 in the ducts, immunohistochemical staining on sections of adult murine pancreas was performed. As expected, CD133 was expressed in the ductal structures (Fig. 1C; dotted line). CD71 was found to co-localize with CD133 in the ducts (Fig. 1C). For additional confirmation, ductal cells that expressed Sox9 also stained positive for CD71 (Fig. 1D), again demonstrating that CD71 was expressed in the ductal region.
Pdx1 protein was detected in the CD133+ ductal cells by double immunostaining analyses (Fig. 1E), although the overall intensities of Pdx1 was lower in ducts compared to that in the islet cells, which are known to express Pdx1 [34]. Interestingly, the staining intensities for Pdx1 or CD133 varied among individual ductal cells (Fig. 1E), again suggesting heterogeneity of ductal cells.
To further characterize R1 and R2 ductal cells, genome-wide gene expression analysis using RNA-seq was performed (Supplementary Fig. 1A). Compared to R1, R2 cells expressed higher levels of several ductal and epithelial cell markers including Krt19 (Fig. 1F), consistent with the above qRT-PCR analysis (Fig. 1B). R2 cells expressed higher levels of cyclin A2, a positive regulator of cell cycle gene (Fig. 1F), suggesting that R2 cells may be ready for proliferation and forming colonies. Indeed, R2 but not R1 ductal cells gave rise to colonies in culture (see below). Pathway analysis of the RNA-seq data revealed that R2 and R1 cells have propensities for cell migration and immune response, respectively (Supplementary Fig. 1B). Taken together, these results demonstrate that, unlike the Sox9/EGFP mice used in our previous study [18], CD71 expression status fractionates pancreatic CD133+ ductal cells into subpopulations in adult mice and that ductal cells are heterogeneous.
Most CD133− cells expressed CD71 at higher levels (Fig. 1A; R4) or at undetectable levels (double negative cells). Approximately 80% of CD133−CD71high (R4) cells expressed TER119 (Supplementary Fig. 2), an erythroid lineage-specific marker [33], suggesting that R4 contains mostly red blood cells or their immediate precursors. Red blood cells were lysed before antibody staining and flow cytometry analysis. This manipulation resulted in a reduced percentage of CD133−CD71high (R4) cells (Supplementary Fig. 3), further confirming that R4 contains red blood cells.
Murine adult pancreatic CD133highCD71low (Region 2) cells are most enriched for colony-forming progenitors
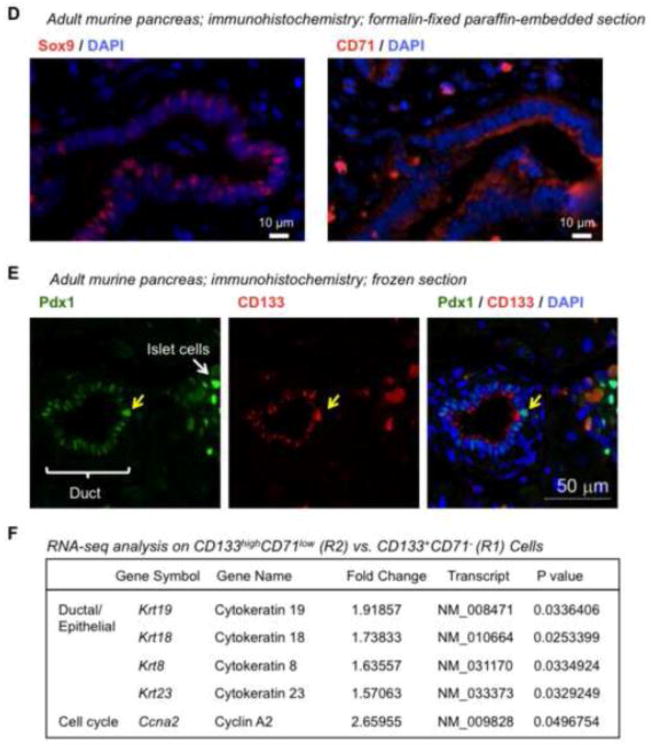
Previously, we devised two pancreatic colony assays, one containing Matrigel and the other a laminin hydrogel, which support the formation of Ring or Endocrine/Acinar colonies, respectively (Supplementary Fig. 4) [18]. These colony types are morphologically distinct. Ring colonies are cystic and contain mostly ductal-like cells [18, 20]; whereas, Endocrine/Acinar colonies are small and compact, composed mostly of endocrine- and acinar-like cells and fewer ductal cells [18]. A single progenitor cell capable of giving rise to a Ring or an Endocrine/Acinar colony is designated as a PCFU-Ring or a PCFU-Endocrine/Acinar, respectively.
Next, we tested which subpopulations contained PCFUs-Ring or PCFUs-Endocrine/Acinar (Fig. 2). Freshly sorted cells were plated into Matrigel or laminin hydrogel colony assays, and the resulting colonies were counted. CD133−CD71− cells did not form Ring or Endocrine/Acinar colonies, consistent with our previous studies showing that CD133− cells are devoid of PCFUs [18, 20]. In contrast, after three weeks in Matrigel colony assay, a plating of 2,500 CD133highCD71low (R2) cells resulted in 816 ± 19 Ring colonies (Fig. 2A & B), demonstrating that 32 ± 1% of the CD133highCD71low cells were PCFUs-Ring (Fig. 2C).
Figure 2. The CD133highCD71low subpopulation is most enriched for colony-forming progenitor cells.
(A & D) Photomicrographs of representative Ring (A) or 1° Endocrine/Acinar (D) colonies viewed under a phase-contrast microscope with visible light illumination. (B & C) Sorted R1 to R4 cells, as indicated in Fig. 1A, were plated into the Matrigel colony assay. After 3 weeks, the resulting Ring colonies were counted and the colony-forming efficiency (number of colonies divided by the total number of cells plated) determined. Data represent mean and standard deviation from triplicate wells. (E & F) Same as in B & C, except that sorted cells were plated in the laminin-hydrogel colony assay. Endocrine/Acinar colonies were counted 10 days post-plating.
In our previous studies, Endocrine/Acinar colonies formed when freshly-sorted CD133+Sox9/EGFP+ cells were first expanded in Matrigel for 3 weeks to generate Ring colonies, which were subsequently dissociated and re-plated into laminin hydrogel for 10 days [18]. These Endocrine/Acinar colonies grown in the secondary laminin-hydrogel culture were designated as secondary (2°) Endocrine/Acinar colonies. Similarly, 2° Endocrine/Acinar colonies formed after Ring colonies derived from CD133highCD71low cells were dissociated and re-plated into laminin hydrogel (Supplementary Fig. 5). When sorted CD133highCD71low cells were directly plated into the laminin-hydrogel assay at 25,000 cells per well, 256 ± 18 Endocrine/Acinar colonies were detected (Fig. 2D & E). These Endocrine/Acinar colonies grown in the primary laminin-hydrogel culture were designated as primary (1°) Endocrine/Acinar colonies. The colony-forming efficiency of CD133highCD71low (R2) cells for 1° Endocrine/Acinar colonies was 1 ± 0.1% (Fig. 2F). In contrast, R1, R3 and R4 cells did not have significant colony-forming capacity compared to unsorted cells (Fig. 2B & E). Together, these results demonstrate that both PCFUs-Ring and 1° PCFUs-Endocrine/Acinar progenitors are enriched in the CD133highCD71low (R2) cell fraction. The CD133highCD71low (R2) cells represented 2.4 ± 1.9% (n=7; ranges 0.6 – 6.0%) of total dissociated pancreatic cells.
Ring colonies derived from the CD133highCD71low (R2) cells are ductal-like, express progenitor markers, and contain self-renewing colony-forming cells
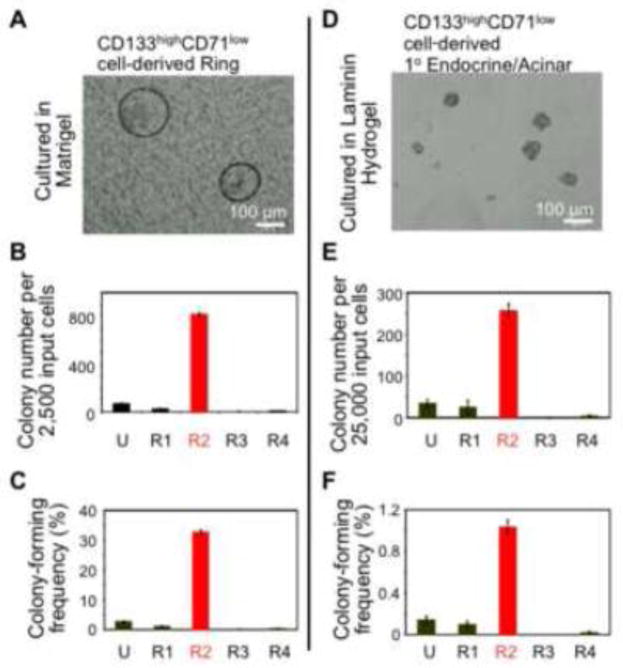
To test the lineage compositions of individual Ring or 1° Endocrine/Acinar colonies, microfluidic qRT-PCR analyses were performed (Fig. 3A & B). Microfluidic qRT-PCR has a reaction volume in the nano-liter range, permitting gene expression analysis from a single colony [18, 35]. Colonies were individually handpicked under direct microscope visualization (n=21 per group). Expression of a panel of genes indicative of pancreatic progenitor, ductal, endocrine, and acinar lineages was analyzed.
Figure 3. CD133highCD71low cell-derived Ring or 1° Endocrine/Acinar colonies express higher levels of duct and progenitor or endocrine and acinar cell markers, respectively.
(A & B) Microfluidic qRT-PCR analyses on individually handpicked colonies. The results in (A) are expressed as heat maps; whereas, (B) shows expression levels relative to β-actin. Each column or bar represents a single colony.
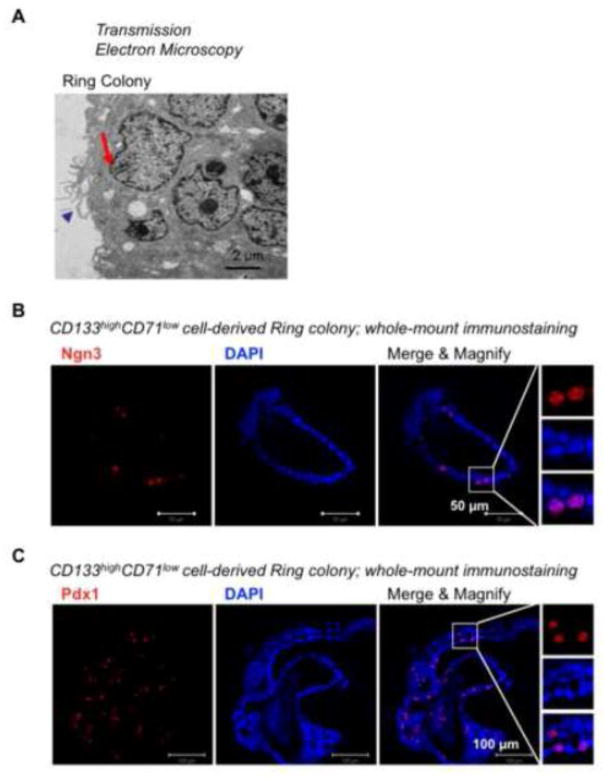
Individual Ring colonies expressed higher levels of ductal (Sox9 and Muc1) and progenitor cell markers (Ngn3 and Pdx1) (Fig. 3A & B), compared to 1° Endocrine/Acinar colonies. Transmission electron microscopy revealed multi-lobed nuclei (arrow) and microvilli (arrow head) (Fig. 4A), indicating a ductal cell phenotype. Whole-mount immunostaining analysis confirmed protein expression of Ngn3 and Pdx1 in Ring colonies derived from CD133highCD71low (R2) cells (Fig. 4B & C; nucleus staining). These results are consistent with our prior findings that Ring colonies, derived either from CD133+Sox9/EGFP+ from CD1 outbred mice [18] or CD133+ cells from B6 inbred mice [20], are predominantly ductal-like cells that express progenitor cell markers.
Figure 4. Cells in Ring colonies display ultra-structures consistent with ductal phenotype, and some cells express progenitor markers.
(A) Transmission electron microscopy of a Ring colony derived from CD133highCD71low cells. Arrow indicates indentation of the nuclear membrane. Arrow head indicates microvilli. These fine structures are ductal characteristics. (B & C) Whole-mount immunostaining of pooled Ring colonies showing protein expression of Ngn3 (B) and Pdx1 (C).
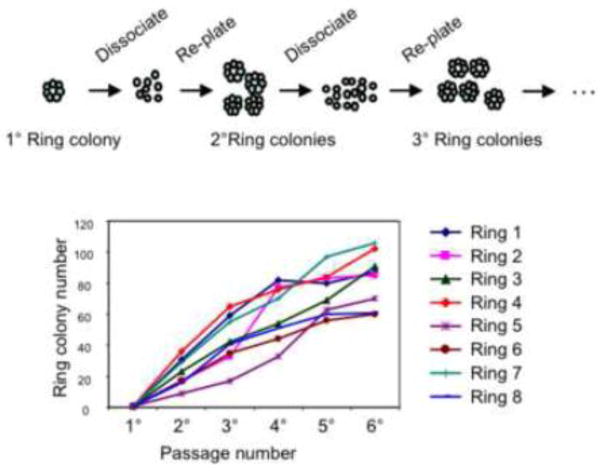
Three-week-old Ring colonies continued to express CD133 and CD71 (Fig. 3A & B), suggesting that the originating CD133highCD71low (R2) cells may have either given rise to ductal cells or self-renewed as progenitors. To test whether the freshly sorted CD133highCD71low (R2) cells could self-renew, 3-week-old primary Ring colonies (n=8) were individually handpicked, dissociated, and re-plated into Matrigel assays for a total of six passages (Fig. 5). All eight primary Ring colonies initiated secondary and subsequent colonies (Fig. 5), indicating that single PCFUs-Ring contained in the CD133highCD71low (R2) population were capable of self-renewal in vitro.
Figure 5. Single PCFU-Ring self-renews and generates secondary and subsequent Ring colonies in vitro.
Upper panel: A schematic representation of a re-plating experiment. Sorted CD133highCD71low cells were plated into Matrigel-containing culture. Three weeks later, a primary Ring colony was hand-picked, dissociated into single cell suspension, and re-plated into Matrigel-containing culture for a total of 6 passages. Lower panel: Serial re-plating of an individually hand-picked primary Ring colony (n=8) generated secondary and subsequent Ring colonies in Matrigel-containing culture.
Primary “Endocrine/Acinar” colonies derived from the CD133highCD71low population express higher levels of endocrine and acinar markers, and lower levels of ductal markers compared to Ring colonies
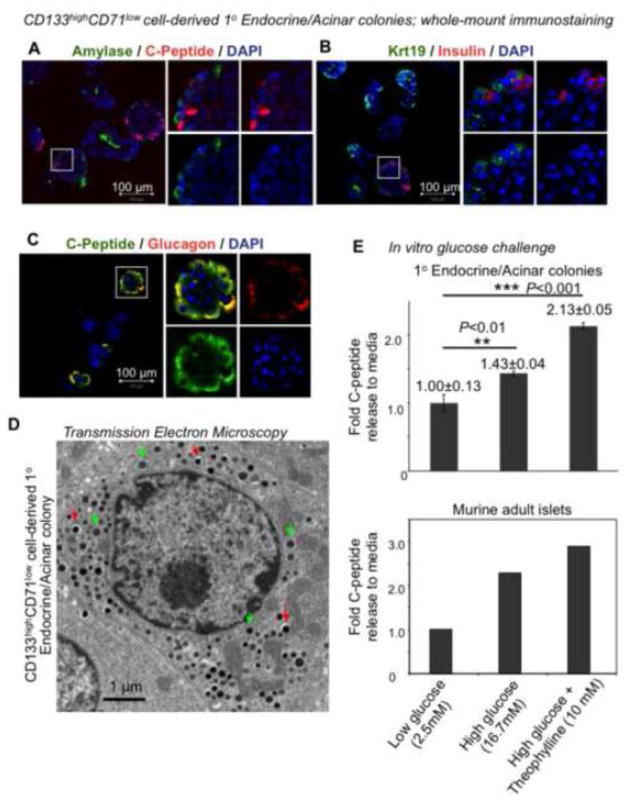
In contrast to Ring colonies, 1° Endocrine/Acinar colonies expressed higher levels of endocrine (Insulin1, Insulin2, Glucagon) and acinar (Amylase 2A) genes, and lower levels of ductal (Sox9, Mucin1) genes in single-colony RT-PCR analyses (Fig. 3A & B). Protein expression of Amylase (an acinar marker) (Fig. 6A) in whole-mounted 1° Endocrine/Acinar colonies was detected but did not co-localize with C-peptide+ cells (a surrogate marker for de novo synthesized insulin). Similarly, Krt19-expressing ductal cells did not co-stain with insulin (Fig. 6B). Surprisingly, double immunostaining of glucagon and C-peptide in 1° Endocrine/Acinar colonies revealed that cells expressing C-peptide were all positive for glucagon (Fig. 6C; yellow color). This is in contrast to our prior results on the 2° Endocrine/Acinar colonies that express single hormones [18]. Transmission electron microscopy showed both insulin-like and non-insulin granules within cells in CD133highCD71low (R2)-derived 1° Endocrine/Acinar colonies (Fig. 6D), further confirming the double-hormonal phenotype.
Figure 6. Primary Endocrine/Acinar colonies express acinar, ductal, and endocrine markers and are glucose responsive in vitro.
(A–C) Whole-mount immunostaining of 10-day-old 1° Endocrine/Acinar colonies. Note that C-peptide-expressing cells also express glucagon. (D) Transmission electron microscopy of a cell in a 1° Endocrine/Acinar colony that contained two types of granules. Red and green arrows indicate insulin-like and non-insulin granules, respectively. (E) Primary Endocrine/Acinar colonies were pooled and subjected to sequential in vitro incubation with designated levels of D-glucose and/or theophylline, a cAMP potentiator. Murine adult islets are positive controls.
CD133highCD71low (R2)-derived 1° Endocrine/Acinar colonies expressed higher levels of several beta cell maturation markers, such as Glut2, Glucokinase, Pcsk1 and Pcsk2, compared to Ring colonies (Fig. 3A & B). Therefore, we tested whether these 1° Endocrine/Acinar colonies were glucose responsive in C-peptide secretion in vitro (Fig. 6E). Pooled (~1,000) 1° Endocrine/Acinar colonies released 1.43-fold more C-peptide into the media when presented with 16.7 mM compared to 2.5 mM D-glucose (Fig. 6E). When colonies were bathed with 16.7 mM D-glucose plus 10 mM theophylline (a cAMP potentiator), 2.13-fold more C-peptide was released (Fig. 6E). Intracellular C-peptide levels in these colonies (0.65±0.32 μg/g proteins) were ~3,700 fold lower than that in adult murine islets (2,427±1,395 μg/g proteins). Thus, despite the simultaneous expression of insulin and glucagon as well as the low intracellular C-peptide levels, CD133highCD71low (R2)-derived 1° Endocrine/Acinar colonies are functional in C-peptide secretion in vitro.
Primary Endocrine/Acinar colonies give rise to ductal, acinar, C-Peptide+Glucagon−, and C-Peptide−Glucagon+ cells in vivo
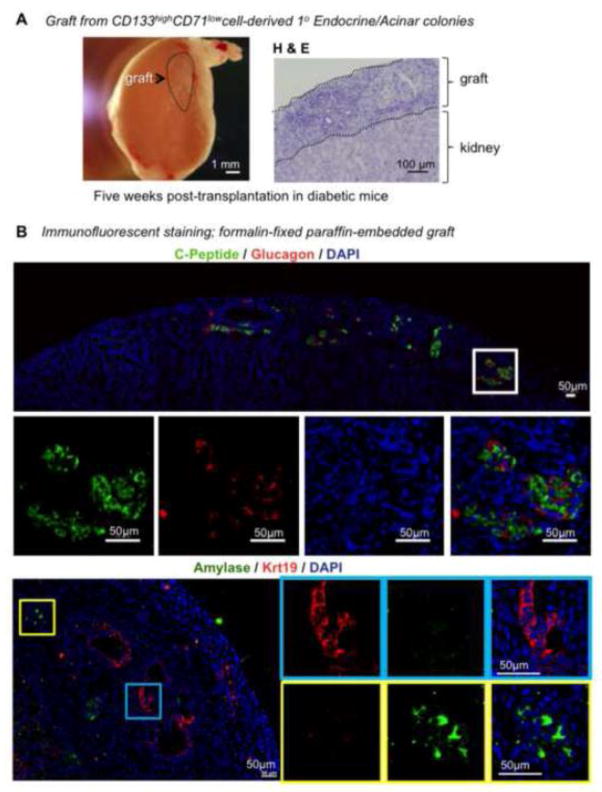
Attempts to dissociate and re-plate 1° Endocrine/Acinar colonies into 2° laminin hydrogel culture did not result in the formation of 2° Endocrine/Acinar colonies. In the positive control experiment, dissociated Ring colonies did form 2° Endocrine/Acinar colonies. These results suggest that the current laminin hydrogel culture condition may not support the development of the 2° Endocrine/Acinar colonies from the 1° Endocrine/Acinar colonies. To test whether an in vivo environment may allow further development of pancreatic lineages, 10-day-old 1° Endocrine/Acinar colonies were placed underneath the renal capsules of streptozotocin-induced diabetic mice (~12,000 colonies per kidney; n=2). The grafts were examined 5 weeks post-transplantation (Fig. 7A). By week 5, cells were either insulin+glucagon− or insulin−glucagon+ as shown by immunohistochemical staining (Fig. 7B). In addition, Krt19+ ductal cells and Amylase+ acinar cells were also detected in the grafts (Fig. 7B). The ductal-like cells were most likely originated from donor cells because they were located inside the grafts (Fig. 7A, H&E staining and Fig. 7B, blue box); however, further confirmation is required because kidney tubules are known to express KRT19 [36]. Taken together, these results demonstrate that 1° Endocrine/Acinar colonies derived from the CD133highCD71low population have tri-lineage differentiation potential in vivo and are capable of giving rise to insulin+glucagon− cells.
Figure 7. Primary Endocrine/Acinar colonies give rise to acinar, ductal, insulin+glucagon−, and insulin−glucagon+ cells in diabetic mice.
(A) Left: Photomicrograph of a kidney 5 weeks after transplantation of 12,000 1° Endocrine/Acinar colonies. Right: Hematoxylin & Eosin (H & E) staining of the graft. (B) Double immunohistochemical staining of the graft with antibodies against C-peptide and Glucagon or Amylase and Krt19.
DISCUSSION
We report that sorting for CD133highCD71low cells enriches PCFU progenitors that form Ring or 1° Endocrine/Acinar colonies in Matrigel or laminin hydrogel, respectively. The enrichment of these PCFUs is achieved using a strategy that has been successful in purifying adult hematopoietic stem cells (HSCs) [37]. Murine HSCs are often designated as lineage negative (Lin−), indicating that they do not express the cell-surface markers CD4, CD8, B220, Mac-1, Gr-1, or TER119 for differentiated blood cells [37]. Additional markers, including stem cell antigen (Sca-1) [37], CD117 (c-Kit) [33], CD48, and CD150 [38], have been identified to either positively or negatively select HSCs. About one in two Lin−Sca-1+c-kit+CD48−CD150+ bone marrow cells is a HSC [38].
CD133 has been shown to positively enrich progenitors from embryos and neonates of murine [26, 28, 29, 39] and human [29] pancreas, as well as from adult human pancreas [23]. We [20] and others [22] have previously shown that pancreatic progenitor cells from adult mice can be enriched by CD133 selection. However, the cell culture techniques reported by others were more difficult to enumerate colonies. Cells were mixed in high concentrations (>33% vol/vol) of Matrigel and placed only at the edge of the culture well. After solidification, the Matrigel was thicker at the periphery than towards the center of the culture dish. When viewed under a phase-contrast light microscope, the images of the resulting colonies were largely overlapping, preventing effective counting. Quantification of colony was achieved by sparse organoid plating or single cell deposition methods [22], which were labor-intensive and resource-consuming. In contrast, the methylcellulose-containing semi-solid media that we used allowed us to evenly spread Matrigel and colonies across the culture well. In addition, a grid was attached underneath the culture well to further facilitate accurate numeration by avoiding double counting [40]. Using our quantitative assays, we find that approximately one in three adult pancreatic CD133highCD71low cells is a PCFU-Ring (Fig. 2C), compared to ~1 in 100 pre-sorted pancreatic cells [18, 20] (Fig. 2C), which translates to a 33-fold enrichment. The efficiency of formation of Ring colonies from CD133highCD71low cells has now reached ~30% (Fig. 2C), compared to ~5% from CD133+ selection alone in B6 mice [20] and ~15% from CD133+Sox9/EGFP+ cells from CD1 mice [18]. Importantly, the use of commercially-available antibodies rather than transgenic mice will enable the characterization of adult PCFUs in many laboratories.
The efficiency of formation of 1° Endocrine/Acinar colonies from unsorted pancreatic cells is only ~0.1% (Fig. 2F). CD133highCD71low cells enrich 1° PCFUs-Endocrine/Acinar about 8-fold compared to unsorted cells (Fig. 2F), and the colony-forming efficiency for 1° Endocrine/Acinar colonies from CD133highCD71low cells reaches ~1% (Fig. 2F).
As mentioned, Ring colonies in our previous studies developed from sorted CD133+ [20] or CD133+Sox9/EGFP+ cells [18]. Consistent with the characteristics of those colonies, the Ring colonies derived from CD133highCD71low cells also express higher levels of ductal (Fig. 3B) and pancreatic progenitor cell markers, such as Pdx1 or Ngn3 (Fig. 3B), compared to 1° Endocrine/Acinar colonies. Similar to previous results [20], cells from a handpicked Ring colony derived from CD133highCD71low cells generate secondary and subsequent Ring colonies over six serial passages in Matrigel (Fig. 5), demonstrating the capacity for self-renewal of the originating, single PCFUs-Ring.
A new observation from the current study is that cells in 10-day-old 1° Endocrine/Acinar colonies grown in laminin hydrogel express insulin and glucagon simultaneously (Fig. 6C&D). This is in contrast to the insulin+glucagon− or insulin−glucagon+ cells in the 2° Endocrine/Acinar colonies that we identified in a previous report [18]. Previously, freshly sorted CD133+Sox9/EGFP+ cells were initially plated into the Matrigel assay, with or without exogenous R-spondin1, a Wnt signaling agonist, for 3 weeks to generate Ring colonies. When Ring colonies were collected and dissociated into single cell suspension and re-plated into laminin hydrogel for an additional 10 days, the 2° Endocrine/Acinar colonies that formed expressed either insulin or glucagon [18]. These results suggest that our former two-step culture method, in which the PCFUs were expanded prior to the induction of endocrine differentiation, permitted more time for the progenitor cells to develop and mature into single hormonal positive cells. The 1° Endocrine/Acinar colonies derived from freshly sorted CD133+Sox9/EGFP+ cells were not examined in the previous study [18] because of the low incidence of 1° Endocrine/Acinar colonies.
Five weeks post-transplantation into diabetic mice, CD133highCD71low cell-derived, 10-day-old 1° Endocrine/Acinar colonies gave rise to insulin+glucagon− and insulin−glucagon+ cells as well as ductal and acinar cells (Fig. 7). These results demonstrate that CD133highCD71low cells are progenitor cells with tri-lineage differentiation capacity. An in vivo study reported by Thorel et al. [17] showed that severe beta-cell injury (>99% destruction) led to the formation of insulin+glucagon+ cells, followed by the appearance of insulin+glucagon− cells. Because the regenerated beta cells were lineage-traced from glucagon-expressing cells, it was concluded that alpha cells trans-differentiated into beta cells [17]. It is possible that CD133highCD71low cells described in this study differentiated into insulin+glucagon− cells in vivo via an insulin+glucagon+ intermediate cell stage in vitro. However, further lineage tracing experiments are needed to test this hypothesis.
In summary, we report that adult pancreatic progenitor cells from B6, non-transgenic mice can be highly enriched using two cell-surface markers and live-cell sorting. Approximately 30 and 10-fold enrichment of PCFUs-Ring and 1° PCFUs-Endocrine/Acinar, respectively, in CD133highCD71low cells were achieved compared to unsorted cells. It should be noted that only in vitro self-renewal and multi-lineage differentiation by the CD133highCD71low cells are demonstrated in the current study, and these results do not prove that progenitor cells exist in vivo in the adult pancreas. Further confirmation will require examining multi-lineage reconstitution and self-renewal of CD133highCD71low cells transplanted into an animal model with a severely injured pancreas. Finally, the results presented in this study have direct implications for in vitro generation of glucose-responsive beta-like cells to treat diabetes patients.
MATERIALS AND METHODS
Mice
C57BL/6J (donors; 2–4 months old) (The Jackson Laboratory, Bar Harbor, ME, USA) and NOD-SCID (recipients; 2 months old) (Charles River Laboratory, Wilmington, MA, USA) were used in this study. All mice were maintained under specific pathogen-free conditions, and animal experiments were conducted according to the Institutional Animal Care and Use Committee at the City of Hope.
Preparation of Single Cell Suspensions
Murine pancreata were dissected, cleared of fat tissue under a dissecting microscope, and rinsed three times in cold DPBS containing 0.1% bovine serum albumin, penicillin, and streptomycin (PBS/BSA). Whole pancreata, in a dry petri dish placed on ice, were chopped using spring scissors for approximately 2 min or until finely minced. The triturated tissue was transferred to a 15 ml conical tube, washed once, resuspended in PBS/BSA containing collagenase B (2–4 mg/ml) (Roche, Mannheim, Germany) and DNase (2000 U/ml) (Calbiochem, Darmstadt, Germany), and incubated at 37°C for 20 min to yield a mostly single cell suspension. To hasten the digestion, tissue was gently disrupted every 5–10 min using a 16G syringe needle. Cells were then washed twice in cold PBS/BSA supplemented with 2000 U/ml DNase I, which was used to prevent re-aggregation of dissociated cells, and filtered through a 40 μm nylon mesh (BD Biosciences, San Jose, CA, USA) before antibody staining. Between 3–5 million cells per pancreas can be recovered from adult B6 mice.
Flow Cytometry and Cell Sorting
Single cell suspensions were first incubated with anti-mouse CD16/32 (10 μg/ml) (BioLegend, San Diego, CA, USA) for 5 min on ice to diminish nonspecific binding. Biotin-conjugated anti-mouse CD133 (clone 13A4; 5 μg/ml) (eBioscience, San Diego, CA, USA) and phycoerythrin/Cy7 (PECy7)-conjugated anti-mouse CD71 (clone RI7217; 5 μg/ml) (BioLegend) antibodies were added to cells. Cells were incubated for 20 min on ice, washed twice, and then treated with streptavidin-labeled allophycocyanin (APC) (2 μg/ml) (BioLegend) for 15 min on ice, washed twice, and resuspended in PBS/BSA/DNase I containing DAPI (0.2 μg/ml). Control antibodies were biotin-conjugated rat immunoglobulin (Ig)G1 (5 μg/ml) (eBioscience) and PE/Cy7-conjugated rat IgG1 (5 μg/ml) (BioLegend). For Supplementary Fig. 1 & 2, fluorescein isothiocyanate (FITC)-conjugated anti-mouse TER119 (clone TER119; 5 μg/ml) (Biolegend) was added to the primary antibody mix. Flow cytometry analysis was performed on an Accuri C6 or an LSRFortessa Cell Analyzer (Becton Dickinson, San Jose, CA, USA). Acquired flow data were analyzed with software provided by Accuri or FloJo (TreeStar, Ashland, OR, USA). Cell sorting was performed on an Aria-special order research product (SORP) (Becton Dickinson). All analyses included an initial gating of forward (FSC) and side (SSC) scatter to exclude debris. In cell sorting experiments, doublets were excluded by gating out high pulse-width cells, and live cells were selected by DAPI negative staining (Supplementary Fig. 6). The purity of the sorted population was routinely > 95%.
Colony Assays
Sorted cells were resuspended at a density of 2.5×103 cells/well/0.5 ml for the Matrigel assay or 2.5×104 cells/well/0.5 ml for the laminin-hydrogel assay as described previously [18]. Culture media contained DMEM/F12 media, 1% methylcellulose (Sinetsu Chemical, Tokyo, Japan), 50% conditioned media from mouse embryonic stem cell derived pancreatic-like cells, 5% fetal calf serum (FCS), 10 mmol/l nicotinamide (Sigma, St. Louis, MO, USA), 10 ng/ml human recombinant activin B, 0.1 nmol/l exendin-4 (Sigma), and 1 ng/ml vascular endothelial growth factor-A (VEGF) (Sigma). When indicated, either 5% Matrigel or 100 μg/ml of laminin hydrogel [18] was added to media to generate Ring or Endocrine/Acinar colonies, respectively. Cells were plated in 24-well ultra-low protein-binding plates (Corning, Corning, NY, USA) and incubated in a humidified 5% CO2 atmosphere. Ring or Endocrine/Acinar colony numbers were counted 3 weeks or 10 days after plating, respectively. For serial re-plating experiments, individual Ring colonies were handpicked and dissociated into single cells by incubating with 0.25% trypsin-EDTA at 37°C for 5 min, followed by gentle pipetting. Single cells were then re-plated in the Matrigel assay as described above.
Conventional or Microfluidic Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA extraction, reverse transcription, and conventional qRT-PCR analyses were performed as previously described [18]. Duplicate samples were used in all analyses. Microfluidic qRT-PCR was performed using the BioMark™ 48.48 Dynamic Array system (Fluidigm, South San Francisco, CA, USA). Single colonies were lifted one by one from the methylcellulose-containing medium under direct microscopic visualization using a 10 μl Eppendorf pipette, collected in reaction buffer (10 μl), and pre-amplified (14 cycles) according to the manufacturer’s instructions (Fluidigm). Amplified cDNA was loaded onto a 48.48 DynamicArray using the NanoFlex integrated fluidic circuit (IFC) controller (Fluidigm). Threshold cycle (Ct), as a measure of fluorescence intensity, was determined by the BioMark PCR analysis software (Fluidigm) and expressed as a heat map or delta Ct compared to β-actin. All experiments were performed with negative (water) and positive (adult C57BL/6J pancreatic cells) controls. Taqman probes (Life Technologies, Grand Island, NY, USA) and their catalog numbers are listed in Supplementary Table 2.
Electron Microscopy
Single colonies were collected, pooled, and fixed in 2% glutaraldehyde + 0.1 M Cacodylate buffer [Na(CH3)2AsO2·3H2O; pH7.2] at 4°C overnight. Colonies were washed three times with 0.1 M Cacodylate buffer (pH 7.2), post-fixed with 1% OsO4 in 0.1 M Cacodylate buffer for 30 min, and washed three times with 0.1 M Cacodylate buffer. The samples were then dehydrated, embedded in Eponate, and polymerized at 64°C for 48 h. Ultrathin sections (70-nm thickness) were cut using a Leica ultramicrotome (Leica Biosystems, Wetzlar, Germany) with a diamond knife, transferred to 200-mesh EM grids, and stained with 2% uranyl acetate in 70% ethanol for 1 min followed by treatment with Reynold’s lead citrate for 1 min. Electron microscopy was performed on a Tecnai 12 transmission electron microscope (FEI, Hillsboro, OR, USA) equipped with an Ultrascan 2K CCD camera (Gatan, Pleasanton, CA, USA).
Immunostaining
For frozen sections, prefixed-pancreata were cryoprotected in 30% sucrose in PBS, embedded in O.C.T. (Sakura Finetek, Torrance, CA, USA), and sliced at 10 μm thickness. For paraffin-embedded formalin-fixed tissues, samples were cut to 5 μm thickness, de-waxed in xylene bath for 15 minutes, rehydrated in ethanol, and antigens retrieved by heating in a microwave oven for 10 min in 200 ml of sodium citrate buffer. Samples were incubated with blocking buffer supplemented with 5% donkey serum and 0.1% TritonX-100 at 4°C overnight before addition of primary antibodies. For whole-mount immunohistochemistry, colonies were manually picked, pooled, fixed in 4% paraformaldehyde at 4°C overnight, and incubated with blocking buffer supplemented with 5% donkey serum and 0.1% TritonX-100 at 4°C overnight. Primary antibodies were as listed in Supplementary Table 3 and detected with donkey-raised secondary antibodies conjugated to Cy3, Cy5, DyLight488, or AlexaFlour647 (Jackson Immunoresearch, West Grove, PA, USA). Images were captured on a Zeiss LSM510 META NLO Axiovert 200M inverted microscope, and figures prepared with LSM Image Browser software (Carl Zeiss, Germany).
In Vitro Glucose Challenge Assay and Intracellular C-peptide measurement
Primary Endocrine/Acinar colonies were pooled and incubated overnight with Krebs-Ringer solution (KRBH; 129 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5 mM NaHCO3, 10 mM HEPES, 0.1% bovine serum albumin) containing 10% FCS and 2.5 mM D-glucose. The next day, colonies were washed three times with KRBH containing 2% FCS and 2.5 mM D-glucose and then sequentially incubated with 2.5 mM D-glucose, 16.7 mM D-glucose, and 16.7 mM D-glucose plus 10 mM theophylline (37°C, 0.5 ml/well, 2 h). The C-peptide concentration in the buffer was measured using the murine C-peptide ELISA kit, U-Type (Shibayaji Co., Gunma, Japan), which has a detection limit of 30 pg/ml. The stimulation was expressed as the fold change of C-peptide concentrations in buffer from the second and third treatments compared to the first treatment of the same well. To measure intracellular C-peptide, cells were lysed in RIPA buffer (Sigma) and the resulting lysate was analyzed for levels of total protein by BCA protein assay (ThermoFisher Scientific, Waltham, MA, USA) and C-peptide by ELISA as described above.
In Vivo Transplantation
Primary Endocrine/Acinar colonies were handpicked, pooled, and incubated in recovery solution (BD Bioscience) for 1 h on ice. Intact colonies released from the semisolid media were washed and collected for transplantation. NOD-SCID mice (8 week-old males) were injected with streptozotocin (STZ; 50 mg/kg body weight; freshly made in 0.05 M citrate buffer, pH 4.5) for 3 consecutive days to induce diabetes [41]. Hyperglycemia was defined as >200 mg/dl and measured by OneTouch Ultra2 glucometer (LifeScan, Milpitas, CA, USA) starting 1 week after the last STZ injection. Approximately 12,000 Endocrine/Acinar colonies were placed under the renal capsule per mouse (n=2). Grafts were examined 5 weeks post-transplantation by immunohistochemical staining of formalin-fixed paraffin-embedded tissue sections.
RNA-seq analysis
Reads were aligned to the mouse genome (build mm10). Reads per kilobase per million (RPKM) [42] were calculated for RefSeq transcripts [43] using an expectation-maximization algorithm in Partek Genomics Suite (Version 6.6) (Partek, St. Louis, MI, USA) [44]. RPKM values were then further normalized via log2 transformation following the addition of a scaling factor of 0.1 [45]. Fold-change values were calculated on a linear scale based on the least-squares mean. P values were calculated by one-way ANOVA using the normalized RPKM values. False discovery rate (FDR) values were calculated using the method of Benjamini and Hochberg [46]. Transcripts were defined as differentially expressed if they showed a fold-change value higher than 1.5 and FDR lower than 0.05. Potential functional significance was assessed by Gene Ontology enrichment terms (Genomics Suite™) (Partek). Genes differentially expressed by the CD133highCD71low compared to the CD133+CD71− cells are listed in Supplementary Table 1. Data were deposited to the repository system Gene Expression Omnibus.
Statistical Analysis
All values were shown as mean ± standard deviation. Significance was determined by Student’s two-tailed t-test and P<0.05 was considered significant.
Supplementary Material
Highlights.
CD133, a cell-surface marker expressed by pancreatic ducts, enriches colony-forming progenitor cells from adult mice but at a low efficiency (~5%).
CD71 (transferrin receptor) expression status fractionates pancreatic CD133+ ductal cells into 3 major sub-populations: CD133+CD71−, CD133highCD71low, and CD133lowCD71low cells, suggesting that ductal cells are heterogeneous.
CD133highCD71low cells are most enriched for colony-forming progenitor cells with a colony-forming efficiency up to 30%.
CD133highCD71low cells are capable of self-renewal and tri-lineage differentiation into duct, acinar, and endocrine cells in vitro—these are stem cell-like behaviors.
CD133highCD71low cells give rise to insulin-expressing cells in vitro in the presence of a defined extracellular matrix protein. These insulin-expressing cells express glucagon simultaneously and secrete insulin in response to glucose.
Transplantation into diabetic mice of colonies grown in the presence of a defined extracellular matrix protein leads to the formation of duct, acinar, and endocrine (e.g., insulin+glucagon− and insulin−glucagon+) cells.
Acknowledgments
We thank Lucy Brown and Alexander Spalla from the Analytical Cytometry Core, Ricardo Zerda and Zhuo Li from the Electron Microscopy Core, and Dr. Xiwei Wu and Charles Warden from the Integrative Genomics Core of City of Hope for assistance in sorting, transmission electron microscopy, and RNA-seq analyses, respectively. We thank Dr. Maike Sander of University of California-San Diego for useful discussion. This work is supported in part by National Institutes of Health (NIH) grants R01DK081587 and R01DK099734 to H.T.K. and U01DK089533 to A.D.R., National Science Foundation grant NSF-DMR-1206121 and California Institute for Regenerative Medicine grant RB5-07398 to D.A.T., and Office of Naval Research ONR-N00014-02-1 0958 and NSF-DBI-9970143 to the Electron Microscopy Core facility at City of Hope. Support from the Joseph J. Jacobs Institute for Molecular Engineering for Medicine at Caltech is also gratefully acknowledged. L. J. is supported by National High Technology Research and Development Program of China (863 Program, No.2015AA020314), Excellent Youth Foundation of Jiangsu Scientific Committee (BK20140029), the Fundamental Research Funds for the Central Universities (Z114037), Priority Academic Program Development of Jiangsu Higher Education Institutions, and National Natural Science Foundation of China (No. 81570696).
10. Abbreviation
- PCFU
pancreatic colony-forming unit
- CD
cluster of differentiation
- R2
region 2 cells
Footnotes
7. Author Responsibilities:
Liang Jin, Dan Gao: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript.
Tao Feng, Angela Luo, Jeffery Rawson, Janine Quijano, Jing Chai, Lena Wedeken, Nadiah Ghazalli, Jasper Hsu, Jeanne LeBon, Stephanie Walker, Jacob Tremblay: acquisition of data, analysis and interpretation of data.
Alborz Mahdavi, Hung-Ping Shih; technical or material support.
David A. Tirrell, Arthur D. Riggs: technical or material support, critical revision of the manuscript for important intellectual content, obtained funding.
Hsun Teresa Ku: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtained funding.
8. Disclosure: The authors have nothing to disclose.
9. Study sponsor: The sponsor did not participate in the study design, collection, analysis, or interpretation of data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Developmental cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 5.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1111. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5(Suppl 2):16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 7.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 10.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 11.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC developmental biology. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140:751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stange G, Shemer R, Nord C, Scheel DW, Pan FC, Ahlgren U, Gu G, Stoffers DA, Dor Y, Ferrer J, Gradwohl G, Wright CV, Van de Casteele M, German MS, Bouwens L, Heimberg H. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin L, Feng T, Shih HP, Zerda R, Luo A, Hsu J, Mahdavi A, Sander M, Tirrell DA, Riggs AD, Ku HT. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A. 2013;110:3907–3912. doi: 10.1073/pnas.1301889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu X, Jin L, Wang X, Luo A, Hu J, Zheng X, Tsark WM, Riggs AD, Ku HT, Huang W. MicroRNA-26a targets ten eleven translocation enzymes and is regulated during pancreatic cell differentiation. Proc Natl Acad Sci U S A. 2013;110:17892–17897. doi: 10.1073/pnas.1317397110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin L, Feng T, Zerda R, Chen CC, Riggs AD, Ku HT. In Vitro Multilineage Differentiation and Self-Renewal of Single Pancreatic Colony-Forming Cells from Adult C57Bl/6 Mice. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, Ringnalda F, Begthel H, Hamer K, Mulder J, van Es JH, de Koning E, Vries RG, Heimberg H, Clevers H. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. Embo J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorrell C, Tarlow B, Wang Y, Canaday PS, Haft A, Schug J, Streeter PR, Finegold MJ, Shenje LT, Kaestner KH, Grompe M. The organoid-initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Res. 2014;13:275–283. doi: 10.1016/j.scr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki J, Szot GL, Bottino R, Kim SK. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940. doi: 10.7554/eLife.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1108–1118. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 26.Hori Y, Fukumoto M, Kuroda Y. Enrichment of putative pancreatic progenitor cells from mice by sorting for prominin1 (CD133) and platelet-derived growth factor receptor beta. Stem Cells. 2008;26:2912–2920. doi: 10.1634/stemcells.2008-0192. [DOI] [PubMed] [Google Scholar]
- 27.Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas. 2008;36:e1–6. doi: 10.1097/mpa.0b013e318149f2dc. [DOI] [PubMed] [Google Scholar]
- 28.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci U S A. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keysar SB, Jimeno A. More than markers: biological significance of cancer stem cell-defining molecules. Mol Cancer Ther. 2010;9:2450–2457. doi: 10.1158/1535-7163.MCT-10-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983;36:539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood. 1991;78:1706–1112. [PubMed] [Google Scholar]
- 34.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. Embo J. 1993;12:4251–4429. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, Jan T, Wilson KD, Leong D, Rosenberg J, Yao M, Robbins RC, Wu JC. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121:1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P, Young A, de Peralta-Venturina M, Amin MB. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol. 2005;29:747–754. doi: 10.1097/01.pas.0000163362.78475.63. [DOI] [PubMed] [Google Scholar]
- 37.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 38.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Ghazalli N, Mahdavi A, Feng T, Jin L, Kozlowski MT, Hsu J, Riggs AD, Tirrell DA, Ku HT. Postnatal Pancreas of Mice Contains Tripotent Progenitors Capable of Giving Rise to Duct, Acinar, and Endocrine Cells In Vitro. Stem Cells Dev. 2015 doi: 10.1089/scd.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler M, Trieu N, Feng T, Jin L, Walker S, Singh L, Ku HT. A quantitative assay for insulin-expressing colony-forming progenitors. J Vis Exp. 2011:e3148. doi: 10.3791/3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerling IC, Friedman H, Greiner DL, Shultz LD, Leiter EH. Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes. 1994;43:433–440. doi: 10.2337/diab.43.3.433. [DOI] [PubMed] [Google Scholar]
- 42.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 43.Pruitt KD, Tatusova T, Brown GR, Maglott DR. NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 2012;40:D130–135. doi: 10.1093/nar/gkr1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing Y, Yu T, Wu YN, Roy M, Kim J, Lee C. An expectation-maximization algorithm for probabilistic reconstructions of full-length isoforms from splice graphs. Nucleic Acids Res. 2006;34:3150–3160. doi: 10.1093/nar/gkl396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warden CD, Yuan YC, Wu X. Optimal calculation of RNA-seq fold-change values. Int J Comput Bioinfor In Silico Model. 2014;2:285–292. [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.